Abstract
Resveratrol (1), a naturally occurring stilbene compound, has been suggested as a potential whitening agent with strong inhibitory activity on melanin synthesis. However, the use of resveratrol in cosmetics has been limited due to its chemical instability and poor bioavailability. Therefore, resveratrol derivatives were prepared to improve bioavailability and anti-melanogenesis activity. Nine resveratrol derivatives including five alkyl ether derivatives with C2H5, C4H9, C5H11, C6H13, and C8H17 (2a–2e) and four ester derivatives with CH3, CH=C(CH3)2, CH(C2H5)C4H9, C7H15 (3a–3d) were newly synthesized and their effect on melanin synthesis were assessed. All the synthetic derivatives efficiently reduced the melanin content in α-MSH stimulated B16F10 melanoma cells. Further investigation showed that the inhibitory effect of 2a on melanin synthesis was achieved not by the inhibition of tyrosinase activity but by the inhibition of melanogenic enzyme expressions such as tyrosinase and tyrosinase-related protein (TRP)-1. Our synthetic resveratrol derivatives have more lipophilic properties than resveratrol by the addition of alkyl or acyl chains to free hydroxyl moiety of resveratrol; thus, they are expected to show better bioavailability in skin application. Therefore, we suggest that our synthetic resveratrol derivatives might be promising candidates for better practical application to skin-whitening cosmetics.
Keywords: melanin, resveratrol derivatives, tyrosinase, tyrosinase-related protein (TRP)-1
1. Introduction
Melanin is produced in melanocytes by melanogenesis and determines skin and hair color. It also plays an important role in skin protection from UV radiation. However, excessive accumulation by pathological and environmental factors induces pigmentation problems and has become a critical issue in the cosmetic field [1,2]. Therefore, melanogenesis inhibitors have become important constituents in cosmetic products for depigmentation [3,4,5].
Melanogenesis is a complex biosynthetic process regulated by enzymatic and chemical reactions. Exposure of the skin to UV radiation stimulates keratinocytes to secrete α-melanocyte stimulating hormone (α-MSH). α-MSH then binds to melanocortin 1 receptor (MC1R) on melanocyte and induces melanogenesis. Microphthalmia-associated transcriptional factor (MITF) is a key transcriptional factor in melanogenesis that involved in the regulation of melanogenic enzymes such as tyrosinase, tyrosinase-related protein (TRP)-1 and TRP-2. Tyrosinase catalyzes the conversion of l-tyrosine to dopaquinone, the first rate-limiting step in the melanogenesis. TRP-1 and TRP-2 are also involved in the major steps of melanin synthesis. Therefore, tyrosinase, TRP-1 and TRP-2 have become important targets in the development of depigmenting agents, such as skin-whitening cosmetics [6,7,8,9].
Resveratrol, 3,4′,5-trihydroxy-trans-stilbene, has diverse biological activities, such as antioxidant, anti-inflammatory and cardiovascular protective effects [10,11,12]. Resveratrol also has been reported to reduce melanin synthesis [13,14,15]. However, its utilization and development in products are limited due to the chemical stability, poor solubility, and low bioavailability [16,17]. Therefore, attempts to increase the bioavailability of resveratrol have been made in many fields. Drug delivery systems using new formulations, such as encapsulation are suggested to enhance bioavailability [18,19,20]. The resveratrol derivatives, such as resveratrol dimers or synthetic derivatives, also have been reported to have better biological activity with fewer drawbacks [21,22,23,24,25]. Recently, the acetylated derivative of resveratrol has been synthesized with more efficient anti-melanogenic activity and better stability [26]. For the purpose of developing anti-melanogenesis inhibitory resveratrol derivatives with better bioavailability, nine resveratrol derivatives, five alkyl ether derivatives and four alkyl ester derivatives by chemical reaction and were synthesized in the present study. Effect on melanogenesis and the mode of action were also investigated.
2. Results and Discussion
2.1. Chemistry
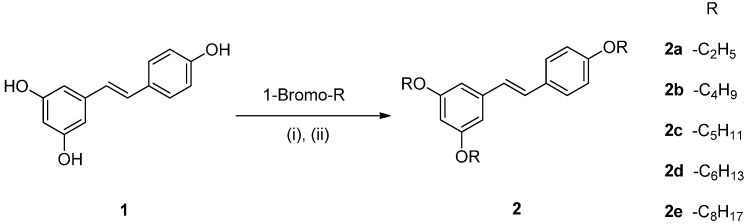
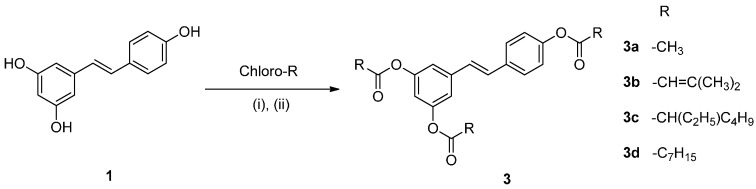
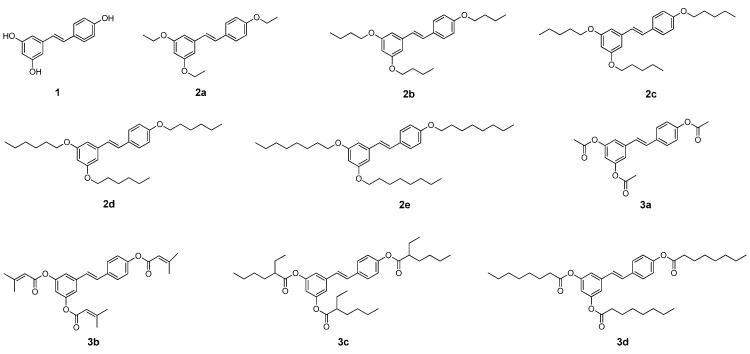
The resveratrol ether derivatives were synthesized as shown in Scheme 1. Resveratrol (1) was converted to the corresponding ether derivatives (2a–2e) in the presence of corresponding alkyl bromides. The synthesis of resveratrol ester derivatives is shown in Scheme 2. Resveratrol ester derivatives (3a–3d) were synthesized by the addition of corresponding acyl chlorides. The structures of synthetic derivatives were confirmed by spectroscopic analysis including NMR, IR and MS analysis (Figure 1).
