Abstract
The plant Aeschynomene fascicularis (Fabaceae) has been used in Mayan traditional medicine in the Yucatan peninsula. However, the compounds present in the plant responsible for its curative properties have not yet been investigated. Aeschynomene fascicularis root bark was extracted with 100% methanol to obtain a crude extract. The methanol extract was partitioned successively with solvents with increasing polarity to obtain the corresponding hexane (Hx), dichloromethane (DCM) and ethyl acetate fractions (EtOAc), as well as a residual water-alcoholic fraction. These fractions were tested for their cytotoxic activities using an MTT assay against Hep-2 cancer cell lines. The Hx fraction led to the isolation of spinochalcone C (1), spinochalcone A (2), isocordoin (3) and secundiflorol G (4). Their structures were identified based on spectroscopic evidence and chemical properties. All compounds were subjected to cytotoxicity and antiproliferative assays against a panel of seven cell lines, including one normal-type cell line. Spinochalcone A (2) exhibited cytotoxic activity against DU-145 cell line and antiproliferative activity against the KB cell line. Secundiflorol G (4) showed strong cytotoxic activity towards KB and Hep-2 cell lines. In addition, isocordoin (3) showed moderate activity on KB, Hep-2 and DU-145 cell lines. The active Compounds 2, 3 and 4 are potential therapeutic entities against cancer.
Keywords: Fabaceae, flavonoids, Mayan medicinal plant, cytotoxic activity, antiproliferative activity
1. Introduction
The flora of the Yucatan peninsula is rich in vascular plants, including 2600–3000 species [1]. Some of these species have been used in traditional medicine by local communities for the treatment of a large number of diseases [2,3,4]. Therefore, based on their traditional uses, these plants constitute good sources of new molecules that can be used for the treatment of different ailments. Furthermore, 50% of anticancer drugs used in clinical trials have been isolated from plants [5]. Consequently, traditional medicinal plants can serve as potential sources in the development of new, more effective anticancer agents for future therapy.
In the Yucatan, medicinal plants have been used in urban and rural communities as a common practice for the control of many types of diseases, including cancer. Moreover, they are used in the treatment of conditions consistent with the symptoms of cancer: abscesses, calluses, corns, hard lumps, polyps, tumors or warts. In a preliminary screening for cytotoxic constituents from plants used in Mayan traditional medicine to treat cancer-like symptoms, 21 species were collected, and the crude methanol extracts of various plant parts were subjected to a cytotoxicity assay against a panel of cancer cell lines [6]. The results indicated that Aeschynomene fascicularis root bark has a pronounced cytotoxic activity on cancer cell lines.
Aeschynomene fascicularis Cham. and Schltdl. (Fabaceae) is a shrub extensively distributed from Mexico to Colombia. This plant is commonly known as kabal pich by the ancient inhabitants of the Yucatan Peninsula, and it has been used in Mayan traditional medicine to treat superficial tumors [2]. Few chemical studies on species of Aeschynomene genus have been done [7]. Recently, our group has isolated pterocarpans from the methanol extract of A. fascicularis [8]. Therefore, there are no studies yet on the cytotoxic active principles of the medicinal plant A. fascicularis.
Continuing our effort to search for novel anti-cancer agents from Mayan medicinal plants of the Yucatan peninsula, we performed a guided fractionation of the root bark extract to isolate and identify the cytotoxic compound(s) from this plant. Thus, the aim of this study was to isolate and identify the active cytotoxic compounds from A. fascicularis.
2. Results and Discussion
2.1. Identification of Compounds 1–4
The active hexane fraction obtained from the methanol extract of A. fascicularis root bark was subjected to chromatographic purification, resulting in the isolation of four compounds. The chemical structures of all compounds were determined by the analysis of their NMR spectra (1H, 13C, HSQC and HMBC) and mass spectra and by comparison of these data with those published in the literature.
