Abstract
Tilorone dihydrochloride (1) has great potential for inducing interferon against pathogenic infection. In this paper, we describe a convenient preparation method for 2,7-dihydroxyfluoren-9-one (2), which is a usual pharmaceutical intermediate for preparing tilorone dihydrochloride (1). In the novel method, methyl esterification of 4,4′-dihydroxy-[1,1′-biphenyl]-2-carboxylic acid (4) was carried out under milder conditions with higher yield and played an important role in the preparation of compound 2. The structures of the relative intermediates and target compound were characterized by melting point, IR, MS, and 1H-NMR. Furthermore, the synthesized tilorone dihydrochloride exhibited an obvious effect on induction of interferon-α (IFN-α) in mice within 12 h, and the peak level was observed until 24 h. This fruitful work has resulted in tilorone dihydrochloride becoming available in large-scale and wide application in clinics, which has a good pharmaceutical development prospects.
Keywords: synthesis; 2,7-dihydroxyfluoren-9-one; tilorone; methyl esterification; IFN-α
1. Introduction
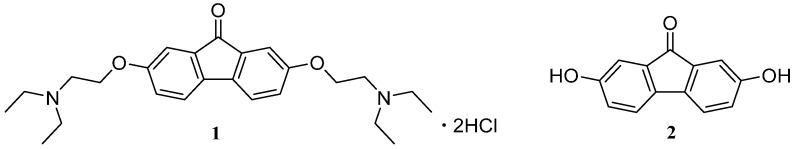
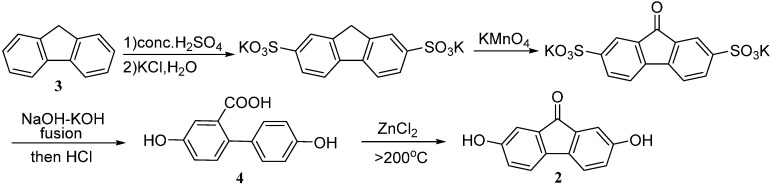
Tilorone dihydrochloride (1, Figure 1) was first reported to have antivirus activity in mice in 1970 [1,2]. Moreover, it has been gradually proved to possess extensive pharmacological functions, such as enhanced phagocytic activity of natural killer cells [3], anti-pyretic [4], anti-fibrotic [5], anti-inflammatory [6,7,8,9,10], anti-virus [11,12], anti-tumor [13,14,15], as well as interferon induction [16,17,18]. All the effects are attributed to its planar structure of a modification of the fluorenone skeleton and amino side chain [15,19,20,21]. The skeleton comes straight from 2,7-dihydroxyfluoren-9-one (2, Figure 1), which can be substituted by appropriate dialkylaminoalkyl halides to give tilorone and other compounds [15]. Compound 2 has been maturely synthesized by 9H-fluorene (3) in several steps as outlined in Scheme 1 [22,23,24]. However, the straight cyclization in the last step is hardly carried out by a solid-solid reaction, which will be not suitable for manufacture on a large scale. Meanwhile, a temperature higher than 200 °C is required, resulting in low purity, a great deal of energy consumption, a tendency to get out of control to burst, and the yield of pure compound 2 is not over 80%. Therefore, there is still a strong demand for developing a more effective approach to produce the intermediate for tilorone under milder conditions.
Figure 1.
Structures of tilorone dihydrochloride (1) and 2,7-dihydroxyfluoren-9-one (2).
Scheme 1.
Traditional synthetic route of 2,7-dihydroxyfluoren-9-one (2).
The antiviral effect of tilorone has been extensively investigated. Many studies had demonstrated the broad-spectrum antiviral activity of tilorone, which performed well with both oral administration and intravenous injection [1,25]. Additionally, some other studies had proved that the antiviral property was attributed to the induction of interferon in serum against viral infection. In addition, they also described the method of interferon assay—the plaque reduction assay measured as U/mL [16]—but it was not simple and was time-consuming. To improve the efficiency, rapid and accurate detections of interferon measured as pg/mL or ppm need to be developed and play an important role, especially the enzyme-linked immunosorbent assay (ELISA), which is most commonly used in the determination of different antigens, antibodies, cytokines, etc. [26,27,28].
In the present study, we successfully prepared tilorone dihydrochloride (1) via improving the traditional method in great yield. In contrast, we creatively employed methanol to synthesize ester so that we could obtain purer intermediate in a larger number with a low cost, which was suitable for industrial production. We also carried out an ELISA test to investigate the specific induction of IFN-α by the synthesized tilorone dihydrochloride to analyze kinetic features.
