Abstract
In this review, a comprehensive overview of advances in the supramolecular complexes of carbohydrates and poorly soluble drugs is presented. Through the complexation process, poorly soluble drugs could be efficiently delivered to their desired destinations. Carbohydrates, the most abundant biomolecules, have diverse physicochemical properties owing to their inherent three-dimensional structures, hydrogen bonding, and molecular recognition abilities. In this regard, oligosaccharides and their derivatives have been utilized for the bioavailability enhancement of hydrophobic drugs via increasing the solubility or stability. By extension, polysaccharides and their derivatives can form self-assembled architectures with poorly soluble drugs and have shown increased bioavailability in terms of the sustained or controlled drug release. These supramolecular systems using carbohydrate will be developed consistently in the field of pharmaceutical and medical application.
Keywords: poorly soluble drugs, carbohydrates, supramolecular complexation, bioavailability enhancement
1. Introduction
Poorly soluble drugs belong to Biopharmaceutics Classification System (BCS) class II (low solubility/high permeability) and IV (low solubility/low permeability) materials [1]. The poor aqueous solubility of drugs has been a main obstacle in drug discovery and development, since it results in poor bioavailability at the active site [2]. With increasing molecular weight and log p values, the solubility of drugs decreases. Actually, 40% of drug candidates have been listed as practically insoluble (<100 μg/mL), whereas only 8% of new drug candidates have shown both high solubility and permeability [1,3]. To enhance the bioavailability of insoluble drugs, various techniques, including use of co-solvents, micronization, salt formation, and supramolecular complexation have been reported [4,5]. In the case of most non-intravenous administrations, the bioavailability is much less than 100%, because all drugs may not be adsorbed and metabolized before the delivery at the target site. This review aims to shed light on the supramolecular complexation of drugs with carbohydrates for bioavailability enhancement with flexibility and simplicity in the design and resulting in a considerable increase in the solubility and dissolution of drugs.
Supramolecular chemistry is defined as “chemistry beyond the molecule” and its importance has been established by the award of the 1987 Nobel Prize in chemistry [6]. For biochemical interactions, the classical lock-and-key model has been changed and extended. Biomolecular architectures such as proteins, DNA, and bio-membranes are rather formed by supramolecular interactions, and they regulate the biological processes [7,8]. A lot of bioinspired materials have also been developed using supramolecular chemistry [9]. Self-assembly, molecular recognition, metal coordination, folding, and host-guest chemistry are the sophisticated concepts of supramolecular chemistry [10,11]. They are mainly composed of various noncovalent interactions including hydrogen bonding, dipole-dipole interactions, van der Waals forces, pi-pi interactions, and electrostatic interactions between molecules [12].
Hydrogen bonding plays crucial roles in biological molecular recognition together with other noncovalent interactions [13]. Carbohydrates (CnH2nOn), the key molecules in Nature, are polyhydroxyaldoses or ketoses, which are the possible hydrogen bonding donors and/or acceptors. They are also coated onto all the cells and involved in various types of supramolecular interactions. Through cooperative hydrogen bonding, carbohydrate recognition mediates cell-cell interactions, pathogenesis, and immune responses [14,15]. To the supramolecular chemist, the recognition ability of carbohydrates can be useful for the biomedical application such as drug delivery and development. Therefore, carbohydrate-based drug development has grown rapidly as a promising and exciting research field. In particular, the supramolecular association of carbohydrates with drugs could enhance the bioavailability of poorly soluble drugs. The strategies are varied, depending on the carbohydrate types such as monosaccharides, oligosaccharides, and polysaccharides.