Scheme 1.
Synthesis of resveratrol ether derivatives 2a–e.
Reagents and conditions: (i) DMF, NaOH, 10 min; (ii) 1-bromoethane, 1-bromobutane, 1-bromopentane, 1-bromohexane, or 1-bromooctane, 40 °C, 24 h.
Scheme 2.
Synthesis of resveratrol ester derivatives 3a–d.
Reagents and conditions: (i) CH2Cl2, 10 °C, 5 min; (ii) TEA, DMAP, 10 °C; (iii) acetyl chloride, 3,3-dimethylacryloyl chloride, 2-ethylhexanoyl chloride, or octanoyl chloride, 10 °C; 1 h.
Figure 1.
Chemical structure of resveratrol (1) and its derivatives (2a–e and 3a–d).
2.2. Effect on Melanogenesis
2.2.1. Effect on Melanin Content in B16F10 Melanoma Cells
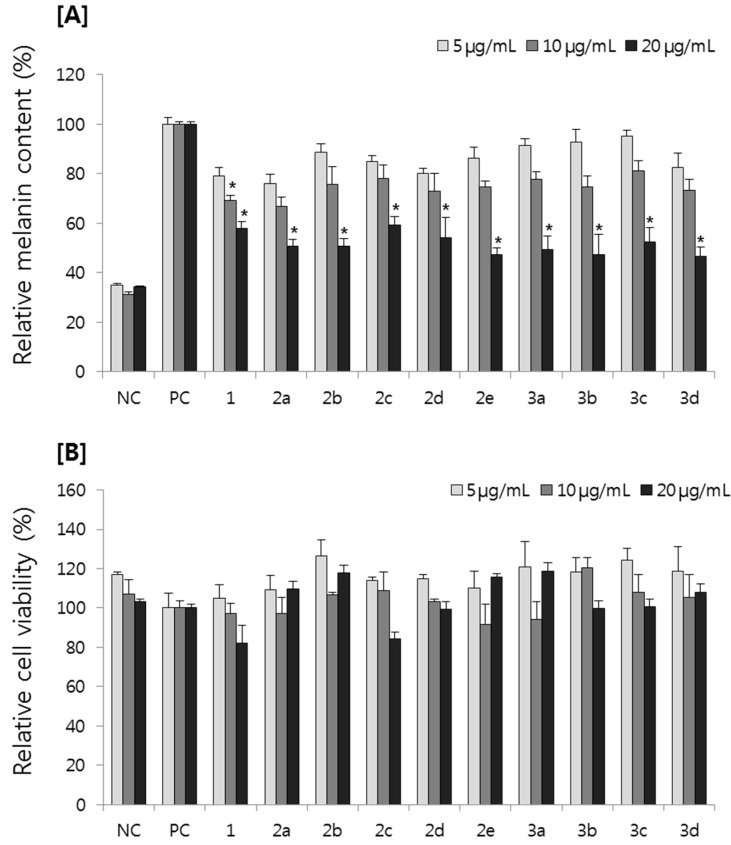
The effect of resveratrol derivatives on melanogenesis and cell viability was first investigated using B16F10 melanoma cells. Stimulation of B16F10 melanoma cells with 100 nM α-MSH for 72 h significantly increased the melanin synthesis. Resveratrol derivatives dose-dependently reduced the melanin content concentration from 5 to 20 µg/mL without any cytotoxicity (Figure 2A,B). Although the inhibition was slightly increased compared to resveratrol in some derivatives, there was no significant difference among resveratrol and synthetic derivatives. In addition, all the synthetic derivatives showed similar inhibition regardless of side chains.
Figure 2.
Effects of resveratrol derivatives on (A) melanin content and (B) cell viability in B16F10 melanoma cells. NC: vehicle treated normal control; PC: α-MSH stimulated positive control. * p < 0.05 compared with PC group.
2.2.2. Effect on Tyrosinase Activity
Inhibition of melanin synthesis can be achieved either by inhibiting tyrosinase activity or by reducing melanogenic enzyme expression [8,9]. Therefore, the effect of resveratrol derivatives on tyrosinase activity and the expression of melanogenic enzymes were investigated.
