Abstract
Background: Due to its high antioxidant activity, baicalein, a kind of flavonoid present in Radical Scutellariae, has various pharmacological effects. However, the protective effect against oxidative stress in Schwann cells, which plays an important role in peripheral neuropathy, has not yet been studied. In this study, the effects of baicalein on hydrogen peroxide (H2O2)-induced DNA damage and apoptosis in RT4-D6P2T Schwann cells were evaluated.
Methods: Cell viability assay was performed using MTT assay and colony formation assay. Apoptosis was assessed by flow cytometry analysis and DNA fragmentation assay. The effects on DNA damage and ATP content were analyzed by comet method and luminometer. In addition, changes in protein expression were observed by Western blotting.
Results: Our results show that baicalein significantly inhibits H2O2-induced cytotoxicity through blocking reactive oxygen species (ROS) generation. We also demonstrate that baicalein is to block H2O2-induced DNA damage as evidenced by inhibition of DNA tail formation and γH2AX phosphorylation. Moreover, baicalein significantly attenuated H2O2-induced apoptosis and mitochondrial dysfunction, and restored inhibition of ATP production. The suppression of apoptosis by baicalein in H2O2-stimulated cells was associated with reduction of increased Bax/Bcl-2 ratio, activation of caspase-9 and -3, and degradation of poly (ADP-ribose) polymerase.
Conclusions: These results demonstrate that baicalein eliminates H2O2-induced apoptosis through conservation of mitochondrial function by the removal of ROS. Therefore, it is suggested that baicalein protects Schwann cells from oxidative stress, and may be beneficial for the prevention and treatment of peripheral neuropathy induced by oxidative stress.
Keywords: Baicalein, Schwann cells, oxidative stress, DNA damage, apoptosis
Introduction
Oxidative stress, characterized by overwhelming reactive oxygen species (ROS), is a crucial initiating factor in many chronic diseases, including peripheral neuropathy 1,2. Schwann cells are the major glial cells of the peripheral nervous system, and support the normal physiological functions of neurons 3,4. Mitochondria are the major organelle involved in ROS production by various oxidative stimuli in cells. Although at low levels, ROS plays the role of a second messenger in cellular signal transduction and homeostasis, the overproduction of ROS damages cellular biomolecules, such as proteins, lipids and nucleic acids, and induces DNA damage and apoptosis in multiple types of cells, including Schwann cells 5-9. In particular, Schwann cell apoptosis can enhance axonal degeneration, which is an important cause of peripheral neuropathy induction, due to reduced neurotrophic support from Schwann cells 5,10. Therefore, it is essential to inhibit excessive ROS production, in order to maintain the nerve fiber regeneration function of Schwann cells.
Recent data have shown that the antioxidants present in various natural products can be effective in suppressing and curing many diseases, including peripheral neuropathy 11-14. Among them, baicalein is one of the flavonoids found mainly in Radix Scutellariae, the root of Scutellaria baicalensis Georgi, which has been used in Korea, China, and Japan in the traditional treatment of various diseases 15,16. A number of studies, including our previous results, have shown that baicalein has a variety of pharmacological activities, including anti-inflammatory, antioxidant, and anti-cancer effects 14,17-25. However, the protective effects and mechanisms of baicalein against oxidative stress in Schwann cells have not yet been studied. Therefore, in this study, we investigate the inhibitory potential of baicalein on cellular injury by oxidative stress using RT4-D6P2T Schwann cells. For this purpose, hydrogen peroxide (H2O2), pro-oxidant agent, is used to mimic the in vitro oxidation, and the effects of baicalein on H2O2- induced DNA damage and apoptosis are investigated.
Materials and Methods
Reagents and antibodies
Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), and antibiotic mixtures were purchased from WelGENE Inc. (Daegu, Republic of Korea). Baicalein, H2O2, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), N-acetyl cysteine (NAC), 5,6-carboxy-2',7'-dichlorofluorescin diacetate (DCF-DA), propidium iodide (PI), 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethyl-imidacarbocyanine iodide (JC-1), ethidium bromide (EtBr), 4',6-diamidino-2-phenylindole (DAPI), and annexin V-fluorescein isothiocyanate (FITC) were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Bio-Rad protein assay kit and mitochondrial protein isolation kit were purchased from Bio-Rad Lab (Hercules, CA, USA) and Active Motif (Carlsbad, CA, USA), respectively. Polyvinylidene difluoride (PVDF) membranes and enhanced chemiluminescence (ECL) solution were obtained from Schleicher and Schuell (Keene, NH, USA) and Amersham Corp. (Arlington Heights, IL, USA), respectively. ATP assay kit was purchased from Abcam Inc. (Cambridge, UK). The primary antibodies against actin, Bax, Bcl-2, cytochrome c, cytochrome oxidase subunit 4 (COX IV), caspase-9, caspase-3 and poly(ADP-ribose) polymerase (PARP) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Anti-histone variant H2AX (γH2AX) and p-γH2AX were obtained from Cell Signaling Technology Inc. (Beverly, MA, USA). Appropriate horseradish-peroxidase (HRP)-linked secondary antibodies were purchased from Santa Cruz Biotechnology Inc. All reagents that were not specifically identified were purchased from Sigma-Aldrich Chemical Co.