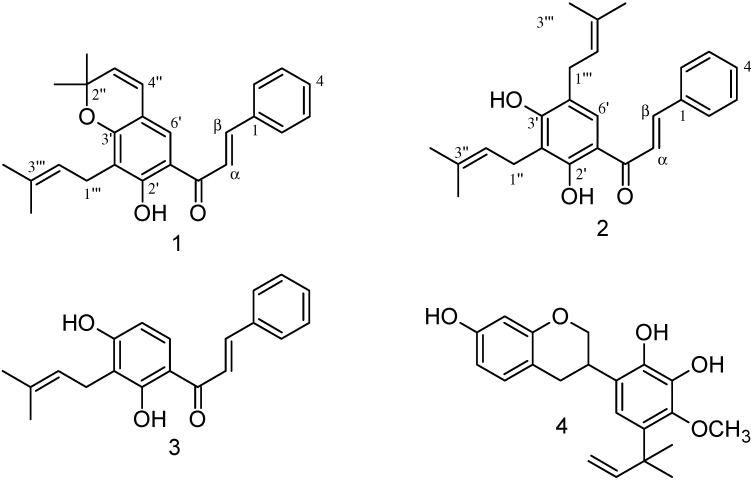
Compound 1: yellow oil. UV λmax (CHCl3) nm: 239, 308; FTIR (film) νmax cm−1: 3385, 1649, 1600, 1450, 1332, 1122; EI-MS 70 eV, m/z (relative intensity %): 374 ([M]+, 30), 359 (100), 303 (25), 255 (20), 131 (15), 103 (17). For 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3) data, see Table 1. Compound 1 showed a molecular mass of 374 a.m.u. Its FTIR spectrum showed bands at 3385 and 1649 cm−1, which in conjunction with the 1H-NMR signal of a chelated proton at δ 13.74 (1H, s), signals due to two trans-olefinic protons at δ 7.57 (1H, d, J = 15.4 Hz) and δ 7.86 (1H, d, J = 15.4 Hz), and an α,β-unsaturated carbonyl signal at δ 191.9 in the 13C-NMR spectrum, confirmed the structure of a chalcone. Additionally, the 1H-NMR spectrum showed signals in the aromatic region belonging to a singlet at δ 7.40 and two sets of multiplets at δ 7.43 (3H) and δ 7.65 (2H) characteristic of protons in penta-substituted and mono-substituted rings, respectively. The presence of two cis-olefinic protons at δ 5.59 (1H, d, J = 9.8 Hz) and δ 6.32 (1H, d, J = 9.8 Hz) along with two signals for two methyls (δ 25.9 and 18.0) and a signal at δ 77.8 due to a quaternary carbon in the 13C-NMR spectrum are consistent with the occurrence of a 2,2-dimethyl-pyran ring. Furthermore, signals at δ 1.46 (6H), δ 3.36 (1H, d, J = 7.3 Hz) and a multiplet at δ 5.26 (1H) indicate the presence of an isoprenyl group. The position of all protons and carbons was established by combining the two-dimensional HSQC and HMBC experiments. The assigned structure (Figure 1) corresponds to spinochalcone C, previously reported from Tephrosia spinosa [9].
Table 1.
NMR spectra of compounds 1 and 2.
| Position | Spinochalcone C (1) | Spinochalcone A (2) | ||
|---|---|---|---|---|
| δH (J = Hz) | δC | δH (J = Hz) | δC | |
| 1 | 135.0 | 135.2 | ||
| 2 | 7.65 m | 128.6 | 7.62 m | 128.5 |
| 3 | 7.43 m | 129.0 | 7.40 m | 129.0 |
| 4 | 7.43 m | 130.6 | 7.40 m | 130.6 |
| 5 | 7.43 m | 129.0 | 7.40 m | 129.0 |
| 6 | 7.65 m | 128.6 | 7.62 m | 128.5 |
| A | 7.57 d (15.4) | 120.6 | 7.56 d (15.4) | 120.7 |
| B | 7.86 d (15.4) | 144.0 | 7.86 d (15.4) | 144.0 |
| 1′ | 113.8 | 113.7 | ||
| 2′ | 164.4 | 162.6 | ||
| 3′ | 117.1 | 114.5 | ||
| 4′ | 158.0 | 160.4 | ||
| 5′ | 113.2 | 119.0 | ||
| 6′ | 7.40 s | 125.2 | 7.55 s | 128.6 |
| 1′′ | 3.46 d (7.0) | 22.0 | ||
| 2′′ | 77.8 | 5.30 m | 121.4 | |
| 2′′-(CH3)2 | 1.46 s | 28.6 | ||
| 3′′ | 5.59 d (9.8) | 128.7 | 135.0 | |
| 3′′-(CH3)2 | 1.76 s | 25.9 | ||
| 1.84 s | 18.0 | |||
| 4′′ | 6.32 d (9.8) | 121.7 | ||
| 1′′′ | 3.36 d (7.3) | 21.6 | 3.33 d (7.0) | 29.3 |
| 2′′′ | 5.26 m | 122.0 | 5.30 m | 122.0 |
| 3′′′ | 131.7 | 134.8 | ||
| 3′′′-(CH3)2 | 1.69 s | 25.9 | 1.79 s | 25.9 |
| 1.82 s | 18.0 | 1.80 s | 18.0 | |
| 2′-OH | 13.74 s | 13.63 s | ||
| 4′-OH | 6.31 s | |||
| C=O | 191.9 | 192.1 | ||
Figure 1.
Chemical structures from flavonoids isolated from Aeschynomene fascicularis 1–4.
