Abstract
A new synthesis of 2-oxa-7-azaspiro[3.5]nonane is described. Spirocyclic oxetanes, including 2-oxa-6-azaspiro[3.3]heptane were converted into o-cycloalkylaminoacetanilides for oxidative cyclizations using Oxone® in formic acid. The expanded spirocyclic oxetane successfully gave the [1,2-a] ring-fused benzimidazole. X-ray crystal structure of the resultant new tetracyclic system, 1ʹ,2ʹ-dihydro-4ʹH-spiro[oxetane-3,3ʹ-pyrido[1,2-a]benzimidazole] and the azetidine ring-opened adduct, N-(2-acetamido-4-bromophenyl)-N-{[3-(chloromethyl)oxetan-3-yl]methyl}acetamide are disclosed.
Keywords: annulation, cyclization, diazole, heterocycle, 4-membered rings
1. Introduction
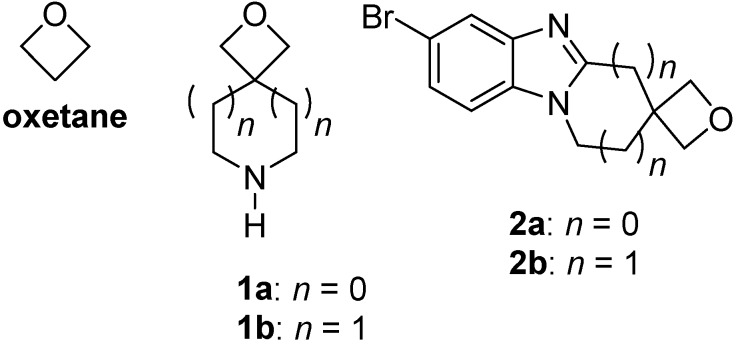
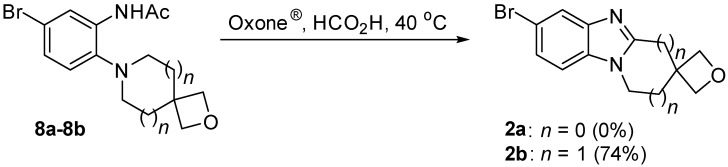
Oxetane is considered as a polar equivalent of the gem-dimethyl group (Figure 1) [1,2,3]. Spirocyclic oxetanes such as 2-oxa-6-azaspiro[3.3]heptane (1a) and 2-oxa-7-azaspiro[3.5]nonane (1b) were proposed as valuable structural alternatives to ubiquitous morpholine in medicinal chemistry [4]. A drug discovery project within our group demanded a substituent that enabled higher binding affinities to the NAD(P)H:quinone oxidoreductase 1 (NQO1) active site [5]. NQO1 is an enzyme over-expressed in cancer cell lines. Our attention turned to oxetane due to its heralded metabolic robustness in comparison to carbonyl alternatives, while offering the hydrogen bonding capacity necessary for efficient binding to the His194 residue of NQO1 enabling more efficient reduction of benzimidazolequinone and imidazobenzimidazolequinone substrates. The following article describes the first fusion of the spirocyclic oxetane motif onto heterocycles in attempts to prepare [1,2-a] alicyclic ring-fused benzimidazoles 2a and 2b.
Figure 1.
Rationale design of synthetic targets.
2. Results and Discussion
2.1. Synthesis of Spirocyclic Oxetanes
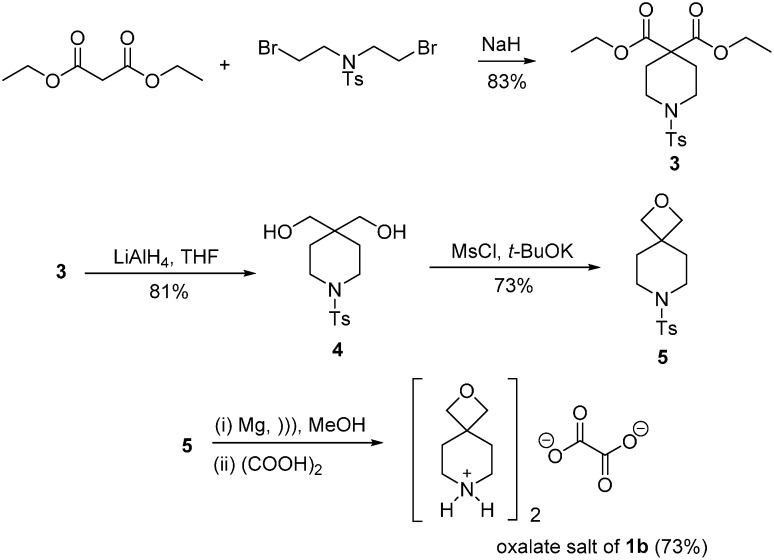
The synthesis of the oxalate salt of 2-oxa-6-azaspiro[3.3]heptane 1a starting from the condensation of 3-bromo-2,2-bis(bromomethyl)propan-1-ol with p-tosylamide has been described [4]. The expanded analogue 1b is commercially available but expensive, and a new synthetic pathway is now reported (Scheme 1). N-tosyl-piperidine-4,4-diethyl ester 3 was prepared using the reaction of diethyl malonate with N-tosylbis(2-bromoethyl)amine, and similar transformations are available in patents [6,7]. Lithium aluminum hydride reduction provides diol 4, which is subjected to a one-pot mesylation and ring closure to give the previously unreported oxetane 5. The tosyl group was removed and the oxalate salt of 2-oxa-7-azaspiro[3.5]nonane 1b formed. An alternative synthesis appears in a patent via the ring-closure of 2,2′-(oxetane-3,3-diyl)bis(ethan-1-ol) to give 1b [8]. The latter multi-step synthesis begins with a Wittig reaction on 3-oxetanone, and represents a longer more costly preparation of 1b.