Cell culture and baicalein treatment
The immortalized human vestibular schwannoma RT4-D6P2T cells were kindly provided by Dr. Hwan Tae Park (Department of Physiology, College of Medicine, Dong-A University, Busan, Republic of Korea). The cells were cultured in DMEM containing 10 % FBS and 100 U/ml penicillin and streptomycin at 37°C in humidified air with 5 % CO2, and were subcultured every three days. Baicalein was dissolved in dimethyl sulfoxide (DMSO), and the final concentrations were adjusted by dilution with a complete culture medium. The final DMSO concentration was <0.05% in all experiments (i.e., a non-cytotoxic range).
MTT assay
For the cell viability study, RT4-D6P2T cells were cultured in 96-well plates at a density of 1×104 cells per well. After 24 h incubation, the cells were treated with various concentrations of baicalein or H2O2 (1 mM) alone, or pretreated with different concentrations of baicalein for 1 h before H2O2 treatment. After 24 h, the medium was replaced with MTT (0.5 mg/ml) solution and reacted for 3 h at 37°C. The formazan crystals were dissolved by replacing the supernatant with DMSO. After 10 min, the optical density was measured at a wavelength of 540 nm by enzyme-linked immunosorbent assay (ELISA) microplate reader (Dynatech Laboratories, Chantilly, VA, USA).
Detection of the intracellular ROS levels
To measure ROS production using DCF-DA, RT4-D6P2T cells were pretreated with 100 µM baicalein for 1 h, and then incubated for 1 h in the presence or absence of 1 mM H2O2. Following the termination of the treatment period, the cells were stained with 10 µM DCF-DA for 15 min, rinsed twice with phosphate buffered saline (PBS), and then immediately analyzed using a flow cytometer (Becton Dickinson, San Jose, CA, USA) with an excitation wavelength of 480 nm and an emission wavelength of 525 nm.
Comet assay
To investigate DNA damage using comet assay, the cells were harvested by trypsinization, and mixed with 0.5 % low-melting-point agarose. The mixture was spread on a slide at 37°C and solidified using an ice pack for 5 minutes, and then immersed in a lysis solution [2.5 M sodium chloride (NaCl), 100 mM Na-ethylenediaminetetraacetic acid (EDTA), 10 mM Tris, 1 % Triton X100, and 10 % DMSO (pH 10)] for 1 h at 4°C. After electrophoresis, the slides were rinsed with a neutralizing buffer (0.4 M Tris, pH 7.5), dehydrated in absolute ethanol at 4°C, and allowed to dry. After staining the cells with PI solution (20 µg/ml), images were captured by fluorescence microscopy (Carl Zeiss, Oberkochen, Germany) according to the previous method 26.
Protein isolation and Western blot analysis
To extract whole-cellular proteins, the cells were collected, washed twice with ice-cold PBS, and then lysed using the cell lysis buffer [25 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM Na-EDTA, 1 % nonidet-P40, 1 mM phenylmethylsulfonyl fluoride, and 5 mM dithiothreitol] for 1 h. The mitochondrial and cytosolic proteins were prepared using a mitochondria isolation kit, in accordance with the instructions of the manufacturer. Protein concentration was measured according to the Bio-Rad protein assay kit and the same amount of protein was separated by electrophoresis in sodium dodecyl sulfate (SDS)-polyacrylamide gel and then transferred to PVDF membrane. After blocking with 5% non-fat dry milk for 1 h at room temperature, the membranes were probed overnight with primary antibodies at 4°C. The membranes were washed with Tris buffered saline containing 0.1% Tween-20 for 5 min, then incubated for 2 h at room temperature with the corresponding HRP-conjugated secondary antibody, and visualized with ECL solution. Bands were quantified using ImageJ (Ver. 1.46; NIH, Bethesda, MD, USA) and normalized to actin and the ratio was determined.
Detection of nuclear morphological changes
To observe the nuclear morphological changes, the harvested cells were fixed with 3.7 % paraformaldehyde in PBS for 10 min at 25°C. The cells were washed with PBS and stained with DAPI solution (1 mg/ml) for 10 min in the dark. After washing with PBS, the morphological changes in the nucleus were examined by fluorescence microscopy at ×400 magnification.
DNA fragmentation assay
The collected cells were dissolved in lysis buffer [10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, 0.5 % Triton X-100, and 0.1 mg/ml proteinase K] for 30 min at room temperature. DNA from the supernatant was extracted by chloroform/phenol/ isoamyl alcohol (24/25/1, v/v/v) and was precipitated by ethanol. DNA was then transferred to 1.5 % agarose gel containing 0.1 µg/ml EtBr, and electrophoresis was carried out at 70 V.
Colony formation assay
Cells treated with H2O2 in the presence or absence of baicalein were washed with PBS. The single cell suspensions were prepared by trypsin treatment, and the cells were inoculated on 6-well plates (500 cells/well). The cells were further cultured for two weeks to form colonies. The colonies were fixed with 3.7 % paraformaldehyde, and stained with a 0.1 % purple-violet solution for 10 min. After washing by PBS, the cell colonies were photographed under inverted microscopy (Carl Zeiss).