Compound 2: yellow amorphous solid. UV λmax (CHCl3) nm: 238, 322; FTIR (film) νmax cm−1: 3390, 1634, 1608, 1567, 1362, 1147; 1H- (400 MHz, CDCl3) and 13C- (100 MHz, CDCl3) NMR: see Table 1; EI-MS 70 eV, m/z (relative intensity %): 376 ([M]+, 100), 333 (40), 305 (45), 265 (30), 201 (40), 161 (60), 131 (40), 103 (35). Compound 2 has a molecular mass of 376 a.m.u. A set of signals in the 1H, 13C-NMR and FTIR spectra indicated the presence of a chalcone skeleton similar to Compound 1. However, 2 presents two isoprenyl groups confirmed by the presence of methyl signals at δ 1.76, 1.79, 1.80 and 1.84 (3H each), two signals at δ 3.33 and 3.46 (2H, J = 7.0 Hz) and a multiplet at δ 5.30 (2H). The location of all protons and carbons was established by combining the two-dimensional HSQC and HMBC experiments. The assigned structure (Figure 1) corresponds to spinochalcone A, previously reported from Tephrosia spinosa [10]. Complete NMR assignments of 1 and 2 are reported here for the first time.
Compound 3: yellow amorphous solid. FTIR (film) νmax cm−1: 3231, 1629, 1613, 1480, 1444, 1362, 1234; EI-MS 70 eV, m/z (relative intensity %): 308 ([M]+, 55), 265 (90), 231 (12), 149 (100), 131 (37), 103 (40), 77 (20). 1H-NMR (400 MHz, CDCl3): δ 1.78 (3H, s, 3′′-Me), 1.85 (3H, s, 3′′-Me), 3.49 (2H, d, J = 7.0 Hz, H-1′′), 5.31 (1H, t, J = 7.0 Hz, H-2′′), 6.44 (1H, d, J = 8.9 Hz, H-5′), 7.43 (5H, m, B ring protons), 7.60 (1H, d, J = 15.4 Hz, H-α), 7.74 (1H, d, J = 8.9 Hz, H-6′), 7.89 (1H, d, J = 15.4 Hz, H-β), 13.76 (1H, s, OH). 13C-NMR (100 MHz, CDCl3): δ 18.2 (3′′-Me), 22.0 (C-1′′), 26.0 (3′′-Me), 108.1 (C-5′), 114.3 (C-1′), 114.4 (C-3′), 120.8 (C-α), 121.3 (C-2′′), 128.7 (C-2/C-6), 129.2 (C-3/C-5), 129.6 (C-6′), 130.8 (C-4), 135.1 (C-1) 136.1 (C-3′′), 144.5 (C-β), 162.0 (C-4′), 164.2 (C-2′), 192.3 (C=O). Compound 3 gave identical spectral features with those described for isocordoin, which has previously been isolated from Cordoa piaca [11] and from the root of Lonchocarpus xuul [12].
Compound 4: brown-red amorphous solid. +9.5 (c 0.02, CHCl3); UV λmax (CHCl3) nm: 238, 283; FTIR (film) νmax cm−1: 3385, 1618, 1598, 1506, 1454, 1275, 1157, 1116. In the 1H-NMR spectrum, a set of mutually-coupled five protons (δ 2.88 (1H, dd, J = 16.0, 4.0 Hz), 3.02 (1H, ddd, J = 16.0, 10.0, 3.0 Hz), 3.52 (1H, m), 4.09 (1H, t, J = 10.0 Hz) and 4.36 (1H, d, J = 10.0 Hz)) suggested the presence of an isoflavan skeleton. The 1H-NMR spectrum further exhibited the presence of a methoxyl at δ 3.74 (s), and signals of a typical α,α-dimethyl-allyl group were observed (δ 1.37 (3H, s), 1.51 (3H, s), 4.97 (1H, dd, J = 18.0, 1.0 Hz), 5.02 (1H, dd, J = 11.0, 1.0 Hz), and 6.13 (1H, ddd, J = 18.0, 11.0, 3.0 Hz). An isolated aromatic proton at δ 6.56 (1H, s) and three protons in an ABC system (δ 6.36 (1H, d, J = 3.0 Hz), 6.38 (1H, dd, J = 8.0, 3.0 Hz) and 6.92 (1H, d, J = 8.0 Hz)) indicate that an aromatic ring is penta-substituted, and another ring is tri-substituted. Characterization of Compound 4 was made by comparing the 1H-NMR spectrum to that reported for secundiflorol G, a flavan previously described as isolated from Sophora secundiflora [13]. This study is the first report on the presence of chalcones and flavans in the genus Aeschynomene and, particularly, on the contribution to the phytochemistry of the species A. fascicularis.