Scheme 1.
Synthesis of the oxalate salt of 2-oxa-7-azaspiro[3.5]nonane (1b).
2.2. Synthesis of o-Cycloalkylaminoacetanilides
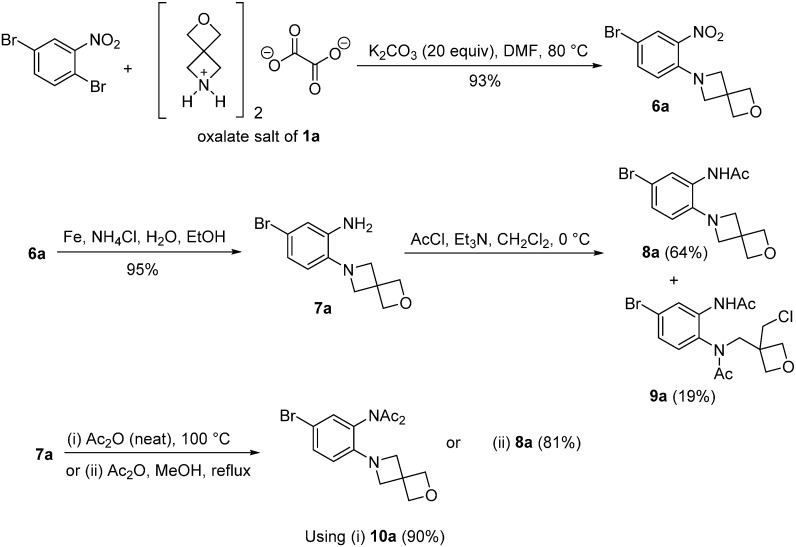
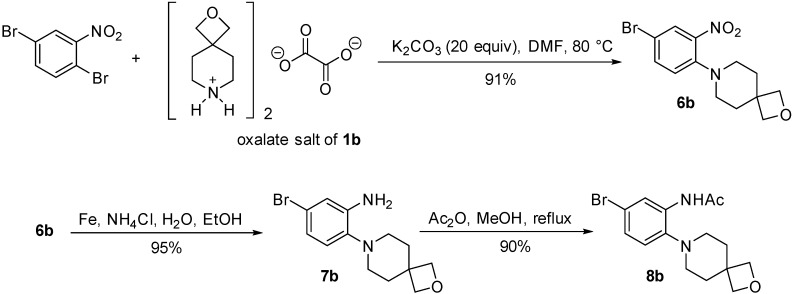
Nucleophilic aromatic substitution onto 1,4-dibromo-2-nitrobenzene using the oxalate salts of spirocyclic oxetanes 1a and 1b was possible in yields of >90% when using a large excess of potassium carbonate in DMF (Scheme 2 and Scheme 3). Reduction of the nitro group of 6a and 6b using iron and aqueous ammonium chloride yielded the corresponding anilines 7a and 7b in 95% yield. The acetylation was optimized with aniline 7a, where the initial attempt using acetyl chloride gave the desired acetamide 8a in 64% yield together with adduct 9a of ring-opening of the azetidine in 19% yield (Scheme 2). By-product 9a was confirmed by X-ray crystallography (Figure 2). As pointed out by the Reviewer this may have occurred using the chloride ion liberated from the first acetylation. The ring-opening of azetidines by the nucleophilic attack of halide ions on azetidinium ions has been previously described [9]. Acetylations using acetic anhydride were in contrast regioselective giving diacetylated adduct 10a in 90% yield with neat acetic anhydride, while acetylation with acetic anhydride in methanol gave the desired product 8a in 81% yield. The latter conditions gave the analogous acetamide of 2-oxa-7-azaspiro[3.5]nonane 8b in 90% yield (Scheme 3).
Scheme 2.
Synthesis of N-[5-bromo-2-(2-oxa-6-azaspiro[3.3]heptan-6-yl)phenyl]acetamide (8a).
Scheme 3.
Synthesis of N-[5-bromo-2-(2-oxa-7-azaspiro[3.5]nonan-7-yl)phenyl]acetamide (8b).
Figure 2.