Detection of apoptosis by annexin V staining
Following the termination of treatment, the cells were harvested and suspension was made in binding buffer (Becton Dickinson). And then, the staining of the cells was conducted using an Annexin V-FITC Apoptosis Detection Kit (Becton Dickinson) for 20 min in the dark, according to the manufacturer's instructions. The cells were immediately analyzed from each sample using a flow cytometer, and the degree of apoptosis was quantified as a percentage of the annexin V-positive cells.
Measurement of the mitochondrial membrane potential (MMP)
The changes in the MMP (Δψm) were assessed using JC-1, following the manufacturer's protocol. In brief, the collected cells were rinsed with cold PBS, and then stained with 10 µM JC-1 for 30 min at 37 °C in the dark. After washing with PBS to remove the unbound dye, the green fluorescence intensities from the JC1 monomer and the red fluorescence intensities from the aggregated form of JC1 in the cells were measured by flow cytometry (Becton Dickinson), as recommended by the manufacturer's guidelines.
Measurement of ATP content
The ATP content of cells cultured with different stimuli was measured lumimetrically using a commercially available ATP assay kit. Briefly, after lysing the cells with the buffer provided, the supernatant was mixed with the reaction buffer at a ratio of 1:10, and measured by GLOMAX luminometer (Promega Co., Madison, WI, USA). Subsequently, the cellular ATP content from three replicate experiments was measured from the ATP standard curve, according to the manufacturer's instructions. The results were expressed as percentage, and the ATP content of the untreated control cells was assumed to be 100%.
Statistical analysis
All the experiments reported in this study were performed independently at least three times. The results are presented as the mean ± SD. Statistical significance was assessed by one-way ANOVA. A p value of < 0.05 was considered statistically significant.
Results
Suppression of H2O2-induced RT4-D6P2T cell cytotoxicity by baicalein
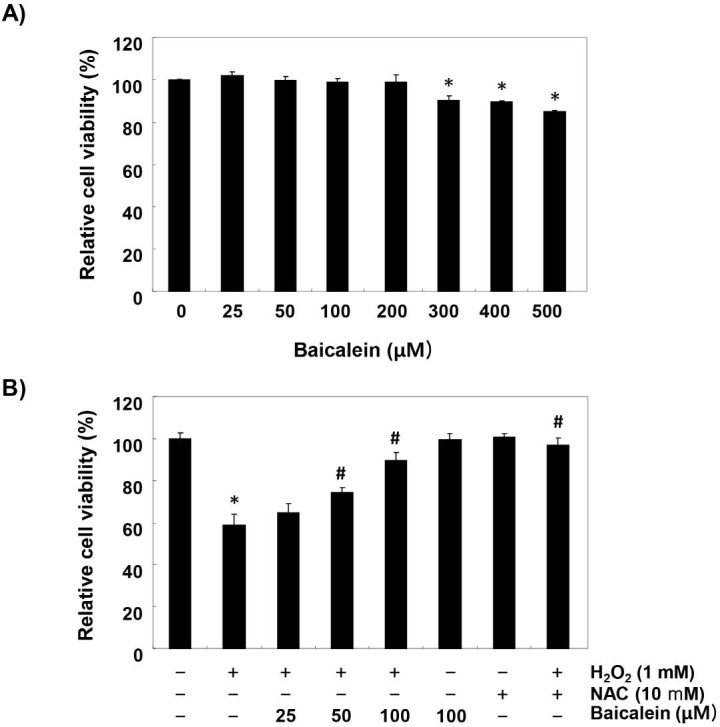
To establish the experimental conditions, RT4-D6P2T cells were treated with a wide range of concentrations of baicalein for 24 h, and MTT assay was performed. Figure 1A shows that the cytotoxic effect of baicalein was not induced at concentrations up to 200 µM, but the cell viability was gradually suppressed at concentrations above 300 µM, as compared to the control cells that had received no treatment. Therefore, the maximum concentration of baicalein to 100 µM was chosen to investigate study the inhibitory effect of baicalein on H2O2-induced cell damage. Our results indicated that pretreatment with baicalein concentration-dependently prevented the reduction of cell viability in H2O2-treated cells (Figure 1B). Moreover, H2O2-induced cell viability reduction was completely suppressed in cells pretreated with an antioxidant NAC, as a positive control (Figure 1B).
Figure 1.
Inhibition of H2O2-induced cytotoxicity by baicalein in RT4-D6P2T cells. Cells were (A) treated with various concentrations of baicalein for 24 h, or (B) pretreated with or without baicalein for 1 h, and then stimulated with 1 mM H2O2 for 24 h. NAC was used for cells as a positive control. Cell viability was assessed by MTT reduction assay. The results are the mean ± SD obtained from three independent experiments (*p < 0.05 compared with the control group, #p < 0.05 compared with the H2O2-treated group).
