Abstract
Parasitic flatworms cause serious infectious diseases that affect humans and livestock in vast regions of the world, yet there are few effective drugs to treat them. Thioredoxin glutathione reductase (TGR) is an essential enzyme for redox homeostasis in flatworm parasites and a promising pharmacological target. We purified to homogeneity and characterized the TGR from the tapeworm Mesocestoides vogae (syn. M. corti). This purification revealed absence of conventional TR and GR. The glutathione reductase activity of the purified TGR exhibits a hysteretic behavior typical of flatworm TGRs. Consistently, M. vogae genome analysis revealed the presence of a selenocysteine-containing TGR and absence of conventional TR and GR. M. vogae thioredoxin and glutathione reductase activities were inhibited by 3,4-bis(phenylsulfonyl)-1,2,5-oxadiazole N2-oxide (VL16E), an oxadiazole N-oxide previously identified as an inhibitor of fluke and tapeworm TGRs. Finally, we show that mice experimentally infected with M. vogae tetrathyridia and treated with either praziquantel, the reference drug for flatworm infections, or VL16E exhibited a 28% reduction of intraperitoneal larvae numbers compared to vehicle treated mice. Our results show that oxadiazole N-oxide is a promising chemotype in vivo and highlights the convenience of M. vogae as a model for rapid assessment of tapeworm infections in vivo.
Keywords: thioredoxin reductase, glutathione reductase, thioredoxin glutathione reductase, oxadiazole N-oxide, Mesocestoides, tapeworms
1. Introduction
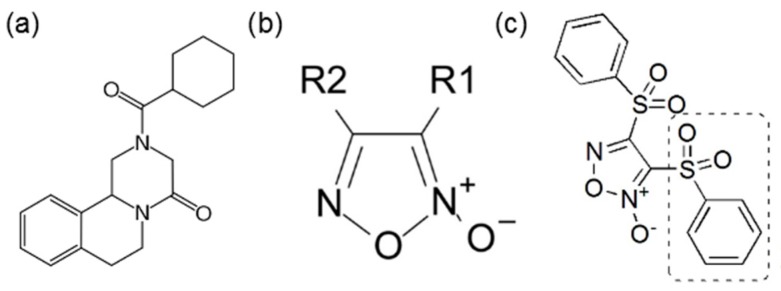
Parasitic flatworms from two main classes, Cestoda (or tapeworms) and Trematoda (or flukes), cause chronic human and livestock infections and are a major cause of disability, mortality and significant economic losses in most developing countries [1,2]. There is no vaccine available for any human flatworm infection and large-scale treatment of flatworm infections relies on very few drugs [3,4,5]. A single drug, praziquantel (Figure 1a), is currently available for treatment of Schistosomasis, a flatworm disease that affects 200 million people worldwide. Benzimidazoles, like albendazole or mebendazole, are drugs partially effective for human infections caused by Echinococcus spp [6]. Furthermore, the emergence and spread of drug resistance against parasites is a serious issue nowadays [7,8,9]. The problem is aggravated since there is little research by pharmaceutical companies, due to the limited commercial interest of drugs that target flatworm infections. Thus, the identification of novel drugs remains as an important goal in public health, particularly in the poorest regions of the world. A rational target-based approach to the discovery of drug candidates holds promise to accelerate the process. A unique aspect of flatworm biochemistry is that these parasites are entirely dependent on the enzyme thioredoxin glutathione reductase (TGR, EC 1.8.1.B1) for the control of redox homeostasis and other essential processes. This enzyme functionally replaces both canonical thioredoxin reductase (EC 1.8.1.9) and glutathione reductase (EC 1.8.1.7) [10,11,12,13], and thus constitutes a redox bottleneck for flatworm metabolism. RNAi studies and assays with TGR specific inhibitors have shown that TGR is essential for the trematodes Schistosoma mansoni and Schistosoma japonicum, and validated this molecule as a novel drug target [14,15]. Subsequent high throughput screening of TGR inhibitors identified oxadiazole N-oxide (Figure 1b) as a drug hit for the control of schistosomiasis [16,17,18]. One of the oxadiazole N-oxides identified in this study partially cured S. mansoni experimental infection in mice [14]. TGR has also been found to be essential for the cestode flatworm Echinococcus granulosus [19]. It has been recently shown that oxadiazole N-oxides also inhibited E. granulosus TGR and killed both major classes of flatworm parasites: tapeworms and flukes [20]. That study showed that 3,4-bis(phenylsulfonyl)-1,2,5-oxadiazole N2-oxide (VL16E, Figure 1c) was among the most potent oxadiazole N-oxides tested and it was suggested that the phenylsulfonyl group of VL16E (Figure 1c) may be a potential chemotype in itself [20]. However, whether VL16E or other oxadiazole N-oxides are active in vivo against cestode infections has not been addressed yet. The lack of an established and convenient cestode model that allows cestocidal drugs to be tested in experimental infections has hampered research in this area. Mesocestoides vogae (syn. corti) [21,22], a rodent cestode parasite that does not infect humans has been proposed as an important in vitro-in vivo model to test and study cestocidal and anthelmintic drugs [23,24,25], due to its rapid mouse infection and its close relationship to cestodes of medical relevance, such as those from Echinococcus or Taenia genera. Here, we used M. vogae as a cestode experimental infection model to assess the efficacy of the previously identified TGR inhibitor VL16E. The results indicate that M. vogae thiol-redox network relies exclusively on a selenocysteine-containing TGR, a biochemical scenario similar to other flatworm parasites. We found that VL16E was as good as praziquantel in reducing asexual reproduction in M. vogae infection with tetrathyridial metacestodes. Our results highlight that M. vogae is a good model for flatworm parasite redox metabolism and provides further evidence that is a convenient model to examine cestocidal drugs in vivo.
Figure 1.
Molecular formula of (a) praziquantel; (b) a generic oxadiazole N-oxide and (c) 3,4-bis(phenylsulfonyl)-1,2,5-oxadiazole N2-oxide (VL16E). One of the phenylsulfonyl structural motifs of VL16E is marked.