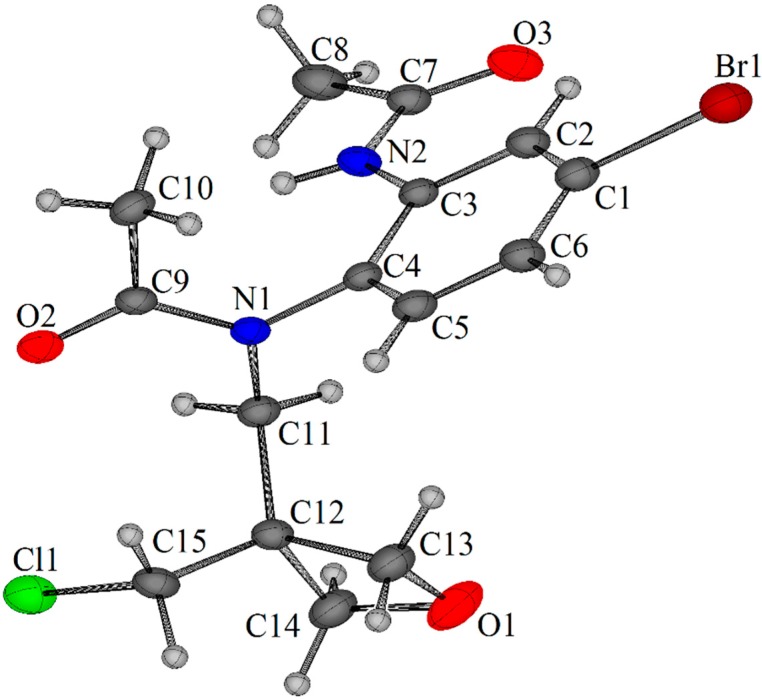
X-ray crystal structure of N-(2-acetamido-4-bromophenyl)-N-{[3-(chloromethyl)oxetan-3-yl]methyl}acetamide (9a) [10].
2.3. Fusion of the Spirocyclic Oxetanes
In order to fuse the spirocyclic oxetane of 8a and 8b, the well-established oxidative cyclization reaction of acetamide onto the neighbouring cyclic amine was used [11,12,13]. Oxone® in formic acid is as an expedient means of converting o-cycloalkylaminoacetanilides into [1,2-a] alicyclic ring-fused benzimidazoles and double annulated imidazobenzimidazoles in high yields [13]. Using the latter conditions (Scheme 4), acetanilide 8a gave a mixture of products indicating degradation of the spirocyclic oxetane and an absence of the desired benzimidazole 2a. In contrast the 2-oxa-7-azaspiro[3.5]nonane fused benzimidazole 2b was isolated in 74% yield at the end of the reaction of 8b without the requirement for chromatography by simple organic extraction from the basified aqueous mixture. The structure of the novel tetracyclic system 2b was confirmed by X-ray crystallography (Figure 3).
Scheme 4.
Oxidative cyclizations to give spirocyclic oxetane fused benzimidazoles.
Figure 3.
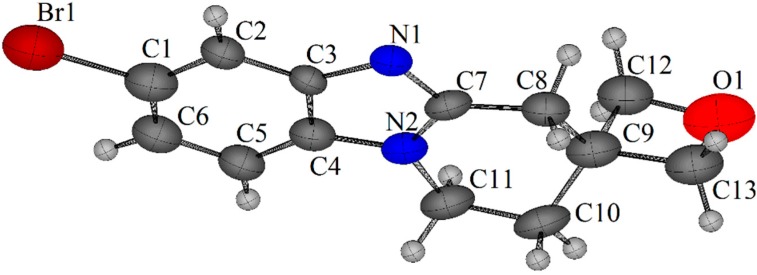
X-ray crystal structure of 7ʹ-bromo-1ʹ,2ʹ-dihydro-4ʹH-spiro[oxetane-3,3ʹ-pyrido [1,2-a]benzimidazole] (2b) [10].
3. Experimental Section
3.1. General
All chemicals were obtained from commercial sources and used without further purification. Thin layer chromatography (TLC) was performed on TLC silica gel 60 F254 plates. Dry vacuum column chromatography was carried out on silica gel (Apollo Scientific ZEOprep 60/15–35 microns). Melting points were measured on a Stuart Scientific melting point apparatus SMP1. Infrared spectra were recorded using a Perkin-Elmer Spec 1 with ATR attached. 1H-NMR spectra were recorded using a Joel ECX 400 MHz instrument equipped with a DEC AXP 300 computer workstation. The chemical shifts were recorded in ppm relative to tetramethylsilane. 13C-NMR data were collected at 100 MHz with complete proton decoupling. High resolution mass spectra (HRMS) were carried out using ESI time-of-flight mass spectrometer (TOFMS). The precision of all accurate mass measurements were better than 5 ppm. NMR spectra of all compounds are available in the Supplementary Materials document accompanying this article.
3.2. Experimental Procedures
3.2.1. Synthesis of Spirocyclic Oxetanes
Bis(2-oxa-6-azaspiro[3.3]heptan-6-ium) ethanedioate (oxalate salt of 1a) was prepared according to the literature [4].
Diethyl 1-(4-methylbenzene-1-sulfonyl)piperidine-4,4-dicarboxylate (3). Diethyl malonate (0.500 g, 3.1 mmol) and NaH (0.165 g, 6.9 mmol) in DMF (50 mL) were heated at 80 °C for 30 min. N,N-bis(2-bromoethyl)-4-methyl-1-sulfonamide [14] (1.320 g, 3.4 mmol) was added and heating at 80 °C continued for 12 h. The cooled mixture was evaporated, EtOAc (50 mL) added, and washed with aqueous LiCl (5%) solution (3 × 50 mL). The organic extracts were dried (Na2SO4) and evaporated to dryness, and the residue purified by column chromatography using gradient elution of petroleum ether/EtOAc to yield the title compound (0.987 g, 83%) as a white solid; Rf 0.32 (1:4 EtOAc/Pet); mp 90–92 °C; νmax (neat, cm−1) 2987, 2851, 1742 (C=O), 1598, 1467, 1444, 1367, 1350 (S=O), 1327, 1305, 1261, 1247, 1192, 1162 (S=O), 1092, 1052, 1072, 1015; δH (400 MHz, CDCl3) 1.16 (t, J = 7.3 Hz, 6H, CH3), 2.17 (t, J = 5.5 Hz, 4H), 2.41 (s, 3H), 3.03 (t, J = 5.5 Hz, 4H), 4.13 (q, J = 7.3 Hz, 4H, OCH2), 7.29 (d, J = 8.0 Hz, 2H), 7.59 (d, J = 8.0 Hz, 2H); δC (100 MHz, CDCl3) 14.0, 21.6 (CH3), 30.2, 43.2 (CH2), 52.4 (C), 61.8 (OCH2), 127.7, 129.8 (CH), 133.1, 143.7 (C), 170.4 (C=O); HRMS (ESI) m/z [M + H]+, C18H26NO6S calcd. 384.1481, observed 384.1483.