2.2. Biological Activities
This study was conducted towards the isolation of active compounds from the root bark of A. fascicularis. Our guided fractionation study suggests that flavan 4 and chalcones 2 and 3 are the main compounds responsible for the observed biological activity in the original extract and the active hexane fraction. The results of the bioassays towards six human cancer cell lines and their selectivity indices (SI) compared with a normal cell line are presented in Table 2, Table 3, Table 4 and Table 5.
Table 2.
Cytotoxic activity (CC50) of extract, fractions and compounds isolated from A. fascicularis root bark.
| Fractions/Compound | Cell Lines CC50 µg/mL (µM) | ||||||
|---|---|---|---|---|---|---|---|
| MDCK | Hep-2 | KB | HeLa | SiHa | DU-145 | PC-3 | |
| Crude extract | 150.3 (ND) | 55.3 (ND) | 14.0 (ND) | 16.7 (ND) | 24.0 (ND) | 23.5 (ND) | 50.2 (ND) |
| Hexane fraction | 175.4 (ND) | 33.7 (ND) | 21.5 (ND) | 18.9 (ND) | 30.1 (ND) | 29.1 (ND) | 21.6 (ND) |
| Dichloromethane fraction | - a | - | - | - | - | - | - |
| Ethyl acetate fraction | - | - | - | - | - | - | - |
| Aqueous fraction | - | - | - | - | - | - | - |
| 1 | - | - | - | - | - | - | - |
| 2 | 92.1 (245.0) | - | - | - | - | 6.1 (16.3) | 9.7 (26.0) |
| 3 | 4.2 (14.0) | 6.1 (20.0) | 11.0 (27.0) | - | - | 7.0 (23.1) | 18.4 (60.3) |
| 4 | 29.7 (83.4) | 3.9 (11.0) | 5.3 (15.0) | 8.1 (23.0) | 14.7 (41.3) | 21.5 (60.6) | 16.5 (46.4) |
| Docetaxel | 1.1 (1.4) | 0.07 (0.1) | 0.2 (0.3) | 0.19 (0.25) | 0.17 (0.22) | 0.007 (0.01) | 0.07 (0.1) |
a >100 µg/mL. ND = not determined; range of activity in µg/mL: <5 = very strong; 5–10 = strong; 10–20 = moderate; 20–100 = weak; >100 = not active [16].
Table 3.
Selectivity index (SI) of cytotoxic activity of compounds isolated from A. fascicularis root bark.
| Compound | Cell Lines SI | |||||
|---|---|---|---|---|---|---|
| Hep-2 | KB | HeLa | SiHa | DU-145 | PC-3 | |
| Crude extract | 2.7 | 10.7 | 9.0 | 6.3 | 6.3 | 3.0 |
| Hexane fraction | 5.2 | 8.1 | 9.2 | 5.8 | 6.0 | 8.1 |
| Dichloromethane fraction | - | - | - | - | - | - |
| Ethyl acetate fraction | - | - | - | - | - | - |
| Aqueous fraction | - | - | - | - | - | - |
| 1 | - a | - | - | - | - | - |
| 2 | - | - | - | - | 15.0 | 9.4 |
| 3 | 0.7 | 0.5 | - | - | 0.6 | 0.23 |
| 4 | 7.6 | 5.5 | 3.6 | 2.0 | 1.3 | 1.8 |
| Docetaxel | 14.0 | 4.6 | 5.6 | 6.3 | 140 | 14.0 |
a Undetermined; not selective when SI < 10 [12].
Table 4.
Antiproliferative activity (IC50) of compounds isolated from A. fascicularis root bark.
| Compound | Cell Lines IC50a µg/mL (µM) | ||||||
|---|---|---|---|---|---|---|---|
| MDCK | Hep-2 | KB | HeLa | SiHa | DU-145 | PC-3 | |
| 1 | - a | - | - | - | - | - | - |
| 2 | 18.7 (50.0) | - | 6.0 (16.0) | - | - | - | - |
| 3 | 18.4 (60.8) | - | - | - | - | 13.3 (43.8) | - |
| 4 | 25.4 (72.0) | - | - | - | 21.2 (60.0) | - | - |
| Docetaxel | 0.11 (0.14) | 0.05 (0.07) | 0.04 (0.06) | 0.03 (0.04) | 0.07 (0.1) | 0.01 (0.02) | 0.007 (0.01) |
a >100 µM; range of activity in µg/mL: <5 = very strong; 5–10 = strong; 10–20 = moderate; 20–100 = weak; >100 = not active [16].
Table 5.
