Abstract
The aim of this study was to construct a nanostructured lipid system as a strategy to improve the in vitro antibacterial activity of copper(II) complexes. New compounds with the general formulae [CuX2(INH)2]·nH2O (X = Cl− and n = 1 (1); X = NCS− and n = 5 (2); X = NCO− and n = 4 (3); INH = isoniazid, a drug widely used to treat tuberculosis) derived from the reaction between the copper(II) chloride and isoniazid in the presence or absence of pseudohalide ions (NCS− or NCO−) were synthesized and characterized by infrared spectrometry, electronic absorption spectroscopy, electron paramagnetic resonance (EPR) spectroscopy, elemental analysis, melting points and complexometry with 2,2′,2′′,2′′′-(Ethane-1,2-diyldinitrilo)tetraacetic acid (EDTA). The characterization techniques allowed us to confirm the formation of the copper(II) complexes. The Cu(II) complexes were loaded into microemulsion (MEs) composed of 10% phase oil (cholesterol), 10% surfactant [soy oleate and Brij® 58 (1:2)] and 80% aqueous phase (phosphate buffer pH = 7.4) prepared by sonication. The Cu(II) complex-loaded MEs displayed sizes ranging from 158.0 ± 1.060 to 212.6 ± 1.539 nm, whereas the polydispersity index (PDI) ranged from 0.218 ± 0.007 to 0.284 ± 0.034. The antibacterial activity of the free compounds and those that were loaded into the MEs against Staphylococcus aureus ATCC® 25923 and Escherichia coli ATCC® 25922, as evaluated by a microdilution technique, and the cytotoxicity index (IC50) against the Vero cell line (ATCC® CCL-81TM) were used to calculate the selectivity index (SI). Among the free compounds, only compound 2 (MIC 500 μg/mL) showed activity for S. aureus. After loading the compounds into the MEs, the antibacterial activity of compounds 1, 2 and 3 was significantly increased against E. coli (MIC’s 125, 125 and 500 μg/mL, respectively) and S. aureus (MICs 250, 500 and 125 μg/mL, respectively). The loaded compounds were less toxic against the Vero cell line, especially compound 1 (IC50 from 109.5 to 319.3 μg/mL). The compound 2- and 3-loaded MEs displayed the best SI for E. coli and S. aureus, respectively. These results indicated that the Cu(II) complex-loaded MEs were considerably more selective than the free compounds, in some cases, up to 40 times higher.
Keywords: copper(II) complexes, antibacterial activity, Staphylococcus aureus, Escherichia coli, nanostructured lipid system
1. Introduction
The ability of bacteria to acquire resistance to drugs that are used as therapeutic agents has become a significant problem because there is a daily increase in the microbial resistance to the currently used antibiotics [1]. The prospects for the use of antimicrobial drugs in the future are still uncertain and require the search for new compounds with potential activity against pathogenic bacteria and fungi [2].
Copper is the third most abundant transition element in the human body and is present in the active site of enzymes that participate in oxidative reactions, such as cytochrome oxidase and superoxide dismutase. Furthermore, it has long been recognized that copper compounds are capable of inhibiting a wide variety of fungi and bacteria [3,4].
The Cu(II) ion has a tendency to interact with molecules to yield coordination compounds with different properties, including antibacterial activity. A coordination compound or metallic complex is the product of a Lewis acid-base reaction in which neutral molecules or anions (called ligands) bind to a central metal atom (or ion) by coordinate covalent bonds [5].
There has been an increasing interest in the literature on the investigation of copper(II) complexes with antimicrobial activity [6]. The compound [Cu(TTA)2], TTA = 2-tenoyltrifluoroacetone, which was synthesized and characterized by Xu et al. [7], showed antibacterial activity against Escherichia coli and Staphylococcus aureus with MICs (minimum inhibitory concentrations) of 180 and 150 μg/mL, respectively. Pahontu et al. [8] studied the antibacterial activity of the copper(II) compound [Cu(L)(ClO4)(H2O)] (HL = 4-[(E)-(2-hydroxy-4-methoxyphenyl)methyleneamino]benzoate) against E. coli and S. aureus and showed MICs of 500 μg/mL for both bacteria.
The low solubility of metallic compounds in water makes the majority of biological activity assays impossible, particularly in vivo experiments. Therefore, researchers have sought alternatives to produce these metallic compounds through technological strategies to effectively compartmentalize this diverse group of molecules and modify their behavior in the body [9]. Innovations in the field of drug delivery systems have emerged at a much faster pace over the past two decades. The improvements in therapeutic adherence and the efficacy of the treatment are important benefits in the search for innovative pharmacological formulations [5]. The microemulsion (ME) drug delivery system has been very important in the pharmaceutical field as it can provide a modern, more efficient therapeutic alternative that can be used for substances that are normally of limited use due to their hydrophobicity, toxicity or inability to access the site of action [10,11,12].