[1-(4-Methylbenzene-1-sulfonyl)]piperidine-4,4-diyl]dimethanol (4). LiAlH4 (5.2 mL of a 1 M solution in THF, 5.2 mmol) was added over 5 min to a solution of diethyl ester 3 (1.000 g, 2.6 mmol) in THF (20 mL), and the solution stirred for 16 h at room temperature. The reaction was quenched with ethanol (3 mL), a saturated sodium potassium tartrate solution (40 mL), and stirred vigorously for 1 h. The resulting biphasic mixture was extracted with EtOAc (2 × 50 mL), washed with brine (2 × 30 mL) and dried (Na2SO4). The solution was evaporated to dryness and the residue purified by column chromatography using gradient elution of petroleum ether/EtOAc to yield the title compound (0.628 g, 81%) as a white solid; Rf 0.45 (EtOAc); mp 110–114 °C; νmax (neat, cm−1) 3524 (OH), 3257, 2919, 2869, 1725, 1597, 1340, 1320 (S=O), 1248, 1184, 1156 (S=O), 1132, 1090, 1053, 1012; δH (400 MHz, CDCl3) 1.57 (t, J = 5.7 Hz, 4H), 2.43 (s, 3H), 2.56 (t, J = 4.9 Hz, 2H, OH, disappears with D2O), 2.97 (t, J = 5.7 Hz, 4H), 3.49 (d, J = 4.9 Hz, 4H, CH2OH), 7.31 (d, J = 7.8 Hz, 2H), 7.61 (d, J = 7.8 Hz, 2H); δC (100 MHz, CDCl3) 21.6 (CH3), 28.4 (CH2), 36.6 (C), 42.1 (CH2), 68.7 (OCH2), 127.8, 129.8 (CH), 132.9, 143.8 (C); HRMS (ESI) m/z [M + H]+, C14H22NO4S calcd. 300.1270, observed 300.1269.
7-(4-Methylbenzene-1-sulfonyl)-2-oxa-7-azaspiro[3.5]nonane (5). Methanesulfonyl chloride (0.4 mL, 5.2 mmol) in THF (5 mL) was added to diol 4 (1.700 g, 5.7 mmol) in THF (50 mL) via syringe pump at a rate of 1.7 mL/h, while potassium tert-butoxide (0.636 g, 5.7 mmol) was added in portions (one third per h). The mixture was stirred at room temperature for 1 h with further potassium tert-butoxide (1.900 g, 17.0 mmol) added, and stirred for 2 h. The mixture was evaporated, EtOAc (50 mL) added, washed with water (2 × 20 mL), and dried (Na2SO4). The solution was evaporated to dryness and the residue purified by column chromatography using gradient elution of petroleum ether/EtOAc to yield the title compound (1.160 g, 73%) as a white solid; Rf 0.44 (1:1 EtOAc/Pet); mp 168–172 °C; νmax (neat, cm−1) 2928, 2868, 1597, 1597, 1345, 1331 (S=O), 1241, 1163 (S=O), 1140, 1089; δH (400 MHz, CDCl3) 1.93 (bs, 4H), 2.42 (s, 3H), 2.90 (bs, 4H), 4.29 (s, 4H, OCH2), 7.30 (d, J = 7.3 Hz, 2H), 7.60 (d, J = 7.3 Hz, 2H); δC (100 MHz, CDCl3), 21.7 (CH3) 34.1 (CH2), 38.1 (C), 43.3 (CH2), 81.1 (OCH2), 127.7, 129.8 (CH), 133.1, 143.8 (C); HRMS (ESI) m/z [M + H]+, C14H20NO3S calcd. 282.1164, observed 282.1163.
Bis(2-oxa-7-azaspiro[3.5]nonan-7-ium) ethanedioate (oxalate salt of 1b). 2-Oxa-7azaspiro[3.5]nonane 5 (1.300 g, 4.6 mmol) and Mg turnings (0.780 g, 32.3 mmol) in MeOH (50 mL) were sonicated for 1 h. The mixture was evaporated to give a viscous grey residue to which Et2O (50 mL) and Na2SO4·10H2O (2.000 g) were added. After 30 min of stirring, the mixture was filtered, and anhydrous oxalic acid (0.210 g, 2.3 mmol) added to the filtrate to form a precipitate. The precipitate was collected and dried under vacuum to yield the title compound (0.580 g, 73%) as a white solid; mp 148–152 °C; νmax (neat, cm−1) 3389 (NH), 3124, 2931, 2866, 1713, 1610 (C=O), 1475, 1444, 1397, 1171; δH (400 MHz, D2O) 1.94 (t, J = 5.7 Hz, 4H), 2.98 (t, J = 5.7 Hz, 4H), 4.38 (s, 4H, OCH2); δC (100 MHz, D2O) 30.2 (CH2), 36.6 (C), 41.1 (CH2), 81.1 (OCH2), 165.7 (C=O).