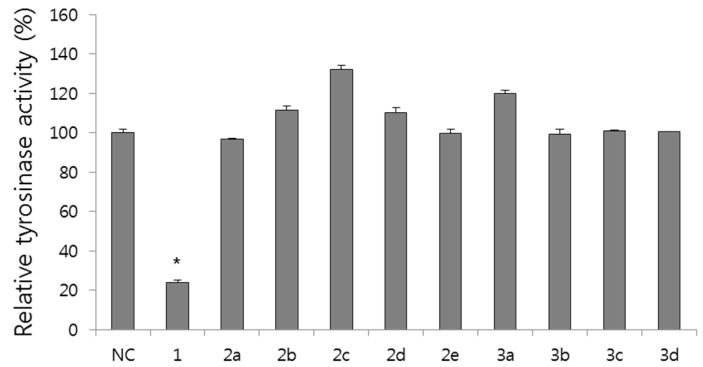
Tyrosinase catalyzes the first rate-limiting step in the melanogenesis and plays a pivotal role in melanin synthesis [6,7]. The effect of resveratrol derivatives on tyrosinase activity was first evaluated in vitro using mushroom tyrosinase. Although resveratrol effectively inhibited the tyrosinase activity, both alkyl ether (2a–2e) and ester derivatives (3a–3d) showed little inhibition (Figure 3). These results suggest that free hydroxyl groups of resveratrol are important for the inhibition of tyrosinase activity, which is consistent with previous reports [27].
Figure 3.
Effects of resveratrol derivatives (100 µg/mL) on tyrosinase activity. NC: vehicle treated normal control. * p < 0.05 compared with NC group.
2.2.3. Effect on Melanin Synthesis in B16F10 Melanoma Cells
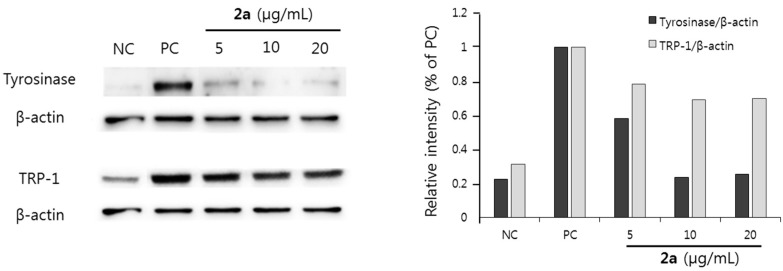
Melanin synthesis is also regulated by the expression of melanogenic enzymes. Tyrosinase and TRP-1 are key enzymes involved in the major steps of melanin synthesis [8,9]. Therefore, the effect of the resveratrol derivative 2a on the expressions of tyrosinase and TRP-1 was determined. The expression of tyrosinase was dramatically reduced by the treatment of compound 2a (Figure 4). Treatment of 2a also inhibited the expression of TRP-1 expression. These results suggest that 2a efficiently inhibited the melanogenic enzyme expression.
Figure 4.
Effect of resveratrol derivative 2a on the expression of tyrosinase and TRP-1 in B16F10 melanoma cells. NC: vehicle treated normal control; PC: α-MSH stimulated positive control.
2.3. Discussion
Botanical ingredients are good sources of medicine, functional foods and cosmetics. They provide numerous compounds with diverse skeletons and biological activities. However, their applications are often limited due to their small amounts, poor bioavailability, etc. Resveratrol is well known for its potential biological activities. As a cosmetic ingredient, it has antioxidant and melanogenesis inhibitory activities. However, it has limitations for cosmetic development, such as chemical instability and low solubility. In addition, the hydroxyl moiety of resveratrol contributes to poor skin absorption. Many attempts have been made to overcome its limitations, and the synthetic derivatives of resveratrol have been suggested as effective in increasing stability and bioavailability [26,28,29,30].
In our present study, we synthesized nine resveratrol derivatives, including five ether derivatives (2a–2e) and four ester derivatives (3a–3d) and then evaluated melanogenesis inhibitory activity. Our present study showed that all the synthetic ether and ester derivatives of resveratrol inhibited melanin synthesis in melanoma cells. Further study also showed that resveratrol derivative 2a inhibited melanin synthesis in melanoma cells by inhibiting the expression of melanogenic enzymes, tyrosinase and TRP-1 (Figure 2 and Figure 4). However, it showed little effect on tyrosinase activity (Figure 3). Taken together, we suggest that 2a reduced melanin synthesis by the inhibition of melanogenic enzyme expressions rather than direct inhibition on tyrosinase activity.
Skin is a barrier of our body with lipophilic membrane. Therefore, skin absorption of chemical is determined by its physicochemical properties. Lipophilicity is one of the important factors that affect the skin permeation process. Melanocytes and keratinocytes exist in epidermis, the outermost layers of the skin. Thus, lipophilic compounds penetrate deeper in the skin layers [31]. Our synthetic resveratrol derivatives have alkyl or acyl chains instead of free hydroxyl moiety of resveratrol. They exhibit more lipophilic properties than resveratrol, thus these synthetic derivatives are expected to show better bioavailability in skin application. Consistent with our suggestion, triacetyl resveratrol has been reported to have anti-melanogenic activity with better stability [26]. Moreover, resveratrol triacetate reduced hyperpigmented spots in human skin models without any skin irritation [28]. Although the exact mechanism of our synthetic derivatives needs to be confirmed in further animal study, these derivatives might be promising candidates for skin-whitening cosmetics with better practical applications.
3. Experimental Section
3.1. General Information
NMR spectra were recorded using a Bruker DRX 500 MHz NMR spectrometer (Bruker, Karlsruhe, Germany). EI-mass spectra were obtained using VG Autospec Ultima mass spectrometers (Waters, Milford, MA, USA). Semipreparative HPLC was performed using a Waters HPLC system equipped with Waters 600 Q-pumps (Waters, Milford, MA, USA), a 996 photodiode array detector Waters, Milford, MA, USA), and Waters Empower software using a Gemini-NX ODS-column (5 μm, 10 mm × 150 mm) (Phenominex, Inc., Torrance, CA, USA). Silica gel (70–230 mesh, Merck, Darmstadt, Germany) was used for open column chromatography (CC). Thin-layer chromatography (TLC) was performed on a precoated silica gel 60 F254 (0.25 mm, Merck). All other chemicals and reagents were analytical grade.