2. Results and Discussion
2.1. Analysis of M. vogae Genome Revealed the Presence of One TGR Gene, and Absence of Conventional TR and GR Genes
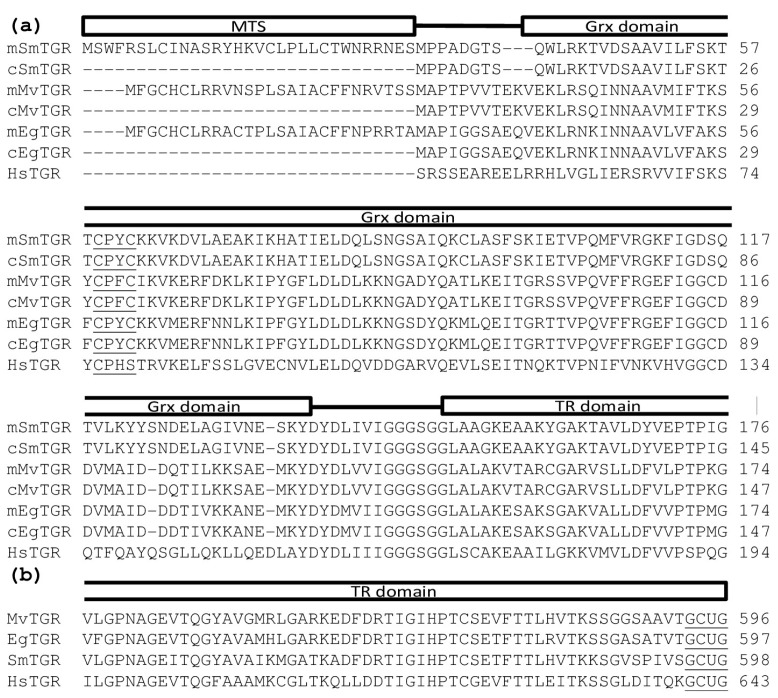
M. vogae has recently been sequenced [26], however, the genes and proteins have not been annotated yet. Thus, we analyzed M. vogae genome for the presence of TGR and conventional TR and GR by tblastn using parasitic flatworm TGRs and conventional TR and GR from free-living flatworms as queries. This analysis revealed the presence of a TGR gene only. The deduced amino acid sequence indicates that M. vogae TGR contains an N-terminal glutaredoxin domain fused to a conventional thioredoxin reductase, similar to what has been observed for all other TGRs. The glutaredoxin domain contains a CX2C redox active center, typical of flatworm TGRs (Figure 2a). The TR domains contain a characteristic CX4C redox center and a C-terminal redox motif containing cysteine and selenocysteine (GCUG, where U denotes selenocysteine) (Figure 2a,b). The nucleotide sequence also revealed the existence of a selenocysteine insertion sequence element at the 3ʹUTR (data not shown) needed for recoding the UGA codon as selenocysteine. M. vogae TGR displays 81% identity to E. granulosus TGR. The genomic organization (number and size of exons and introns) of M. vogae TGR gene was very similar to the E. granulosus TGR gene. Interestingly, the gene model of M. vogae TGR predicts the existence of two transcript variants of TGR that would encode cytosolic and mitochondrial isoforms (Figure 2a). These variants would be identical in sequence except for an additional exon encoding a leader peptide that would give rise to the mitochondrial variant. The existence of two transcripts from a single TGR gene has been demonstrated for E. granulosus and S. mansoni [11].
Figure 2.
Protein sequence alignment of human and flatworm TGRs. Partial sequences from M. vogae (Mv), S. mansoni (Sm, AAK85233.1), E. granulosus (Eg, AAN63052.1) and H. sapiens (Hs, NP_443115.1) TGRs are aligned and N-terminus and C-terminus of the proteins are shown in (a,b), respectively. Conserved domains and mitochondrial targeting signal are represented by boxes above the alignment. Mitochondrial and cytosolic variants (m and c) for flatworm TGRs are shown. Grx domain active site and selenocysteine-containing TR domain are underlined.
2.2. M. vogae Thioredoxin Reductase and Glutathione Reductase Activities Co-Purified
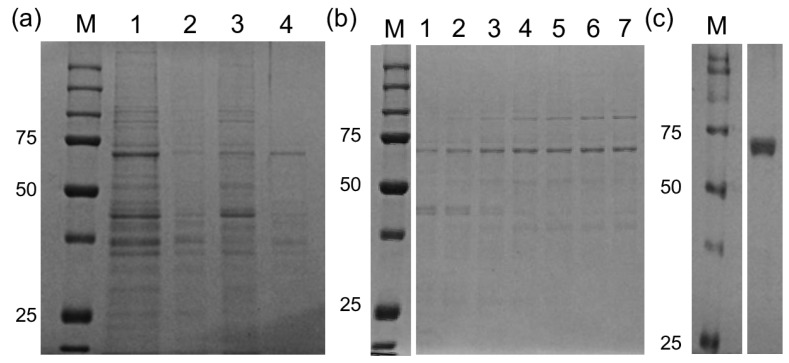
Both TR and GR activities were traced in purification schemes from a total aqueous extract derived from 6 mL of packed M. vogae tetrathiridium larvae. In an optimized three step-wise ammonium sulfate fractionation (25%, 50%, 85% saturation solutions), 80% of both activities were recovered in the 25%–50% saturated ammonium sulfate fraction with approximately a two-fold enrichment (Figure 3a). Approximately 15% of TR and GR activities were recovered on the 50%–85% saturated ammonium sulfate fraction. The TR/GR activity ratio in these fractions was identical to the ratio of the initial total aqueous extract, suggesting the distribution of one enzyme in two fractions. The 25%–50% fraction was re-suspended, desalted and then chromatographed on a HiLoad 16/600 Superdex 200 PG. The size exclusion chromatography led to a nearly complete recovery (90%) of both activities and a 24-fold enrichment (Figure 3b). The GR and TR activities co-eluted in fractions corresponding to 120–140 kDa approximately, consistent with a dimeric holoprotein. The active fractions were pooled, desalted and chromatographed on anion exchange Mono Q column. Following anion exchange, a 68% recovery and a 25-fold enrichment were achieved (Figure 3c). An SDS-PAGE revealed the presence of a single band of approximately 65 kDa in the final step of purification (Figure 3c), consistent with the Mr of TGR. It is important to emphasize that both activities co-purified in all the purification steps. Furthermore, the same TR/GR activity ratio as the original extract was maintained in the purification process. Altogether, the data from the purification of native thioredoxin and glutathione reductases activities indicate that only TGR is responsible for both activities.