2. Monosaccharides
2.1. Prodrug System
Using monosaccharides, drug-monosaccharide conjugates can be used as a type of prodrug. An anticancer drug, dodetaxel, was conjugated with the glucose moiety, resulting in 52-fold increase in its solubility compared to the original drug [16]. Glucuronide prodrugs of doxorubicin and glycosyllonidamine have been reported [17,18,19]. Several antitumor agents such as bleomycin, anthracycline, and mithramycin also have a glycosidic moiety in their intrinsic structures. Their water solubility can be increased by the modulation of their hydrophilicity, and the glycosylated drugs can take advantage of the recognition by glucose transporters [20]. As another example, when a neurotransmitter for antiparkinsonian agents is glycosylated, the glycoconjugate could be delivered into the central nervous system across the blood brain barrier [21,22]. Some peptide drugs have been glycosylated, and their physicochemical and pharmacological properties also changed [23,24]. They undergo enzymatic hydrolysis in plasma, and the free drugs can be released and become active for in vivo investigation.
2.2. Glycosylated Carrier
For drug delivery, various carriers such as liposomes, micelles, dendrimers, micro/nanoparticles, and micro/nanocapsules are available [25,26,27]. The use of monosaccharides offers the characteristic effects for the advanced carrier system of poorly soluble drugs [28]. Since carbohydrate binding proteins are present on different cell surfaces, glycosylated carriers can be designed for targeted delivery [29]. Furthermore, galactose-, fucose-, and mannose-conjugated carriers show bioadhesive properties, stability/solubility enhancement, and reduced immunogenicity/toxicity [30,31,32]. In terms of solubility of poorly soluble drugs, the limited effect of monosaccharides can be overcome by the use of oligosaccharides.
3. Oligosaccharides
3.1. Binary Systems
3.1.1. Cyclic Oligosaccharides and the Derivatives
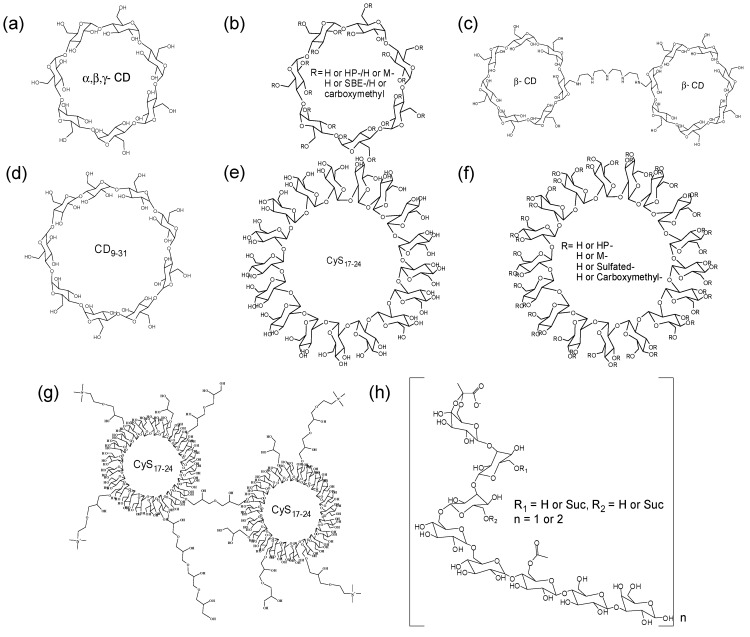
Cyclodextrin (CD): In 1896, the use of CD glucosyltransferase (CGTase) allowed a practical production of CD from starch. CDs are cyclic α-d-1,4 glucans, most commonly composed of 6, 7 and 8 glucosidic units called α-, β-, and γ-CD, respectively (Figure 1a). The macrocycles can maintain a conformationally constrained structure compared to linear structures. The respective cavity diameters of α-, β-, and γ-CDs are in the ranges 4.7–5.3, 6.0–6.5, and 7.5–8.3 Å, and the height of the torus is 7.9 Å in all types [33,34]. In 1911, the first noncovalent host-guest inclusion complexation of CD and other molecules was reported, and the first drug/CD patent was disclosed in 1953 [35,36]. With the characteristic cone or torus shape, various drugs containing ibuprofen, itraconazole, diclofenac, or atenolol have been incorporated into the cavity of β-CD even now, with a significant enhancement in the stability or solubility [37,38,39,40]. This is reasonable because CD can prevent crystallization, increase the dissolution of hydrophobic drugs, and protect the degradation of labile drugs by shielding guests with the hydrophobic cavity and the external layer of hydrophilic carbohydrates. They may also decrease the drug toxicity by making the drug more effective at lower doses. Furthermore, the native CD has Generally Regarded as Safe (GRAS) status in the United States and is found in many pharmaceutical and biomedical products.