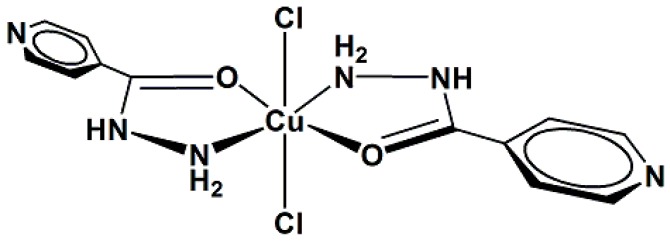
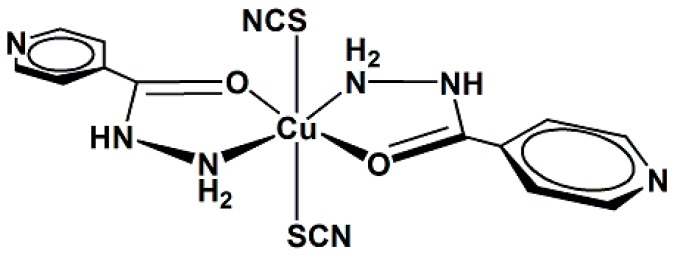
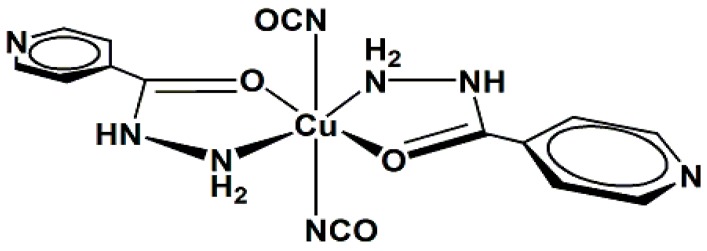
This study reports the preparation and spectroscopic characterization of three copper(II) complexes containing isoniazid (INH) as a ligand, Figure 1: [CuCl2(INH)2]·H2O (1), Figure 2, [Cu(NCS)2(INH)2]·5H2O (2), Figure 3 and [Cu(NCO)2(INH)2]·4H2O (3), Figure 4, and the incorporation of these compounds into a nanostructured lipid system consisting of 10% phase oil (cholesterol), 10% surfactant [soy oleate and Brij® 58 (1:2)] and 80% aqueous phase (phosphate buffer pH = 7.4). Then, the antimicrobial activity of the Cu(II) compounds was evaluated on two different bacterial strains (Staphylococcus aureus and Escherichia coli) and tested on VERO cells (a normal eukaryotic cell) to determine their cytotoxicity (IC50).
Figure 1.
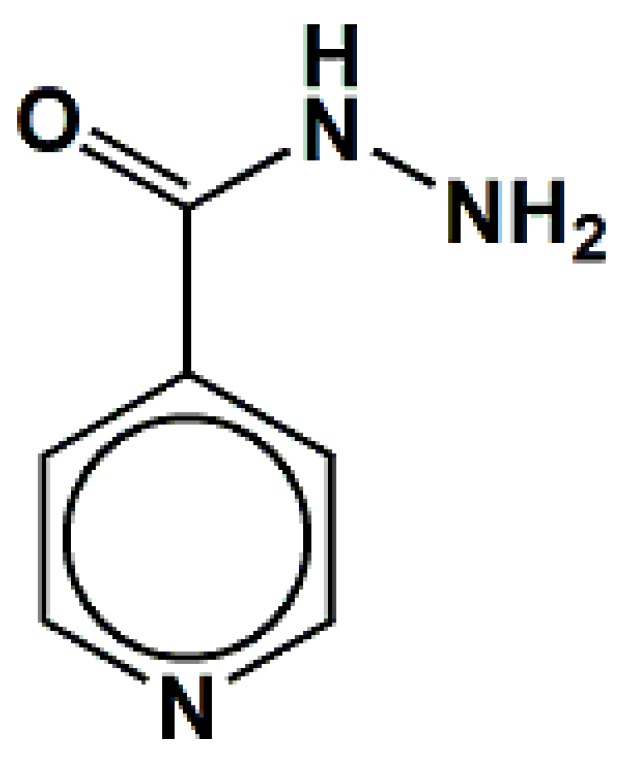
Structure for the isoniazid (INH).
Figure 2.
Proposed structure for the [CuCl2(INH)2]·H2O complex (1).
Figure 3.
Proposed structure for the [Cu(NCS)2(INH)2]·5H2O complex (2).
Figure 4.
Proposed structure for the [Cu(NCO)2(INH)2]·4H2O complex (3).
2. Results and Discussion
2.1. Chemistry
2.1.1. Infrared (IR) Spectroscopy Studies
IR spectroscopy is one of the most widely used physical methods for inferring the binding mode of the isoniazid ligand. The most important IR frequencies of the new copper(II) complexes and their assignments are presented in Table 1.
Table 1.
Selected vibrational data (cm−1) and assignments for isoniazid (INH) and the Cu(II) compounds (1–3).
| Compound | Assignment | |||
|---|---|---|---|---|
| INH | 1 | 2 | 3 | |
| - | 3437 m | 3434 m | 3449 w | νOH |
| 3306 m, 3213 sh | 3222 s | 3241 s | 3236 w | νasNH2 |
| 3112 s | 3098 s | 3101 sh | 3108 w | νsiNH2 |
| 3035 m, 3014 m | 3048 s, 3019 m | 3057 sh | 3061 sh, 3017 w | νCH |
| - | - | - | 2212 s | νasNCO |
| - | - | 2130 s | - | νasNCS |
| 1667 s | 1653 m | 1606 w | 1615 m | νC=O |
| 1635 m | 1648 s | 1648 m | 1655 sh | Scissoring NH2 |
| 1603 m | 1590 s | 1603 s | 1589 sh | νring + δCH +δHOH |
| 1556 s | 1547 s | 1554 m | 1537 m | νring |
| 1492 w | 1494 w | 1498 w | 1499 w | δCH + νring + δNH |
| 1412 m | 1415 m | 1415 m | 1416 m | δCH + νring |
| - | - | - | 1317 w | νsNCO |
| 1335 s | 1332 m | - | - | Rocking NH2 |
| - | 1284 w | 1268 w | 1283 w | νring |
| 1221 m | 1225 m, 1202 s | 1233 w, 1208 w | 1239 w | δCH + νring |
| 1141 m | 1139 m | 1131 w, 1098 w | 1131 w, 1103 w | δCH + νring + νCN |
| 1060 s | 1065 w | 1058 m | 1058 w | νring + δCH + δring |
| 996 m | 996 w | 988 w | 987 sh | νring + δring |
| 943 s | - | 941 w, 919 w | 945 w, 924 w | νNN + twisting NH2 + νNH2 |
| 888 w, 845 m | 906 w, 881 w, 858 m | 876 w | 868 sh | γCH + γC-CO-NH-NH2 |
| 747 w | 761 w | 755 w | 775 w, 756 w | γCH + τring + γCO +ν NC-S |
| - | 712 m | 715 m | 719 w | τring + γCO + γCH |
| 675 s | - | 690 m | 692 w | δring + νC-CO-NH-NH2 |
| 659 s | - | 664 sh | 658 w | δring + δCH |
| - | - | - | 620 w | δNCO |
| 504 w | 538 w | 504 w | 501 sh | τHNNH + γNH + δNCC + δC-CO-NH-NH2 + δOCC |
| 436 w | 463 w | 466 w, 430 w | 420 w | γNH + τHNNH + γCH + τring + δNCS |
ν = stretching; δ = in-plane bending; γ = out-of-plane bending; as = asymmetric; si =symmetric; s = strong, m = medium, w = weak, sh = shoulder.