Figure 3.
Purification of TGR from M. vogae tetrathyridium larvae. (a) Ammonium sulfate precipitation of a total aqueous extract from tetrathyridium larvae was carried out in three steps (0%–25%, 25%–50% and 50%–85% ammonium sulfate saturation) and analyzed by SDS-PAGE under reducing conditions (lane 1 corresponds to the total extract, lanes 2, 3 and 4 to 0%–25%, 25%–50% and 50%–85% ammonium sulfate saturation fractions, respectively); (b) The 25%–50% ammonium sulfate saturation fraction was further purified by size exclusion chromatography (SEC) on a 16/600 Superdex 200 column; lanes 1 to 7 show fractions were the TR and GR activities eluted; (c) SEC fractions containing TR and GR activities were pooled and subjected to a anionic chromatography on a MonoQ column. Bound proteins were eluted using a NaCl 10–1000 mM gradient and the fractions obtained were analyzed by SDS-PAGE under reducing conditions. The lane shown corresponds to the fraction with maximal TR and GR activity, which eluted at NaCl 300 mM. The gels shown in (a,b) were coomassie stained and the gel shown in (c) was silver stained. M denotes molecular weight markers.
2.3. The GR Activity of M. vogae Exhibits Hysteresis towards Glutathione and is Inhibited by Auranofin
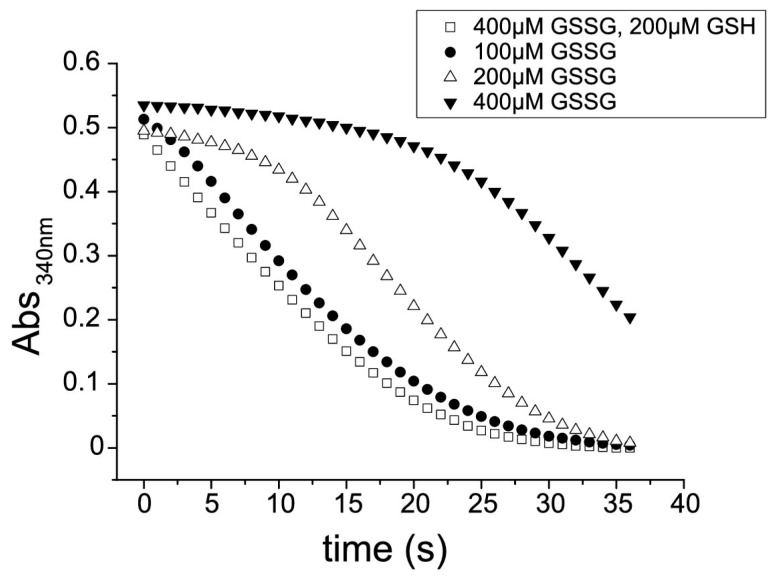
A hysteretic behavior (i.e., the existence of a lag time before full catalytic activity takes place) of GR activity at high concentration of oxidized glutathione is a footprint of flatworm TGRs and it has not been observed in canonical GR. Thus, we examined whether the GR activity of the purified enzyme exhibited hysteresis towards oxidized glutathione. A typical hysteretical behavior was observed: (i) hysteresis lag time increased as oxidized glutathione concentration increased; and (ii) the maximal slope of the curves did not change once the enzyme was fully active (Figure 4). Furthermore, addition of reduced glutathione to the GR assay mix abolished hysteresis (Figure 4), a conspicuous feature of TGR hysteretical behavior, which is controlled by the [GSSG]/[GSH] ratio [19,27,28]. Thus, these experiments provide further evidence that TGR is responsible for the GR activity in M. vogae. Auranofin is a known gold inhibitor of selenocysteine-containing TRs [29] and TGRs [10,14,15,27,30,31]. Both TR and GR activities present in the purified enzyme were inhibited by nanomolar concentrations of auranofin (Figure 5). Since conventional GRs do not possess selenocysteine at their active site, these results indicated that the GR activity is due to TGR and that selenocysteine is present at the TGR active site.
Figure 4.
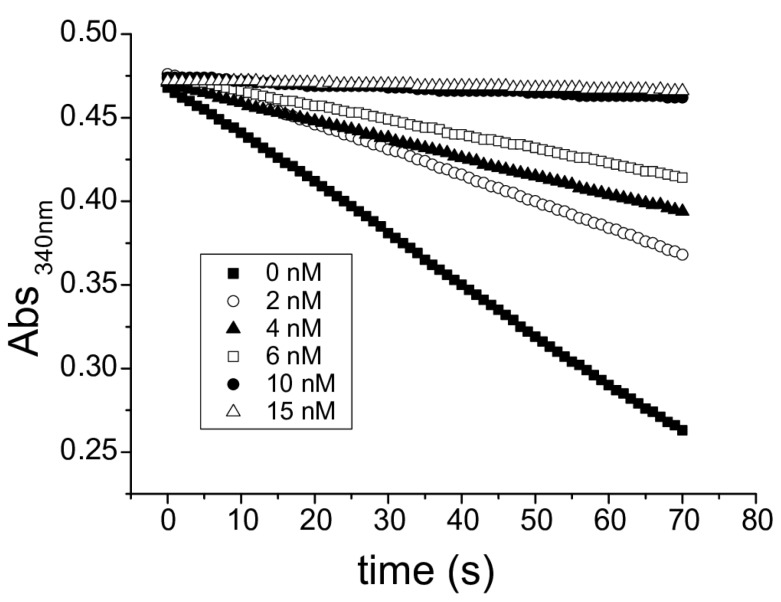
Hysteretic behavior of GR activity of TGR. Full-time courses obtained at different conditions are shown. In all cases constant nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) and enzyme concentrations were used and reactions were started by the addition of enzyme. Oxidized glutathione (GSSG) and reduced glutathione (GSH) concentrations varied. GR assays were carried out with the purified TGR at a 2 nM concentration.
