Abstract
Amine-functionalized ZnO nanosheets were prepared through a one-step hydrothermal method by using monoethanolamine, which has a hydroxyl group, for covalent attachment on ZnO and a primary amine group to supply the amine-functionalization. We demonstrate that the terminal amine groups on ZnO surfaces substantially increase the capability of CO2 capture via chemisorption, resulting in effective CO2 activation. As a result, the photogenerated electrons from excited ZnO can more readily reduce the surface-activated CO2, which thereby enhances the activity for photocatalytic CO2 reduction.
Keywords: photocatalysis, CO2 reduction, solar fuels, zinc oxide
1. Introduction
Nowadays, the increasing level of CO2 concentration from the use of fossil fuels has become the most serious environmental concern related to global warming and climate change. Researchers have realized that photocatalytic conversion of CO2 into hydrocarbons by means of solar energy would be an ideal way to lower down the CO2 concentration in the atmosphere and generate sustainable chemical fuels in the meantime. A pioneering study on the reduction of CO2 was demonstrated by Inoue et al. in 1979 by using semiconductor photocatalysts [1]. Afterwards, many scientific studies have been devoted to the development of efficient photocatalysts for CO2 photoreduction [2,3,4,5,6,7,8,9,10,11,12,13,14]. Among them, ZnO-based nanostructures with different sizes and morphologies have been extensively reported as photocatalysts since they are stable, non-toxic, and low cost [15,16,17,18,19,20]. However, the efficiencies for CO2 photoreduction of these ZnO-based photocatalysts were usually unsatisfactory [21,22], which might be ascribed to the low affinity between CO2 and ZnO surfaces [23].
As is well known, the amine functionalization can effectively assist immobilization of CO2 and has been extensively used for CO2 capture in industry [24,25]. Tremendous efforts have been made to introduce amine groups onto solid material surfaces to promote CO2 capture [26,27]. The chemical interactions between amine groups and CO2 lead to the formation of carbamate (or bicarbamate) that can transform into carbonate upon hydrolysis [28]. In this work, we report a straightforward method to prepare amine-functionalized ZnO nanosheets by using monoethanolamine (MEA) that possesses a hydroxyl (-OH) group for covalent attachment on ZnO and a primary amine (-NH2) group to endow an amine-functionalized surface. We demonstrate that the terminal amine groups on ZnO surfaces substantially improve the CO2 adsorption, which consequently results in significantly enhanced photocatalytic activity for CO2 reduction as compared to the clean ZnO without surface amine groups.
2. Results and Discussion
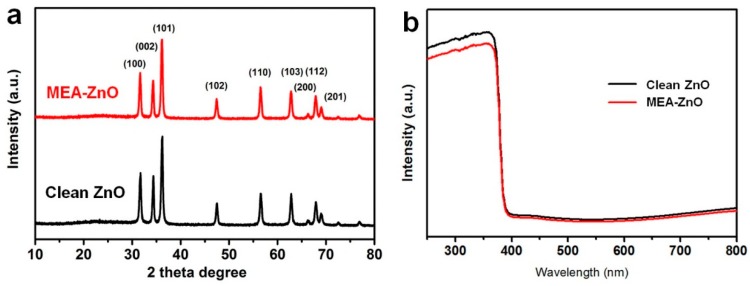
Figure 1a shows the X-ray diffraction (XRD) patterns of the prepared MEA–ZnO sample and clean ZnO sample. These two samples exhibit almost the same diffraction lines which can be indexed to wurtzite ZnO. This indicates that the involvement of MEA into the reaction did not alter the crystal structure of ZnO. The absorption spectra of both samples also appear to have almost no difference, as shown in Figure 1b. The SEM image (Figure 2a) of the MEA–ZnO sample confirms the nanosheet morphology, and the high-resolution TEM (HRTEM) image (Figure 2a inset) shows a lattice fringe of d = 0.28 nm, corresponding to the (100) interplanar spacing of wurtzite ZnO [29]. In comparison, the clean ZnO sample prepared without MEA appears as irregular particles, as shown in Figure 2b. The sheet-like growth of ZnO is probably due to attached MEA molecules that suppress the intrinsically anisotropic growth of ZnO along the (0001) direction. As such, the ZnO crystal growth would intend to proceed sideways towards a nanosheet [15].
Figure 1.