Figure 1.
Oligosaccharide hosts in binary system. (a) CD; (b) CD derivatives; (c) CD dimer; (d) Large CD; (e) CyS; (f) CyS derivatives; (g) Cys dimer; and (h) Linear oligosaccharides.
CD derivatives: β-CD is generally used owing to its easy availability and appropriate size, however its solubility in water is relatively low (1.8%) because of the intramolecular hydrogen bonding and strong order on the surrounding water [41]. To improve the complexation ability and aqueous solubility, CD derivatives have been synthesized (Figure 1b), and the aqueous solubilities of hydroxypropyl (HP-), methyl (M-), and sulfobutylether (SBE-) β-CDs are >60%, 50%, and 50%, respectively [34]. Using HP-β-CD, nonpolar drugs such as itraconazole, indomethacin, hydrocortisone, cisapride, and mitomycin were marketed in the complex form in Europe and the United States [42,43]. HP-β-CD is also much more toxicologically benign than the natural β-CD [44]. In the case of M-β-CD or SBE-β-CD derivatives, the antibacterial chloramphenicol or the antifungal voriconazole have been introduced as complex solution formulations. However, because M-β-CD has hemolytic activity based on the complexation with cholesterol from membranes, its oral administration is limited [45]. Using ionic interactions, carboxymethyl- and (2-hydroxy-3-(trimethylammonio)propyl) β-CDs have been investigated for their solubilizing effect with oppositely charged drugs [46]. Finally, dimeric or oligomeric β-CD have attracted much attention as versatile receptors for molecular recognition [47]. Two or more hydrophobic complexation sites can enhance the stability, selectivity, and flexibility towards various nonpolar drugs. For example, the complexes of titanocene or paclitaxel with CD dimer are fairly soluble in water (Figure 1c), and the bioavailability is dramatically improved compared to the original drug [48,49].
Large CDs: The large CD comprising more than eight glucose units was isolated from the reaction mixture of CGTase and starch in 1957 by French and co-workers (Figure 1d) [50]. The CDs containing nine to 13 glucoses are sequentially called as δ-, ε-, ζ-, η-, and θ-CD. δ-CD (CD9) is found to be not a doughnut-shaped but rather elliptic boat-shaped, and has shown a solubilization effect for large guests such as digitoxin and spironolactone [51,52]. CD10, CD14 and CD26 also display distorted structures containing double band-flip motifs owing to their inherent flexibility [53,54]. Although the complex itself is less stable than the rigid α-, β-, and γ-CDs, the flexible complex may be better for the effective release of drugs [55]. Furthermore, a variety of cavity sizes can be useful for special guests not geometrically suitable for α-, β-, and γ-CDs.
Cyclosophoraoses (CyS) and the derivatives: CyS isolated from Rhizobium species are cyclic β-1,2 glucans containing 17 and 40 glucose residues (Figure 1e). In 1984, CyS17 were found to exhibit the complexation ability with hydrophobic guests including indomethacin, propericiazine, reserpine, and steroids [56]. The cavity was considered to be able to accommodate three-dimensionally extended guest molecules, and the diameter and depth of the cavities were estimated as 9 and 15 Å according to the CPK models. Besides, CyS17-24 efficiently complexed with paclitaxel, indomethacin, or luteolin [57,58,59]. The molecules are shaped such as a distorted and flexible ring, and the possibility of a different molecular mechanism for the complexation from the typical inclusion complexation by CD was suggested [60,61]. They could also be substituted by various functional groups including carboxymethyl, hydroxypropyl, sulfated, or methyl groups (Figure 1f) [62,63,64,65]. CyS oligomers were also designed (Figure 1g), and the cooperative complexation enhanced solubility and bioavailability of fisetin [66]. Because the functionalized CyS17-24 provide the additional space and property for guests, they are expected to behave as promising solubilizers for poorly soluble drugs.