3.2. Synthesis of Resveratrol Derivatives
(E)-1,3-Diethoxy-5-(4-ethoxystyryl)benzene (2a). Resveratrol (1) (15.0 g, 65.7 mmol) was dissolved in a solution of DMF (150 mL) and NaOH (8.9 g), and stirred for 10 min. Then, 1-bromoethane (25.8 g, 236.6 mmol) was added and the reaction was kept for 24 h at 40 °C. After the removal of DMF by evaporation under vacuum, the reaction mixture was extracted with toluene (45 mL × 2). The combined organic layer were dried over anhydrous MgSO4 and evaporated under vacuum. The crude product was purified by silica gel column chromatography with n-hexane–EtOAc (95:5) as an eluent to give compound 2a (15.4 g, 75.0% yield). Compound 2a: IR νmax cm−1: 1587, 1509, 1247; 1H-NMR (400 MHz, CDCl3) δ 7.45 (2H, d, J = 8.4 Hz), 7.05 (1H, d, J = 16.4 Hz), 6.91 (2H, J = 8.4 Hz), 6.92 (1H, d, J = 16.0 Hz), 6.66 (2H, d, J = 2.4 Hz), 6.39 (1H, t, J = 2.0 Hz), 4.08 (6H, m), 1.45 (9H, m); 13C-NMR (100 MHz, CDCl3) δ 160.3, 158.8, 139.6, 129.8, 128.6, 127.8, 126.6, 114.7, 104.9, 100.5, 63.5, 14.9, 14.8; ESI-MS (positive mode) m/z: 313 [M + H]+, HRESIMS: m/z 313.1798 (calcd. for C20H25O3, 313.1798).
(E)-1,3-Dibutoxy-5-(4-butoxystyryl)benzene (2b). Resveratrol (1) (15.0 g, 65.7 mmol) was dissolved in a solution of DMF (150 mL) and NaOH (8.9 g), and stirred for 10 min. Then, 1-bromobuthane (30.6 g, 223.5 mmol) was added and the reaction was kept for 24 h at 40 °C. After the removal of DMF by evaporation under vacuum, the reaction mixture was extracted with toluene (45 mL × 2). The combined organic layer were dried over anhydrous MgSO4 and evaporated under vacuum. The crude product was purified by silica gel column chromatography with n-hexane–EtOAc (95:5) as an eluent to give compound 2b (21.2 g, 81.5% yield). Compound 2b: IR νmax cm−1: 1589, 1509, 1385, 1294; 1H-NMR (400 MHz, CDCl3) δ 7.45 (2H, d, J = 8.4 Hz), 7.08 (1H, d, J = 16.4 Hz), 6.93 (2H, J = 8.4 Hz), 6.94 (1H, d, J = 16.0 Hz), 6.69 (2H, d, J = 2.4 Hz), 6.43 (1H, t, J = 2.0 Hz), 4.02 (6H, m), 1.83 (6H, m), 1.57 (6H, m), 1.05 (9H, m); 13C-NMR (100 MHz, CDCl3) δ 160.5, 159.0, 139.6, 129.8, 128.6, 127.7, 126.6, 114.7, 104.9, 100.5, 67.7, 31.5, 31.4, 19.4, 19.3, 13.9; ESI-MS (positive mode) m/z: 397 [M + H]+, HRESIMS: m/z 435.2296 (calcd. for C26H36KO3, 435.2296).
(E)-1,3-Bis(pentyloxy)-5-(4-(pentyloxy)styryl)benzene (2c). Resveratrol (1) (50.0 g, 219.1 mmol) was dissolved in a solution of DMF (200 mL). After adding NaOH (28.0 g) and KI (1.8 g), the reaction mixture was stirred for 5 min at RT. Then, 1-bromopentane (109.2 g, 223.5 mmol) was added and the reaction was kept for 12 h at 45 °C. After the removal of DMF by evaporation under vacuum, the reaction mixture was extracted with toluene (45 mL × 2). The combined organic layer were dried over anhydrous MgSO4 and evaporated under vacuum. The crude product was purified by silica gel column chromatography with n-hexane–EtOAc (95:5) as an eluent to give compound 2b (21.2 g, 81.5% yield). Compound 2c: IR νmax cm−1: 1692, 1385, 1344; 1H-NMR (400 MHz, CDCl3) δ 7.47 (2H, d, J = 8.4 Hz), 7.07 (1H, d, J = 16.4 Hz), 6.93 (2H, J = 8.4 Hz), 6.94 (1H, d, J = 16.0 Hz), 6.68 (2H, d, J = 2.4 Hz), 6.42 (1H, brs), 4.00 (6H, m), 1.85 (6H, m), 1.48 (12H, m), 0.99 (9H, m); 13C-NMR (100 MHz, CDCl3) δ 160.5, 159.0, 139.6, 129.8, 128.6, 127.7, 126.6, 114.7, 104.9, 100.5, 68.0, 29.1, 29.0, 28.3, 28.2, 22.5, 14.1; ESI-MS (positive mode) m/z: 439 [M + H]+, HRESIMS: m/z 477.2761 (calcd. for C29H42KO3, 477.2765).