The isoniazid (INH) molecule can be coordinated by the nitrogen atoms of the -NH2 group and/or pyridine ring as well as by the carbonyl oxygen. IR spectroscopy allows us to obtain valuable information regarding the participation of these groups in the coordination. When the nitrogen of the -NH2 group is involved in the coordination, there is a negative shift in the νNH mode (Δ = νligand − νcomplex) [13]. The coordination by the oxygen of the carbonyl group is suggested by the positive shift in the ν(C = O) mode (Δ = νligand − νcomplex) [14]. Some of the observed vibrations of the pyridine ring in the range of 1600–1400 cm−1 exhibit small shifts to higher frequencies after the formation of the complex [15].
The IR spectra of complexes 1–3 revealed that the νNH band showed a shift of 3213 cm−1 (ligand free) to 3222 ( = −9 cm−1), 3241 ( = −28 cm−1) and 3236 cm−1 ( = −23 cm−1) in compounds 1–3, respectively. This alteration is typical of the coordination of the metal to the nitrogen atom of the -NH2 group. The absorptions in the IR spectra that were related to the vibrations associated with the pyridine ring of the INH did not exhibit significant shifts to higher frequencies after coordination, suggesting that the heterocyclic nitrogen atom does not participate in coordination. The ν(C = O) band in compounds 1–3 was displaced to a lower frequency, from 1667 (free ligand) to 1653, 1606 and 1615 cm−1, respectively, suggesting coordination by the oxygen atom of the carbonyl group. The presence of hydration water in compounds 1–3 is clearly detected by the appearance of its characteristic absorptions at 3500–3600 cm−1 (νOH) and 1655 cm−1 (δHOH) [14].
The IR spectra of the compound 2 exhibit a very intense band at approximately 2130 cm−1, which was attributed to the νas(NCS) vibrational mode, the typical spectral range of the terminal coordination by the sulfur atom [16].
The shift of the 2212, 1317 and 620 cm−1 bands of the cyanate group related to the νas(CN), νs(CO) and δ(NCO) vibrational modes, respectively, for compound 3 confirms the coordination of the pseudohalide of terminal mode by the nitrogen atom [17].
2.1.2. Ultraviolet-Visible (UV-Vis) Spectroscopy Studies
The electronic absorption data of the Cu(II) compounds and their attributions are listed in Table 2. The strong bands observed at 249–273 nm were assigned to the Intraligand Transition (IL) of the isoniazid ligand. The Ligand to Metal Charge Transfer (LMCT) transitions of the Cl−→Cu(II) [18], NCS−→Cu(II) [19] and NCO−→Cu(II) [20] types were detected at ~352, 353 and 356 nm, respectively. The appearance of a broad and very low intensity band at 678–688 nm was assigned to the spin-allowed 2Eg←2T2g transition, which is typical of octahedral Cu(II) compounds (d9 electronic systems) [21].
Table 2.
UV-Vis spectral data a of INH and the Cu(II) complexes (1.0 × 10−3 mol·L−1).
| Compound | IL Transition | LMCT Transition | LF Transition | Ref. |
|---|---|---|---|---|
| INH | 249 (3120) | - | - | This work |
| [CuCl2(INH)2]·H2O (1) | 259 (2930) | 352 (1170) | 678 (228) | This work |
| [Cu(NCS)2(INH)2]·5H2O (2) | 249 (3000) | 353 (1000) | 688 (184) | This work |
| [Cu(NCO)2(INH)2]·4H2O (3) | 273 (1440) | 356 (1120) | 678 (233) | This work |
a absorption maximum in nm and ε in L·mol−1·cm−1; IL = intraligand; LMCT = Ligand to metal charge transfer; LF = ligand field.
2.1.3. Electronic Paramagnetic Resonance (EPR) Studies and Proposed Structures for the New Complexes
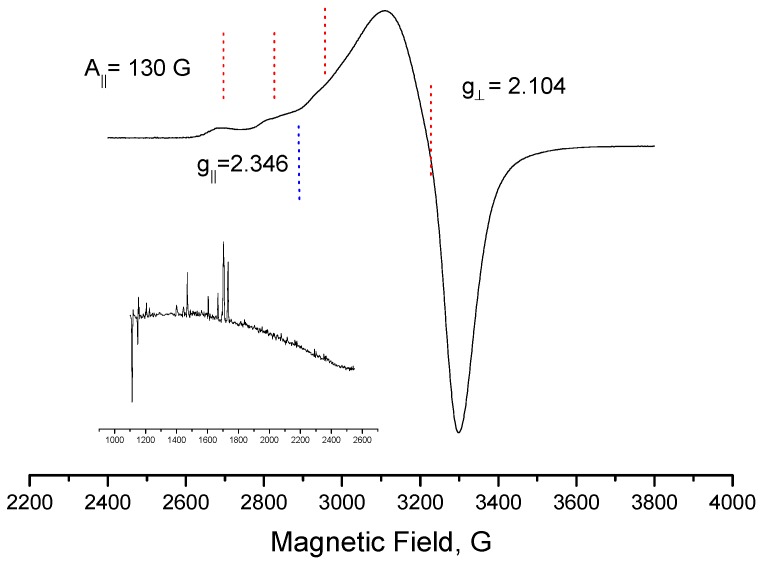
An analysis of the EPR result (Figure 5) of compound 1 allows us to suggest that the Cu(II) ions were octahedrally coordinated, with the octahedron elongated in the axial direction, because neither of the g values is equal to 2.00 [22]. The literature reports that the EPR spectra of divalent copper species obtained in solution at a temperature of 77 K, which have g║ > g⊥ > g0, are typical Cu(II) (d9) in axial symmetry, with the unpaired electron in the orbital [23]
Figure 5.