Reduction of H2O2-induced ROS generation by baicalein in RT4-D6P2T cells
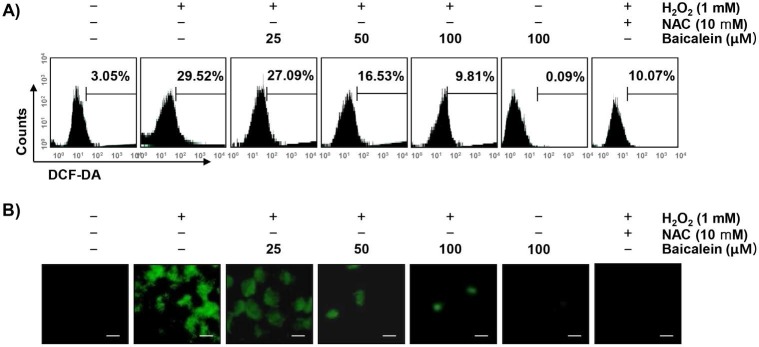
To examine whether the cytoprotective effect of baicalein on oxidative stress in RT4-D6P2T cells was correlated with antioxidant activity, the effect of baicalein on H2O2-induced excessive ROS production was investigated. Our results showed that the level of ROS gradually increased with the treatment of H2O2, peaked at 1 h (data not shown). However, the treatment with baicalein alone did not induce ROS production, and the pretreatment with baicalein effectively attenuated the level of ROS released by H2O2 treatment (Figure 2A). As in the fluorescence microscope observation, we further confirmed that baicalein had a powerful ROS scavenging effect (Figure 2B). NAC also significantly inhibited the H2O2-induced production of ROS.
Figure 2.
Attenuation of H2O2-induced ROS generation by baicalein in RT4-D6P2T cells. Cells were pretreated with the indicated concentration of baicalein or 10 mM NAC for 1 h, and then stimulated with or without 1 mM H2O2 for 1 h. (A) The cells were incubated with DCF-DA, and DCF fluorescence was measured by flow cytometry. The values represent the means of two independent experiments. (B) After staining with DCF-DA, images were obtained by fluorescence microscopy (original magnification, ×200). These images are representative of at least three independent experiments. Scale bars, 10 μm.
Attenuation of H2O2-induced DNA damage by baicalein in RT4-D6P2T cells
To assess whether the inhibitory effects of baicalein on H2O2-induced cytotoxicity and ROS accumulation were associated with the protection of DNA damage, the comet and immunoblotting assays were performed. As indicated in Figure 3A, no smeared pattern of nuclear DNA was observed in the cells treated with baicalein alone, similar to the control cells. However, DNA tails, which imply DNA damage, were clearly increased in H2O2-treated cells, while under the baicalein pretreatment conditions, DNA tails were hardly observed. In addition, immunoblotting results showed a marked increase in γH2AX phosphorylation (at serine 139) in H2O2-stimulated cells, compared to the untreated control cells. However, phosphorylation of γH2AX by H2O2 was almost inhibited in baicalein-pretreated cells (Figure 3B and C).
Figure 3.
Protection of H2O2-induced DNA damage by baicalein in RT4-D6P2T cells. Cells were pretreated with 100 µM baicalein for 1 h, and then stimulated with or without 1 mM H2O2 for 24 h. (A) To detect cellular DNA damage, the comet assay was performed, and representative photographs of the comets were taken by fluorescence microscopy (original magnification, ×200). Scale bars, 10 μm. (B) Equal amounts of cell lysates were separated on SDS-polyacrylamide gels, and transferred to membranes. The membranes were probed with specific antibodies against γH2AX and p-γH2AX, and the proteins were visualized using an ECL detection system. Actin was used as an internal control. (C) Bands were quantified using ImageJ and normalized to actin, and the ratio was determined. Data are expressed as mean ± SD. All experiments were repeated three times (#p<0.05 in comparison to the control group; *p<0.05 compared with the H2O2 group).
Inhibition of H2O2-induced apoptosis by baicalein in RT4-D6P2T cells
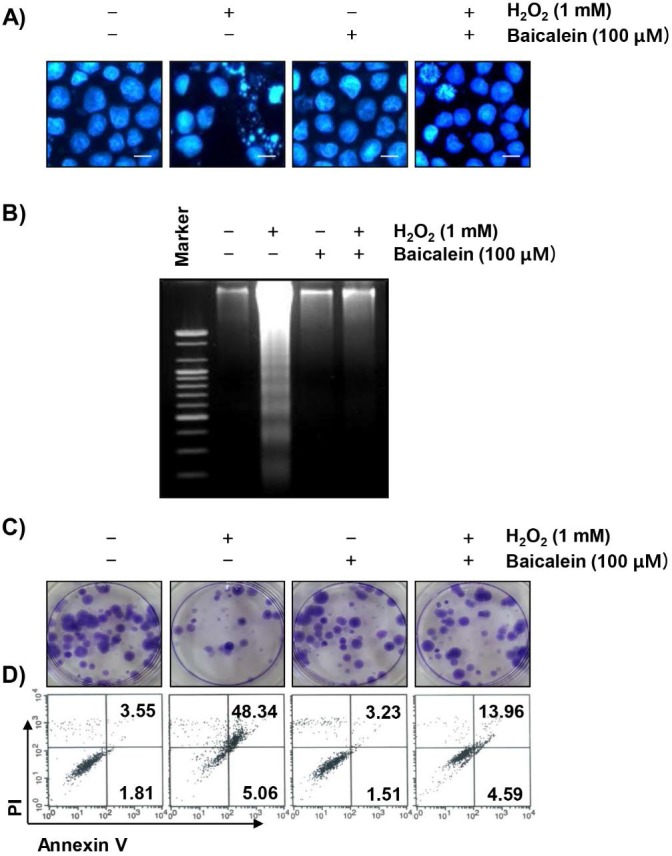
We next examined whether the protective effect of baicalein on the ROS production and DNA damage by H2O2 is related to the inhibition of apoptosis. The fluorescent images using DAPI staining show that the formation of chromatin condensation, which is observed in the apoptosis-induced cells, was greatly increased in the H2O2-treated RT4-D6P2T cells, and baicalein reliably weakened this effect (Figure 4A). In addition, the results of agarose gel electrophoresis showed that H2O2-induced DNA fragmentation, another evidence of the induction of apoptosis, was completely attenuated by the pretreatment of baicalein (Figure 4B). Furthermore, in cells treated with baicalein prior to H2O2 exposure, the inhibition of colony formation by H2O2 was significantly reduced (Figure 4C), and baicalein pretreatment also reduced the increased frequency of apoptotic cells in H2O2-treated cells (Figure 4D).