Selectivity index (SI) of antiproliferative activity of compounds isolated from A. fascicularis root bark.
| Compound | Cell Lines IC50 SI | |||||
|---|---|---|---|---|---|---|
| Hep-2 | KB | HeLa | SiHa | DU-145 | PC-3 | |
| 1 | - a | - | - | - | - | - |
| 2 | - | 3.1 | - | - | - | - |
| 3 | - | - | - | - | 1.4 | - |
| 4 | - | - | - | 1.2 | - | - |
| Docetaxel | 2.0 | 2.3 | 3.5 | 1.4 | 7 | 14 |
a Undetermined; not selective when SI < 10 [17].
Compound 1 did not show any activity on tested cell lines to the maximum concentration (100 µM).
Compound 2 showed good cytotoxic activity against the DU-145 and PC-3 cell line; moreover, this compound showed high selectivity compared with a normal cell line. Interestingly, only this compound showed good antiproliferative activity toward the KB cell line (Table 4).
Compound 3 showed cytotoxic activities on Hep-2, KB and DU-145 cell lines; however, this compound showed poor selectivity (SI) towards all tested cell lines. Cytotoxic activity of isocordoin (3) on ovarian, prostate and leukemia human cancer cell lines has been documented [14,15]. Furthermore, isocordoin has been shown to be active against the parasites Leishmania mexicana and Trypanosoma cruzi [14]. The present work contributes to showing isocordoin to be cytotoxic against Hep-2, HeLa and SiHa cancer cell lines, in which this molecule had not been tested before.
Secundiflorol G (4) showed potent activity and moderate selectivity towards oral-laryngeal cancer cell lines Hep-2 and KB. Previously, the cytotoxic activity of various isoflavans had been reported against mouth cancer cell lines [18,19] and is consistent with the results obtained in this work for secundiflorol G (4) towards KB and Hep-2 lines. Therefore, we propose the potential use of secundiflorol G in oral-laryngeal cancer chemotherapy.
This is the first report on the pharmacology of Compounds 1, 2 and 4. Furthermore, it can be deduced that cytotoxic activity observed for the methanol extract and hexane fraction can be attributed to Compounds 2, 3 and 4. The mechanism of action by which chalcones and isoflavans exert their cytotoxic activity remains undiscovered; however, Sabzevari [20] described chalcone-type compounds to have a mechanism of free radical toxicity. In addition to this, it has been elucidated that the main mechanism of action of chalcones is inducing in vitro and in vivo apoptosis [21,22,23]. By contrast, the mechanism by which isoflavans exert their cytotoxicity has not been well elucidated.
Finally, spinochalcone A (2) is the most abundant compound present, as much as 30% of the hexane fraction of A. fascicularis root bark (measured by GC-MS), and this could be exploited to develop 2 as a potential marker molecule (together with 3 and 4) to standardize the hexane fraction of A. fascicularis as a phytomedicine. In addition to this, it is worth highlighting the antiproliferative effect that 2 presented against the KB cell line, providing an added value to the development of A. fascicularis as a phytomedicine for the treatment of oral-laryngeal cancer. At present, cancer therapies are using multiple cytotoxic drugs acting synergistically, in order to enhance the curative potency of all substances together. This emphasizes the effectiveness of a phytomedicine prepared from the hexane fraction of A. fascicularis.
The present study suggests that chalcones 2 and 3 and flavan 4 are the main compounds responsible for the observed biological activity in the original extract of A. fascicularis and are potential candidates for drug development based on their effective cytotoxic and antiproliferative activities.
3. Experimental Section
3.1. General
UV spectra were taken in CH2Cl2 using a Beckman Coulter DU-650 spectrophotometer (Brea, CA, USA). The FTIR spectra were measured with a Nicolet Protégé spectrophotometer (Madison, WI, USA). NMR spectra (1H, 13C, DEPT, COSY, HSQC and HMBC) were acquired on a Bruker Avance (Billerica, MA, USA) 400 Ultra Shield spectrometer and TMS was used as an internal standard. Low-resolution mass spectra were taken on an Agilent Technologies (Santa Clara, CA, USA) 6890N gas chromatograph coupled to a 5975B mass spectrometer (GC-MS). Column chromatography was carried out on silica gel 60 (70–230 mesh) from Merck (Darmstadt, Germany) and Sephadex LH-20 from Sigma (St. Louis, MO, USA). TLC was performed on precoated silica gel 60 F254 (0.25 mm) (Merck). Spots on TLC were visualized under UV light and by spraying phosphomolybdic acid reagent, followed by heating. The optical density of the developed plates with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma) and sulforhodamine B (SRB, Sigma) was read in a ThermoSpectronic spectrophotometer from Bio-Rad (Hercules, CA, USA).
