Abstract
Objectives: Meconopsis integrifolia (M. integrifolia) is one of the most popular members in Traditional Tibetan Medicine. This study aimed to investigate the anticancer effect of M. integrifolia and to detect the underlying mechanisms of these effects. Methods: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and trypan blue assay were used to evaluate the cytotoxicity of M. integrifolia. Changes in cell nuclear morphology and reactive oxygen species (ROS) level were observed by fluorescent microscopy. Apoptosis ratio, DNA damage and mitochondrial membrane potential (MMP) loss were analyzed by flow cytometry. Western blotting assay was adopted to detect the proteins related to apoptosis. Immunofluorescence was used to observe the release of cytochrome C. Results: The obtained data revealed that M. integrifolia could significantly inhibit K562 cell viability, mainly by targeting apoptosis induction and cell cycle arrest in G2/M phase. Collapse in cell morphology, chromatin condensation, DNA damage and ROS accumulation were observed. Further mechanism detection revealed that mitochondrion might be a key factor in M. integrifolia-induced apoptosis. Conclusions: M. integrifolia could induce mitochondria mediated apoptosis and cell cycle arrest in G2/M phase with little damage to normal cells, suggesting that M. integrifolia might be a potential and efficient anticancer agent that deserves further investigation.
Keywords: M. integrifolia ethanol extract, K562 cells, apoptosis, mitochondria, G2/M phase arrest
1. Introduction
Tibetan medicine is one of the most important ethnic drugs in traditional Chinese medicine and has been popular worldwide, especially in India and Europe. Its usage has grown significantly in recent years because of the trend of international movement towards natural products. Tibetan medical herbs grow in extreme alpine environments that include high altitude, hypothermic and hypoxic conditions, considerable temperature ranges and strong exposure to sun and ultraviolet radiation, which makes these medical plants more effective, especially on cardiovascular and rheumatism diseases. The genus Papaver (Papaveraceae), one of the most popular stars in Tibetan medicine, comprises approximately 80 types of annual, biennial and perennial herbs, mainly distributed in Central and Southwestern Asia, Central and Southern Europe and North Africa [1,2]. Meconopsis, an endangered genus of ornamental flowers, belongs to the Papaveraceae family. It has been used to treat multiple diseases for hundreds of years in Tibet [3]. Genus Meconopsis comprises approximately 50 species, wherein, 43 species are mainly distributed in the Qinghai-Tibetan Plateau (QPT) and the neighboring mountains, except for a few that inhabit in Europe [4,5]. Meconopsis species have been increasingly attractive for scholars not only because of their beautiful flowers but also their medicinal functions. Some Meconopsis species have been reported to have anti-inflammatory and antioxidant effects [6,7]. For example, Meconopsis integrifolia (M. integrifolia) Franch, belonging to the Meconopsis species, was firstly reported to treat hepatitis, pneumonia, and edema in the eighth century. M. integrifolia is a flagship species of the alpine scree in the Qinghai–Tibetan Plateau. The bright yellow flowers and leaf blade margins of the species make it distinguishable from others [5].
Modern pharmacological research on M. integrifolia has mainly focused on the hepatoprotective effects in rats. G. Zhou has proved that ethanol extract of Meconopsis integrifolia exhibited excellent hepatoprotective effects in vitro and antioxidant activity in rats with CCl4-induced liver injury in vivo [8]. To our best knowledge, this research uniquely addresses its biological function. Furthermore, it has been reported that alkaloids, flavonoids, and phenylpropanoids are abundant in the Meconopsis species including M. integrifolia [9]. Studies have also shown that flavonoids are relatively high in M. integrifolia [10]. It is well established that many flavonoids have outstanding anticancer effects. For example, studies revealed that quercetin could induce apoptosis in tumor cells [11,12]. Taken together, in this study, we chose K562 cells, which have been reported to be quite resistant to conventional anti-tumor drugs, as the subject to study the tumor inhibition activity of M. integrifolia extract. Interestingly, our results showed that K562 cells displayed a relatively high sensitivity to M. integrifolia. Our data indicated that M. integrifolia could induce K562 cell apoptosis and cell cycle arrest in G2/M phase. In addition, the role of mitochondria in M. integrifolia- induced apoptosis was elucidated in this study.
2. Results and Discussion
2.1. Cytotoxicity of M. integrifolia
In this paper, we first investigated the in vitro effects of M. integrifolia on the apoptotic cascade in K562 cells. After incubation with M. integrifolia, the cells were analyzed for their proliferation rate, nuclear alteration, cell cycle distribution, caspase activity and mitochondrial damage with the goal of elucidating the underlying mechanism of M. integrifolia induced-apoptosis.