2. Results and Discussion
2.1 Synthesis of Tilorone Dihydrochloride
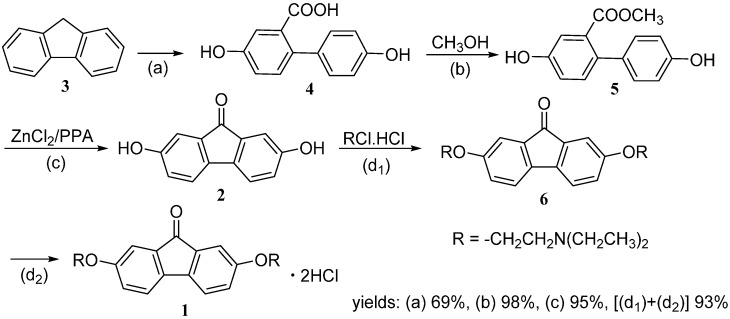
As shown in Scheme 2, the target compound 1 was prepared from 2. According to the method of Andrews [22], 4,4′-dihydroxy-[1,1′-biphenyl]-2-carboxylic acid (4) was synthesized by the reaction of 3 in the same reaction conditions shown in Scheme 1. By examining the reaction behavior of compound 4 and ZnCl2 (Scheme 1), we found that compound 4 could not be fully mixed with ZnCl2 and converted into 2 at 200 °C, especially when the reaction scale was large. To solve the problem, we added polyphosphoric acid (PPA) in the reactants to try to make the mixture more easily, but failed. Problems still existed, including inconvenient practice and the existence of heavy material clashing due to the high temperature. In order to improve the efficiency and practicability of this reaction, we found out the following useful method. Compound 4 was firstly esterified in CH3OH using concentrated H2SO4 as a catalyzer under reflux for 2 h, whereby the yield of corresponding methyl ester 5 reached 98%. Then, the conversion of 5 to 2 was successfully accomplished by treatment with ZnCl2 and PPA at 110–120 °C in 96% yield, which reduced the temperature and improved the yield to a great degree in contrast to the direct cyclization of 4. Finally, compound 2 was reacted with 2-diethylaminoethylchloride hydrochloride in toluene in the presence of KOH under reflux for 24 h to give 6, which was subsequently acidified to afford 1. In this synthetic route of compound 1 using compound 3 as a starting material, the overall yield of 1 achieved 60%.
Scheme 2.
Approach to 2,7-dihydroxyfluoren-9-one (2) and tilorone dihydrochloride (1).
2.2 Evaluation on Induction Activity of IFN-α
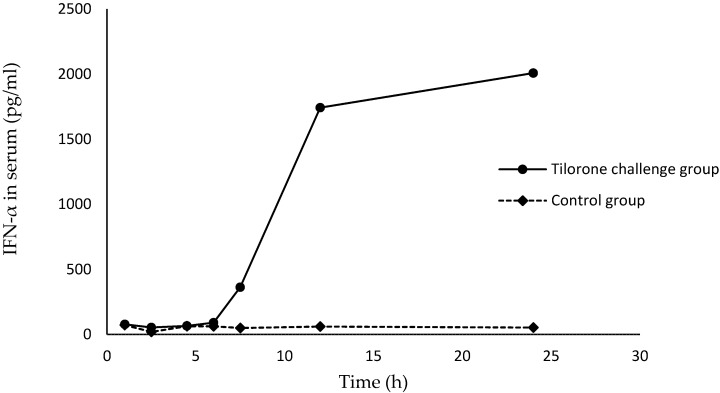
Tilorone dihydrochloride has shown obvious IFN-inducing and antiviral properties. In the present study, our target compound was dissolved in phosphate buffer solution (PBS) at a concentration permitting an inoculum of 250 mg/kg in specific pathogen free (SPF) mice orally. Mice were bled eight times after tilorone dihydrochloride challenge and serum was collected and diluted for determination. The data suggests that tilorone dihydrochloride stimulates a delayed but prolonged response with the probable peak levels, approximately 2000 pg/mL, being observed 12 to 24 h after gavages (Figure 2). The kinetics displayed were similar with variations of the antiviral property.
Figure 2.
Serum IFN-α response of mice to tilorone dihydrochloride (1).