3.2. Plant Material
Root bark of A. fascicularis was collected in May 2009 from Yaxcabá, Yucatan (latitude 20°38′06′′N; longitude 88°48′42′′W) (Mexico). The plant material was identified and authenticated by a taxonomist from the Natural Resources Unit of the Centro de Investigación Científica de Yucatán (CICY). Specimens under the voucher number P. Simá 2997 were deposited at CICY’s U Najil Tikin Xiw herbarium.
3.3. Isolation of Active Compounds
Dried powdered root bark (594 g) was extracted by maceration at room temperature with MeOH (3 × 500 mL) for 24 h. The supernatants were filtered and evaporated under vacuum by means of a rotary evaporator to obtain a dried extract. The MeOH extract (84 g) was suspended in 500 mL MeOH/H2O (1:3) and extracted using solvents of increasing polarity (3 × 1000 mL): hexane, CH2Cl2 and EtOAc. The solvents were removed using a rotary evaporator (40 °C) to obtain the hexane, dichloromethane, ethyl acetate and aqueous fractions. Only the hexane fraction showed cytotoxic activity against the Hep-2 cancer cell line (CC50 = 18 µg/mL).
The active hexane fraction (6.4 g) was submitted to a vacuum liquid chromatography (VLC) on silica gel using mixtures of hexane (Hx) and acetone (An) of ascending polarity to yield 11 fractions, which were pooled into six final fractions (A1–A6), according to their similarity on TLC. Fractions A2 and A3 were each purified by silica gel column chromatography (CC) under isocratic elution with Hx/An (9:1) to yield spinochalcone C (1, 60 mg) and spinochalcone A (2, 65 mg), respectively. Fraction A4 was explored using a Sephadex LH-20 CC to obtained four fractions (B1–B4). Fraction B2 was eluted with MeOH (100%) and was further subjected to CC silica gel using the isocratic system Hx/An (8:2) to obtain Compound 3 (5 mg). Finally, from Fraction A6, Compound 4 (20 mg) was isolated by means of a CC silica gel using the solvent system CH2Cl2/MeOH (9:1).
3.4. Cell Culture
Three types of cancer cell lines obtained from the American Type Culture Collection (ATCC) were cultured: two oral-laryngeal cancer cell lines, nasopharynx carcinoma (KB, ATCC-CCL-17) and laryngeal carcinoma (Hep-2, ATCC-CCL-23); two cervix-uterine cancer cell lines, cervix adenocarcinoma (HeLa, ATCC-CCL-2) and cervix squamous carcinoma (SiHa, ATCC-HTB-35); and two prostate cancer cell lines, prostate carcinoma (DU-145, ATCC-HTB-81) and prostate adenocarcinoma (PC-3, ATCC-CRL-1435); along with one normal cell line (MDCK, ATCC-CCL-34). The cells were cultured in sterile Costar T75 flasks containing DMEM (Gibco, Hong Kong, China), supplemented with fetal bovine serum (10%, v/v), 100 U/mL penicillin G, and 100 mg/mL streptomycin at 37 °C in an atmosphere of 5% CO2 (95% humidity). At 70%–80% confluence, cells were detached from the cultured flask by treatment with 0.05% trypsin-EDTA (Gibco), and a suspension of 5 × 104 cells/mL of viable cells was seeded in a 96-well microtiter plate (Costar, Washington, DC, USA) and incubated.
3.5. Cytotoxicity Assay
Cytotoxicity was assessed by the MTT assay as previously described by Rahman [24]. When cells reached 90% confluence in a microtiter plate, the DMEM was replaced with fresh medium and cells were incubated with compounds at various concentrations. After 72 h of incubation, 10 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma) solution (5 mg/mL) were added to each well and incubated at 37 °C for 4 h. The medium was aspirated, and the formazan product was solubilized with acidified isopropanol (0.4 N HCl). The amount of MTT-formazan is directly proportional to the number of living cells and was determined by measuring the optical density (OD) at 590 nm using a bioassay reader (Bio-Rad). All determinations were performed in triplicate.
3.6. Antiproliferative Assay
The growth inhibition of cancer cells was evaluated by the sulforhodamine B method [25]. When cells reached 70% confluence in a microtiter plate, the medium was replaced with DMEM 5% FBS and samples at various concentrations. After 48 h, the medium was discarded, and cells were fixed by adding 50 μL of 10% trichloroacetic acid (TCA, Sigma-Aldrich Chemical, St. Louis, MO, USA). The cells were then incubated at 4 °C for 30 min; TCA was drained off, and the plates were left to dry. Then, 50 μL of SRB stain (10 mg of 1% acetic acid, Sigma) were added to each well for 30 min. Finally, the plates were washed four times with 1% acetic acid (100 μL). The OD was measured at 540 nm using an ELISA reader (Bio-Rad). Docetaxel was used as a positive control, whereas cells incubated only with 0.05% of DMSO were used as a negative control. All determinations were performed in triplicate.