3.1.2. Linear Oligosaccharides
Further, host-guest complexation was not found to be the exclusive property of cyclic structures. In 1989, it was demonstrated that cell-cell recognition is initiated by direct oligosaccharide-oligosaccharide interactions, indicating hydrophobic effects are responsible for the interaction [67]. In carbohydrate-protein interactions, stacking and non-polar interactions between sugar and tryptophan or phenylalanine residues of proteins were also observed, together with hydrogen bonds or metal coordination [68]. From this point of view, the hydrophobic character of linear carbohydrates was investigated with fluorescence probes, and the host-guest complexation might involve an induced-fit type adjustment [69]. Non-cyclic hexadecasaccharide has been reported as an effective complexing agent for pyrimethamine, haloperidol, or isoflavonoids (Figure 1h) [70,71,72]. In fact, the solubility of the antimalarial drug pyrimethamine and antipsychotic medication haloperidol was increased 42- and 87-fold in water by complexing with succinoglycan dimer D3 [70,71]. Even in octasaccharides, a solubility enhancement of pindolol was described, and their cytotoxic effect was low enough to warrant further study [73]. Recently, an acyclic cucurbit[n]uril molecular container has also been reported to overcome potential limitations such as the structural rigidity and slow dissociation kinetics of macrocyclic cucurbit[n]urils [74]. Acyclic oligosaccharides would be flexible hosts to recognize a broad range of poorly soluble drugs and provide high dissociation kinetics.
3.1.3. Preparation of Inclusion Complexes
To overcome the practical problems, including long processing time, the use of excessive solvent, and multistep synthesis, practical preparation methods have been developed as follows. The preparation can be optimized depending on the substrates.
Kneading [75]: In this method, carbohydrate hosts were placed in a mortar and wetted with little amount of water or hydro-alcoholic solution. Subsequently, the drug was added to the host paste, and the mixture was kneaded for a specified time. Finally, the kneaded sample was dried.
Freeze-drying [76,77]: Drugs and carbohydrate hosts were dissolved in water to achieve equilibrium, and the solutions were frozen by immersion in liquid nitrogen, and the frozen solutions were lyophilized.
Spray-drying [78]: Aqueous solutions of carbohydrate hosts and alcoholic solution of drugs are mixed to produce a clear solution. The solution is then spray-dried using a spray dryer.
3.1.4. Analysis of Inclusion Complex
UV-Vis spectroscopy—Measurement of drug solubility enhancement by complexation.
Nuclear Magnetic Resonance (NMR) spectroscopy—1H-NMR is a suitable method for the evaluation of noncovalent interactions at the molecular level [79]. To elucidate the intermolecular interaction of inclusion complexes, two-dimensional NMR spectroscopy (Nuclear Overhauser enhancement spectroscopy (NOESY) or Rotating frame nuclear Overhauser effect spectroscopy (ROESY)) has also been frequently used, because two protons located within 5 Å induce an NOE crosspeak [80].
Thermogravimetric Analysis (TGA)/Differential Scanning Calorimetry (DSC)—TGA curves describe the weight losses of pure components and the complexes. DSC is performed to characterize the solid-state interactions for the inclusion complexes as compared to the melting points [81].
Fourier Transform Infrared (FTIR) spectroscopy—The analysis of the vibrational changes upon the inclusion of drugs with a host.
X-ray Powder Diffractometry (XRPD)—The powder diffraction patterns of drugs and complex are compared. In principle, drugs displayed sharp peaks, which are the characteristics of an organic molecule with crystallinity, and the complex shows different patterns with crystalline drugs.
Scanning Electron Microscopy (SEM)—The surface morphology of the pure and complexed form is investigated. The morphological changes are frequently analyzed to evaluate the interaction between drugs and host [82,83].