Figure 5.
Inhibition of GR activity with Auranofin. Time-courses obtained using different auranofin concentrations are shown. GR assays were carried out at constant concentrations of TGR (2 nM), GSSG (50 µM), NADPH (100 µM) and without GSH. The enzyme was preincubated with the inhibitor during three minutes and the reaction was started by addition of GSSG.
2.4. An Oxadiazole N-Oxide that Inhibits TGR Reduces M. vogae Infection Burden in Mice
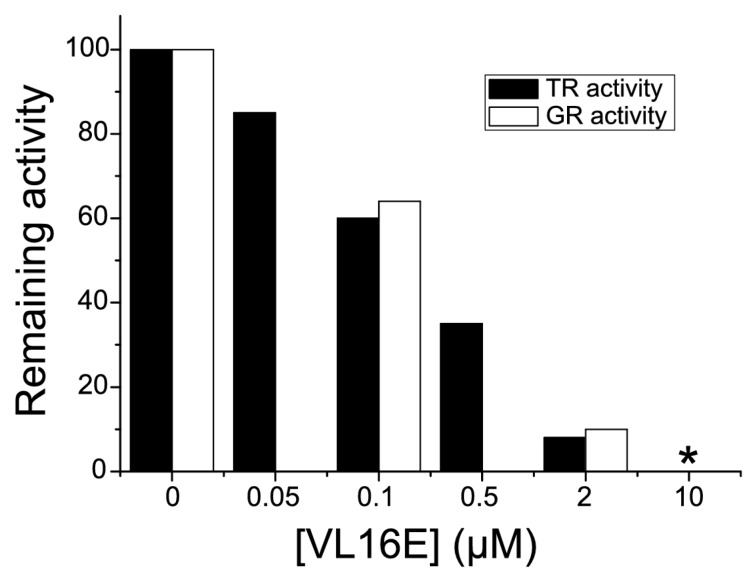
We have recently shown that 3,4-bis(phenylsulfonyl)-1,2,5-oxadiazole N2-oxide (VL16E, Figure 1c) is a potent TGR inhibitor that killed in vitro larval worms of the cestode parasite E. granulosus [20]. Whether VL16E is capable of cure infection in cestodes has not been addressed. M. vogae provides an excellent model to test this inhibitor in vivo. We first examined whether VL16E inhibits M. vogae TGR. Similar to the results obtained with E. granulosus TGR, the TR and GR activities of the purified TGR were inhibited by VL16E (Figure 6). Furthermore, VL16E killed tetrathyridium larvae in vitro at 20 µM concentration (data not shown).
Figure 6.
Inhibition of TR and GR activity of purified TGR from M. vogae by VL16E. Residual TR and GR activity as a function of inhibitor concentration is plotted in black and white respectively. Residual GR activity was measured only with 0.01 and 10 µM of inhibitor. (*)TR and GR activities could not be detected. TGR was used at 2nM concentration in all assays.
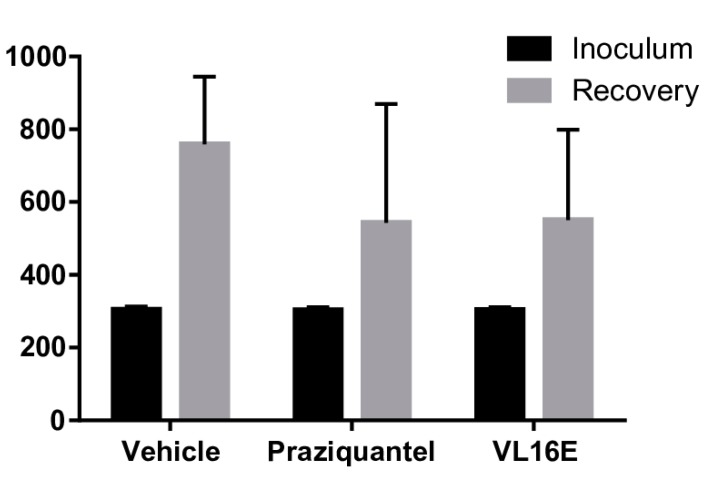
We then examined whether VL16E was active in vivo in M. vogae infection in mice. Three groups of mice (n = 8) were infected with M. vogae tetrathyridium. Infection was allowed to proceed for two weeks and treated during three consecutive days with either VL16E, a reference anthelmintic (praziquantel) or vehicle. The infection burden was assessed at day 17. The results are presented in Figure 7. A statistical analysis (non-parametric Mann-Whitney test) comparing inoculum and recovery within each group was performed. A significant 2.5-fold increase in parasite burden was observed in the vehicle control group. In contrast, only a 1.8-fold increase in parasite burden was observed in both groups treated with either praziquantel or VL16E for three days. A statistical analysis comparing inoculum and recovery within each group indicated that the increase in parasite burden was significant in the control group but not in the treated groups. A 28.5% and 28% reduction in tetrathyridium recovery was observed in the groups treated with praziquantel and VL16E, respectively, compared to tetrathyridium recovery in the vehicle control group (Figure 7). Although under the conditions assayed, VL16E performed equally well to praziquantel, the drug of choice for several flatworm infections, the decrease in parasite burden was not statistically supported using an α = 0.05 (non-parametric test Kruskal-Wallis). Importantly, no evident side effects were observed in mice during treatment with VL16E.
Figure 7.
VL16E reduces infection burden of M. vogae infection in mice. Three groups of eight mice each were infected with approximately 300 M. vogae tetrathyridium larvae by intraperitoneal injection and treated by gavage at days 14, 15 and 16 after infection with vehicle (4% dimethyl sulfoxide in PBS, negative control group), praziquantel (positive control group) and VL16E (oxadiazole N-oxide 3,4-bis(phenylsulfonyl)-1,2,5-oxadiazole N2-oxide). At day 17 mice were sacrificed and the recovered larvae counted. Results are expressed as mean larval count and standard deviations. The increase in tetrathyridium count (recovery vs. inoculum) wss statistically significant in the vehicle group but not in treated groups (either praziquantel or VL16).