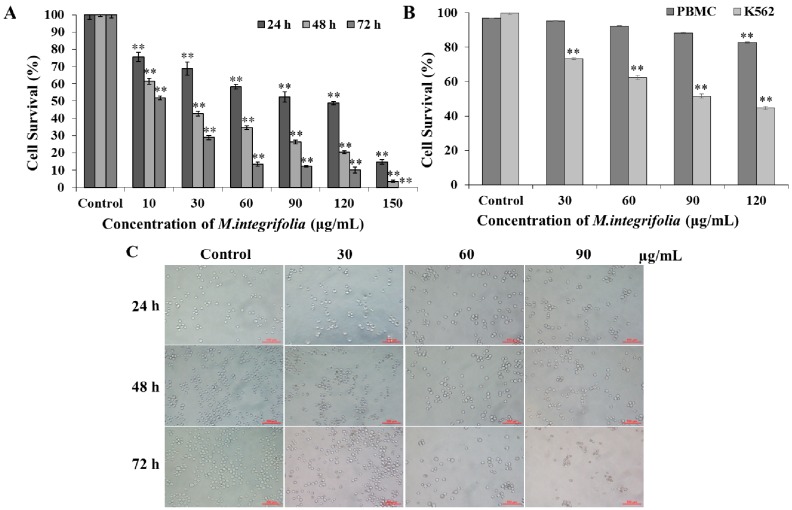
We measured K562 cell viability when treated with various doses of M. integrifolia (0, 10, 30, 60, 90, 120 and 150 μg/mL) at cell densities of 1 × 105 cells/mL. According to Figure 1A, M. integrifolia inhibited K562 cell proliferation in a dose- and incubation time-dependent manner. The IC50 values were calculated as 62.07, 19.62 and 10.85 μg/mL at 24, 48 and 72 h, respectively.
Figure 1.
Cell viability tests. (A) K562 cells viability after M. integrifolia treatment was measured by MTT assay; (B) Cytotoxicity effect of M. integrifolia on PBMCs and K562 cells through Typan blue assay; (C) After treatment with different concentrations of M. integrifolia for 24, 48 and 72 h, cell morphology was observed by a phase-contrast microscopy. Each value was expressed as a mean ± S.D. of at least three independent determinations. One-way ANOVA was used for comparisons of multiple group means followed by Dunnett’s t-test. ** p < 0.01 vs. Control. (Error bars = S.D., n = 3).
For comparison, Figure 1B reflected the cytotoxicity of M. integrifolia on human peripheral blood mononuclear cells (PBMCs), much weaker cell damage was observed in PBMCs compared with cancer cells under the same dose of M. integrifolia, suggesting M. integrifolia has some selective tumor cell killing effect.
According to the cell viability test, three doses of M. integrifolia (0, 30, 60 and 90 μg/mL) were chosen for subsequent experiments in this study. To further confirm the data from cytotoxicity evaluation, alterations in cell number after M. integrifolia extract incubation were observed. As shown in Figure 1C, more serious decrease in cell number after M. integrifolia treatment was observed as the dose of the extract and incubation time increased.
The data presented above demonstrated that M. integrifolia could significantly decrease K562 cell viability as the drug dose and incubation time increased without damaging the normal cells.
2.2. M. integrifolia Causes Cell Cycle Arrest in K562 Cells
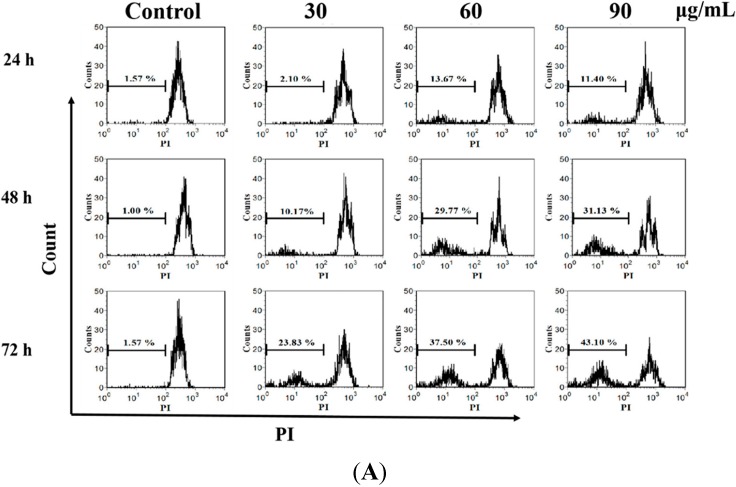
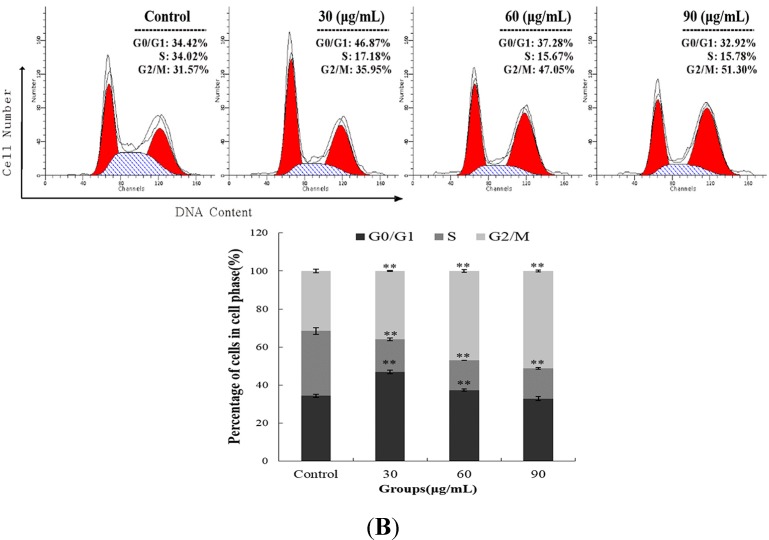
DNA damage causes genomic instability and ultimately may lead to cancer. Paradoxically, induction of DNA damage, such as DNA double-strand break (DSB), has been shown to be an effective cancer treatment [13]. Yukyung Karne, a traditional Tibetan medicine was able to cause DNA fragmentation and induce cell cycle arrest in many cancer cells [14]. Thus, DNA damage was evaluated in the present study. As shown in Figure 2A, we found that cells treated by M. integrifolia extract displayed an increase in DNA damage in a dose- and time-dependent manner. M. Integrifolia at 90 μg/mL triggered a 7.32-fold increase in DNA damage in K562 cells after 24 h treatment, a 27.45-fold (p < 0.01) increase for 48 h, and a 31.13-fold (p < 0.01) increase for 72 h. Moreover, preventing tumorigenesis often involves signal transduction pathway modulation, resulting in cell cycle arrest and, eventually, apoptosis [15,16,17]. Therefore, cell-cycle analyses