3.2.2. Synthesis of Acetanilide Cyclization Precursors
General Procedure for the Synthesis of Nitrobenzenes 6a and 6b
1,4-Dibromo-2-nitrobenzene (1.000 g, 3.6 mmol), K2CO3 (9.820 g, 71.2 mmol) and spirocyclic oxetane 1a–1b oxalate salts (3.6 mmol) were heated in DMF (50 mL) at 80 °C for 4 h. The cooled mixture was evaporated, EtOAc (50 mL) added, and washed with aqueous LiCl (5%) solution (3 × 20 mL). The organic extracts were dried (Na2SO4), evaporated to dryness, and purified by column chromatography using gradient elution of petroleum ether/EtOAc to yield:
6-(4-Bromo-2-nitrophenyl)-2-oxa-6-azaspiro[3.3]heptane (6a). (0.987 g, 93%) as an orange solid; Rf 0.49 (1:1 EtOAc/Pet); mp 148–150 °C; νmax (neat, cm−1) 2955, 2861, 1672, 1602, 1558 (NO2), 1502, 1484, 1458, 1373, 1362, 1348 (NO2), 1276, 1246, 1203, 1123, 1070; δH (400 MHz, CDCl3) 4.11 (s, 4H, NCH2), 4.81 (s, 4H, OCH2), 6.47 (d, J = 9.2 Hz, 1H, 6-H), 7.45 (dd, J = 9.2, 2.3 Hz, 1H, 5-H), 7.93 (d, J = 2.3 Hz, 1H, 3-H); δC (100 MHz, CDCl3) 38.3 (C), 62.8 (NCH2), 80.9 (OCH2), 108.3 (C), 117.4 (6-CH), 128.8 (3-CH), 136.2 (C), 136.5 (5-CH), 143.8 (C); HRMS (ESI) m/z [M + H]+, C11H12N2O379Br calcd. 299.0031, observed 299.0038.
7-(4-Bromo-2-nitrophenyl)-2-oxa-7-azaspiro[3.5]nonane (6b). (1.061 g, 91%) as an orange oil; Rf 0.58 (1:4 EtOAc/Pet); νmax (neat, cm−1) 2931, 2861, 1599, 1519 (NO2), 1485, 1462, 1384, 1331 (NO2), 1271, 1231, 1168, 1131, 1091; δH (400 MHz, CDCl3) 1.99–2.02 (m, 4H), 2.90–2.93 (m, 4H, NCH2), 4.46 (s, 4H, OCH2), 6.97 (d, J = 8.7 Hz, 1H, 6-H), 7.53 (dd, J = 8.7, 2.3 Hz, 1H, 5-H), 7.89 (d, J = 2.3 Hz, 1H, 3-H); δC (100 MHz, CDCl3) 34.9 (CH2) 38.3 (C), 49.2 (NCH2), 81.6 (OCH2), 113.3 (C), 122.8 (6-CH), 128.7 (3-CH), 136.4 (5-CH), 143.3, 145.5 (C); HRMS (ESI) m/z [M + H]+, C13H16N2O379Br calcd. 327.0344, observed 327.0355.
General Procedure for the Synthesis of Anilines 7a and 7b
Nitrobenzene 6a or 6b (2.00 mmol), Fe powder (0.370 g, 6.6 mmol), NH4Cl (59 mg, 1.0 mmol) and water (1.5 mL) in EtOH (5 mL) were heated at 80 °C for 5 h. EtOAc (50 mL) was added to the cooled mixture, which was filtered, washed with water (50 mL), and dried to yield:
5-Bromo-2-(2-oxa-6-azaspiro[3.3]heptan-6-yl)aniline (7a). (0.508 g, 95%) as a brown oil; νmax (neat, cm−1) 3414, 3334, 2940, 2870, 1614, 1575, 1497, 1292, 1255, 1134, 1046; δH (400 MHz, CDCl3) 3.50 (bs, 2H, NH2), 3.91 (s, 4H, NCH2), 4.82 (s, 4H, OCH2), 6.41 (d, J = 8.4 Hz, 1H, 3-H), 6.75 (d, J = 2.0 Hz, 1H, 6-H), 6.82 (dd, J = 8.4, 2.0 Hz, 1H, 4-H); δC (100 MHz, CDCl3) 39.1 (C), 62.1 (NCH2), 81.2 (OCH2), 114.1 (C), 115.8 (3-CH), 118.4 (6-CH), 121.7 (4-CH), 137.8 (C), 139.1 (C); HRMS (ESI) m/z [M + H]+, C11H14N2O79Br calcd. 269.0289, observed 269.0290.
5-Bromo-2-(2-oxa-7-azaspiro[3.5]nonan-7-yl)aniline (7b). (0.564 g, 95%) as a brown solid; mp 160–162 °C; νmax (neat, cm−1) 3400, 3317, 2931, 2865, 2811, 1618, 1577, 1493, 1459, 1231, 1210, 1133, 1122; δH (400 MHz, CDCl3) 1.98 (bs, 4H), 2.72 (bs, 4H, NCH2), 3.99 (bs, 2H, NH2), 4.47 (s, 4H, OCH2), 6.76–6.84 (m, 3H); δC (100 MHz, CDCl3) 35.8 (CH2), 38.5 (C), 48.7 (NCH2), 81.9 (OCH2), 117.6 (C & CH), 121.2, 121.4 (CH), 138.7, 143.1 (C); HRMS (ESI) m/z [M − H]−, C13H16N2O79Br calcd. 295.0446, observed 295.0448.