2.5. Discussion
The redox systems of several parasitic flatworms are unique in that conventional TR and GR have been replaced by TGR. TGR is currently one of the most attractive drug targets for flatworm infections, since it is an essential and “druggable” enzyme whose inhibition leads to redox homeostasis disruption and death of flatworm parasites [14,15,20,32]. However, it is not known whether this pathway setup is common to all parasitic flatworms, particularly considering that free-living flatworms have TR, GR and TGR [11]. In this article, we demonstrate that the cestode M. vogae shares the same biochemical scenario as other parasitic flatworms regarding thioredoxin and glutathione pathways. The results are clearly indicative of the existence of TGR, and absence of conventional TR and GR. The genome mining of M. vogae, which has recently been published, but not annotated, indicated that this parasite lacks TR and GR genes and possesses a selenocysteine-containing TGR gene. The selenocysteine residue at the prototypical carboxy terminal GCUG redox center is coded by an in frame UGA codon and the presence of a SECIS element in the 3ʹUTR allows UGA to be recoded as selenocysteine. Furthermore, the predicted domains of M. vogae TGR are identical to that of other TGRs; with a C-terminal TR module that would be responsible for the TR activity and an N-terminal Grx domain that, in combination with the TR module would be responsible for the GR activity. We purified to homogeneity the TR and GR activities from M. vogae extracts. Both enzymatic activities co-purified at a constant GR/TR ratio in the purification scheme. After salting in, size exclusion chromatography and ion exchange, a single protein band was isolated and its size corresponded to the molecular weight of TGR (65 kDa). No bands corresponding to conventional TR (54 kDa) or conventional GR (52 kDa) were observed, in full agreement with the genome data mining. Furthermore, the biochemical characterization revealed that the purified activities correspond, in all likelihood to TGR. The hysteretical behavior of the GR activity observed at high concentrations of oxidized glutathione (Figure 4) is an “enzymatic signature” of TGRs that contain a dithiolic redox active site at the N-terminal glutaredoxin domain, as the one deduced from M. vogae TGR gene. Indeed this phenomenon is not observed in conventional GR and, it is a conspicuous feature so far observed exclusively in flatworm TGRs [19,27,28,33,34]. Finally, the inhibition of M. vogae TGR with auranofin affected both GR and TR activities indicating that the purified TGR contains selenocysteine at its redox active site. These results highlight that M. vogae is a particularly relevant model to test compounds that target the thioredoxin and glutathione pathways, since this parasite shares the same metabolism with other parasitic flatworms. Furthermore, TGR inhibitors will target cytosolic and mitochondrial redox homeostasis: similar to other flatworms, the TGR gene organization predicts the existence of two TGR variants, cytosolic and mitochondrial, derived from the same gene and differing exclusively in the presence of an additional leader peptide in the mitochondrial isoform.
The TGR inhibitors oxadiazole N-oxides have been shown to be a potential drug hit for flatworm infections and have been shown to be effective in treating trematode (fluke) experimental infections [16]. We have previously described that oxadiazoles were able to kill the cestode E. granulosus larval worms in vitro [19]. However, whether oxadiazole N-oxides are effective in vivo against cestodes (tapeworms), the other major class of parasitic flatworms, had not been assessed yet. Testing E. granulosus experimental infection in mice takes at least nine months [35] and assessment of infection by cyst number and size is not reliable due to low numbers and variability in infection rates. Thus, we addressed this issue by using M. vogae as a cestode model for infections. M vogae is a convenient cestode parasite to assess potential drug hits in vivo. M. vogae possesses an unparalleled proliferation rate of its larval form, tetrathyridium, in experimental infections in mice, and therefore allows parasite growth rate to be tested in two weeks. We tested the cestocidal activity of oxadiazole N-oxide 3,4-bis(phenylsulfonyl)-1,2,5-oxadiazole N2-oxide (VL16E) since it was one of the most potent inhibitors in vitro and because the phenylsulfonyl moiety was suggested as an important determinant for the activity. Before testing VL16E in infection studies, we show that VL16E inhibited both thioredoxin and glutathione reductase activities of the purified M. vogae TGR. We then examined whether VL16E cured M. vogae infection in mice. In parallel to the vehicle control group and VL16E, we used a control group with praziquantel, the reference drug for cestodes. The results obtained indicated that after two weeks of infection and three days of treatment, the increase in tetrathiridium larval count during infection was statistically significant (p ≤ 0.05) in the vehicle control group, but not in the VL16E or praziquantel treated groups. In other words, the analysis of inoculum vs. recovery within each group revealed that only the non-treated group showed a statistically significant increase in parasite burden. Infection recoveries were very similar in the groups treated with either drugs, and showed a 28% average reduction of parasitaemia in comparison to the vehicle control group. Although this reduction in parasite recovery was not statistically supported the results are promising. It is important to highlight that the most strict positive control group was included in the experimental design (i.e., praziquantel). It should also be noted that this effect is associated with a three-day treatment only. The doses of either drug used in the present study were similar to those used in other studies [14,16]. Importantly, VL16E was administered by gavage, indicating that VL16E is active by this route. It is also relevant to highlight that no notorious side effects were observed in the mice group treated with VL16E. Thus, our results showed a good correlation of TGR inhibition, in vitro and in vivo antiparasitic activity for VL16E. Overall, these results reinforce the idea that oxadiazole N-oxides are promising chemotype for flatworm infections that deserve to be further explored. This is particularly relevant considering that flatworm infections are chronic and debilitating diseases and there is a very restricted set of drugs and a few rational drug targets and supports TGR as a valid target in the development of drugs against tapeworm and fluke parasites.
3. Experimental Section
3.1. Mesocestoides vogae Tetrathyridia Infection
Two months old CD1 mice were infected with fresh M. vogae tetrathyridia in saline solution (0.3 mL, ca. 300 tetrathyridia) by intraperitoneal inoculation, according to [25,36]. Infection was allowed to proceed for a variable time and then the mice were killed by cervical dislocation. The animal protocol complied with Uruguayan Law No. 18.611 [37] and it was harmonized with The Canadian Guidelines on Animal Care. Experimental protocol n 02-05-10 of the study was reviewed and approved by IACUC of Facultad de Química, Universidad de la República, Uruguay.