(a) XRD patterns of MEA–ZnO and clean ZnO. Both samples can be indexed as wurtzite ZnO; (b) UV-Vis spectra of MEA–ZnO and clean ZnO.
Figure 2.
(a) SEM images of MEA–ZnO; (b) SEM image of clean ZnO; (c) TEM image of MEA–ZnO nanosheets; (d) HRTEM image of a representative MEA–ZnO nanosheet.
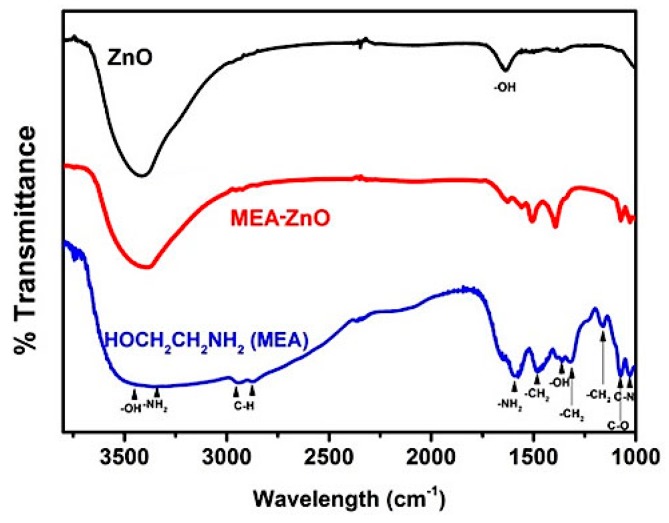
For the MEA–ZnO sample, the nature of covalent attachment of MEA on the ZnO surface is verified by Fourier transform infrared (FT-IR) spectroscopy. Prior to the measurement, the as-prepared MEA–ZnO sample was annealed at 250 °C under Ar atmosphere to remove the physisorbed MEA since the boiling point of MEA is ~170 °C. The FT-IR spectrum of the MEA–ZnO sample after annealing (Figure 3) still shows most of the signature peaks of MEA. The bands at 2964, 2919, and 1399 cm−1 can be assigned to the C–H stretch of MEA. The peaks at 1073 and 1028 cm−1 are attributed to the C–O stretch and C–N stretch, respectively, and the band at 1560 cm−1 is owing to the N-H stretch of MEA on the ZnO surface [28,30]. These IR results indicate that MEA molecules are successfully covalently binding on ZnO surfaces.
Figure 3.
FT-IR spectra of clean ZnO, MEA–ZnO, and the pure MEA molecule.
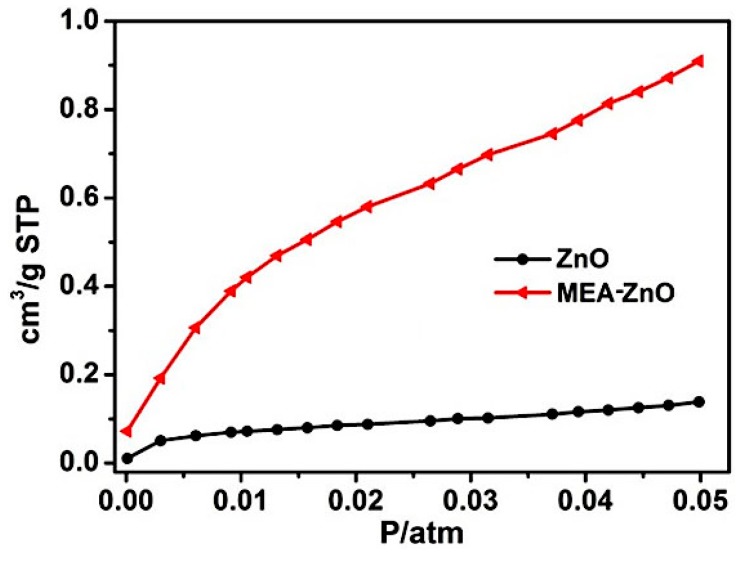
The effect of amine functionalization by MEA is examined through the CO2 adsorption test. As shown in Figure 4, the MEA–ZnO exhibits much higher (approximately six times) CO2 uptake capability (0.87 cm3·g−1) than the clean ZnO (0.13 cm3·g−1), while in comparison, the difference in their surface area is quite minor (15.1 m2·g−1 for MEA–ZnO vs. 9.6 m2·g−1 for clean ZnO). This result proves that the amine-functionalized ZnO surface can greatly enhance CO2 adsorption. Moreover, the MEA–ZnO shows a dramatic rise in CO2 uptake along with elevated CO2 pressure, while the clean ZnO shows almost linearly increased CO2 adsorption. This observation suggests that specific interactions between CO2 and MEA–ZnO occur during the CO2 adsorption process.