were performed to determine whether the suppression of the proliferative effect of M. integrifolia was associated with uncoupling of the cell-cycle progression profile. The data presented in Figure 2B demonstrate that M. integrifolia can arrest K562 cells in G2/M phase. After 90 μg/mL M. Integrifolia treatment for 24 h, the accumulation of K562 cells in the G2/M phase increased from 30.98% to 51.58% (p < 0.01), while the proportion of cells in G1 phase decreased from 35.36% to 32.34% and the proportion of cells in S phase were decreased from 32.01% to 16.05% (p < 0.01).
Figure 2.
DNA damage and cell cycle arrest post M. integrifolia treatment. (A) Effect of M. integrifolia on DNA fragmentation of K562 cells. K562 cells were treated with M. integrifolia for 24, 48 and 72 h, then stained with propidium iodide (PI) and analyzed by flow cytometry; (B) Cell cycle analysis of M. integrifolia-treated cells. K562 cells were harvested and fixed in 70% alcohol and then stained with PI. Finally the stained cells were analyzed using a flow cytometer. ** p < 0.01 vs. Control. (Error bars = S.D., n = 3).
2.3. M. integrifolia Triggers Cell Apoptosis in K562 Cells
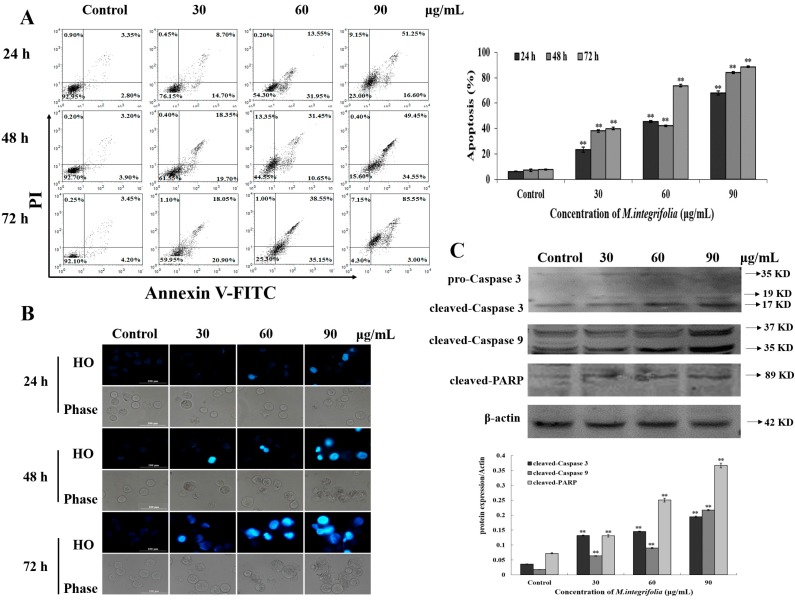
Evidence has shown that alkaloids, flavonoids, and phenylpropanoids are abundant in the Meconopsis species [9]. Additionally, the reports show that flavonoids are relatively higher than alkaloids in M. integrifolia alcohol extract [10], which could represent the functional constituents. At the same time, there are lots of reports demonstrating that flavonoids can induce cancer cell programmed death, which is called apoptosis [18,19]. For example, Kaempferol has recently been reported to suppress proliferation, induce cell cycle arrest and promote apoptosis in various human cancer cell lines. Diosmin was found to be the most potent genotoxic agent in DU145 cells, which resulted in its pro-apoptotic activity [18,19]. Based on these observations, we found that M. integrifolia could significantly inhibit K562 cell viability, and we speculated that the cell death caused by M. integrifolia could be apoptosis. To confirm this hypothesis, Annexin V/FITC was applied to evaluate the apoptosis ratio induced by the extract. As expected, the results (Figure 3A) indicated that M. integrifolia could induce K562 cell apoptosis. The apoptosis ratio increased from 23.4% to 67.8% accompanied by the increase of M. integrifolia from 30 to 90 μg/mL, while the control was 6.15%. A similar trend was observed after treatment for 48 and 72 h. The apoptosis ratio was in accordance with the cell mortality obtained from MTT assay, suggesting that M. integrifolia might primarily trigger K562 cell apoptosis. Membrane blebbing, cellular shrinkage, chromatin condensation and formation of apoptotic bodies are always the main morphological characteristics of apoptosis [20,21]. Therefore, we performed HO (Hoechst 33342) staining to further confirm the apoptosis induced by M. integrifolia. Data in Figure 3B clearly shows that M. integrifolia extract induced