Procedures for the Synthesis of N-[5-Bromo-2-(2-oxa-6-azaspiro[3.3]heptan-6-yl)phenyl]acetamide (8a)
AcCl (0.2 mL, 2.5 mmol) was added over 5 min to aniline 7a (0.420 g, 1.6 mmol) and Et3N (0.4 mL, 2.7 mmol) in CH2Cl2 (10 mL) at 0 °C, and stirred for 3 h at room temperature. The solution was evaporated, EtOAc (50 mL) added, and washed with water (50 mL). The organic extracts were dried (Na2SO4), evaporated, and purified by column chromatography using gradient elution of petroleum ether/EtOAc to yield:
N-(2-acetylamido-4-bromophenyl)-N-{[3-(chloromethyl)oxetan-3-yl]methyl}acetamide (9a). 0.114 g, 19%; brown solid; mp 160–164 °C; Rf 0.25 (EtOAc); νmax (neat, cm−1) 3296, 3262, 2953, 2876, 1691 (C=O), 1657 (C=O), 1578, 1518, 1403, 1364, 1313, 1259, 1193, 1089; δH (400 MHz, CDCl3) 1.85 (s, 3H), 2.12 (s, 3H), 3.57 (d, J = 14.2 Hz, 1H), 3.67 (d, J = 11.2 Hz, 1H), 3.79 (d, J = 11.2 Hz, 1H), 4.12 (d, J = 7.1 Hz, 1H, CHHO), 4.42 (d, J = 14.2 Hz, 1H), 4.59–4.63 (m, 2H, OCH2), 4.68 (d, J = 7.1 Hz, 1H, CHHO), 7.09 (d, J = 8.5 Hz, 1H, 6-H), 7.32 (dd, J = 8.5, 2.1 Hz, 1H, 5-H), 8.43 (d, J = 2.1 Hz, 1H, 3-H), 9.18 (bs, 1H, NH); δC 22.3, 24.2 (CH3), 45.9 (C), 48.3, 53.2 (CH2), 76.8, 77.2 (OCH2), 122.8 (C), 127.0 (3-CH), 128.8 (2 × CH), 133.9, 137.2 (C), 169.3, 173.7 (C=O); HRMS (ESI) m/z [M + H]+, C15H19N2O335Cl79Br calcd. 389.0268, observed 389.0271 and N-[5-bromo-2-(2-oxa-6-azaspiro[3.3]heptan-6-yl)phenyl]acetamide (8a). (0.312 g, 64%) as a white solid; Rf 0.18 (EtOAc); mp 185–189 °C; νmax (neat, cm−1) 3218, 2929, 2864 1652 (C=O), 1591, 1569, 1520, 1485, 1408, 1368, 1317, 1279, 1254, 1132, 1060, 1011; δH (400 MHz, (CD3)2SO) 1.99 (s, 3H, CH3), 3.94 (s, 4H, NCH2), 4.65 (s, 4H, OCH2), 6.43 (d, J = 8.7 Hz, 1H, 3-H), 7.15 (dd, J = 8.7, 2.3 Hz, 1H, 4-H), 7.25 (d, J = 2.3 Hz, 1H, 6-H), 9.23 (s, 1H, NH); δC (100 MHz, (CD3)2SO) 23.7 (CH3), 39.0 (C), 62.4 (NCH2), 80.3 (OCH2), 109.3 (C), 115.9 (3-CH), 126.5 (C), 129.0 (4-CH), 130.4 (6-CH), 145.7 (C), 169.2 (C=O); HRMS (ESI) m/z [M − H]−, C13H14N2O279Br calcd. 309.0239, observed 309.0249.
N-Acetyl-N-[5-bromo-2-(2-oxa-6-azaspiro[3.3]heptan-6-yl)phenyl]acetamide (10a). Aniline 7a (0.850 g, 3.2 mmol) in Ac2O (30 mL) was heated at 100 °C for 2 h. The cooled solution was evaporated, ice-water (50 mL) added, and stirred for 3 h. The resultant precipitate was purified by column chromatography using gradient elution of petroleum ether/EtOAc to yield the title compound (1.020 g, 90%) as a white solid; Rf 0.34 (1:1 EtOAc/Pet); mp 152–156 °C; νmax (neat, cm−1) 2932, 2861, 1707 (C=O), 1702 (C=O), 1591, 1493, 1479, 1458, 1408, 1366, 1334, 1300, 1284, 1225, 1156, 1126, 1073, 1022; δH (400 MHz, CDCl3) 2.29 (s, 6H, CH3), 3.96 (s, 4H, NCH2), 4.76 (s, 4H, OCH2), 6.41 (d, J = 8.7 Hz, 1H, 3-H), 7.05 (d, J = 2.3 Hz, 1H, 6-H), 7.34 (dd, J = 8.7, 2.3 Hz, 1H, 4-H); δC 26.5 (CH3), 39.0 (C), 62.2 (NCH2), 80.9 (OCH2), 111.1 (C), 115.9 (3-CH), 126.7 (C) , 132.7, 133.0 (CH), 146.4 (C), 174.0 (C=O); HRMS (ESI) m/z [M + H]+, C15H18N2O379Br calcd. 353.0501, observed 353.0501.