Figure 4.
CO2 adsorption isotherms of MEA–ZnO and clean ZnO.
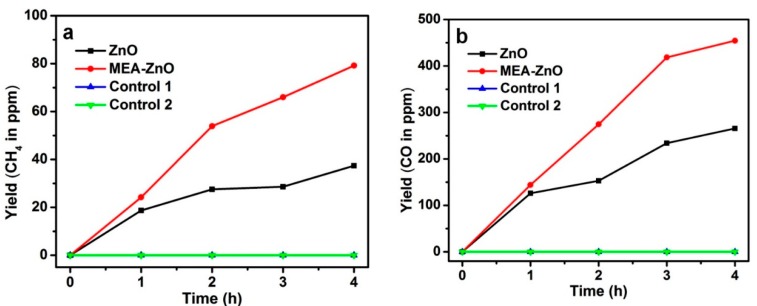
We further carried out photocatalytic CO2 reduction by irradiating the prepared sample (MEA–ZnO or clean ZnO) with UV-Vis light in the presence of CO2 and water vapor. As shown in Figure 5, the clean ZnO sample exhibits very low generation rates of methane (2.1 μmol/g) and CO (14.8 μmol/g). In comparison, the amine-functionalized sample (MEA–ZnO) showed a much higher production rate for methane (4.4 μmol/g) and CO (25.3 μmol/g). This comparison indicates that the amine functionalization by MEA can not only enhance the capture of CO2 molecules but also facilitates their photoreduction into CO and CH4. Control experiments were carried out in the absence of light or CO2 (in O2 atmosphere), which did not lead to generation of CO or CH4. This suggests that excitation of ZnO is required to obtain the products (CO and CH4) which come from CO2 photoreduction rather than the oxidation of MEA by the photogenerated holes of ZnO.
Figure 5.
The amount of generated (a) CH4 and (b) CO as a function of irradiation time over the sample of MEA–ZnO or clean ZnO. Control experiments without light irradiation (control 1) or without CO2 (control 2) showed no generation of CO and CH4.
To measure the amount of O2 formed during the photocatalytic reaction, we performed a 12 h photocatalytic reaction for the MEA–ZnO catalyst under the reaction conditions described above. The detected amount of H2, O2, CO, and CH4 were 81 ppm, 1145 ppm, 1520 ppm, and 146 ppm, respectively. According to the stoichiometry of the involved reactions (1) to (4) and the amount of gaseous products (H2/CO/CH4), the O2 evolution amount should be around 1092 ppm, which is slightly lower than the actual detected O2 amount (1145 ppm). This might be due to the possible generation of liquid hydrocarbon products (e.g., CH3OH, HCOOH) from the reactions (5) to (7), which could not be effectively analyzed by the TCD (thermal conductivity detector) of GC (Gas Chromatography). Nevertheless, this result still can prove that the attached MEA on ZnO surfaces are stable against oxidation by the photogenerated holes from ZnO.