several features of apoptosis, such as condensed chromatin and fragmented punctate blue nuclear fluorescence in K562 cells. Caspase-3, one of the key executioners of apoptosis, is responsible for the proteolytic cleavage of many key proteins, such as the nuclear enzyme poly (ADP-ribose) polymerase (PARP) [22]. Herein, our results (Figure 3C) showed cleaved-Caspase 3 and cleaved-PARP expression was up-regulated after M. integrifolia extract incubation. Taken together, M. integrifolia-induced apoptosis might initiate in a caspase-dependent manner.
Figure 3.
Apoptosis detection in M. integrifolia-treated cells. (A) The quantification of apoptotic cells. K562 Cells were double-stained with Annexin V-FITC and PI, and then analyzed by flow cytometry; (B) Effects of M. integrifolia on cell morphology and nucleus of K562 cells. Cells treated for 24, 48 and 72 h were stained with Hoechst 33342. Morphological changes were observed under fluorescent microscope. All experiments were done independently in triplicate per experimental point, and representative results were shown. The results represented the mean ± S.D. of three independent experiments. ** p < 0.01 indicated statistically significant differences vs. Control; (C) Effects of M. integrifolia on the expression of some key apoptotic proteins in K562 cells. K562 cells were treated with M. integrifolia for 24 h. Western blot analysis was performed in triplicate per experimental point; β-actin was used as reference control.
2.4. Mitochondria Play a Key Role Following M. integrifolia Incubation
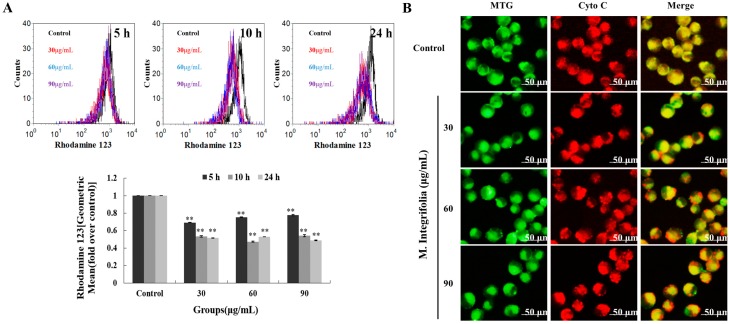
Mitochondria are thought to be the major pathway for apoptosis, and, therefore, targeting them is a novel strategy for cancer therapy [23,24]. Mitochondria mediated apoptosis is highly regulated by the balance between the expression of pro- and anti-apoptotic proteins in the Bcl-2 family proteins [25,26]. If this balance is broken, the direct consequence is a loss of the mitochondrial transmembrane potential (Δψm) [27,28]. Thus, MMP (mitochondria membrane potential) loss was first analyzed. As shown in Figure 4A, significant MMP loss was detected after M. integrifolia incubation. The reduction of membrane potential leads to the release of mitochondrial cytochrome C, which is the key factor that results in the formation of apoptosomes. Our investigation revealed that M. integrifolia could significantly induce the release of mitochondrial cytochrome C (Figure 4B), which was in accordance with the results above. In addition, Caspase-9, another key factor involved in the mitochondria-mediated apoptosis, is always activated by cytochrome C. In our study, the protein activated the effector Caspase-3 and subsequently increased the cleavage of PARP expression and, finally, induced cell apoptosis. Western blot analysis (Figure 3C) also showed that Caspase-9/3 and PARP were all involved in M. integrifolia induced apoptosis in K562 cells. These results clearly indicated that M. integrifolia induced apoptosis via mitochondrial pathways.