N-[5-Bromo-2-(2-oxa-7-azaspiro[3.5]nonan-7-yl)phenyl]acetamide (8b). Ac2O (0.9 mL, 10.0 mmol) and aniline 7b (0.594 g, 2.0 mmol) in methanol (20 mL) were heated at reflux for 1 h. The cooled mixture was evaporated, ice-water added (40 mL), and stirred for 3 h. The resultant precipitate was washed with water, and dried under vacuum to yield the title compound (0.611 g, 90%) as a white solid; mp 164–166 °C; νmax (neat, cm−1) 3353, 2934, 2860, 2811, 1687 (C=O) 1578, 1507, 1445, 1410, 1372, 1224, 1132, 1109; δH (400 MHz, CDCl3) 2.02 (bs, 4H), 2.18 (s, 3H), 2.69 (bs, 4H, NCH2), 4.48 (s, 4H, OCH2), 6.92 (d, J = 8.2 Hz, 1H, 3-H), 7.12 (dd, J = 8.2, 1.8 Hz, 1H, 4-H), 8.36 (bs, 1H, NH), 8.53 (d, J = 1.8 Hz, 1H, 6-H); δC (100 MHz, CDCl3) 25.0 (CH3), 35.8 (CH2), 38.3 (C), 49.9 (NCH2), 81.6 (OCH2), 118.8 (C), 121.8 (3-CH), 122.3 (6-CH), 126.6 (4-CH), 134.6, 140.2 (C), 168.1 (C=O); HRMS (ESI) m/z [M + H]+, C15H20N2O279Br calcd. 339.0708, observed 339.0707.
3.2.3. Oxidative Cyclization to Give the Spirocyclic Oxetane Fused Benzimidazole
7ʹ-Bromo-1ʹ,2ʹ-dihydro-4ʹH-spiro[oxetane-3,3ʹ-pyrido[1,2-a]benzimidazole] (2b). Acetanilide 8b (0.150 g, 0.44 mmol) and Oxone® (0.820 g, 1.32 mmol) were stirred in formic acid (20 mL) at 40 °C for 6 h. The mixture was evaporated, water added (30 mL), neutralized with solid Na2CO3, and extracted with CH2Cl2 (3 × 10 mL). The organic extracts were dried (Na2SO4), and evaporated dryness to yield the title compound (96 mg, 74%) as a white solid; mp 178–181 °C; νmax (neat, cm−1) 2926, 2856, 1721, 1511, 1481, 1450, 1406, 1307, 1269, 1163, 1047; δH (400 MHz, CDCl3) 2.44 (t, J = 6.4 Hz, 2H, 2ʹ-CH2), 3.36 (s, 2H, 4ʹ-CH2), 4.09 (t, J = 6.4 Hz, 2H, 1ʹ-CH2), 4.54–4.59 (m, 4H, 2,4-CH2), 7.14 (d, J = 8.7 Hz, 1H, 9ʹ-H), 7.32 (dd, J = 8.7, 1.8 Hz, 1H, 8ʹ-H), 7.79 (d, J = 1.8 Hz, 1H, 6ʹ-H); δC (100 MHz, CDCl3) 30.6 (2ʹ-CH2), 34.9 (4ʹ-CH2), 37.9 (C), 39.0 (1'-CH2), 80.6 (2,4-CH2), 110.2 (9ʹ-CH), 115.6 (C), 122.1 (6ʹ-CH), 125.2 (8ʹ-CH), 133.3, 144.5, 150.8 (C); HRMS (ESI) m/z [M + H]+, C13H14N2O79Br calcd. 293.0289, observed 293.0287.
4. Conclusions
The new spirocyclic oxetane fused system, 1ʹ,2ʹ-dihydro-4ʹH-spiro[oxetane-3,3ʹ-pyrido[1,2-a]benzimidazole] has been prepared through oxidative cyclization of the o-cycloalkylaminoacetanilide, where 2-oxa-7-azaspiro[3.5]nonane is the cycloamino substituent. Future work will focus on preparing quinone analogues for anti-cancer studies, as well as investigating conditions for the synthesis of the more strained tetracycle 2a. The acetanilide containing 2-oxa-6-azaspiro[3.3]heptane system proved to be unstable under the presented oxidative cyclization conditions giving an intractable mixture. Research is on-going in the group to establish milder conditions for oxidative cyclizations of o-cyclic amine substituted anilines [15] and acetamides to give ring-fused benzimidazoles.
Acknowledgments
Michael Gurry is funded by the College of Science, National University of Ireland Galway.
Supplementary Materials
Supplementary materials can be accessed at: http:www.mdpi.com/1420-3049/20/08/13864/s1.
Author Contributions
Michael Gurry and Patrick McArdle performed experimental and X-ray crystallography work respectively. Fawaz Aldabbagh conceived the project and directed the research. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all compounds are available from the authors upon request.