EPR spectra for the [CuCl2(INH)2]·H2O complex (1) registered in a frozen DMSO solution at 77 K.
Thus, it is suggested that complex 1 displays an octahedral symmetry around the copper ion with strong tetragonal distortion due to the Jahn Teller effect. Its coordination sites are occupied by two chloro atoms at the axial positions and two molecules of isoniazid in the equatorial plane via the nitrogen atom of the -NH2 group and the oxygen atom of the carbonyl group to form five-membered chelate rings (Figure 2).
For complexes 2 and 3, it is suggested that the compounds consist of monomeric species, in which the environment around the copper ion is octahedral, with some tetragonal distortion. As shown in Figure 3 and Figure 4 (the water molecules are omitted), it is suggested that the coordination sphere of the compounds, with the minimum formula [CuX2(INH)2] (X = SNC− (2) and NCO− (3)), consists of two isoniazid ligands coordinated by the nitrogen atom of the -NH2 group and the carbonyl oxygen atom, forming two five-membered chelate rings and two pseudohalide groups. For compound 2, the two thiocyanate groups are likely terminally coordinated by the sulfur atoms, whereas the cyanate pseudohalides are terminally linked by the nitrogen atoms in compound 3.
2.2. Nanostructured Lipid System
Table 3 shows the mean values and standard deviation of the particle size and polydispersity index (PDI) for the nanostructured lipid system (ME) and the copper(II) compounds loaded into the microemulsion. PDI measures the homogeneity between the average particle size in relation to the standard deviation, where smaller PDI values represent more uniform particles [24].
Table 3.
Determination of the droplet size and polydispersity index of the nanostructured lipid system (MEs) using light scattering.
| Formulation | Mean Diameter ± S.D. (nm) * | Mean PDI ± S.D. * |
|---|---|---|
| ME | 125.6 ± 1.804 | 0.246 ± 0.008 |
| 1-Loaded | 158.0 ± 1.060 | 0.228 ± 0.011 |
| 2-Loaded | 212.6 ± 1.539 | 0.284 ± 0.034 |
| 3-Loaded | 171.7 ± 1.947 | 0.218 ± 0.007 |
* Standard deviation (S.D.), polydispersity index (PDI).
The diameter of the ME particles was 125.6 ± 1.804 nm. After the incorporation of the complexes, there was a small variation in the particle diameter size, ranging from 158.0 ± 1.060 to 212.6 ± 1.539 nm. All values are smaller than 1.0 μm, which is characteristic of one ME [25]. When comparing the MEs and the formulations containing the complexes, there was a small increase in the particle diameter, a strong indication that the complexes were incorporated into the nanostructured lipid system. The PDI values of the nanostructured lipid system before and after the incorporation of the coordination compounds varied between 0.218 ± 0.007 and 0.284 ± 0.034, indicating a good size distribution of the droplets in the ME system.
2.3. In Vitro Biological Activity
The in vitro results (i.e., the MIC and IC50 values) obtained for the free copper(II) complexes and those loaded into the microemulsions are shown in Table 4.
Table 4.
Results of biological assessments (MIC and IC50) and determination of the selectivity index (SI) of the free copper(II) complexes or those loaded into the nanostructured lipid system against S. aureus and E. coli.
| Complexes | Parameters | |||||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | |||||
| MIC (μg/mL) | IC50 (μg/mL) | SI | MIC (μg/mL) | IC50 (μg/mL) | SI | |
| 1-Not loaded | 1000 | 109.5 | 0.1095 | 2000 | 109.5 | 0.05475 |
| 1-Loaded | 250 | 319.3 | 1.288 | 125 | 319.3 | 2.554 |
| 2-Not loaded | 500 | 314.3 | 0.6286 | 2000 | 314.3 | 0.1571 |
| 2-Loaded | 500 | 329.3 | 0.6586 | 125 | 329.3 | 2.634 |
| 3-Not loaded | 1000 | 325.3 | 0.3253 | 8000 | 325.3 | 0.040 |
| 3-Loaded | 125 | >500 | 4.000 | 500 | >500 | 1.000 |
Before loading into the nanostructured lipid system, the compounds had shown moderate activity against S. aureus; compounds 1 and 3 showed MICs of 1000 μg/mL, and compound 2 displayed an MIC of 500 μg/mL. Their activity against E. coli was even lower as compounds 1, 2 (MIC 2000 μg/mL) and 3 (8000 μg/mL) showed higher MIC values. After being loaded into the nanosystem, compounds 1 and 3 displayed improved antibacterial activity against S. aureus as their MIC values were reduced eight-fold; compound 2 did not display improved activity, even after incorporation. We highlighted the results against E. coli as all the compounds displayed improved antibacterial activity, with MIC values that were 16-fold lower than those before incorporation. Compounds 1 and 2 displayed MIC values from 2000 to 125 μg/mL and compound 3 displayed MIC values from 8000 to 500 μg/mL.