3. Experimental Section
3.1. Synthesis of Tilorone Dihydrochloride (1)
General
Every reaction was monitored, and the endpoint of the reaction was checked by TLC performed on GF-254 silica gel plates with visualization by UV light. Melting points were measured on an YRT-3 temperature apparatus (Tianjin Jing Tuo instrument technology, Tianjin, China) and uncorrected. IR spectra were performed on a IR spectra were recorded on a VERTEX 80 instrument (Bruker Corporation (Beijing, China). Mass spectra were determined on a VG Auto Spec-3000 spectrometer (VG instruments, Kuala Lumpur, Malaysia) and reported as m/z. The 1H-NMR spectral data were recorded on a Bruker Avance 400 NMR spectrometer (Bruker Corporation (Beijing), Beijing, China) and chemical shifts were reported in ppm (δ) relative to TMS as an internal standard.
4,4′-dihydroxy-[1,1′-biphenyl]-2-carboxylic acid (4). Compound 3 (200 g, 1.20 mol) was dissolved in concentrated H2SO4 (570 mL) at 110–115 °C. After 2 h of vigorous stirring, the solution was allowed to cool and water (1.7 L) was added. Then the solution was filtered. To the filtrate KCl (400 g) was added and the solution was stirred at room temperature for 30 min. Then the precipitate was filtered and dried to give a solid (396 g, 82%), which was added in batches to a stirred solution of KMnO4 (208 g, 1.32 mol) in water (7.1 L), maintained at 20–30 °C for 2 h, and then filtered. The filtrate was concentrated in vacuum to 4.5 L, and cooled to 5 °C. The precipitate was filtered and dried to obtain a solid (378 g, 89%), 105 g of which (0.24 mol) was added to a mixture of NaOH (210 g, 5.25 mol) and KOH (56 g, 1.00 mol). After 1 h of stirring at 270–300 °C, the melt was allowed to cool and dissolved in water (1.5 L). The solution was acidified by addition of concentrated HCl to pH 3.0, and then the precipitate was filtered and dried. Compound 4 was obtained (52 g, 95%) in an overall yield of 69%; m.p. 269–270 °C (Lit. 270–273 °C [22]); IR (KBr), υ (cm−1): 3145.4–2491.3 (OH), 1693.3 (C=O); ESI-MS (m/z): 229.0 [M − H]−, C13H10O4 (MW. = 230.1); 1H-NMR (400 MHz, DMSO-d6), δ (ppm): 12.60 (br, 1H), 9.73 (s,1H), 9.40 (s, 1H), 7.37 (d, J = 8.5 Hz, 1H), 7.13 (d, J = 8.4 Hz, 1H), 7.06 (d, J = 8.4 Hz, 2H), 7.03 (d, J = 2.5 Hz, 1H), 6.91 (dd, J = 8.3, 2.5 Hz, 1H), 6.74 (d, J = 8.4 Hz, 2H).
Methyl 4,4′-Dihydroxy-[1,1′-biphenyl]-2-carboxylic acid (5). A mixture of 4 (50 g, 0.22 mol), concentrated H2SO4 (5 mL), and CH3OH (400 mL) was refluxed for 2 h and then cooled to r.t., precipitated. The precipitate was filtered and dried to give 5 (52 g, 98%); m.p. 224–226 °C; IR (KBr), υ (cm−1): 3291.0, 3202.8 (OH) 1692.5 (C=O), 1435.5 (CH3); ESI-MS (m/z): 245.0 [M + H]+, C14H12O4 (MW. = 244.1); 1H-NMR (400 MHz, DMSO-d6), δ (ppm): 9.80 (s, 1H), 9.42 (s, 1H), 7.18 (d, J = 8.3 Hz, 1H), 7.02 (d, J = 2.0 Hz, 2H), 7.00 (d, J = 2.0 Hz, 1H), 6.95 (dd, J = 8.4, 2.6 Hz, 1H), 6.76 (d, J = 2.0 Hz, 1H), 6.75 (d, J = 2.0 Hz, 1H), 3.58 (s, 3H).