3.2. Enzyme Purification from Mesocestoides vogae
Tetrathyridia larvae were collected from the peritoneum of three month infected mice, washed by sedimentation with a saline solution and frozen at −80 °C until use. Six mL of tetrathyridia larvae were grounded to a powder under liquid nitrogen using a mortar and a pestle. Five mL of phosphate saline buffer (PBS) pH 7.6, containing 50 mM NaH2PO4, 100 mM NaCl, 0.05% tween, 1 mM EDTA, 1 mM PMSF and 100 µg/mL DNAse, were added to the thawed suspension and sonicated. The suspension obtained was centrifuged at 20,000 g during 30 min and the supernatant containing the aqueous protein extract was used for purification. Ammonium sulfate precipitation of the extract was carried out in three steps of 25%, 50% and 85% saturation. Most of the thioredoxin reductase (TR) and glutathione reductase (GR) activities (see enzymatic assays below) were present in the 25%–50% fraction; less than 10% of TR and GR activities were present in the 50%–85% fraction. The 25%–50% fraction was re-suspended in water and immediately dialyzed against PBS and applied to a HiLoad 16/600 Superdex 200 pg (GE Healthcare Life Sciences, Buckinghamshire, UK). All fractions were analyzed for TR and GR activities. Fractions containing TR and GR activities were pooled and dialyzed against Tris 20 mM, NaCl 10 mM, pH 8. The dialyzed fraction was applied to a MonoQ™ 5/50 GL column (GE Healthcare Life Sciences) previously equilibrated in Tris 20 mM, NaCl 10 mM, pH 8. After washing with equilibration buffer, the retained material was eluted by a salt concentration gradient (10–1000 mM NaCl in 15 column volumes). Enzyme activities were measured in the unbound and bound fractions. The fractions were analyzed in 10% SDS-PAGE gels under reducing conditions according to standard techniques. Gels were coomassie or silver stained (the latter staining for the final purification step). Precision plus Protein™ (BIO_RAD) molecular weight markers were used. Protein concentration was determined using Bradford reagent [38,39].
3.3. DTNB Reduction Assay for TR Activity
The reduction of 5,5ʹ-dithiobis(2-dinitrobenzoic acid) (DTNB) with concomitant NADPH oxidation was determined by the increase in absorbance at 412 nm due to the formation of 5ʹ-thionitrobenzoic acid (TNB) (ε = 13,600 M−1·cm−1) [40]. The reaction mixtures contained 100 μM NADPH and 5 mM DTNB in phosphate buffer 50 mM, EDTA 1 mM, pH 7. The reaction was followed for three minutes. Control experiments without NADPH were performed.
3.4. GSSG Reduction Assay
The GR activity was assayed as the NADPH-dependent reduction of oxidized glutathione (GSSG), which is followed by the decrease in absorbance at 340 nm due to NADPH oxidation (ε = 6200 M−1·cm−1) [41]. The reaction mixtures contained 100 μM NADPH, 1 mM GSSG and 1 mM GSH to avoid conditions of hysteresis in phosphate buffer 50 mM, EDTA 1 mM, pH 7 [19,27,28,33]. The hysteresis of glutathione reductase was studied by varying the concentration of GSH from 0 to 1 mM GSH in the assay. The reaction was recorded until depletion of NADPH.
3.5. Inhibition Studies
In all cases, fractions containing TGR were preincubated during three minutes with NADPH and the inhibitor VL16E or auranofin, a specific inhibitor of selenocysteine-containing TRs and TGRs. The reaction was started by the addition of DTNB for thioredoxin reductase assay or GSSG for glutathione reductase assay (see above). All assays were performed in triplicate. In every case, controls for reaction without enzyme and without inhibitor were carried out in parallel. The residual TR or GR activity after inhibition was calculated as: (vi/vo) × 100, where vi and vo correspond to the initial velocities of TNB formation (TR assay) or NADPH consumption (GR assay) with and without inhibitor, respectively. A range of inhibitor concentrations from 10 nm to 10 µM was assayed.
3.6. Cestocidal Effect of VL16E in Tetrathyridia Infected Mice
Cestocidal effect of VL16E was tested using mice infected by the same procedure described above. Three groups of mice (eight mice each) were separated and treated with VL16E, the reference drug praziquantel or vehicle (4% dimethyl sulfoxide in PBS). On days 14, 15 and 16 after infection, mice received a daily dose of praziquantel (20 mg/kg body weight in a 0.2 mL volume), VL16E (equimolar dose to praziquantel, in a 0.2 mL volume) or vehicle (0.2 mL) administered by gavage. At day 17, post-infection, the animals were killed by cervical dislocation and the larvae collected from their peritoneum, washed and counted. Parasite recovery (larval count) from control and treated groups was determined and the efficacy of chemotherapy estimated. The efficacy of chemotherapy was estimated through the percentage of reduction tetrathyridium recovery, calculated as: % reduction = 100 × (mean from control group − mean from treated group)/mean from control group (where mean refers to the mean number of recovered parasites). The differences between the mean number of recovered and inoculated parasites in each group were tested for statistical significance using the non-parametric Mann-Whitney test (α = 0.05). The statistical analysis to compare parasite recovery among the three groups was performed using the non-parametric test Kruskal-Wallis.
3.7. Sequence Analysis
In order to obtain information on putative TR, GR and TGR sequences from M vogae the 50 Helminth Genomes Initiative database [26] was searched by tblastn using the amino acid sequences of TGR from E. granulosus (a parasitic flatworm) and GR and TR from Schmidtea mediterranea (a free-living flatworm) as queries. Since proteins and gene models have not been predicted for M. vogae, the coding sequence of M. vogae TGR was mapped using the exon and intron information of E. granulosus and S. mansoni TGRs. Final adjustments of intron-exon boundaries were performed based on a multiple alignment of TGRs from E. granulosus Echinococcuus multilocularis, Hymenolepis microstoma and Taenia solium. The multiple alignment of flatworm TGRs was carried out using ClustalW2 [42]. The selenocysteine insertion sequence (SECIS) elements present in M. vogae TGR was identified using the SECISearch program, version 2-19 [43,44] using the default energy cutoffs and the canonical pattern. Topology prediction of the polypeptides was carried out using signal P [45] and ipSORT [46].