Figure 4.
The alterations of mitochondria triggered by M. integrifolia. (A) Effect of M. integrifolia on mitochondrial membrane potential of K562 cells. Cells were treated with M. integrifolia for 5, 10 and 24 h. Then the cells were labeled with Rhodamine 123 and analyzed by flow cytometry. Histograms show number of cell channel (vertical axis) vs. Rhodamine 123 fluorescence (horizontal axis). ** p < 0.01 vs. Control. (Error bars = S.D., n = 3); (B) Detection of release of cytochrome C from mitochondria in K562 cells after M. integrifolia treatment.
2.5. M. integrifolia Initiates ROS Accumulation in K562 Cells
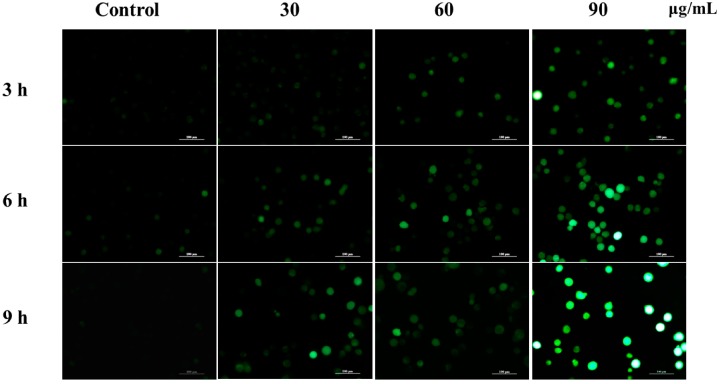
ROS plays an important role in the apoptosis investigation and is mainly produced in mitochondria. Mounting evidence proved that the increasing ratio of Bax and Bcl-2 could decrease MMP, induce the release of cytochrome C, and eventually result in ROS accumulation. However, excessive amounts of ROS can cause oxidative damage to lipids, proteins, and DNA leading to tumorigenesis or cell death. To address the role that ROS played in M. integrifolia extract-induced apoptosis, ROS level was analyzed. In our experiment, M. integrifolia could significantly elevate ROS level in K562 cells, which indicated that ROS might have a function in the apoptosis process (Figure 5).
Figure 5.
ROS generation induced by M. integrifolia. K562 cells were treated with M. intergrifolia for 3, 6 and 9 h. The intracellular ROS level was observed under fluorescent microscope.
3. Experimental Section
3.1. Preparation for the M. integrifolia Extract
Fresh M. integrifolia plants were collected in Yushu County, Tibetan Autonomous Prefecture of Yushu Qinghai Province in August 2011 (altitude 4639 m). The plant species identities were authenticated by Associate Prof Jun-Hua Du at the School of Life and Geographical Sciences, Qinghai Normal University.
Dried M. integrifolia powder was homogenized in 95% ethanol at 45 °C using ultrasonic-assisted extraction (UAE) three times. The ethanol extract was obtained by vacuum filter, and the supernatant was collected and concentrated with a rotary evaporator (RE-2000 A; Belong, Shanghai, China). The ethanol fraction was homogenized in 75% ethanol and the supernatant was filtered with 0.22 μm filters.
3.2. Cell Cultures
K562 cells were obtained from the Institute of Chinese Academy of Medical Sciences, Beijing, China. K562 cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS), 1% penicillin–streptomycin (100 U/mL penicillin and 100 μg/mL streptomycin) and 1% glutamine in cell culture flasks under a humidified 5% CO2 and 95% air atmosphere at 37 °C.