(E)-1,3-Bis(hexyloxy)-5-(4-(hexyloxy)styryl)benzene (2d). Resveratrol (1) (10.0 g, 43.8 mmol) was dissolved in a solution of DMF (100 mL) and NaOH (5.96 g), and stirred for 10 min. Then, 1-bromohexane (25.8 g, 236.6 mmol) was added and the reaction was kept for 24 h at 40 °C. After the removal of DMF by evaporation under vacuum, the reaction mixture was extracted with toluene (45 mL × 2). The combined organic layer were dried over anhydrous MgSO4 and evaporated under vacuum. The crude product was purified by silica gel column chromatography with n-hexane–EtOAc (95:5) as an eluent to give compound 2d (16.3 g, 77.3% yield). Compound 2d: IR νmax cm−1: 1698, 1593, 1457, 1162; 1H-NMR (400 MHz, CDCl3) δ 7.45 (2H, d, J = 8.4 Hz), 7.06 (1H, d, J = 16.4 Hz), 6.91 (2H, J = 8.4 Hz), 6.92 (1H, d, J = 16.0 Hz), 6.67 (2H, d, J = 2.4 Hz), 6.42 (1H, t, J = 2.0 Hz), 4.00 (6H, m), 1.82 (6H, m), 1.52 (6H, m), 1.39 (12H, m), 0.97 (9H, m); 13C-NMR (100 MHz, CDCl3) δ 160.5, 158.9, 139.6, 129.8, 128.6, 127.7, 126.6, 114.7, 104.8, 100.5, 68.0, 31.6, 29.4, 29.3, 25.8, 25.7, 22.6, 14.0; ESI-MS (positive mode) m/z: 481 [M + H]+, HRESIMS: m/z 519.3235 (calcd. for C32H48KO3, 519.3235).
(E)-1,3-Bis(octyloxy)-5-(4-(octyloxy)styryl)benzene (2e). Resveratrol (1) (10.0 g, 43.8 mmol) was dissolved in a solution of DMF (100 mL) and NaOH (5.9 g), and stirred for 10 min. Then, 1-bromooctane (25.8 g, 236.59 mmol) was added and the reaction was kept for 24 h at 40 °C. After the removal of DMF by evaporation under vacuum, the reaction mixture was extracted with toluene (45 mL × 2). The combined organic layer were dried over anhydrous MgSO4 and evaporated under vacuum. The crude product was purified by silica gel column chromatography with n-hexane–EtOAc (95:5) as an eluent to give compound 2e (21.0 g, 84.7% yield). Compound 2e: IR νmax cm−1: 1694, 1507, 1383, 1298; 1H-NMR (400 MHz, CDCl3) δ 7.45 (2H, d, J = 8.4 Hz), 7.06 (1H, d, J = 16.4 Hz), 6.91 (2H, J = 8.4 Hz), 6.92 (1H, d, J = 16.0 Hz), 6.66 (2H, d, J = 2.4 Hz), 6.40 (1H, t, J = 2.0 Hz), 4.00 (6H, m), 1.83 (6H, m), 1.50 (6H, m), 1.36 (24H, m), 0.94 (9H, m); 13C-NMR (100 MHz, CDCl3) δ 160.5, 158.9, 139.6, 129.8, 128.6, 127.7, 126.5, 114.7, 104.8, 100.5, 68.0, 31.8, 29.4, 29.3, 29.2, 26.1, 26.0, 22.6, 14.1; ESI-MS (positive mode) m/z: 565 [M + H]+, HRESIMS: m/z 603.4176 (calcd. for C38H60KO3, 603.4174).
(E)-5-(4-Acetoxystyryl)-1,3-phenylene diacetate (3a). Resveratrol (1) (5.0 g, 21.9 mmol) was dissolved in CH2Cl2 (40 mL) and kept cool at 10 °C. TEA (7.1 g, 70.1 mmol) and DMAP (0.26 g, 2.19 mmol) were added to the reaction mixture at 10 °C. Acetyl chloride (5.3 g, 67.9 mmol) was added carefully and the reaction was kept for 1 h at 10 °C. After adding distilled water (40 mL) to the reaction mixture, the CH2Cl2 fraction was extracted. The CH2Cl2 layers were dried over anhydrous MgSO4 and evaporated under vacuum. The crude product was purified by silica gel column chromatography with n-hexane–EtOAc (30:1) as an eluent to give compound 3a (6.9 g, 88.2% yield). Compound 3a: IR νmax cm−1: 1759, 1707, 1532, 1124; 1H-NMR (500 MHz, CDCl3) δ 7.54 (2H, d, J = 8.5 Hz), 7.12 (4H, m), 7.08 (1H, J = 16.0 Hz), 6.98 (1H, d, J = 16.0 Hz), 6.85 (1H, d, J = 2.0 Hz), 2.33 (9H, s); 13C-NMR (100 MHz, CDCl3) δ 169.3, 169.0, 151.3, 150.4, 139.5, 134.4, 129.6, 127.6, 127.2, 121.9, 116.9, 114.4, 21.1; ESI-MS (positive mode) m/z: 377 [M + Na]+, HRESIMS: m/z 393.0734 (calcd. for C20H18KO6, 393.0734).