Acknowledgements
This work was supported by FIRCA-NIH (Grant RO3TW008588) to GS and Universidad de la República, CSIC (Grant CSIC 625), FOCEM (MERCOSUR Structural Convergence Fund, [COF 03/11]) and Seeding Labs. We thank Hugo Cerecetto, Mercedes González and Mariana Boiani from the Medicinal Chemistry Lab, Facultad de Ciencias, Universidad de la República, Uruguay for helpful discussions.
Author Contributions
V.P. performed enzymatic assays and enzyme purification; H.B. data mining and analysis of tapeworm genomes, contributed to experimental design and writing, G.V.L. synthesized VL16E, J.S. performed infection experiments, M.B. general assistance in enzymatic assays, and contributed to experimental design, L.R.-C. performed statistical analysis and contributed to the interpretation and analysis of results, G.S. conceived the study, performed enzymatic assays and enzyme purification and wrote the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compound VL16E are available from the authors.
References
- 1.Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobson J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO The World Health Report-Changing History. [(accessed on 12 March 2015)]. Available online: http://www.who.int/whr/2004/en/report04_en.pdf.
- 3.Hotez P.J., Brown A.S. Neglected tropical disease vaccines. Biologicals. 2009;37:160–164. doi: 10.1016/j.biologicals.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Mansour T. Chemotherapeutic Targets in Parasites. Contemporary strategies. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- 5.Cioli D., Valle C., Angelucci F., Miele A.E. Will new antischistosomal drugs finally emerge? Trends Parasitol. 2008;24:379–382. doi: 10.1016/j.pt.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Kern P. Clinical features and treatment of alveolar echinococcosis. Curr. Opin. Infect. Dis. 2010;23:505–512. doi: 10.1097/QCO.0b013e32833d7516. [DOI] [PubMed] [Google Scholar]
- 7.Doenhoff M.J., Kusel J.R., Coles G.C., Cioli D. Resistance of Schistosoma mansoni to praziquantel: Is there a problem? Trans. R. Soc. Trop. Med. Hyg. 2002;96:465–469. doi: 10.1016/S0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- 8.Doenhoff M.J., Cioli D., Utzinger J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 9.Valentim C.L.L., Cioli D., Chevalier F.D., Cao X., Taylor A.B., Holloway S.P., Pica-Mattoccia L., Guidi A., Basso A., Tsai I.J., et al. Genetic and molecular basis of drug resistance and species-specific drug action in Schistosome parasites. Science. 2013;342:1385–1389. doi: 10.1126/science.1243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alger H.M., Williams D.L. The disulfide redox system of Schistosoma mansoni and the importance of a multifunctional enzyme, thioredoxin glutathione reductase. Mol. Biochem. Parasitol. 2002;121:129–139. doi: 10.1016/S0166-6851(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 11.Otero L., Bonilla M., Protasio A.V., Fernandez C., Gladyshev V.N., Salinas G. Thioredoxin and glutathione systems differ in parasitic and free-living platyhelminths. BMC Genomics. 2010;11 doi: 10.1186/1471-2164-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prast-Nielsen S., Huang H.-H., Williams D.L. Thioredoxin-glutathione reductase: Its role in redox biology and potential as a target for drugs against neglected diseases. Biochim. Biophys. Acta. 2011;1810:1262–1271. doi: 10.1016/j.bbagen.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams D.L., Bonilla M., Gladyshev V.N., Salinas G. Thioredoxin glutathione reductase-dependent redox networks in platyhelminth parasites. Antiox. Redox Signal. 2012;19:735–745. doi: 10.1089/ars.2012.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuntz A.N., Davioud-Charvet E., Sayed A.A., Califf L.L., Dessolin J., Arner E.S., Williams D.L. Thioredoxin glutathione reductase from Schistosoma mansoni: An essential parasite enzyme and a key drug target. PLoS Med. 2007;4:e206. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song L., Li J., Xie S., Qian C., Wang J., Zhang W., Yin X., Hua Z., Yu C. Thioredoxin glutathione reductase as a novel drug target: Evidence from Schistosoma japonicum. PLoS ONE. 2012;7:e31456. doi: 10.1371/journal.pone.0031456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayed A.A., Simeonov A., Thomas C.J., Inglese J., Austin C.P., Williams D.L. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat. Med. 2008;14:407–412. doi: 10.1038/nm1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simeonov A., Jadhav A., Sayed A.A., Wang Y., Nelson M.E., Thomas C.J., Inglese J., Williams D.L., Austin C.P. Quantitative high-throughput screen identifies inhibitors of the Schistosoma mansoni redox cascade. PLoS Negl. Trop. Dis. 2008;2:e127. doi: 10.1371/journal.pntd.0000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rai G., Sayed A.A., Lea W.A., Luecke H.F., Chakrapani H., Prast-Nielsen S., Jadhav A., Leister W., Shen M., Inglese J., et al. Structure mechanism insights and the role of nitric oxide donation guide the development of oxadiazole-2-oxides as therapeutic agents against schistosomiasis. J. Med. Chem. 2009;52:6474–6483. doi: 10.1021/jm901021k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonilla M., Denicola A., Novoselov S.V., Turanov A.A., Protasio A., Izmendi D., Gladyshev V.N., Salinas G. Platyhelminth mitochondrial and cytosolic redox homeostasis is controlled by a single thioredoxin glutathione reductase and dependent on selenium and glutathione. J. Biol. Chem. 2008;283:17898–17907. doi: 10.1074/jbc.M710609200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross F., Hernández P., Porcal W., López G.V., Cerecetto H., González M., Basika T., Carmona C., Fló M., Maggioli G., et al. Identification of thioredoxin glutathione reductase inhibitors that kill cestode and trematode parasites. PLoS ONE. 2012;7:e35033. doi: 10.1371/journal.pone.0035033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Specht D., Voge M. Asexual multiplication of Mesocestoides tetrathyridia in laboratory animals. J. Parasitol. 1956;51:268–272. doi: 10.2307/3276097. [DOI] [PubMed] [Google Scholar]