The compound 1 and 2-loaded MEs showed an MIC of 125 μg/mL against E. coli, which is better than the [Cu(TTA)2], TTA = 2-tenoyltrifluoroacetone compound studied by Xu et al. [7] that showed an MIC of 180 μg/mL.
Regarding the activity against S. aureus, when compound 3 was loaded into the nanosystem, it exhibited an MIC of 125 μg/mL, with better antibacterial activity than [Cu(L)(ClO4)(H2O)] (HL = 4-[(E)-(2-hydroxy-4-methoxyphenyl)methyleneamino]benzoate), which exhibited an MIC of 500 μg/mL [6]. There are no reports of the use of a nanostrucutured lipid system as a strategy to improve the antibacterial activity of copper(II) complexes against E. coli and S. aureus.
Moreover, as shown in Table 2, the cytotoxicity results showed that the cytotoxicity of all compounds was after they were loaded into the nanosystem, particularly compound 3 (IC50 from 325.3 to higher than 500). The compounds also exhibited higher selectivity index values, even though they still are not ideal (SI higher than 10).
3. Experimental Section
3.1. General Information
Amphotericin-B (AMB), cholesterol (CHO), CuCl2·2H2O, isoniazid (INH), polyoxyethylene-20 cetyl ether (Brij® 58), potassium cyanate and rezasurin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol, dimethylsulfoxide (DMSO), dimethylformamide (DMF) and tetrahydrofuran (THF) were purchased from Merck Sharp & Dohme Ltd. (Darmstadt, Hesse, Germany). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum, gentamicin sulfate, and trypsin/EDTA were purchased from Vitrocell Embriolife (Campinas, SP, Brazil). Soy phosphatidylcholine was purchased from Lipoid (Newark, NJ, USA). All the solutions were prepared using deionized water from a Millipore instrument (Darmstadt, Hesse, Germany).
3.2. Synthesis of the Copper(II) Complexes
3.2.1. [CuCl2(INH)2]·H2O (1)
Approximately 80.4 mg (0.586 mmol) of isoniazid was dissolved in 5 cm3 of CH3OH and added dropwise to a green solution of CuCl2·2H2O (200 mg; 0.993 mmol) in 15 cm3 of the same solvent, producing a light green suspension. After stirring for 3 h, the solid was filtered, washed with methanol and vacuum-dried at room temperature. Yield: 52%. The compound is soluble in dimethylsulfoxide and dimethylformamide. M.p. = 153 °C (dec.). Anal. Calcd for C12N6H14O2Cl2Cu (%): C, 33.8; N, 19.7; H, 2.38; Cu, 15.6. Observed: C, 31.8; N, 21.4; H, 2.47; Cu, 15.3 [26].
3.2.2. [Cu(NCS)2(INH)2]·5H2O (2)
Approximately 161 mg (1.17 mmol) of isoniazid was dissolved in 10 cm3 of CH3OH and added to a solution of 50 mg (0.293 mmol) of CuCl2·2H2O in 15 cm3 of the same solvent. After stirring this suspension for 5 min, 47.5 mg (0.0586 mmol) of sodium thiocyanate (NaSCN), dissolved in 5 cm3 of water was added to the mixture and stirred for 3 h. The dark green solid was isolated by filtration, washed with methanol and vacuum-dried at room temperature. The compound is soluble in methanol, dimethylsulfoxide, dimethylformamide and tetrahydrofuran. Yield: 40%. M.p. = 149 °C. Anal. Calcd for C14N8H24O7S2Cu (%): C, 30.9; N, 20.6; H, 4.45; Cu, 11.7. Observed: C, 31.5; N, 20.2; H, 2.46; Cu, 11.0.
3.2.3. [Cu(NCO)2(INH)2]·4H2O (3)
A solution of 161 mg (1.17 mmol) of isoniazid in 10 cm3 of CH3OH was added to a solution of 50 mg (0.293 mmol) of CuCl2 2H2O in 15 cm3 of the same solvent. After stirring this suspension for 5 min, 47.6 mg (0.0587 mmol) of potassium cyanate (KNCO) dissolved in 5 cm3 of water was added to reaction medium, producing a dark blue suspension. The mixture was stirred for 5 h, and the resulting precipitate was isolated by filtration. The product was thoroughly washed with methanol and dried in vacuum at room temperature. The compound is soluble in methanol, dimethylsulfoxide, dimethylformamide and tetrahydrofuran. Yield: 33%. M.p. ≥ 250 °C. Anal. Calcd. for C14N8H22O8Cu (%): C, 34.0; N, 22.7; H, 4.49; Cu, 13.0. Observed: C, 34.0; N, 18.0; H, 3.06; Cu, 13.6.
3.3. Characterization of the Copper(II) Complexes
3.3.1. IR Spectroscopy
The vibrational spectra in the infrared region were registered on a Nicolet FTIR-Impact 400 spectrometer (Thermo Scientific, San Jose, CA, USA), in the range 400–4000 cm−1. The samples were prepared as KBr pellets (~10 mg complex/100 mg KBr) that had previously been dried at 120 °C.
3.3.2. UV-Vis Spectroscopy
The electronic spectra were recorded on a Lambda 14P spectrophotometer (Perkin Elmer, Boston, MA, USA) using quartz cells with a 1.00 cm optical length and 1.0 × 10−3 mol·L−1 solutions of the complexes in methanol.
3.3.3. EPR Spectroscopy
The EPR spectra were recorded on a Bruker EMX instrument (Bruker, Karlsruhe, Germany) working at X-band (9.65 GHz frequency, 20 mW power, 100 kHz modulation frequency). DPPH (α,α′-diphenyl-β-picrylhydrazyl) was used as a magnetic field calibrator (g = 2.0036), and the measurements were performed with a frozen dimethylsulfoxide solution of complex 1 in Wilmad quartz tubes (4 mm internal diameter). Usually, the standard conditions for recording the spectra were 15 G modulation amplitude, 7.96 × 103 or 3.56 × 104 receiver gain, and 2 or 4 scans at 77 K.