(E)-5-(4-(3-Methylbut-2-enoyloxy)styryl)-1,3-phenylene bis(3-methylbut-2-enoate) (3b). Resveratrol (1) (5.0 g, 21.9 mmol) was dissolved in CH2Cl2 (40 mL) and kept cool at 10 °C. TEA (7.3 g, 72.3 mmol) and DMAP (0.26 g, 2.2 mmol) were added to the reaction mixture at 10 °C. 3,3-Dimethylacroyl chloride (8.3 g, 70.1 mmol) was added carefully and the reaction was kept for 1 h at 10 °C. After adding distilled water (50 mL) to reaction mixture, the CH2Cl2 fraction was extracted. The CH2Cl2 layers were dried over anhydrous MgSO4 and evaporated under vacuum. The crude product was purified by silica gel column chromatography with n-hexane–EtOAc (95:5) as an eluent to give compound 3b (6.9 g, 92.5% yield). Compound 3b: IR νmax cm−1: 1738, 1645, 1379, 1343; 1H-NMR (400 MHz, CDCl3) δ 7.50 (2H, dd, J = 7.2, 2.0 Hz), 7.11 (4H, m), 7.01 (1H, d, J = 16.4 Hz), 6.86 (1H, t, J = 2.0 Hz), 2.26 (9H, d, J = 1.2 Hz), 2.02 (9H, s); 13C-NMR (100 MHz, CDCl3) δ 164.7, 164.4, 160.4, 160.1, 151.4, 150.4, 139.3, 134.3, 129.4, 127.5, 127.2, 122.0, 116.8, 115.1, 115.0, 114.8, 27.7, 27.6, 20.6, 20.5; ESI-MS (positive mode) m/z: 497 [M + Na]+, HRESIMS: m/z 513.1672 (calcd. for C29H30KO6, 513.1673).
(E)-5-(4-(2-Ethylhexanoyloxy)styryl)-1,3-phenylene bis(2-ethylhexanoate) (3c). Resveratrol (1) (10.0 g, 43.8 mmol) was dissolved in CH2Cl2 (50 mL) and kept to cool at 10 °C. TEA (7.3 g, 72.3 mmol) and DMAP (0.54 g, 4.4 mmol) were added to the reaction mixture. After stirring at 10 °C, 2-ethylnexanoyl chloride (22.8 g, 140.2 mmol) was added carefully and the reaction was kept for 1 h at 10 °C. After adding distilled water (50 mL) to reaction mixture, the CH2Cl2 fraction was extracted. The CH2Cl2 layers were dried over anhydrous MgSO4 and evaporated under vacuum. The crude product was purified by silica gel column chromatography with n-hexane–EtOAc (50:1) as an eluent to give compound 3c (25.2 g, 94.8% yield). Compound 3c: IR νmax cm−1: 1754, 1644, 1376, 1201; 1H-NMR (500 MHz, CDCl3) δ 7.52 (2H, d, J = 8.5 Hz), 7.11 (4H, m), 7.03 (1H, J = 16.0 Hz), 6.98 (1H, d, J = 16.0 Hz), 6.78 (1H, d, J = 2.0 Hz), 2.56 (3H, m), 1.87 (6H, m), 1.76 (8H, m), 1.07 (9H, m), 0.97 (12H, m); 13C-NMR (100 MHz, CDCl3) δ174.7, 174.4, 151.5, 150.5, 139.4, 134.3, 129.6, 127.6, 127.2, 121.9, 116.8, 114.3, 47.4, 47.3, 31.7, 31.6, 29.6, 25.5, 25.4, 22.6, 13.9, 11.9; ESI-MS (positive mode) m/z : 629 [M + Na]+, HRESIMS: m/z 645.3550 (calcd. for C38H54KO6, 645.3551).
(E)-5-(4-(Octanoyloxy)styryl)-1,3-phenylene dioctanoate (3d). Resveratrol (1) (5.0 g, 21.9 mmol) was dissolved in CH2Cl2 (40 mL) and kept cool at 10 °C. TEA (7.1 g, 70.1 mmol) and DMAP (0.26 g, 2.2 mmol) were added to the reaction mixture. After stirring at 10 °C, octanoyl chloride (11.0 g, 67.9 mmol) was added carefully and the reaction was kept for 1 h at 10 °C. After adding distilled water (40 mL) to reaction mixture, the CH2Cl2 fraction was extracted. The CH2Cl2 layer were dried over anhydrous MgSO4 and evaporated under vacuum. The crude product was purified by silica gel column chromatography with n-hexane–EtOAc (30:1) as an eluent to give compound 3d (12.9 g, 96.7% yield). Compound 3d: IR νmax cm−1: 1758, 1644, 1367, 1267; 1H-NMR (500 MHz, CDCl3) δ 7.51 (2H, d, J = 8.5 Hz), 7.11 (4H, m), 7.08 (1H, J = 16.0 Hz), 6.99 (1H, d, J = 16.0 Hz), 6.82 (1H, d, J = 2.0 Hz), 2.58 (6H, m), 1.79 (6H, m), 1.34-1.42 (24H, m), 0.94 (12H, m); 13C-NMR (100 MHz, CDCl3) δ 172.2, 171.8, 151.4, 150.5, 139.4, 134.3, 129.6, 127.6, 127.2, 121.9, 116.8, 114.4, 34.4, 34.4, 31.6, 29.0, 28.9, 24.9, 24.8, 22.6, 14.0; ESI-MS (positive mode) m/z: 629 [M + Na]+, HRESIMS: m/z 645.3550 (calcd. for C38H54KO6, 645.3551).