Figure 4.
Suppression of H2O2-induced apoptosis by baicalein in RT4-D6P2T cells. Cells were treated with 100 µM baicalein for 1 h, and then stimulated with or without 1 mM H2O2 for 24 h. (A) The cells were fixed and stained with DAPI solution. The stained nuclei were observed by fluorescence microscopy (original magnification, ×400). Scale bars, 10 μm. (B) DNA fragmentation was analyzed by extracting genomic DNA, electrophoresis in a 1.5 % agarose gel, and then visualizing by EtBr staining. (C) After treatment, the cells were further cultured for two weeks to form colonies. The cells were stained with a 0.1 % purple-violet solution, and then imaged under inverted microscopy. Representative photographs are shown. (D) The cells were collected and stained with annexin-V and PI, and the percentages of apoptotic cells were then analyzed by flow cytometry. The results are the means of two independent experiments.
Inhibition of H2O2-induced mitochondrial dysfunction by baicalein in RT4-D6P2T cells
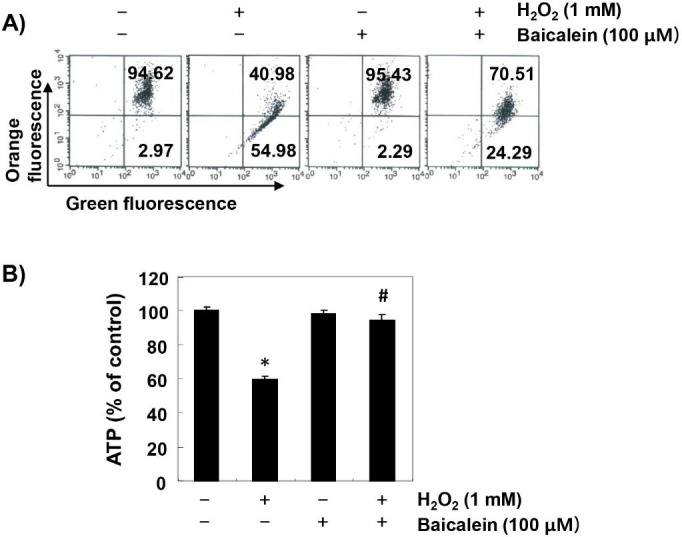
To investigate the effect of baicalein on the mitochondrial dysfunction caused by oxidative stress, MMP values and intracellular ATP levels were evaluated. According to the results of JC1 staining, the loss of MMP was markedly increased in H2O2-exposed cells, while this phenomenon was significantly reduced in baicalein-pretreated cells (Figure 5A). In addition, compared with cells cultured in normal medium, the concentration of ATP in cells exposed to H2O2 was significantly decreased (Figure 5B). However, the content of ATP in H2O2-treated cells in the presence of baicalein was maintained almost at the control level.
Figure 5.
Prevention of H2O2-induced mitochondrial dysfunction by baicalein in RT4-D6P2T cells. Cells were treated with 100 µM baicalein for 1 h, and then stimulated with or without 1 mM H2O2 for 24 h. (A) The cells were collected and incubated with 10 µM JC-1 for 20 min at 37 °C in the dark. The values of MMP were evaluated by flow cytometry. The data are the means of the two different experiments. (B) To monitor the ATP production using a luminometer, a commercially available kit was used. The results are the mean ± SD obtained from three independent experiments (*p < 0.05 compared with the control group, #p < 0.05 compared with the H2O2-treated group).
Effects of baicalein on the alteration of the apoptosis regulatory genes by H2O2 in RT4-D6P2T cells
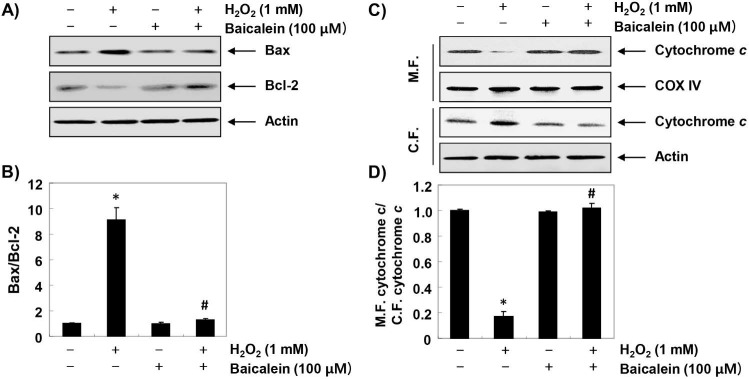
Furthermore, we investigated the effect of baicalein on changes in apoptosis-regulating genes expression in H2O2-treated RT4-D6P2T cells. The immunoblotting results of Figure 6A and B show that the expression of pro-apoptotic Bax was increased, whereas in H2O2-treated cells, the expression of anti-apoptotic Bcl-2 was decreased. Further, the expression of cytochrome c in H2O2-stimulated cells was increased in the cytoplasmic fraction compared to the mitochondrial fraction, indicating that cytochrome c was released from the mitochondria into the cytoplasm (Figure 6C and D). However, in the cells pretreated with baicalein, these changes were not observed. In addition, the expression of pro-caspase-9 and -3 was reduced in H2O2-treated cells, and the expression of truncated PARP, a representative substrate protein degraded by activated caspase-3, was increased, indicating that the intrinsic pathway was activated. In contrast, these changes by H2O2 treatment were relatively conserved in the baicalein-pretreated (Figure 7).