3.3.4. Melting Point Determination
The melting points of the samples (~5 mg complex) were determined using the MQAPF-302 instrument (Microcriquímica Equipamentos Ltda, Palhoça, Santa Catarina, Brazil), which reaches a maximum temperature of 350 °C.
3.3.5. Elemental Analysis
The elemental analyses were performed at the Central Analitica at IQ University of São Paulo, Brasil using a CNH 2400 Perkin-Elmer instrument (Perkin Elmer), which determines the percentage of carbon, hydrogen and nitrogen in the samples with a precision of ±0.5%.
3.3.6. Complexometry with 2,2′,2′′,2′′′-(Ethane-1,2-diyldinitrilo)tetraacetic Acid (EDTA)
To analyze the copper content, 5.00 mg of the samples were weighed on an analytical balance with an uncertainty of 0.01 mg. The sample was digested by the addition of five drops of a hot HNO3 solution (70% w/w). After cooling, 1.0 mL ammonium acetate buffer solution (0.2 mol·L−1) and a known excess quantity of EDTA solution that was sufficient to complex all the metal present in the sample were added. The pH was adjusted to 5.0 (±0.1) and the xylenol orange indicator was added in a sufficient quantity to observe a yellow color. Then, the solution was subjected to titration with a ZnCl2 solution (0.01 mol·L−1), which was previously standardized with EDTA. The turning point was observed by the appearance of pink color. The copper content was calculated using the following equation:
| CCu = MCu × (CEDTA × VEDTA − CZnCl2 × VZnCl2)/msample |
where CCu = the copper content; MCu = the molar mass of copper; CEDTA = the concentration of the EDTA solution; VEDTA = the volume of the EDTA solution; CZnCl2 = the concentration of the ZnCl2 solution; VZnCl2 = the volume of the ZnCl2 solution; and msample = the mass of the weighed sample [27].
3.4. Nanostructured Lipid System Preparation
The MEs were prepared as described by Bonifácio et al. [28], with the following composition: 10% oil phase (i.e., cholesterol), 10% surfactant (i.e., polyoxyethylene-20 cetyl ether (Brij® 58) and soy phosphatidylcholine in a proportion of 2:1) and 80% aqueous phase (i.e., phosphate buffer, pH 7.4). The mixture was sonicated using a rod sonicator (Q700 of QSonica®, Newtown, CT, USA) at 700 watts in discontinuous mode for 10 min with 30 s incubations in an ice bath every two minutes during the sonication process. After sonication, the MEs were centrifuged at 11,180× g for 15 min to eliminate the waste released by the titanium rod sonicator.
3.5. Nanostructured Lipid System Characterization: Mean Diameter and Polydispersity Index (PDI)
The microemulsion droplet size and distribution were determined by an optical particle analyzer (Zetasizer Nano-ZS ZEN3600, Malvern Instruments, San Diego, CA, USA) using dynamic light scattering. All samples (MEs with and without inorganic compounds) were diluted in Milli-Q water (100 μL of sample in 900 μL of deionized water) and placed in the instrument. The analyses were performed in triplicate.
3.6. Preparation of the Coordination Compound-Loaded Nanostructured Lipid System
After obtaining the MEs, the copper(II) complexes were loaded into the nanostructured lipid system. A complex (0.0100 g) was added to the MEs (2 mL) and the mixture was homogenized and sonicated for 5 min at room temperature in discontinuous mode to facilitate the incorporation of the material into the microemulsion at a concentration of 5000 μg/mL. The inorganic compound-loaded nanostructured lipid systems were characterized by measuring the mean diameter and polydispersity index.
3.7. Antibacterial Activity
The antibacterial activity against Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922) was determined by the microdilution method, according to the standard reference method M7-A6 CLSI (2006) [29]. Both of the strains used in this study were obtained from the American Type Culture Collection (Rockville, MD, USA). Compounds 1, 2 and 3 were loaded into the microemulsions at an initial concentration of 2000 μg/mL. At first, 100 μL of each compound loaded into the nanosystem were added to 96-well microplates containing 80 μL/well of Mueller-Hinton broth, and then a two-fold serial dilution was performed to obtain concentrations ranging from 7.81 μg/mL to 8000 μg/mL. Ampicillin and the microemulsions without the compound were used as positive and negative controls, respectively. The bacterial inocula were standardized at 1.0 × 107 CFU/mL and the microplates were incubated at 37 °C for 24 h. Then, 30 μL/well of a resazurin solution (0.01%) were added, and, after incubation at 37 °C for 2 h, the minimum inhibitory concentration (MIC) was determined. Bacterial growth changes the blue resazurin dye to pink, and the blue color indicates growth inhibition. MIC was defined as the lowest concentration that is able to inhibit at least 90% of bacterial growth. All tests were performed in triplicate.
3.8. In Vitro Cytotoxic Activity
The cytotoxicity of the free complexes diluted in DMSO and those incorporated into the nanostructured lipid systems was measured on normal epithelial cells (VERO ATCC CCL-81) as described by Pavan et al. [30]. The cells were seeded on plates with a surface area of 12.50 cm2 in 10 mL DMEM supplemented with 10% fetal bovine serum, gentamicin sulfate (50 mg/L) and amphotericin B (2 mg/L) and incubated at 37 °C and 5% CO2.