3.3. Evaluation of Anti-Melanogenesis Activity
3.3.1. Assessment of Tyrosinase Activity
Tyrosinase inhibitory assays were performed using enzyme solution, which was prepared by the reconstitution of mushroom tyrosinase (Sigma, St. Louis, MO, USA) in 0.1 U/mL phosphate buffer (pH 6.5). A test sample was mixed with 50 μL enzyme buffer, and incubated for 5 min at 37 °C. Then, 50 μL of tyrosine solution, which was diluted with phosphate buffer to 1 mM, was added and the enzyme reaction was allowed to proceed for 20 min at 37 °C. After incubation, the amount of dopachrome formed in the reaction mixture was determined by measuring the absorbance at 490 nm in an ELISA reader (Bio-Tek Synergy HT, Winooski, VT, USA).
3.3.2. Cell Culture
B16F10 mouse melanoma cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin and 100 μg/mL streptomycin. Cells were maintained at 37 °C in a humidified atmosphere of 95% air-5% CO2.
3.3.3. Measurement of Cellular Melanin Contents
B16F10 cells were stimulated with α-MSH and then treated with resveratrol derivatives at the concentration of 5, 10 and 20 μg/mL for 72 h. After washing with PBS, the cells were harvested and solubilized the melanin by vortexing in 1N NaOH-10% DMSO at 80 °C. The melanin contents were measured by absorbance value at 490 nm with synthetic melanin as a standard.
3.3.4. Cell Viability
B16F10 melanoma were treated with resveratrol derivatives at the concentration of 5, 10 and 20 µg/mL for 72 h. Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in an ELISA plate reader.
3.3.5. Western Blot Analysis
Stimulated B16F10 cells were treated with resveratrol derivative 2a for 72 h. Cells were lysed with an SDS lysis buffer containing a protease inhibitor cocktail. The lysates were centrifuged and the supernatants were collected. Proteins were separated in an SDS–polyacrylamide gel and transferred to a PVDF membrane. After blocking in TBST with 5% non-fat dry milk, the membrane was washed and incubated with the primary antibodies including tyrosinase, TRP-1, or β-actin (Santa Cruz Biotechnology Inc., Dallas, TX, USA) at 4 °C overnight. After washing, the membrane was incubated with horseradish peroxidase-conjugated IgG secondary antibody (Cell Signaling Technology Inc., Danvers, MA, USA). The membrane was detected by chemiluminescent reaction, and then exposure to X-ray Kodak film. The proteins of tyrosinase and TRP-1 were normalized by β-actin and the intensities of bands were quantified using a Scion-Image for Windows program (Informer Technologies, Inc., Shingle Springs, CA, USA).
3.3.6. Statistical Analysis
Evaluation of statistical significance was determined by one-way ANOVA test with a value of p < 0.05 considered statistically significant.
4. Conclusions
Nine resveratrol derivatives including five ether derivatives (2a–2e) and four ester derivatives (3a–3d) were newly synthesized and evaluated for melanogenesis inhibitory activity. All the synthetic ether and ester derivatives of resveratrol inhibited melanin synthesis in melanoma cells. Further Western blot analysis showed that resveratrol derivative 2a inhibited melanin synthesis by the inhibition of melanogenic enzyme expressions rather than direct tyrosinase activity. Our synthetic resveratrol derivatives have more lipophilic properties than resveratrol by the addition of alkyl or acyl chains to free hydroxyl moiety of resveratrol, thus are expected to show better bioavailability in skin application. Taken together, we suggest that our synthetic resveratrol derivatives might be promising candidates for better practical application to skin-whitening cosmetics.
Acknowledgments
This work was supported by the Ministry of Trade, Industry and Energy (MOTIE) and the Korea Institute for Advancement of Technology (KIAT) through the Promoting Regional Specialized Industry (A006500037) and Medical Research Center program (2008-0062275) of the National Research Foundation of Korea.
Author Contributions
Qing Liu, Yang Hee Jo and Seon Beom Kim designed the experiments and performed the biological assay. Cheong Taek Kim executed synthesis and identification of derivatives. Bang Yeon Hwang and Mi Kyeong Lee analyzed the data and wrote the paper. All authors discussed the results and commented the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Rees J.L. Genetics of hair and skin color. Annu. Rev. Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- 2.Prota G. Progress in the chemistry of melanins and related metabolites. Med. Res. Rev. 1988;8:525–556. doi: 10.1002/med.2610080405. [DOI] [PubMed] [Google Scholar]
- 3.Chang T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y.J., Uyana H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005;62:1707–1723. doi: 10.1007/s00018-005-5054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parvez S., Kang M., Chung H.W., Bae H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007;21:805–816. doi: 10.1002/ptr.2184. [DOI] [PubMed] [Google Scholar]
- 6.Mayer A.M. Polyphenol oxidases in plant: Recent progress. Phytochemistry. 1987;26:11–20. [Google Scholar]
- 7.Seo S.Y., Sharma V.K., Sharma N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem. 2003;51:2837–2853. doi: 10.1021/jf020826f. [DOI] [PubMed] [Google Scholar]
- 8.Park S.Y., Jin M.L., Kim Y.H., Kim Y., Lee S.J. Aromatic-turmerone inhibits α-MSH and IBMX-induced melanogenesis by inactivating CREB and MITF signaling pathways. Arch. Dermatol. Res. 2011;303:737–744. doi: 10.1007/s00403-011-1155-7. [DOI] [PubMed] [Google Scholar]