Figure 6.
Effects of baicalein on H2O2-induced changes of Bax and Bcl-2 expression in RT4-D6P2T cells. (A and C) Cells were treated with 100 µM baicalein for 1 h, and then stimulated with or without 1 mM H2O2 for 24 h. The cellular proteins were separated by SDS-polyacrylamide gel electrophoresis, and then transferred to membranes. The membranes were probed with the indicated antibodies. Proteins were visualized using an ECL detection system. Actin was used as an internal control. (C) The mitochondrial and cytosolic proteins isolated from cells cultured under the same conditions were separated by SDS polyacrylamide gel electrophoresis, and transferred to the membranes. The membranes were probed with anti-cytochrome c antibody. The proteins were visualized using an ECL detection system. Equal protein loading was confirmed by the analysis of COX VI and actin in each protein extract. (B and D) Bands were quantified using ImageJ and normalized to actin or COX IV, and the ratio was determined. Data are expressed as mean ± SD. All experiments were repeated three times (#p<0.05 in comparison to the control group; *p<0.05 compared with the H2O2 group).
Figure 7.
Effects of baicalein on H2O2-induced activation of caspases and degradation of PARP in RT4-D6P2T cells. (A) The cellular proteins extracted from cells grown under the same condition as in Fig. 6 were separated by SDS-polyacrylamide gel electrophoresis, and then transferred to membranes. The membranes were probed with the indicated antibodies. Proteins were visualized using an ECL detection system. Actin was used as an internal control. (B) The proteins were visualized using an ECL detection system. Equal protein loading was confirmed by the analysis of COX VI and actin in each protein extract. (B and D) Bands were quantified using ImageJ and normalized to actin or COX IV, and the ratio was determined. Data are expressed as mean ± SD. All experiments were repeated three times (#p<0.05 in comparison to the control group; *p<0.05 compared with the H2O2 group).
Discussion
Schwann cells are the main target cells of oxidative stress at the onset of neurodegenerative diseases, and several natural antioxidants have been reported to prevent functional damage of these cells by oxidative stress 27-31. However, since the antioxidant efficacy of baicalein, a major flavonoid isolated from Radix Scutellariae, in Schwann cells has not yet been studied, this study investigated the effect of baicalein on cell injury by oxidative stress using the RT4-D6P2T cell model. Our results indicated that baicalein significantly protected RT4-D6P2T cells from oxidative stress, which was associated with the inhibition of ROS generation. We also demonstrated that baicalein inhibited DNA damage by oxidative stress, as evidenced by attenuation of H2O2-induced DNA tail formation and γH2AX phosphorylation. In addition, we confirmed through DAPI staining, agarose gel electrophoresis, and flow cytometry assays that baicalein significantly inhibited H2O2-induced apoptosis. These results suggest that inhibition of excessive ROS production by baicalein contributed to blocking of H2O2-induced proliferation reduction, DNA damage, and apoptosis in RT4-D6P2T Schwann cells.
Excessive ROS production by oxidative stress has been recognized to be one of the mechanisms leading to apoptosis, following DNA damage associated with mitochondrial dysfunction 32-34. Apoptosis can be categorized into two pathways, generally mitochondria-dependent intrinsic and death receptor-mediated extrinsic apoptotic signaling pathways. Unlike the extrinsic pathway activated by the binding of apoptotic ligands to death receptors on cell membranes, the overload of ROS by oxidative stress results in the loss of MMP, which is considered to be a characteristic of the onset of the intrinsic apoptotic pathway 32,33. At the same time, mitochondrial dysfunction due to excessive ROS production associated with the destruction of MMP causes an abnormality in the electron transport pathway of the mitochondrial respiratory chain 32-34. The impairment of energy metabolism ultimately interferes with the production of intracellular ATP 33,35. According to current studies, the levels of MMP values and ATP contents were significantly reduced in H2O2-treated RT4-D6P2T cells. However, they were significantly inhibited in the baicalein-pretreated cells, and were almost preserved at the control levels, indicating that mitochondrial dysfunction due to oxidative stress was blocked by baicalein.