2,7-Dihydroxy-9H-fluoren-9-one (2). A mixture of 5 (50 g, 0.20 mol), ZnCl2 (50 g, 0.37 mol), PPA (33 mL) was stirred at 110–120 °C for 2 h and then cooled to r.t., precipitated by addition of water (500 mL). The precipitate was filtered and dried to obtain 40 g red-brown crystallized powder (2). Yield 95%; m.p. 336–337 °C (Lit. 338 °C [24]); IR (KBr), υ (cm−1): 3388.9, 3360.6 (OH), 1700.7 (C=O); ESI-MS (m/z): 211.0 [M − H]−, C13H8O3 (MW. = 212.0); 1H-NMR (300 MHz, DMSO-d6), δ (ppm): 9.89 (s, 2H), 7.37 (d, J = 4.8 Hz, 2H), 6.90 (dd, J = 4.9, 2.4 Hz, 2H), 6.86 (d, J = 2.4 Hz, 2H).
2,7-bis[2-(Diethylamino)ethoxy]-9H-fluoren-9-one hydrochloride (1). A mixture of 2 (100 g, 0.47 mol), 2-diethylaminoethylchloride hydrochloride (300 g, 1.74 mol), KOH (225 g, 4.01 mol), water (320 mL) and toluene (1.5 L) was refluxed for 24 h. The emulsion was cooled, and then organic layer was separated, washed with KOH aq. (3 mol/L, 500 mL) and concentrated up to dryness in vacuum. The residue was dissolved in anhydrous ethanol (420 g) and acidified with gasified HCl to pH 3.0. The orange product was recrystallized with ethanol, filtered, and dried to give 200 g of compound 1 in a yield of 93%; m.p. 231–233 °C (Lit. 235–236 °C [22]); IR (KBr), υ (cm−1): 2943.3, 2596.5 (CH), 1708.9 (C=O); ESI-MS (m/z): 411.0 [M + H]+, C25H34N2O3 (MW. = 410.3); 1H-NMR (400 MHz, D2O), δ (ppm): 7.12 (d, J = 6.5 Hz, 2H), 6.99 (dd, J = 6.5, 1.6 Hz, 2H), 6.89 (d, J = 1.6 Hz, 2H), 4.39 (t, J = 3.6 Hz,4H), 3.68 (t, J = 3.6 Hz, 4H), 3.42 (q, J = 5.6 Hz, 8H), 1.44 (t, J = 5.8 Hz, 12H).
3.2. Determination of Serum IFN-α Induced by Tilorone Dihydrochloride by ELISA
SPF mice were housed under conditions of constant temperature and a 12-h light cycle, with food and water provided ad libitum in the Animal Experimental Center, Nanjing Agricultural University. Interferon levels were recorded on a Thermo Multiskan Ascent enzyme-labeled instrument (Thermo Fisher Scientific (Beijing, China). After tilorone dihydrochloride challenge, mice were alive just like the control group, with normal activity levels, appetite, and growth. Sample serum diluent and different concentrations of the standard were added to a micro-well plate (96 tests), which was put at 37 °C for 2 h. Biotin-conjugate diluent prepared before 30 min was added after dumping the liquid out of the plate, which would be put at 37 °C for another 1 h. The plate was washed five times after dumping the liquid and Streptavidin-HRP diluent prepared beforehand was added to the plate that would be put in the thermostat again for 1 h with no light. Substrate solution was added for a 20 min reaction after washing the plate five times. Straight after that stop solution was added to the plate, which was immediately detected for OD450 values.
4. Conclusions
We successfully synthesized tilorone dihydrochloride (1) in a great overall yield of 60%. During the reactions, the creative methyl esterification process made the conversion from 5 to 2 much more easily accomplished and it was operated under much milder conditions (acquired temperature reduced from 200 °C to 110–120 °C), whereby the 2 produced was of higher purity and in greater yield so that tilorone dihydrochloride of better quality might be obtained. On the other hand, the synthesized tilorone dihydrochloride exhibited the significant property of inducing a delayed but prolonged interferon response in mice. In summary, esterification can be a good choice for preparing an intermediate of tilorone dihydrochloride, which can be an effective medicine for clinical applications.
Acknowledgments
We are thankful for the valuable comments from Dawei Guo, Nanjing Agricultural University. This work was supported by the grant from the Natural Science Foundation of Jiangsu Province in China (BK20140716).
Author Contributions
Zuliang Liu initiated and led the project. Junren Zhang performed the experiments. Qizheng Yao analyzed the data. Junren Zhang wrote the manuscript. The manuscript was finalized through contributions from all authors, and all authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: All the samples of the compounds are available from the authors.