- 9.Nakashima S., Matsuda H., Oda Y., Nakamura S., Xu F., Yoshikawa M. Melanogenesis inhibitors from the desert plant Anastatica hierochuntica in B16 melanoma cells. Bioorg. Med. Chem. 2010;18:2337–2345. doi: 10.1016/j.bmc.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre L., Fernandez-Quintela A., Arias N., Portillo M.P. Resveratrol: Anti-obesity mechanisms of action. Molecules. 2014;19:18632–18655. doi: 10.3390/molecules191118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj P., Louis X.L., Thandapilly S.J., Movahed A., Zieroth S., Netticadan T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014;95:63–71. doi: 10.1016/j.lfs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Poulsen M.M., Jorgensen J.O., Jessen M., Richelsen B., Pedersen S.B. Ann. N. Y. Acad. Sci. 2013;1290:74–82. doi: 10.1111/nyas.12141. [DOI] [PubMed] [Google Scholar]
- 13.Farris P., Krutmann J., Li Y.H., McDaniel D., Krol Y. Resveratrol: A unique antioxidant offering a multi-mechanistic approach for treating aging skin. J. Drugs Dermatol. 2013;12:1389–1394. [PubMed] [Google Scholar]
- 14.Sattoka H., Kubo I. Resveratrol as a kcat type inhibitor for tyrosinase: Potentiated melanogenesis inhibitor. Bioorg. Med. Chem. 2012;20:1090–1099. doi: 10.1016/j.bmc.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Newton R.A., Cook A.L., Roberts D.W., Leonard J.H., Sturm R.A. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J. Investig. Dermatol. 2007;127:2216–2227. doi: 10.1038/sj.jid.5700840. [DOI] [PubMed] [Google Scholar]
- 16.Zupančič Š., Lavrič Z., Kristl J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015;93:196–204. doi: 10.1016/j.ejpb.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel E., Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 18.Davidov-Pardo G., McClements D.J. Nutraceutical delivery systems: Resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem. 2015;167:205–212. doi: 10.1016/j.foodchem.2014.06.082. [DOI] [PubMed] [Google Scholar]
- 19.Pangeni R., Sahni J.K., Ali J., Sharma S., Baboota S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery Expert Opin. Drug Deliv. 2014;11:1285–1298. doi: 10.1517/17425247.2014.919253. [DOI] [PubMed] [Google Scholar]
- 20.Davidov-Pardo G., Pérez-Ciordia S., Marín-Arroyo M.R., McClements D.J. Improving resveratrol bioaccessibility using biopolymer nanoparticles and complexes: Impact of protein-carbohydrate maillard conjugation. J. Agric. Food Chem. 2015;63:3915–3923. doi: 10.1021/acs.jafc.5b00777. [DOI] [PubMed] [Google Scholar]
- 21.Chalal M., Klinjuer A., Echairi A., Meunier P., Vervandier-Fasseur D., Adrian M. Antimicrobial activity of resveratrol analogues. Molecules. 2014;19:7679–7688. doi: 10.3390/molecules19067679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houillé B., Papon N., Boudesocque L., Bourdeaue E., Besseau S., Courdavault V., Enguehard-Gueiffier C., Delanoue G., Guérin L., Bouchara J.P., et al. Antifungal activity of resveratrol derivatives against Candida species. J. Nat. Prod. 2014;77:1658–1662. doi: 10.1021/np5002576. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.H., Liu Q., Hwang B.Y., Lee M.K. Inhibitory effects of stilbene derivatives from Parthenocissus tricuspidata on adipocyte differentiation and pancreatic lipase. Nat. Prod. Comm. 2013;8:1439–1441. [PubMed] [Google Scholar]
- 24.Silva F., Figueiras A., Gallardo E., Nerin C., Domingues F.C. Strategies to improve the solubility and stability of stilbene antioxidants: A comparative study between cyclodextrins and bile acids. Food Chem. 2012;145:115–125. doi: 10.1016/j.foodchem.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Francioso A., Mastromarino P., Masci A., D’Erme M., Mosca L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014;10:237–245. doi: 10.2174/15734064113096660053. [DOI] [PubMed] [Google Scholar]
- 26.Park J., Park J.H., Suh H.J., Lee I.C., Koh J., Yoo Y.C. Effect of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014;306:475–487. doi: 10.1007/s00403-014-1440-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y.M., Yun J., Lee C.K., Lee H., Min K.R., Kim Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J. Biol. Chem. 2002;277:16340–16344. doi: 10.1074/jbc.M200678200. [DOI] [PubMed] [Google Scholar]
- 28.Ryu J.H., Seok J.K., An S.M., Baek J.H., Koh J.S., Boo Y.C. A study of the human skin-whitening effects of resveratryl triacetate. Arch. Dermatol. Res. 2015;307:239–247. doi: 10.1007/s00403-015-1556-0. [DOI] [PubMed] [Google Scholar]
- 29.Franco D.C., de Carvalho G.S., Rocha P.R., da Silva T.R., da Silva A.D., Raposo N.R. Inhibitory effects of resveratrol analogs on mushroom tyrosinase activity. Molecules. 2012;17:11816–11825. doi: 10.3390/molecules171011816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Y.M., Ha Y.M., Kim J.A., Chung K.W., Uehara Y., Lee K.J., Chun P., Byun Y., Chung H.Y., Moon H.R. Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2012;22:7451–7455. doi: 10.1016/j.bmcl.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 31.Alonso C., Rubio L., Touriño S., Martí M., Barba C., Fernández-Campos F., Coderch L., Parra J.L. Antioxidative effects and percutaneous absorption of five polyphenols. Free Radic. Biol. Med. 2014;75:149–155. doi: 10.1016/j.freeradbiomed.2014.07.014. [DOI] [PubMed] [Google Scholar]