| 2H2O + 4h+ → 4H+ + O2 | (1) |
| 2H+ + 2e− → H2 | (2) |
| CO2 + 2H+ + 2e− → CO + H2O | (3) |
| CO2 + 8H+ + 8e− → CH4 + 2H2O | (4) |
| CO2 + H+ + 2e− → HCOO− + H2O | (5) |
| CO2 + 4H+ + 4e− → HCHO + H2O | (6) |
| CO2 + 6H+ + 6e− → CH3OH+ H2O | (7) |
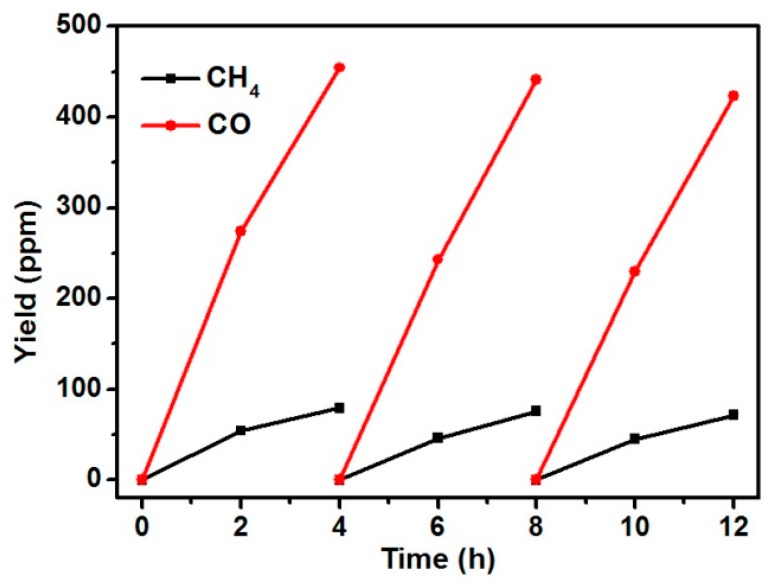
We have also evaluated the long-term stability of MEA–ZnO for CO2 photoreduction through a cycling test. After each cycle of the four-hour test, the reactor was degassed by CO2 and the sample was re-used for the next test. As shown in Figure 6, no obvious decrease of the yield of CH4 and CO was observed after three cycles, suggesting that the MEA–ZnO sample is very stable during the process of CO2 photoreduction. After the cycling test, the MEA–ZnO sample was examined again by FT-IR spectroscopy, and no change was observed in the IR spectrum, which further confirmed the good stability of MEA–ZnO during the reaction.
Figure 6.
The cycling test of CO2 photoreduction over 12 h by using the MEA–ZnO sample.
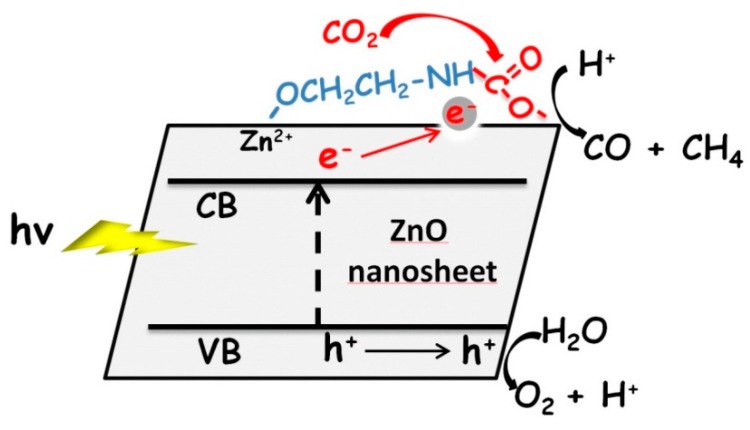
Based on the above results, we conclude that the amine functionalization of ZnO can effectively facilitate the capture of CO2 as well as its further photoconversion into CH4 and CO. The principle can be illustrated in Figure 7. The terminal amine groups enable the creation of “C–N” bonding with CO2 by forming carbamate, which acts like an activation process of CO2 due to higher reactivity of carbamate than that of linear CO2 [31,32,33,34]. Further, these active carbamates are close to the ZnO surface and tend to establish direct interactions with Zn2+, which allows for receiving electrons from excited ZnO to implement reduction reactions towards CO and CH4 production. By this method, the overall CO2 photoreduction process can be promoted.
Figure 7.
Schematic illustration of adsorption and photoreduction of CO2 on the MEA-functionalized ZnO nanosheet.
3. Experimental Section
3.1. Preparation of Amine-Functionalized ZnO (MEA–ZnO)
The amine-functionalized ZnO sample was prepared by a hydrothermal method. In a typical procedure, a mixture aqueous solution (20 mL) containing 0.1 M Zn(Ac)2, 0.2 M NaOH, and 0.005 M MEA was put in a Teflon-lined autoclave and heated at 90 °C for 12 h. Then the solid product was collected and annealed at 250 °C under Ar atmosphere for 3 h to remove physisorbed MEA on ZnO surfaces. For comparison, the clean ZnO sample was also prepared as the control sample under the same condition without using MEA.