On the other hand, the loss of MMP enhances the release of death-promoting factors such as cytochrome c from the mitochondria to the cytoplasm, and cytochrome c in the cytoplasm forms apoptosomes by binding to apoptosis protein activation factor 1 36,37. Apoptosomes sequentially activate caspase-9, a potent stimulant of intrinsic apoptosis pathway. Activated caspase-9 triggers activation of effector caspases such as caspase-3 and -7, which promotes degradation of a variety of substrate proteins, including PARP necessary for cell survival, and eventually induces apoptosis. In this process, Bcl-2 family proteins, which are composed of factors promoting and inhibiting apoptosis, also play an important role 38,39. The pro-apoptotic proteins belonging to the Bcl-2 family members, such as Bax, migrate to the mitochondria, destroying the mitochondrial permeability, and opening the mitochondrial pores to release cytochrome c; while anti-apoptotic proteins, such as Bcl-2, act in reverse manner 38,39. Therefore, the balance between pro-apoptotic proteins and anti-apoptotic proteins in Bcl-2 family members is considered to be a controlling factor of apoptosis induction. The results of this study show that H2O2-induced increase in Bax/Bcl-2 ratio and cytoplasmic release of cytochrome c were effectively reversed by baicalein. In addition, decreased expression of pro-caspase-9 and -3 by H2O2 treatment, which means they were activated, was restored to the control level by pretreatment with baicalein; and degradation of PARP, a biochemical hallmark of apoptosis, was also inhibited. These results imply that baicalein was able to weaken apoptosis through preservation of mitochondrial function in RT4-D6P2T Schwann cells. Although current results may provide a partial understanding of the antioxidant effects of baicalein, further evaluation using primary cultured Schwann cells and in vivo animal models is required. Based on these results, another study should be conducted to discuss metabolism as a functional dietary material of baicalein in human and physiological concentrations in the future.
In conclusion, the current results show that baicalein protects against the H2O2-induced loss of viability, ROS generation, DNA damage, and apoptosis in RT4-D6P2T Schwann cells. The beneficial effects of baicalein are closely related to the maintenance of energy metabolism, by preventing mitochondrial dysfunction. Although further studies between ROS generation inhibition and energy metabolism are needed, the present results suggest that baicalein has potential efficacy in the neuroprotection of peripheral nerves, by potentially protecting Schwann cells from oxidative stress-mediated damage.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korea government (2018R1A2B2005705 and 2016R1A5A2007009).
References
- 1.Ino D, Iino M. Schwann cell mitochondria as key regulators in the development and maintenance of peripheral nerve axons. Cell Mol Life Sci. 2017;74:827–835. doi: 10.1007/s00018-016-2364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tully M, Shi R. New insights in the pathogenesis of multiple sclerosis-role of acrolein in neuronal and myelin damage. Int J Mol Sci. 2013;14:20037–20047. doi: 10.3390/ijms141020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintes S, Brinkmann BG. Transcriptional inhibition in Schwann cell development and nerve regeneration. Neural Regen Res. 2017;12:1241–1246. doi: 10.4103/1673-5374.213537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann HC, Höke A. Use of engineered Schwann cells in peripheral neuropathy: Hopes and hazards. Brain Res. 2016;1638:97–104. doi: 10.1016/j.brainres.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Castelnovo LF, Bonalume V, Melfi S, Ballabio M, Colleoni D, Magnaghi V. Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways. Neural Regen Res. 2017;12:1013–1023. doi: 10.4103/1673-5374.211172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YL, Chen KH, Yin TC, Huang TH, Yuen CM, Chung SY. et al. Extracorporeal shock wave therapy effectively prevented diabetic neuropathy. Am J Transl Res. 2015;7:2543–2560. [PMC free article] [PubMed] [Google Scholar]
- 7.Jirsova K, Mandys V, Gispen WH, Bär PR. Cisplatin-induced apoptosis in cultures of human Schwann cells. Neurosci Lett. 2006;392:22–26. doi: 10.1016/j.neulet.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 8.Park CJ, Park SA, Yoon TG, Lee SJ, Yum KW, Kim HJ. Bupivacaine induces apoptosis via ROS in the Schwann cell line. J Dent Res. 2005;84:852–857. doi: 10.1177/154405910508400914. [DOI] [PubMed] [Google Scholar]
- 9.Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959:368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 10.Fukunaga M, Miyata S, Liu BF, Miyazaki H, Hirota Y, Higo S. et al. Methylglyoxal induces apoptosis through activation of p38 MAPK in rat Schwann cells. Biochem Biophys Res Commun. 2004;320:689–695. doi: 10.1016/j.bbrc.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho LF, Silva AMF, Carvalho AA. The use of antioxidant agents for chemotherapy-induced peripheral neuropathy treatment in animal models. Clin Exp Pharmacol Physiol. 2017;44:971–979. doi: 10.1111/1440-1681.12803. [DOI] [PubMed] [Google Scholar]