3.3. Cell Viability Tests
The cytotoxicity of M. integrifolia extract was evaluated by MTT (Sigma, St. Louis, CA, USA) assay. Briefly, 100 µL of K562 cells suspension was placed in 96-well culture plates at densities of 1 × 105 cells/mL, and incubated with various concentrations of M. integrifolia extract (0, 10, 30, 60, 90, 120 and 150 µg/mL) for various time points (24, 48 and 72 h). Then, 10 μL MTT solution (5 mg/mL in phosphate buffer) was added into the cell suspension and the mixture was incubated for 4 h at 37 °C in a CO2 incubator. The formazan crystals were dissolved in 100 μL 10% SDS, 5% isobutene and 0.01 M HCL solution. Finally, the absorbance at 570 nm was recorded using a microplate reader (ELX800; BIO-TEK Instruments, Inc., Winooski, VT, USA). Cell survival was calculated using the following equation:
| Cell survival (%) = OD treatment group/OD control group × 100% | (1) |
The peripheral blood mononuclear cells (PBMCs) were isolated with the lymphocyte separation kit (Applygen Technologies Inc., Beijing, China) from healthy donors in the hospital of Shaanxi Normal University, China. The whole procedures were performed according to the instructions provided by the manufacturer. In this experiment, PBMCs were used as normal cells to evaluate the cytotoxicity of M. integrifolia. Briefly, K562 cells and PBMCs suspension were seeded at a density of 1 × 105 cells/mL in 24-well culture plates, respectively, and incubated with M. integrifolia at different doses (0, 30, 60, 90, and 120 µg/mL) for 24 h. Two types of cells were then stained with trypan blue and counted under the microscope (Nikon Instech Co., Ltd., Tokyo, Japan). The cell viability was calculated using the following equation:
| Cell viability (%) = number of unstained cells/total cell number × 100% | (2) |
3.4. Phase-Contrast Microscopy Observation
Cells receiving different treatments were observed by the phase-contrast microscopy (Nikon) at a 400× magnification to study the cell number. Representative micrographs were taken using NIS-Elements software.
3.5. Apoptosis Detection by Flow Cytometry
Apoptotic cells were quantified with an Annexin V–FITC Apoptosis Detection Kit (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s protocol. Briefly, cells at 1 × 105 cells/mL were incubated with various concentrations (0, 30, 60 and 90 µg/mL) of M. integrifolia extract for 24, 48 and 72 h at 37 °C. Cells were then harvested and re-suspended in the binding buffer then stained with 2 µL Annexin V–FITC and 2 µL PI (propidium iodide) for 15 min at room temperature in the dark. The apoptotic index was immediately determined by flow cytometry.
3.6. Hoechst 33342 Staining
To detect changes in nuclei morphology of K562 cells after M. integrifolia extract treatment, Hoechst 33342 staining was performed. After treatment with the indicated concentration (0, 30, 60 and 90 μg/mL) of M. integrifolia extract for 24, 48 and 72 h, cells were stained by 10 µM HO for 15 min at room temperature. Then, the stained cells were washed three times with phosphate-buffered saline (PBS) and observed using a fluorescence microscopy with standard excitation filters (Nikon). The excitation wavelength was 346 nm and the emission wavelength was 460 nm.
3.7. Cell Cycle Analysis
The ratio of cells in the G0/G1, S and G2/M phases of cell cycle was determined by examining their DNA content. Cells at 1 × 105 cells/mL were treated with various concentrations (0, 30, 60 and 90 μg/mL) of M. integrifolia extract for 24 h. Then, cells were harvested and washed twice with cold PBS and fixed with 70% ice-cold ethanol at 4 °C overnight. The fixed cells were washed twice with cold PBS and incubated with 100 µg/mL RNase A (Sigma) for 30 min at 37 °C. Cell were then stained with 50 µg/mL propidium iodide (PI; Sigma) for 30 min in the dark and analyzed by flow cytometry (Millipore, Bedford, MA, USA).
3.8. DNA Fragmentation Assay
To analyze DNA fragmentation, flow fluorocytometric detection of DNA hypoploidy after adding PI to the dying cells for permeabilization was performed [29]. The size of DNA fragments appeared as a hypoploid DNA histogram. To investigate the effect of M. integrifolia extract on DNA damage of K562 cells, oligonucleosomal DNA fragmentation by flow cytometry was performed. Cells were treated with various concentrations of M. integrifolia extract (0, 30, 60 and 90 μg/mL) for 24, 48 and 72 h, respectively, and then were stained with 5 µg/mL PI and analyzed for DNA content by flow cytometry.
3.9. Intracellular Reactive Oxygen Species Detection
In this study, we also evaluated changes in the cellular reactive oxygen species (ROS) level through the oxidative conversion of the sensitive fluorescent probe 2′,7′-dichlorofluorescein-diacetate (DCFH-DA) to fluorescent 2′,7′-dichlorofluorescein (DCF). K562 cells were treated with the mentioned concentrations (0, 30, 60 and 90 μg/mL) of M. integrifolia extract for 3, 6 and 9 h. The treated cells were harvested, washed twice with PBS, re-suspended in 500 µL of 10 µM DCFH-DA (purchased from Molecular Probes Inc., Invitrogen) and incubated at 37 °C for 30 min in the dark. The samples were observed using a fluorescence microscopy with standard excitation filters (Nikon).
3.10. Measurement of Mitochondrial Membrane Potential (MMP)
The alterations of MMP were assessed with the fluorescent probe Rhodamine 123 (Rh 123, Sigma). Rh123 selectively enters mitochondria with an intact membrane potential and is retained in the mitochondria. Once the MMP is lost, Rh123 is subsequently washed out of the cells. K526 cells (1 × 105 cells/mL) were incubated with various concentrations of M. integrifolia for 5, 10 and 24 h. Then, the treated cells were stained with 2 μg/mL Rh123 for 20 min at 37 °C. After being washed with PBS, cells were immediately analyzed by flow cytometry.