References
- 1.Krueger R.E., Mayer G.D. Tilorone hydrochloride: An orally active antiviral agent. Science. 1970;169:1213–1214. doi: 10.1126/science.169.3951.1213. [DOI] [PubMed] [Google Scholar]
- 2.Krueger R.F., Mayer G.D., Yoshimura S., Ludwig K.A. In vivo evaluations of tilorone hydrochloride against semliki forest virus. Antimicrob. Agents Chemother. 1970;10:486–490. [PubMed] [Google Scholar]
- 3.Gaforio J.J., Ortega E., Algarra I., Serrano M.J., Alvarez de Cienfuegos G. Nk cells mediate increase of phagocytic activity but not of proinflammatory cytokine (interleukin-6 [IL-6], tumor necrosis factor alpha, and IL-12) production elicited in splenic macrophages by tilorone treatment of mice during acute systemic candidiasis. Clin. Vaccine Immunol. 2002;9:1282–1294. doi: 10.1128/CDLI.9.6.1282-1294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark W.G., Robins J.A. The antipyretic effect of tilorone hydrochloride in the cat. Br. J. Pharmacol. 1978;62:281–287. doi: 10.1111/j.1476-5381.1978.tb08457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepparanta O., Tikkanen J.M., Bespalov M.M., Koli K., Myllarniemi M. Bone morphogenetic protein-inducer tilorone identified by high-throughput screening is antifibrotic in vivo. Am. J. Respir. Cell Mol. Biol. 2013;48:448–455. doi: 10.1165/rcmb.2012-0201OC. [DOI] [PubMed] [Google Scholar]
- 6.Megel H., Raychaudhuri A., Shemano I., Beaver T.H., Thomas L.L. The anti-inflammatory actions of tilorone hydrochloride. Proc. Soc. Exp. Biol. Med. 1975;149:89–93. doi: 10.3181/00379727-149-38749. [DOI] [PubMed] [Google Scholar]
- 7.Kovarik P., Sauer I., Schaljo B. Molecular mechanisms of the anti-inflammatory functions of interferons. Immunobiology. 2007;212:895–901. doi: 10.1016/j.imbio.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skrots’ka O.I., Zholobak N.M., Antonenko S.V., Spivak M., Karpov O.V. Antiherpetic effect of rna-tilorone molecular complex in cell culture. Mikrobiol. Z. 2007;69:62–68. [PubMed] [Google Scholar]
- 9.Zholobak N.M., Kavok N.S., Bogorad-Kobelska O.S., Borovoy I.A., Malyukina M.Y., Spivak M.Y. Effect of tilorone and its analogues on the change of mitochondrial potential of rat hepatocytes. Fiziolohichnyi Zhurnal. 2012;58:39–43. doi: 10.1615/IntJPhysPathophys.v3.i4.60. [DOI] [PubMed] [Google Scholar]
- 10.Briggs C.A., Schrimpf M.R., Anderson D.J., Gubbins E.J., Gronlien J.H., Hakerud M., Ween H., Thorin-Hagene K., Malysz J., Li J., et al. Alpha7 nicotinic acetylcholine receptor agonist properties of tilorone and related tricyclic analogues. Br. J. Pharmacol. 2008;153:1054–1061. doi: 10.1038/sj.bjp.0707649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karpov A.V., Zholobak N.M., Spivak N.Y., Rybalko S.L., Antonenko S.V., Krivokhatskaya L.D. Virus-inhibitory effect of a yeast RNA-tilorone molecular complex in cell cultures. Acta Virol. 2001;45:181–184. [PubMed] [Google Scholar]
- 12.Karpov A.V., Antonenko S.V., Barbasheva E.V., Spivak N. Study of anti-HIV activity of the yeast RNA-tilorone molecular complex. Vopr. Virusol. 1997;42:17–19. [PubMed] [Google Scholar]
- 13.Wissing M.D., Dadon T., Kim E., Piontek K.B., Shim J.S., Kaelber N.S., Liu J.O., Kachhap S.K., Nelkin B.D. Small-molecule screening of pc3 prostate cancer cells identifies tilorone dihydrochloride to selectively inhibit cell growth based on cyclin-dependent kinase 5 expression. Oncol. Rep. 2014;32:419–424. doi: 10.3892/or.2014.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Algarra I., Perez M., Hoglund P., Gaforio J.J., Ljunggren H.G., Garrido F. Generation and control of metastasis in experimental tumor systems; inhibition of experimental metastases by a tilorone analogue. Int. J. Cancer. 1993;54:518–523. doi: 10.1002/ijc.2910540327. [DOI] [PubMed] [Google Scholar]