3.2. Characterizations
X-ray powder diffraction (XRD) analysis was carried out on a Shimadzu XRD-6000 X-ray diffractometer (40 KV, 40 mA, Shimadzu Corp., Kyoto, Japan). The sample morphologies were examined by scanning electron microscopy (SEM, JEOL JSM-7600F, JEOL Ltd., Tokyo, Japan) and transmission electron microscopy (TEM, JEOL JEM-2100F, JEOL Ltd.). UV-Vis diffuse reflectance spectra (DRS) were recorded over the spectral range 320–800 nm on a Lambda 750 UV/Vis/NIR spectrophotometer (Perkin Elmer, Waltham, MA, USA). BaSO4 was used as a reflectance standard. Fourier transform infrared spectra (FT-IR) were collected on a Frontier FT-IR/NIR spectrometer (Perkin Elmer). The Brunauer–Emmett–Teller (BET) surface areas and CO2 adsorption test were measured on a Micro-meritics ASAP 2020M+C system (Micromeritics Instrument Corporation, Norcross, GA, USA).
3.3. Photoreduction of CO2
The photocatalytic CO2 reduction was performed in a 100 mL gastight reactor with a quartz window and two side-sampling ports. In a typical process, the MEA–ZnO sample (20 mg) was suspended in an ethanol solution and the suspension was dispersed on a 6.25 cm2 glass slide upon drying. The glass slide containing MEA–ZnO was then put into the gastight reactor. Prior to the irradiation, the reactor was purged with high purity CO2 for 30 min to remove the residual air and then 0.1 mL ultrapure water was injected into the reaction system. A xenon lamp (MAX-302, Asahi Spectra Co. Ltd., Tokyo, Japan) was used as the light source for the photocatalytic reaction. During the reaction, the gas product was analyzed periodically through a gas chromatograph (GC-7890A, Agilent Technologies Inc., Santa Clara, CA, USA) with a TCD detector.
4. Conclusions
In summary, we have successfully prepared amine-functionalized ZnO nanosheets through a hydrothermal process. The amine functionalization on ZnO surfaces substantially increases the capability of CO2 capture via chemisorption with effective CO2 activation. As such, amine-functionalized ZnO nanosheets exhibit significantly enhanced photocatalytic activity for CO2 reduction into CH4 and CO as compared to the clean ZnO.
Acknowledgments
This work was financially supported by NTU seed funding for Solar Fuels Laboratory, Singapore MOE AcRF-Tier 1 (RG 12/15) and AcRF-Tier 2 (MOE2012-T2-2-041, ARC 5/13).
Author Contributions
Y.L. and C.X. designed the experiments and wrote the paper, Y.L., Z.H., and Q.G. performed the experiments. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the ZnO and MEA–ZnO are available from the authors.
References
- 1.Inoue T., Fujishima A., Konishi S., Honda K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature. 1979;277:637–638. doi: 10.1038/277637a0. [DOI] [Google Scholar]
- 2.Wang C., Ranasingha O., Natesakhawat S., Ohodnicki P.R., Andio M., Lewis J.P., Matranga C. Visible light plasmonic heating of Au-ZnO for the catalytic reduction of CO2. Nanoscale. 2013;5:6968–6974. doi: 10.1039/c3nr02001k. [DOI] [PubMed] [Google Scholar]
- 3.Roy S.C., Varghese O.K., Paulose M., Grimes C.A. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano. 2010;4:1259–1278. doi: 10.1021/nn9015423. [DOI] [PubMed] [Google Scholar]
- 4.Highfield J. Advances and Recent Trends in Heterogeneous Photo(Electro)-Catalysis for Solar Fuels and Chemicals. Molecules. 2015;20:6739–6793. doi: 10.3390/molecules20046739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori K., Yamashita H., Anpo M. Photocatalytic reduction of CO2 with H2O on various titanium oxide photocatalysts. RSC Adv. 2012;2:3165–3172. doi: 10.1039/c2ra01332k. [DOI] [Google Scholar]