- 22.Etges F., Marinakis V. Formation and excretion of calcareous bodies by the metacestode (tetrathyridium) of Mesocestoides vogae. J. Parasitol. 1991;77:595–602. doi: 10.2307/3283166. [DOI] [PubMed] [Google Scholar]
- 23.Hart J.L. Regeneration of tetrathyridia of Mesocestoides (Cestoda: Cyclophyllidea) In vivo and in vitro. J. Parasitology. 1968;54:950–956. doi: 10.2307/3277128. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell G.F., Marchalonis J.J., Smith P.M., Nicholas W.L., Warner N.L. Studies on immune responses to larval cestodes in mice. Immunoglobulins associated with the larvae of Mesocestoides corti. Aust. J. Exp. Biol. Med. 1977;55:187–211. doi: 10.1038/icb.1977.14. [DOI] [PubMed] [Google Scholar]
- 25.Saldaña J., Casaravilla C., Marín M., Fernández C., Domínguez L. The toxicity of praziquantel against Mesocestoides vogae (syn. corti) tetrathyridia can be assessed using a novel in vitro system. Parasitol. Res. 2003;89:467–472. doi: 10.1007/s00436-002-0801-6. [DOI] [PubMed] [Google Scholar]
- 26.50 Helminth Genomes Initiative Database. [(accessed on 25 June 2015)]. Available online: http://www.sanger.ac.uk/research/initiatives/globalhealth/research/helminthgenomes/
- 27.Rendón J.L., del Arenal I.P., Guevara-Flores A., Uribe A., Plancarte A., Mendoza-Hernández G. Purification, characterization and kinetic properties of the multifunctional thioredoxin-glutathione reductase form Taenia crassiceps metacestode (cysticerci) Mol. Biochem. Parasitol. 2004;133:61–69. doi: 10.1016/j.molbiopara.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Guevara-Flores A., Pardo J.P., Rendon J.L. Hysteresis in thioredoxin-glutathione reductase (TGR) from the adult stage of the liver fluke Fasciola hepatica. Parasitol. Int. 2011;60:156–160. doi: 10.1016/j.parint.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Gromer S., Arscott L.D., Williams C.H., Schirmer R.H., Becker K. Human placenta thioredoxin reductase. J. Biol. Chem. 1998;273:20096–20101. doi: 10.1074/jbc.273.32.20096. [DOI] [PubMed] [Google Scholar]
- 30.Agorio A., Chalar C., Cardozo S., Salinas G. Alternative mRNAS arising from trans-splicing code for mitochondrial and cytosolic variants of Echinococcus granulosus thioredoxin glutathione reductase. J. Biol. Chem. 2003;278:12920–12928. doi: 10.1074/jbc.M209266200. [DOI] [PubMed] [Google Scholar]
- 31.Angelucci F., Sayed A.A., Williams D.L., Boumis G., Brunori M., Dimastrogiovanni D., Miele A.E., Pauly F., Bellelli A. Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects. J. Biol. Chem. 2009;284:28977–28985. doi: 10.1074/jbc.M109.020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Gonzalez J.J., Guevara-Flores A., Alvarez G., Rendon-Gomez J.L., Del Arenal I.P. In vitro killing action of auranofin on Taenia crassiceps metacestode (cysticerci) and inactivation of thioredoxin-glutathione reductase (TGR) Parasitol. Res. 2010;107:227–231. doi: 10.1007/s00436-010-1867-1. [DOI] [PubMed] [Google Scholar]
- 33.Huang H.H., Day L., Cass C.L., Ballou D.P., Williams C.H., Williams D.L. Investigations of the catalytic mechanism of thioredoxin glutathione reductase from Schistosoma mansoni. Biochemistry. 2011;50:5870–5882. doi: 10.1021/bi200107n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plancarte A., Nava G. Purification and kinetic analysis of cytosolic and mitochondrial thioredoxin glutathione reductase extracted from Taenia solium cysticerci. Exp. Parasitol. 2015;149:65–73. doi: 10.1016/j.exppara.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Hernández A., Nieto A. Induction of protective immunity against murine secondary hydatidosis. Parasite Immunol. 1994;16:537–544. doi: 10.1111/j.1365-3024.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 36.Saldaña J., Marín M., Fernández C., Domínguez L. In vitro taurocholate-induced segmentation and clustering of Mesocestoides vogae (syn. corti) tetrathyridia (Cestoda)- inhibition by cestocidal drugs. Parasitol. Res. 2001;87:281–286. doi: 10.1007/pl00008579. [DOI] [PubMed] [Google Scholar]
- 37.The Animal Protocol Complied with Uruguayan Law No. 18.611. [(accessed on 25 June 2015)]. Available online: http://archivo.presidencia.gub.uy/_web/leyes/2009/10/EC1395.pdf.
- 38.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 39.Stoscheck C.M. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- 40.Arner E.S.J., Zhong L., Holmgren A. Preparation and assay of mammalian thioredoxin and thioredoxin reductase. Methods Enzymol. 1999;300:226–239. doi: 10.1016/s0076-6879(99)00129-9. [DOI] [PubMed] [Google Scholar]
- 41.Carlberg I., Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 42.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 43.SECISearch Program, Version 2-19. [(accessed on 25 June 2015)]. Available online: http://genome.unl.edu/SECISearch.html.
- 44.Kryukov G.V., Castellano S., Novoselov S.V., Lobanov A.V., Zehtab O., Guigo R., Gladyshev V.N. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 45.Bendtsen J., Nielsen H., von Heijne G., Brunak S. Improved Prediction of Signal Peptides: SignalP 3.0. J. Mol. Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Bannai H., Tamada Y., Maruyama O., Nakai K., Miyano S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics. 2002;18:298–305. doi: 10.1093/bioinformatics/18.2.298. [DOI] [PubMed] [Google Scholar]