- 12.Brami C, Bao T, Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: A systematic review. Crit Rev Oncol Hematol. 2016;98:325–334. doi: 10.1016/j.critrevonc.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyenihi AB, Ayeleso AO, Mukwevho E, Masola B. Antioxidant strategies in the management of diabetic neuropathy. Biomed Res Int. 2015;2015:515042. doi: 10.1155/2015/515042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Q, Chen XY, Martin C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci Bull (Beijing) 2016;61:1391–1398. doi: 10.1007/s11434-016-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaire BP, Moon SK, Kim H. Scutellaria baicalensis in stroke management: nature's blessing in traditional Eastern medicine. Chin J Integr Med. 2014;20:712–720. doi: 10.1007/s11655-014-1347-9. [DOI] [PubMed] [Google Scholar]
- 16.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Cheng CS, Chen J, Tan HY, Wang N, Chen Z, Feng Y. Scutellaria baicalensis and cancer treatment: Recent progress and perspectives in biomedical and clinical studies. Am J Chin Med. 2018;46:25–54. doi: 10.1142/S0192415X18500027. [DOI] [PubMed] [Google Scholar]
- 18.Tian Y, Li X, Xie H, Wang X, Xie Y, Chen C. et al. Protective mechanism of the antioxidant baicalein toward hydroxyl radical-treated bone marrow-derived mesenchymal stem cells. Molecules. 2018;23:223. doi: 10.3390/molecules23010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bie B, Sun J, Guo Y, Li J, Jiang W, Yang J. et al. Baicalein: A review of its anti-cancer effects and mechanisms in hepatocellular carcinoma. Biomed Pharmacother. 2017;93:1285–1291. doi: 10.1016/j.biopha.2017.07.068. [DOI] [PubMed] [Google Scholar]
- 20.Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Baicalein as a potent neuroprotective agent: A review. Biomed Pharmacother. 2017;95:1021–1032. doi: 10.1016/j.biopha.2017.08.135. [DOI] [PubMed] [Google Scholar]
- 21.Choi EO, Cho EJ, Jeong JW, Park C, Hong SH, Hwang HJ. et al. Baicalein inhibits the migration and invasion of B16F10 mouse melanoma cells through inactivation of the PI3K/Akt signaling pathway. Biomol Ther (Seoul) 2017;25:213–221. doi: 10.4062/biomolther.2016.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi EO, Jeong JW, Park C, Hong SH, Kim GY, Hwang HJ. et al. Baicalein protects C6 glial cells against hydrogen peroxide-induced oxidative stress and apoptosis through regulation of the Nrf2 signaling pathway. Int J Mol Med. 2016;37:798–806. doi: 10.3892/ijmm.2016.2460. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Park C, Han MH, Hong SH, Kim GY, Hong SH. et al. Baicalein induces caspase-dependent apoptosis associated with the generation of ROS and the activation of AMPK in human lung carcinoma A549 cells. Drug Dev Res. 2016;77:73–86. doi: 10.1002/ddr.21298. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y, Snyder SA, Smith JN, Chen YC. Anticancer properties of baicalein: a review. Med Chem Res. 2016;25:1515–1523. doi: 10.1007/s00044-016-1607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Oliveira MR, Nabavi SF, Habtemariam S, Erdogan Orhan I, Daglia M, Nabavi SM. The effects of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol Res. 2015;100:296–308. doi: 10.1016/j.phrs.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Collins AR, El Yamani N, Lorenzo Y, Shaposhnikov S, Brunborg G, Azqueta A. Controlling variation in the comet assay. Front Genet. 2014;5:359. doi: 10.3389/fgene.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He B, Wu F, Fan L, Li XH, Liu Y, Liu YJ. et al. Carboxymethylated chitosan protects Schwann cells against hydrogen peroxide-induced apoptosis by inhibiting oxidative stress and mitochondria dependent pathway. Eur J Pharmacol. 2018;825:48–56. doi: 10.1016/j.ejphar.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Xue B, Wang L, Zhang Z, Wang R, Xia XX, Han PP. et al. Puerarin may protect against Schwann cell damage induced by glucose fluctuation. J Nat Med. 2017;71:472–481. doi: 10.1007/s11418-016-1067-0. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Yao W, Shi H, Liu H, Li Y, Gao Y. et al. Paeoniflorin protects Schwann cells against high glucose induced oxidative injury by activating Nrf2/ARE pathway and inhibiting apoptosis. J Ethnopharmacol. 2016;185:361–369. doi: 10.1016/j.jep.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 30.Ju DT, Kuo WW, Ho TJ, Paul CR, Kuo CH, Viswanadha VP. et al. Protocatechuic acid from Alpinia oxyphylla induces Schwann cell migration via ERK1/2, JNK and p38 activation. Am J Chin Med. 2015;43:653–665. doi: 10.1142/S0192415X15500408. [DOI] [PubMed] [Google Scholar]
- 31.Yuan H, Zhang J, Liu H, Li Z. The protective effects of resveratrol on Schwann cells with toxicity induced by ethanol in vitro. Neurochem Int. 2013;63:146–153. doi: 10.1016/j.neuint.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 32.D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 33.Rigoulet M, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H2O2 signaling. Antioxid Redox Signal. 2011;14:459–468. doi: 10.1089/ars.2010.3363. [DOI] [PubMed] [Google Scholar]
- 34.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 35.Valero T. Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des. 2014;20:5507–5509. doi: 10.2174/138161282035140911142118. [DOI] [PubMed] [Google Scholar]
- 36.Wu CC, Bratton SB. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal. 2013;19:546–558. doi: 10.1089/ars.2012.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadenbach B, Arnold S, Lee I, Hüttemann M. The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim Biophys Acta. 2004;1655:400–408. doi: 10.1016/j.bbabio.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Kiraz Y, Adan A, Kartal Yandim M, Baran Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016;37:8471–8486. doi: 10.1007/s13277-016-5035-9. [DOI] [PubMed] [Google Scholar]
- 39.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria-specificity in membrane targeting for death. Biochim Biophys Acta. 2011;1813:532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]