3.7. Data Analysis
The cytotoxic and antiproliferative activities of tested compounds were calculated as the percentage of cells killed or the percentage of inhibition of cell proliferation, respectively, using the equation: growth inhibition (%) = (ODcontrol − ODsample/ODcontrol) × 100. Results are expressed as the concentration of compound that killed 50% of the cells (CC50) and that reduced cell growth by 50% (IC50), respectively, and they were calculated using the software GraphPad Prism 4. In addition, the level of cytotoxicity on normal cells was evaluated by determining the selectivity index (SI) of each compound, which is calculated as the ratio of cytotoxicity or reduced cell growth on normal cells to cancer cells (SI = normal cells/cancer cells) [26]. It is generally considered that biological efficacy is not due to general toxicity when SI ≥ 10 [17].
4. Conclusions
The present study suggests that chalcones 2 and 3 and flavan 4 are the main compounds responsible for the observed biological activity in the original extract of A. fascicularis and are potential candidates for drug development based on their effective cytotoxic and antiproliferative activities.
Acknowledgments
We wish to thank Paulino Simá-Polanco from the Natural Resources Unit (CICY) for his help in the identification and recollection of the plant material. This work was supported by CONACYT Grant Number CB 2010-156755.
Abbreviations
- λmax
wavelength of maximum absorption
- νmax
frequency of the maximum height in the infrared spectrum
- a.m.u.
atomic mass unit
- COSY
correlation spectroscopy
- DMSO
dimethyl sulfoxide
- DEPT
distortionless enhancement by polarization transfer
- EI-MS
electronic impact mass spectrum
- FTIR
Fourier transform infrared spectroscopy
- HSQC
heteronuclear single quantum coherence
- HMBC
heteronuclear multiple bond correlation
- NMR
nuclear magnetic resonance
- OD
optic density
- TLC
thin-layer chromatography
Author Contributions
Edgar E. Caamal-Fuentes, Sergio R. Peraza-Sánchez and Rosa E. Moo-Puc designed research; Edgar E. Caamal-Fuentes, Sergio R. Peraza-Sánchez, Luis W. Torres-Tapia and Rosa E. Moo-Puc performed research and analyzed the data; Edgar E. Caamal-Fuentes wrote the paper. Sergio R. Peraza-Sánchez and Rosa E. Moo-Puc have directed the whole research. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare that they have no conflict of interests.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Vázquez-Domínguez E., Arita H.T. The Yucatan peninsula: Biogeographical history 65 million years in the making. Ecography. 2010;33:212–219. [Google Scholar]
- 2.Osadao R. El Libro del Judío o Medicina Doméstica, Descripción de las Virtudes de las Yerbas Medicinales de Yucatán. 2nd ed. Volume 1. Dra. Dorothy Andrew de Zapata; Mérida, Mexico: 1834. pp. 20–255. [Google Scholar]
- 3.Flores J., Espejel-Carvajal I. Etnoflora Yucatanense: Tipos de Vegetación de la Península de Yucatán. Universidad Autónoma de Yucatán, Sostenibilidad Maya; Mérida, Yucatán, México: 1994. Fascículo 3. [Google Scholar]
- 4.Flores J. Etnoflora Yucatanense: Leguminoseae. Florística, Etnobotánica y Ecología. Volume 18 Universidad Autónoma de Yucatán; Mérida, Yucatán, México: 2001. [Google Scholar]
- 5.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caamal-Fuentes E., Torres-Tapia L.W., Simá-Polanco P., Peraza-Sánchez S.R., Moo-Puc R. Screening of plants used in Mayan traditional medicine to treat cancer-like symptoms. J. Ethnopharmacol. 2011;135:719–724. doi: 10.1016/j.jep.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Fullas F., Kornberg L.J., Wani M.C., Wall M.E., Farnsworth N.R., Chagwedera T.E., Kinghorn A.D. Two new aromatic constituents from the rootwood of Aeschynomene mimosifolia. J. Nat. Prod. 1996;59:190–192. doi: 10.1021/np960052v. [DOI] [PubMed] [Google Scholar]
- 8.Caamal-Fuentes E., Moo-Puc R., Torres-Tapia L.W., Peraza-Sanchez S.R. Pterocarpans from the root bark of Aeschynomene fascicularis. Nat. Prod. Commun. 2013;8:1421–1422. [PubMed] [Google Scholar]