- 6.Fan W., Zhang Q., Wang Y. Semiconductor-based nanocomposites for photocatalytic H2 production and CO2 conversion. Phys. Chem. Chem. Phys. 2013;15:2632–2649. doi: 10.1039/c2cp43524a. [DOI] [PubMed] [Google Scholar]
- 7.Liu S., Xia J., Yu J. Amine-functionalized titanate nanosheet-assembled yolk@shell microspheres for efficient cocatalyst-free visible-light photocatalytic CO2 reduction. ACS Appl. Mater. Interfaces. 2015;7:8166–8175. doi: 10.1021/acsami.5b00982. [DOI] [PubMed] [Google Scholar]
- 8.Yuan Y.P., Ruan L.W., Barber J., Loo S.C.J., Xue C. Hetero-Nanostructured Suspended Photocatalysts for Solar-to-Fuel Conversion. Energy Environ. Sci. 2014;7:3934–3951. doi: 10.1039/C4EE02914C. [DOI] [Google Scholar]
- 9.Indrakanti V.P., Kubicki J.D., Schobert H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009;2:745–758. doi: 10.1039/b822176f. [DOI] [Google Scholar]
- 10.Varghese O.K., Paulose M., LaTempa T.J., Grimes C.A. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett. 2009;9:731–737. doi: 10.1021/nl803258p. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z.Y., Wang Z., Cao S.W., Xue C. Au/Pt Nanoparticle-Decorated TiO2 Nanofibers with Plasmon-Enhanced Photocatalytic Activities for Solar-to-Fuels Conversion. J. Phys. Chem. C. 2013;117:25939–25947. doi: 10.1021/jp409311x. [DOI] [Google Scholar]
- 12.Takeda H., Koike K., Inoue H., Ishitani O. Development of an efficient photocatalytic system for CO2 reduction using rhenium (I) complexes based on mechanistic studies. J. Am. Chem. Soc. 2008;130:2023–2031. doi: 10.1021/ja077752e. [DOI] [PubMed] [Google Scholar]
- 13.Shi H., Chen G., Zhang C., Zou Z. Polymeric g-C3N4 coupled with NaNbO3 nanowires toward enhanced photocatalytic reduction of CO2 into renewable fuel. ACS Catal. 2014;4:3637–3643. doi: 10.1021/cs500848f. [DOI] [Google Scholar]
- 14.Xie S., Wang Y., Zhang Q., Deng W., Wang Y. MgO- and Pt-promoted TiO2 as an efficient photocatalyst for the preferential reduction of carbon dioxide in the presence of water. ACS Catal. 2014;4:3644–3653. doi: 10.1021/cs500648p. [DOI] [Google Scholar]
- 15.Song J., Kulinich S.A., Yan J., Li Z., He J., Kan C., Zeng H. Epitaxial ZnO nanowire-on-nanoplate structures as efficient and transferable field emitters. Adv. Mater. 2013;25:5750–5755. doi: 10.1002/adma.201302293. [DOI] [PubMed] [Google Scholar]
- 16.Reimer T., Paulowicz I., Röder R., Kaps S., Lupan O., Chemnitz S., Benecke W., Ronning C., Adelung R., Mishra Y.K. Single step integration of ZnO nano- and microneedles in Si trenches by novel flame transport approach: Whispering gallery modes and photocatalytic properties. ACS Appl. Mater. Interfaces. 2014;6:7806–7815. doi: 10.1021/am5010877. [DOI] [PubMed] [Google Scholar]
- 17.Mishra Y.M., Modi G., Cretu V., Postica V., Lupan O., Reimer T., Paulowicz I., Hrkac V., Benecke W., Kienle L., et al. Direct growth of freestanding ZnO tetrapod networks for multifunctional applications in photocatalysis, UV photodetection, and gas sensing. ACS Appl. Mater. Interfaces. 2015;7:14303–14316. doi: 10.1021/acsami.5b02816. [DOI] [PubMed] [Google Scholar]
- 18.Jang E.S., Won J.H., Hwang S.J., Choy J.H. Fine tuning of the face orientation of ZnO crystals to optimize their photocatalytic activity. Adv. Mater. 2006;18:3309–3312. doi: 10.1002/adma.200601455. [DOI] [Google Scholar]
- 19.Zeng H., Cai W., Liu P., Xu X., Zhou H., Klingshirn C., Kalt H. ZnO-based hollow nanoparticles by selective etching: Elimination and reconstruction of metal-semiconductor interface, improvement of blue emission and photocatalysis. ACS Nano. 2008;2:1661–1670. doi: 10.1021/nn800353q. [DOI] [PubMed] [Google Scholar]