3.11. Cytochrome C Detection
Cells in each treatment group were incubated with 0.5 mM Mito-tracker Green (MTG) in phosphate-buffered saline at 37 °C for 20 min, fixed in 4% paraformaldehyde at 4 °C for 15 min, and subsequently permeabilized with 0.1% Triton X-100 at 4 °C for 5 min. Between each step, cells were washed with PBS. After permeation, cells were blocked with 5% BSA at 37 °C for 1 h and then incubated with anti-cytochrome C antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4 °C. After being washed with phosphate-buffered saline, the samples were incubated with fluorescein isothiocy-anate-conjugated secondary antibody. Finally, the cells were observed by fluorescence microscope (Nikon E 600).
3.12. Western Blotting Staining
SDS–PAGE and immunoblotting were performed according to standard procedures. Briefly, after treatment with M. integrifolia (0, 30, 60 and 90 μg/mL) for 24 h, K562 cells were lysed by RIPA buffer on ice. The protein samples were separated on a 10% SDS polyacrylamide gel, and the gel was transferred to nitrocellulose membranes (Millipore) and blotted with primary antibodies (Caspase-3, cleaved-Casepase-9, cleaved-PARP were purchased from Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C. The bound primary antibodies were then tagged with IRDye 680-conjugated IgG (Li-Cor Biosciences, Lincoln, NE, USA) at room temperature for 1 h. The infrared fluorescence was detected with the Odyssey infrared imaging system (Li-Cor Bioscience).
3.13. Statistical Analysis
All experiments were performed in triplicate, and data were expressed as the means ± SD. IC50 values were calculated by regression analysis. The data were subjected to an analysis of Duncan’s multiple range tests (SPSS, version 18.0). A significant difference was judged to exist at a level of * p < 0.05 and ** p < 0.01.
4. Conclusions
In conclusion, we first investigated the anticancer effect of M. integrifolia extract, which can block cell cycle processes and induce cell apoptosis in K562 cells. M. integrifolia extract induced-apoptosis might be mediated by the release of cytochrome C, ROS accumulation, and activation of Caspase-3 and Caspase-9. This study could provide the primary proof needed to understand this new function of M. integrifolia. As we know, natural products are increasingly popular due to their potential to improve the efficacy of conventional chemotherapy, thus, in the future, we will try to investigate the combination effect of M. integrifolia with chemotherapy drugs, hoping to apply it in a clinical setting.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81202957), the National Science Foundation for Fostering Talents in Basic Research of the National Natural Science Foundation of China (No. J1103511), Qinghai Province (Application) Basic Research Project (No. 2013-Z-762), and Shaanxi Normal University Training Programs of Innovation and Entrepreneurship for Undergraduates (No. cx15013).
Author Contributions
Jianping Fan and Quanhong Liu are responsible for designing and conducting the study, interpreting the data, and writing the manuscript. Xiaobing Wang, Pan Wang, Wei Tang, Chunliang Liu, Yaqin Wang, Lulu Kong and Wenjuan Yuan contributed to the data collection, analysis, and interpretation.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Carolan J.C., Hook I.L., Chase M.W., Kadereit J.W., Hodkinson T.R. Phylogenetics of Papaver and related genera based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL intron and trnL-F intergenic spacers. Ann. Bot. 2006;98:141–155. doi: 10.1093/aob/mcl079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapoor L.D. Opium Poppy: Botany, Chemistry and Pharmacology. Food Products Press; New York, NY, USA: 1995. [Google Scholar]
- 3.Sulaiman I.M., Hasnain S.E. Randomamplified polymorphic DNA (RAPD) markers reveal genetic homogeneity in the endangered Himalayan species Meconopsis paniculata and M. simplicifolia. Theor. Appl. Genet. 1996;93:91–96. doi: 10.1007/BF00225732. [DOI] [PubMed] [Google Scholar]
- 4.Wu C., Chuang H. A study on the taxonomic system of the genus Meconopsis. Acta Bot. Yunnanica. 1980;2:371–381. [Google Scholar]
- 5.Yang F.S., Qin A.L., Li Y.F., Wang X.Q. Great Genetic Differentiation among Populations of Meconopsis integrifolia and its Implication for Plant Speciation in the Qinghai-Tibetan Plateau. PLoS ONE. 2012;7:e37196. doi: 10.1371/journal.pone.0037196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H.F., Ding I.S., Wang H., Zhang X.F. Advances in the research of phytochemistry and pharmacology of Meconopsis Vig. Nat. Prod. Res. Dev. 2011;23:163–168. [Google Scholar]