References and Notes
- 1.Wuitschik G., Rogers-Evans M., Müller K., Fischer H., Wagner B., Schuler F., Polonchuk L., Carreira E.M. Oxetane as promising modules in drug discovery. Angew. Chem. Int. Ed. 2006;45:7736–7739. doi: 10.1002/anie.200602343. [DOI] [PubMed] [Google Scholar]
- 2.Wuitschik G., Carreira E.M., Wagner B., Fischer H., Parrilla I., Schuler F., Rogers-Evans M., Müller K. Oxetanes in drug discovery: Structural and synthetic insights. J. Med. Chem. 2010;53:3227–3246. doi: 10.1021/jm9018788. [DOI] [PubMed] [Google Scholar]
- 3.Burkhard J.A., Wuitschik G., Rogers-Evans M., Müller K., Carreira E.M. Oxetanes as versatile elements in drug discovery and synthesis. Angew. Chem. Int. Ed. 2010;49:9052–9067. doi: 10.1002/anie.200907155. [DOI] [PubMed] [Google Scholar]
- 4.Wuitschik G., Rogers-Evans M., Buckl A., Bernasconi M., Märki M., Godel T., Fischer H., Wagner B., Parrilla I., Schuler F., et al. Spirocyclic oxetanes: Synthesis and properties. Angew. Chem. Int. Ed. 2008;47:4512–4515. doi: 10.1002/anie.200800450. [DOI] [PubMed] [Google Scholar]
- 5.Fagan V., Bonham S., Carty M.P., Saenz-Méndez P., Eriksson L.A., Aldabbagh F. COMPARE analysis of the toxicity of an iminoquinone derivative of the imidazo[5,4-f]benzimidazoles with NAD(P)H:quinone oxidoreductase 1 (NQO1) activity and computational docking of quinones as NQO1 substrates. Bioorg. Med. Chem. 2012;20:3223–3232. doi: 10.1016/j.bmc.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z., Zhang M., Xiang F. Preparation of 4-substituted and N-substituted piperidine-4-carboxylic acid ethyl ester. CN 101525313. 2009 Sep 09; Scifinder Scholar 2009:1117956.
- 7.Capet M., Levoin N., Berrebi-Bertrand I., Robert P., Schwartz J.C., Lecomte J.M., Aradhye J.D., Pillai M.N., Panchal B.M., Jivani J.K., et al. Dicarboxylic Acid Derivatives, Processes for Preparing Them, Pharmaceutical Compositions Containing Them, and Their Use as Sphingosine-1-Phosphate Receptor Agonists. WO 2008/152149. 2008 Dec 18; Scifinder Scholar 2008:1507568.
- 8.Allen J.R., Chen J.J., Frohn M.J., Hu E., Liu Q., Pickrell A.J., Rumfelt S., Rzasa R.M., Zhong W. Preparation of Nitrogen Heterocyclic Compounds Useful as 3ʹ,5ʹ-Cyclic Nucleotide-Specific Phosphodiesterase 10 (PDE10) Inhibitors. WO 2011/143365. 2011 Nov 17; Scifinder Scholar 2011:145609.
- 9.Kenis S., D’hooghe M., Verniest G., Thi T.A.D., The C.P., Nguyen T.V., de Kimpe N. Synthesis of 1-alkyl-2-(trifluoromethyl)azetidines and their regiospecific ring opening toward diverse α-(trifluoromethyl)amines via intermediate azetidinium salts. J. Org. Chem. 2012;77:5982–5992. doi: 10.1021/jo300694y. [DOI] [PubMed] [Google Scholar]
- 10.Crystallographic data (excluding structure factors) have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication CCDC 1408035 for 9a and CCDC 1408036 for 2b. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/data_request/cif (or from 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-Mail: deposit@ccdc.cam.ac.uk)
- 11.Meth-Cohn O., Suschitzky H. Syntheses of heterocyclic compounds. Part IV. Oxidative cyclisation of aromatic amines and their N-acyl derivatives. J. Chem. Soc. 1963:4666–4669. doi: 10.1039/jr9630004666. [DOI] [Google Scholar]
- 12.Fahey K., Aldabbagh F. Synthesis of seven- and eight-membered [1,2-a] alicyclic ring-fused benzimidazoles and 3-aziridinylazepino[1,2-a]benzimidazolequinone as a potential antitumour agent. Tetrahedron Lett. 2008;49:5235–5237. doi: 10.1016/j.tetlet.2008.06.121. [DOI] [Google Scholar]
- 13.Fagan V., Bonham S., McArdle P., Carty M.P., Aldabbagh F. Synthesis and toxicity of new ring-fused imidazo[5,4-f]benzimidazolequinones and mechanism using amine N-oxide cyclizations. Eur. J. Org. Chem. 2012:1967–1975. doi: 10.1002/ejoc.201101687. [DOI] [Google Scholar]
- 14.Chak B.C.M., McAuley A. The synthesis and characterization of the pendant-armed ligand N,N′-bis(2′-pyridylmethyl)-1,7-dithia-4,11-diazacyclotetradecane (L4) and crystal structures of L4 and the copper(II) complex [Cu(L4)](ClO4)2—Crystal structure of the nickel(II)complex of N-(2′-pyridylmethyl)-1,4,7-trithia-11-azacyclotetradecane (L2), [Ni(L2)(CH3CN)](ClO4)2·CH3CN. Can. J. Chem. 2006;84:187–195. [Google Scholar]
- 15.Gurry M., Sweeney M., McArdle P., Aldabbagh F. One-pot hydrogen peroxide and hydrohalic acid induced ring closure and selective aromatic halogenation to give new ring-fused benzimidazoles. Org. Lett. 2015;17:2856–2859. doi: 10.1021/acs.orglett.5b01317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.