This technique consists of collecting the cells using a solution of trypsin/EDTA, centrifugation (2000 rpm for 5 min), counting the number of cells in a Newbauer chamber and then adjusting the concentration to 3.4 × 105 cells/mL using DMEM [31]. Next, 200 μL of the suspension was deposited into each well of a 96-well microplate to obtain a cell density of 6.8 × 104 cells/well and incubated at 37 °C in an atmosphere of 5% CO2 for 24 h to allow the cells to attach to the plate. Dilutions of the test compounds were prepared to obtain concentrations from 500 to 1.95 μg/mL. The dilutions were added to the cells after the media was removed, and any cells that did not adhere were incubated for an additional 24 h. The cytotoxicity of the compounds was determined by adding 30 μL of resazurin developer and read after a six-hour incubation. The samples were analyzed using a microplate Spectrafluor Plus (TECAN®) reader and excitation and emission wavelengths of 530 and 590 nm, respectively. The cytotoxicity (IC50) was defined as the highest concentration of the compound that retained the viability of at least 50% of the cells.
3.9. Selectivity Index (SI)
The selectivity index (SI) can be calculated by dividing the IC50 value by the MIC value. An SI greater than or equal to 10 indicates that the test compound can be applied at a concentration that is ten-fold higher than the MIC value without exhibiting cytotoxicity [32].
4. Conclusions
The synthesis, characterization, and antibacterial activity of copper(II) complexes bearing an isoniazid ligand that were incorporated into nanostructured lipid system were reported in this work. The nanostructured lipid system was used as a strategy to improve the antibacterial activity against E. coli and S. aureus and decrease the cytotoxicity of the studied compounds. Our findings suggested that the Cu(II) complexes may be promising antibacterial candidates when they are incorporated into the MEs described in this study.
Acknowledgments
This work was financially supported by grant#2013/09265-7 and grant#2013/14957-5 São Paulo Research Foundation (FAPESP), Brazilian National Council for Scientific and Technological Development—CNPq and Programa de Apoio ao Desenvolvimento Científico (PADC) of School of Pharmaceutical Sciences/UNESP, Brazil.
Author Contributions
B.V.B. developed the nanostructures, analyzed the antibacterial activity and assisted in drafting the paper. R.C.G.F. synthesized and characterized the complexes, and assisted in drafting the paper. A.V.G.N. synthesized and characterized the complexes, and assisted in drafting the paper. A.E.M. synthesized and characterized the complexes, and assisted in drafting the paper. A.M.C.F. characterized the complexes and assisted in drafting the paper. É.O.L. performed the cytotoxicity assays and assisted in drafting the paper. F.R.P. performed the cytotoxicity assays and assisted in drafting the paper. M.S.G.R. analyzed the antibacterial activity and assisted in drafting the paper. T.M.B. analyzed the antibacterial activity and assisted in drafting the paper. M.C. supervised the first author (P.B.S.), developed the nanostructure and assisted in drafting the paper.
Conflicts of Interest
The authors declare that there are no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds CuCl2(INH)2]·H2O, [Cu(NCS)2(INH)2]·5H2O and [Cu(NCO)2(INH)2]·4H2O are available from the authors.
References
- 1.Bibi Y., Nisa S., Chaudhary F.M., Zia M. Antibacterial activity of some selected medicinal plants of Pakistan. BMC Complement. Altern. Med. 2011;11:52–58. doi: 10.1186/1472-6882-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikaido H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorenson J., Jr. Copper complexes offer a physiological approach to treatment of chronic diseases. Prog. Med. Chem. 1989;26:437–568. doi: 10.1016/s0079-6468(08)70246-7. [DOI] [PubMed] [Google Scholar]
- 4.Weder J.E., Dillon C.T., Hambley T.W., Kennedy B.J., Lay P.A., Biffin J.R., Regtop H.L., Davies N.M. Copper complexes of non-steroidal anti-inflammatory drugs: An opportunity yet to be realized. Coord. Chem. Rev. 2002;323:95–126. doi: 10.1016/S0010-8545(02)00086-3. [DOI] [Google Scholar]
- 5.Sato M.R., Silva P.B., Souza R.A., Santos K.C., Chorilli M. Recent advances in nanoparticle carriers for coordination complexes. Curr. Top. Med. Chem. 2015;15:287–297. doi: 10.2174/1568026615666150108145614. [DOI] [PubMed] [Google Scholar]
- 6.Patel M.N., Joshi H.N., Patel C.R. Copper(II) complexes with norfloxacin and neutral terpyridines: cytotoxic, antibacterial, superoxide dismutase and DNA-interaction approach. Polyhedron. 2012;40:159–167. doi: 10.1016/j.poly.2012.03.050. [DOI] [Google Scholar]
- 7.Xu D.F., Shen Z.H., Shi Y., He Q., Xia Q.C. Synthesis, characterization, crystal structure, and biological activity of the copper complex. Russ. J. Coord. Chem. 2010;36:458–462. doi: 10.1134/S1070328410060060. [DOI] [Google Scholar]