- 7.Samant S.S. Diversity, endemism and socio-economic values of the Indian Himalayan Papaveraceae and Fumariaceae. J. Indian Bot. Soc. 2005;84:33–44. [Google Scholar]
- 8.Zhou G., Chen Y.X., Liu S., Yao X.C., Wang Y.W. In vitro and in vivo hepatoprotective and antioxidant activity of ethanolic extract from Meconopsis integrifolia (Maxim.) Franch. J. Ethnopharmacol. 2013;148:664–670. doi: 10.1016/j.jep.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Wu H.F., Pan L., Zou D.S., Yang S.J., Zhang X.F. Analysis on volatile oils from three species of Meconopsis by GC-MS. Chin. Pharm. J. 2006;41:1298–1300. [Google Scholar]
- 10.Zhou Y., Song J.Z., Choi F.F.K., Wu H.F., Qiao C.F., Ding L.S., Gesang S.L., Xu H.X. An experimental design approach using response surface techniques to obtain optimal liquid chromatography and mass spectrometry conditions to determine the alkaloids in Meconopsis species. J. Chromatogr. A. 2009;1216:7013–7023. doi: 10.1016/j.chroma.2009.08.058. [DOI] [PubMed] [Google Scholar]
- 11.Ding L., Li J.P. Study on protective effect of Meconopsis quintuplinervia Regel on experimental hepatic injury in mice. Chin. Qinghai J. Anim. Vet. 2007;37:7–8. [Google Scholar]
- 12.Brisdelli F., Coccia C., Cinque B., Cifone M.G., Bozzi A. Induction of apoptosis by quercetin: different response of human chronic myeloid (K562) and acute lymphoblastic (HSB-2) leukemia cells. Mol. Cell. Biochem. 2007;296:137–149. doi: 10.1007/s11010-006-9307-3. [DOI] [PubMed] [Google Scholar]
- 13.Li-Weber M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2013;332:304–312. doi: 10.1016/j.canlet.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Choedon T., Dolma D., Mathan G., Kumar V. Molecular insights into the anti-cancer properties of traditional Tibetan medicine Yukyung Karne. BMC Complement. Altern. Med. 2014;14:380. doi: 10.1186/1472-6882-14-380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Giono L.E., Manfredi J.J. Mdm2 is required for inhibition of CDK2 activity by p21, thereby contributing to p53-dependent cell cycle arrest. Mol. Cell. Biol. 2007;27:4166–4178. doi: 10.1128/MCB.01967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leventis P.A., Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Ann. Rev. Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 17.Hafeez B.B., Siddiqui I.A., Asim M., Malik A., Afaq F., Adhami V.M., Saleem M., Din M., Mukhtar H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: Involvement of nuclear factor-κB signaling. Cancer Res. 2008;68:8564–8572. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ndozangue-Tou riguine O., Hamelin J., Brйard J. Cytoskeleton and apoptosis. Biochem. Pharmacol. 2008;76:11–18. doi: 10.1016/j.bcp.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Naziroglu M., Cihangir Uğuz A., Koçak A., Bal R. Acetaminophen at different doses protects brain microsomal Ca2+ ATPase and the antioxidant redox system in rats. J. Membr. Biol. 2009;231:57–64. doi: 10.1007/s00232-009-9203-3. [DOI] [PubMed] [Google Scholar]
- 20.Lewinska A., Siwak J., Rzeszutek I., Wnuk M. Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol. in Vitro. 2014;29:417–425. doi: 10.1016/j.tiv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Song H., Bao J., Wei Y., Chen Y., Mao X., Li J., Yang Z., Xue Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015;33:868–874. doi: 10.3892/or.2014.3662. [DOI] [PubMed] [Google Scholar]
- 22.Gerald M. Caspases: The executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He N., Shi X., Zhao Y., Tian L., Wang D. Inhibitory effects and molecular mechanisms of selenium-containing tea polysaccharides on human breast cancer mcf-7 cells. J. Agric. Food Chem. 2013;61:579–588. doi: 10.1021/jf3036929. [DOI] [PubMed] [Google Scholar]
- 24.Fulda S., Galluzzi L., Kroemer G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 25.Chidambara Murthy K.N., Jayaprakasha G.K., Kumar V., Rathore K.S., Patil B.S. Citrus limonin and its glucoside inhibit colon adenocarcinoma cell proliferation through apoptosis. J. Agric. Food Chem. 2011;59:2314–2323. doi: 10.1021/jf104498p. [DOI] [PubMed] [Google Scholar]
- 26.Hengartner M.O. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 27.Marzo I., Brenner C., Zamzami N., Jurgensmeier J.M., Susin S.A. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 28.Indran I.R., Tufo G., Pervaiz S., Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Bba-biomembranes. 2011;1807:735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Krysko D.V. Apoptosis and necrosis: Detection, discrimination and phagocytosis. Methods. 2008;44:205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]