- 9.Rao E.V., Prasad Y.R. Prenylated flavonoids from Tephrosia spinosa. Phytochemistry. 1992;32:183–185. [Google Scholar]
- 10.Rao E.V., Prasad Y.R. Two chalcones from Tephrosia spinosa. Phytochemistry. 1992;31:2121–2122. [Google Scholar]
- 11.De Lima O.G., Marini-Bettolo G.B., de Méllo J.F., delle Monache F., de Barros Coélho J.S., de Andrade Lyra F.D., Fernandes de Alburquerque M.M. C- and O-prenylated chalcones from Cordoa piaca. Gazz. Chim. Ital. 1973;103:771–777. [Google Scholar]
- 12.Borges-Argáez R., Peña-Rodríguez L.M., Waterman P. Flavonoids from two Lonchocarpus species of the Yucatan Peninsula. Phytochemistry. 2002;60:533–540. doi: 10.1016/s0031-9422(02)00131-0. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T., Ohyama M., Iinuma M., Shirataki Y., Komatsu M., Charles L.B. Isoflavonoids from Sophora secundiflora, S. arizonica and S. gypsophila. Phytochemistry. 1998;48:1187–1193. [Google Scholar]
- 14.Borges-Argáez R., Balnbury L., Flowers A., Giménez-Turba A., Ruiz G., Waterman P.G., Peña-Rodríguez L.M. Cytotoxic and antiprotozoal activity of flavonoids from Lonchocarpus spp. Phytomedicine. 2007;14:530–533. doi: 10.1016/j.phymed.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Borges-Argáez R., Vela-Catzín T., Yam-Puc A., Chan-Bacab M., Moo-Puc R., Cáceres-Farfán M. Antiprotozoal and cytotoxic studies on some isocordoin derivatives. Planta Med. 2009;75:1336–1338. doi: 10.1055/s-0029-1185670. [DOI] [PubMed] [Google Scholar]
- 16.Wibowo A., Ahmat N., Hamzah A.S., Sufian A.S., Ismail N.H. Malaysianol A, a new trimer resveratrol oligomer from the stem bark of Dryobalanops aromatica. Fitoterapia. 2011;82:676–681. doi: 10.1016/j.fitote.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Vonthron-Sénécheau C., Bernard-Weniger B., Ouattara M., Tra-Bi F., Kamenan A., Lobstein A., Brun R., Anton R. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected Ivorian plants. J. Ethnopharmacol. 2003;87:221–222. doi: 10.1016/s0378-8741(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez L., Rios M.Y., Esquivel C., Chávez M.I., Delgado G., Aguilar M.I., Villarreal M.L., Navarro V. Cytotoxic isoflavans from Eysenhardtia polystachya. J. Nat. Prod. 1998;61:767–770. doi: 10.1021/np970586b. [DOI] [PubMed] [Google Scholar]
- 19.Choi C.W., Choi Y.H., Cha M.R., Kim Y.S., Yon G., Kim Y.K., Choi S.U., Kim Y.H., Ryu S.Y. Antitumor components isolated from the heartwood extract of Dalbergia odorifera. J. Korean Soc. Appl. Biol. Chem. 2009;52:375–379. [Google Scholar]
- 20.Sabzevari O., Galati G., Moridani M.Y., Siraki A., O’Brien P.J. Molecular cytotoxic mechanisms of anticancer hydroxychalcones. Chem. -Biol. Interact. 2004;148:57–67. doi: 10.1016/j.cbi.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Tabata K., Motani K., Takayanagi N., Nishimura R., Asami S., Kimura Y., Ukiya M., Hasegawa D., Akihisa T., Suzuki T. Xanthoangelol, a major chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma and leukemia cells. Biol. Pharm. Bull. 2005;28:1404–1407. doi: 10.1248/bpb.28.1404. [DOI] [PubMed] [Google Scholar]
- 22.Zi X., Simoneau A.R. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005;65:3479–3486. doi: 10.1158/0008-5472.CAN-04-3803. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura R., Tabata K., Arakawa M., Ito Y., Kimura Y., Akihisa T., Nagai H., Sakuma A., Kohno H., Suzuki T. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol. Pharm. Bull. 2007;30:1878–1883. doi: 10.1248/bpb.30.1878. [DOI] [PubMed] [Google Scholar]
- 24.Rahman A., Choudhary M.L., Thonsen W.J. Bioassay Techniques for Drug Development. Harwood Academic Publishers; Amsterdam, The Netherlands: 2001. pp. 28–29. [Google Scholar]
- 25.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 26.Mena-Rejon G., Caamal-Fuentes E., Cantillo-Ciau Z., Cedillo-Rivera R., Flores-Guido J., Moo-Puc R. In vitro cytotoxic activity of nine plants used in Mayan traditional medicine. J. Ethnopharmacol. 2009;21:462–465. doi: 10.1016/j.jep.2008.11.012. [DOI] [PubMed] [Google Scholar]