- 8.Pahontu E., Ilies D.-C., Shova S., Paraschivescu C., Badea M., Gulea A., Rosu T. Synthesis, characteriation, crystal structure and antimicrobial activity of copper(II) complexes with the schiff base derived from 2-hydroxy-4-methoxybenzaldehyde. Molecules. 2015;20:5771–5792. doi: 10.3390/molecules20045771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freitas E.S., Silva P.B., Chorilli M., Batista A.A., Lopes E.O., Silva M.M., Leite C.Q.F., Pavan F.R. Nanostructured lipid systems as a strategy to improve the in vitro cytotoxicity of ruthenium(II) compounds. Molecules. 2014;19:5999–6008. doi: 10.3390/molecules19055999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva P.B., Freitas E.S., Bernegossi J., Gonçalez M.L., Sato M.R., Leite C.Q.F., Pavan F.R., Chorilli M. Nanotechnology-based drug delivery systems for treatment of tuberculosis—A review. J. Biomed. Nanotech. 2016;12:241–260. doi: 10.1166/jbn.2016.2149. [DOI] [PubMed] [Google Scholar]
- 11.Santos F.K., Oyafuso M.H., Kiill C.P., Gremião M.P.D., Chorilli M. Nanotechnology-based drug delivery systems for treatment of hyperproliferative skin diseases—A review. Curr. Nanosci. 2013;9:159–167. [Google Scholar]
- 12.Souza A.L.R., Kiill C.P., Santos F.K., Luz G.M., Chorilli M., Gremião M.P.D. Nanotechnology-based drug delivery systems for dermatomycosis treatment. Curr. Nanosci. 2012;8:512–519. doi: 10.2174/157341312801784311. [DOI] [Google Scholar]
- 13.Yadav H.D.S., Sengupta S.K., Tripathi S.C. Oxozirconium(IV) complexes of terephthalic acid dihydrazide and their reactions with β-diketones. Synth. React. Inorg. Met. Org. Chem. 1987;17:115–127. doi: 10.1080/00945718708059417. [DOI] [Google Scholar]
- 14.Donia A.M., El-Rayes M. Thermochromism of the cobalt(II)-isoniazid complex in the solid state. Thermochim. Acta. 1989;147:65–70. doi: 10.1016/0040-6031(89)85163-9. [DOI] [Google Scholar]
- 15.Akyuz S. The FT-IR spectra of pyrazinamide complexes of transition metal(II) tetracyanonickelate. J. Mol. Struct. 2003;651–653:541–545. doi: 10.1016/S0022-2860(02)00675-0. [DOI] [Google Scholar]
- 16.Nakamnot K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. 4th ed. Wiley; New York, NY, USA: 1986. [Google Scholar]
- 17.Lucca Neto V.A., Mauro A.E., Ananias S.R., Stevanato A., Moro A.C. Synthesis and the use of [Pd(dmba)(NCO)(PPh3)] in catalytic processes involving the preparation of urea derivatives and alkoxycarbonyl complexes. Eclet. Quim. 2006;31:75–81. [Google Scholar]
- 18.Miskowski V.M., Thich J.A., Solomon R., Schugar H.J. Electronic spectra of substituted copper(II) thioether complexes. J. Am. Chem. Soc. 1976;98:8344–8350. doi: 10.1021/ja00442a006. [DOI] [Google Scholar]
- 19.Dockum B.W., Reiff W.M. Thyocyanate-bridged transition-metal polymers. 1. Structure and low-temperature magnetic behavior of (bipyridyl)iron(II) thiocyanate: Fe(2,2′-(bpy)(NCS)2. Inorg. Chem. 1982;21:391–398. doi: 10.1021/ic00131a070. [DOI] [Google Scholar]
- 20.Sargentelli V. Ph.D. Thesis. Chemistry Institute—Universidade Estadual Paulista; Araraquara, Brazil: 1986. Synthesis, Study of Thermal and Eletrochemical Behavior and Reactivity of Pseudohalides Copper(II) Complexes. [Google Scholar]
- 21.Lever A.B.P. Inorganic Electronic Spectroscopy. 2nd ed. Elsevier; New York, NY, USA: 1986. p. 420. [Google Scholar]
- 22.Van Ooijen J.A.C., van der Put P.J., Reedijk J. Compressed tetragonal geometry in Cu(II) doped dichlorobis(pyrazole)cadmium. Chem. Phys. Lett. 1977;51:380–382. doi: 10.1016/0009-2614(77)80425-9. [DOI] [Google Scholar]
- 23.Chandra S., Gupta L.K. EPR and electronic spectral studies on Co(II), Ni(II) and Cu(II) complexes with a new tetradentate [N4] macrocyclic ligand and their biological activity. Spectrochim. Acta A. 2004;60:1563–1571. doi: 10.1016/j.saa.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Goyal U., Arora R., Aggarwal G. Formulation design and evaluation of a self-microemulsifying drug delivery system of lovastatin. Acta Pharm. 2012;62:357–370. doi: 10.2478/v10007-012-0022-1. [DOI] [PubMed] [Google Scholar]
- 25.Cunha Junior A., Fialho S.L., Carneiro L.B., Oréfice F. Microemulsions as drug delivery systems for topical ocular administration. Arq. Bras. Oftalmol. 2003;63:285–391. [Google Scholar]
- 26.Afanaséva G.V., Bychkova T.I., Shtyrlin V.G., Shakirova A.R., Garipov R.R., Zyavkina Y.I., Zakharov A.V. Complexation and ligand exchange in aqueous of Cu(II) and Ni(II) with hydrazides of some aromatic acids. Russ. J. Gen. Chem. 2006;76:346–355. [Google Scholar]
- 27.Oliveira C.N., Ionashiro M., Graner C.A.F. Complexometric titration of zinc, copper and cobalt. Eclet. Quim. 1985;10:7–10. [Google Scholar]
- 28.Bonifácio B.V., Ramos M.A.S., Silva P.B., Negri K.M.S., Lopes E.O., Souza L.P., Vilegas W., Pavan F.R., Chorilli M., Bauab T.M. Nanostructured lipid system as a strategy to improve the anti-Candida albicans activity of Astronium sp. Int. J. Nanomed. 2015;10:5081–5092. doi: 10.2147/IJN.S79684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute (CLSI) Approved Standard. 6th ed. CLSI; Wayne, PA, USA: 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Document M7-A6 Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 30.Pavan F.R., Maia P.I., Leite S.R.A., Deflon V.M., Batista A.A., Sato D.N., Franzblau S.G., Leite C.Q.F. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010;45:1898–1905. doi: 10.1016/j.ejmech.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Valdivieso-Garcia A., Clarke R.C., Rahn K., Durette A., MacLeod D.L., Gyles C.L. Neutral red assay for measurement of quantitative vero cell cytotoxicity. Appl. Environ. Microbiol. 1993;59:1981–1983. doi: 10.1128/aem.59.6.1981-1983.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orme I. Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 2001;45:1943–1946. doi: 10.1128/AAC.45.7.1943-1946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]