- 20.Mishra Y.K., Kaps S., Schuchardt A., Paulowicz I., Jin X., Gedamu D., Freitag S., Claus M., Wille S., Kovalev A., et al. Fabrication of macroscopically flexible and highly porous 3D semiconductor networks from interpenetrating nanostructures by a simple flame transport approach. Part. Part. Syst. Charact. 2013;30:775–783. doi: 10.1002/ppsc.201300197. [DOI] [Google Scholar]
- 21.He Y., Wang Y., Zhang L., Teng B., Fan M. A high-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B. 2015;168:1–8. doi: 10.1016/j.apcatb.2014.12.017. [DOI] [Google Scholar]
- 22.Yu W., Xu D., Peng T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct Z-scheme mechanism. J. Mater. Chem. A. 2015;3:19936–19947. doi: 10.1039/C5TA05503B. [DOI] [Google Scholar]
- 23.Wang J., Burghaus U. Adsorption dynamics of CO2 on Zn–ZnO(0001): A molecular beam study. J. Chem. Phys. 2005;122:044705. doi: 10.1063/1.1834490. [DOI] [PubMed] [Google Scholar]
- 24.Yang H., Xu Z., Fan M., Slimane R., Bland A.E., Wright I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008;20:14–27. doi: 10.1016/S1001-0742(08)60002-9. [DOI] [PubMed] [Google Scholar]
- 25.Goeppert A., Czaun M., May R.B., Prakash G.K.S., Olah G.A., Narayanan S.R. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent. J. Am. Chem. Soc. 2011;133:20164–20167. doi: 10.1021/ja2100005. [DOI] [PubMed] [Google Scholar]
- 26.Qi G., Wang Y., Estevez L., Duan X., Anako N., Park A.-H.A., Li W., Jones C.W., Giannelis E.P. High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules. Energy Environ. Sci. 2011;4:444–452. doi: 10.1039/C0EE00213E. [DOI] [Google Scholar]
- 27.Rochelle G.T. Amine scrubbing for CO2 capture. Science. 2009;325:1652–1654. doi: 10.1126/science.1176731. [DOI] [PubMed] [Google Scholar]
- 28.Liao Y., Cao S.-W., Yuan Y., Gu Q., Zhang Z., Xue C. Efficient CO2 capture and photoreduction by amine-functionalized TiO2. Chem. Eur. J. 2014;20:10220–10222. doi: 10.1002/chem.201403321. [DOI] [PubMed] [Google Scholar]
- 29.Peng Y., Xu A.-W., Deng B., Antonietti M., Colfen H. Polymer-controlled crystallization of zinc oxide hexagonal nanorings and disks. J. Phys. Chem. B. 2006;110:2988–2993. doi: 10.1021/jp056246d. [DOI] [PubMed] [Google Scholar]
- 30.Tseng C.L., Chen Y.K., Wang S.H., Peng Z.W., Lin J.L. 2-Ethanolamine on TiO2 investigated by in situ infrared spectroscopy. Adsorption, photochemistry, and its interaction with CO2. J. Phys. Chem. C. 2010;114:11835–11843. doi: 10.1021/jp9117166. [DOI] [Google Scholar]
- 31.Wang W.-N., An W.-J., Ramalingam B., Mukherjee S., Niedzwiedzki D., Gangopadhyay S., Biswas P. Size and structure matter: Enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J. Am. Chem. Soc. 2012;134:11276–11281. doi: 10.1021/ja304075b. [DOI] [PubMed] [Google Scholar]
- 32.Xie S., Wang Y., Zhang Q., Fan W., Deng W., Wang Y. Photocatalytic reduction of CO2 with H2O: Significant enhancement of the activity of Pt–TiO2 in CH4 formation by addition of MgO. Chem. Commun. 2013;49:2451–2453. doi: 10.1039/c3cc00107e. [DOI] [PubMed] [Google Scholar]
- 33.Teramura K., Okuoka S., Tsuneoka H., Shishido T., Tanaka T. Photocatalytic reduction of CO2 using H2 as reductant over ATaO3 photocatalysts (A = Li, Na, K) Appl. Catal. B. 2010;96:565–568. doi: 10.1016/j.apcatb.2010.03.021. [DOI] [Google Scholar]
- 34.Teramura K., Okuoka S., Tsuneoka H., Shishido T., Tanaka T. Photocatalytic conversion of CO2 in water over layered double hydroxides. Angew. Chem. Int. Ed. 2012;51:8008–8011. doi: 10.1002/anie.201201847. [DOI] [PubMed] [Google Scholar]