- 15.Zhou D., Tuo W., Hu H., Xu J., Chen H., Rao Z., Xiao Y., Hu X., Liu P. Synthesis and activity evaluation of tilorone analogs as potential anticancer agents. Eur. J. Med. Chem. 2013;64:432–441. doi: 10.1016/j.ejmech.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 16.Li L., Sherry B. Ifn-alpha expression and antiviral effects are subtype and cell type specific in the cardiac response to viral infection. Virology. 2010;396:59–68. doi: 10.1016/j.virol.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiyama Y., Kuriyama K. Dissociation between antiinflammatory action of tilorone and its interferon inducing activity. Agents Actions. 1991;33:229–232. doi: 10.1007/BF01986567. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann H., Kunz C. The protective effect of the interferon inducers tilorone hydrochloride and poly i:C on experimental tick-borne encephalitis in mice. Arch. Gesamte Virusforsch. 1972;37:262–266. doi: 10.1007/BF01268009. [DOI] [PubMed] [Google Scholar]
- 19.Lee C.C., Chang D.M., Huang K.F., Chen C.L., Chen T.C., Lo Y., Guh J.H., Huang H.S. Design, synthesis and antiproliferative evaluation of fluorenone analogs with DNA topoisomerase i inhibitory properties. Bioorg. Med. Chem. 2013;21:7125–7133. doi: 10.1016/j.bmc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Cinelli M.A., Morrell A., Dexheimer T.S., Scher E.S., Pommier Y., Cushman M. Design, synthesis, and biological evaluation of 14-substituted aromathecins as topoisomerase i inhibitors. J. Med. Chem. 2008;51:4609–4619. doi: 10.1021/jm800259e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldo J.P., Zhang X., Shi F., Larock R.C. Efficient synthesis of fluoren-9-ones by the palladium-catalyzed annulation of arynes by 2-haloarenecarboxaldehydes. J. Org. Chem. 2008;73:6679–6685. doi: 10.1021/jo8009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews E.R., Fleming R.W., Grisar J.M., Kihm J.C., Wenstrup D.L., Mayer G.D. Bis-basic-substituted polycyclic aromatic compounds. A new class of antiviral agents. 2. Tilorone and related bis-basic ethers of fluorenone, fluorenol, and fluorene. J. Med. Chem. 1974;17:882–886. doi: 10.1021/jm00254a020. [DOI] [PubMed] [Google Scholar]
- 23.Burke H.M., Joullie M.M. New synthetic routes to tilorone dihydrochloride and some of its analogues. J. Med. Chem. 1978;21:1084–1086. doi: 10.1021/jm00208a016. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal K.C. Fluorene derivatives for antitumor activity. J. Med. Chem. 1967;10:99–101. doi: 10.1021/jm00313a023. [DOI] [PubMed] [Google Scholar]
- 25.Balezina T.I., Korneeva L.E., Zemskov V.M., Loidina G.I., Nikolaeva O.V., Fainshtein S.L., Fadeeva L.L., Eromolieva Z.V. Comparison of interferon-inducing activities and antiviral effects of tobacco mosaic virus, tilorone and sodium nucleinate. Acta Virol. 1977;21:338–343. [PubMed] [Google Scholar]
- 26.Hane H., Muro Y., Watanabe K., Ogawa Y., Sugiura K., Akiyama M. Establishment of an ELISA to detect anti-glycyl-tRNA synthetase antibody (anti-EJ), a serological marker of dermatomyositis/polymyositis and interstitial lung disease. Clin. Chim. Acta. 2014;431:9–14. doi: 10.1016/j.cca.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Piccoli L., Meroni V., Genco F., Tamarozzi F., Tinelli C., Filice C., Brunetti E. Serum cytokine profile by ELISA in patients with echinococcal cysts of the liver: A stage-specific approach to assess their biological activity. Clin. Dev. Immunol. 2012;2012:483935. doi: 10.1155/2012/483935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes S.G., Gruffydd-Jones T., Gunn-Moore D., Jahans K. Adaptation of IFN-gamma ELISA and ELISPOT tests for feline tuberculosis. Vet. Immunol. Immunopathol. 2008;124:379–384. doi: 10.1016/j.vetimm.2008.04.006. [DOI] [PubMed] [Google Scholar]