Electrospray mass spectrometry (ESI-MS)—Determination of molecular association of noncovalent bonding.
Computational method (Molecular modeling)—The appropriate binding mode of complex between host and drug can be derived from molecular docking simulations [84].
3.1.5. Phase Solubility Studies
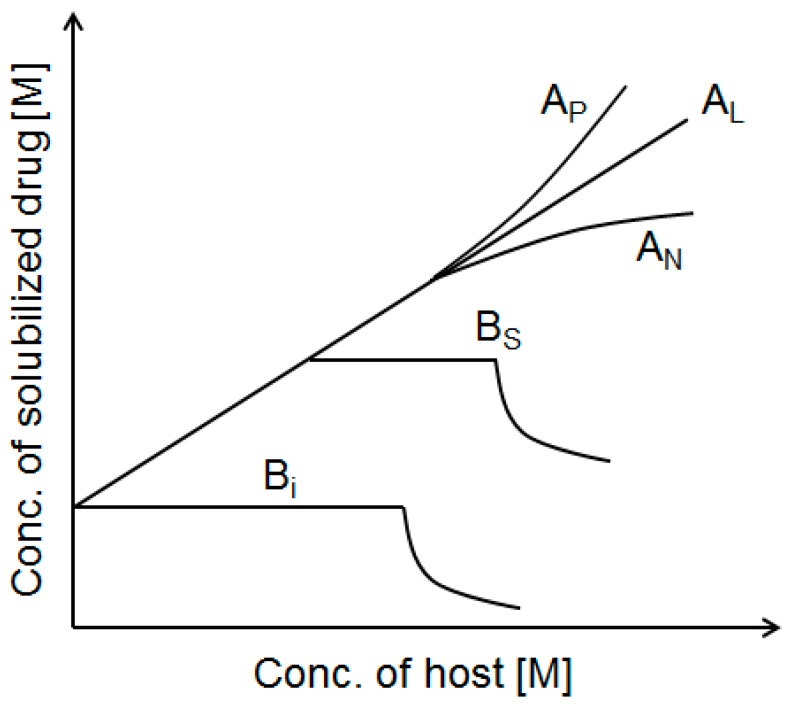
The complexation between drugs and carbohydrates is caused by forming dynamic non-covalent bonds, which increases the aqueous solubility of drugs. In phase-solubility diagram (Figure 2), the solubility enhancement of drugs is assessed as a function of host concentration [85]. Based on the shape of the plot, the diagram is classified as AP, AL, AN, BS, or Bi [42].
Figure 2.
Phase solubility diagram.
A type profile: In this profile, the apparent solubility of drugs increases as a function of host concentration. The AL graph indicates a linear increase in solubility, whereas AP and AN type graphs indicate a positive and negative deviation from linearity, respectively. Although AN systems are not easy to interpret, AP type suggests the formation of a higher-order complex with respect to the host. In AL type, a plot of drug concentration (Dt) vs. host concentration (Ht) for the formation of Dm-H complex gives a linear line with the intercept and the slope is defined as:
| (1) |
where, if m is known, the K value can be calculated from the slope and intercept (S0, the intrinsic solubility of the drug in water).
If the complex has a 1:1 drug:host ratio, the complexation is an equilibrium governed by an equilibrium constant (K1:1) calculated based on the linear phase-solubility profiles (AL type) using the following equation:
| K1:1 (M−1) = slope/S0(1 − slope) | (2) |
In this case, the slope is always less than unity [86]:
In the case of 2:1 drug:host complexation, the slope of the AL type diagram is less than two, and the association constant (K2:1) is determined by the following equation [87]:
| (3) |
B type profile: When the system forms complexes with limited solubility, the diagram is B type, which is usually observed with β-CD. BS type is derived from the initial soluble complex and insoluble complex at the maximum point. Bi style indicates that the complex is too insoluble to increase the isotherm.
3.1.6. Drug Delivery and CD Elimination from the Drug/CD Complexes
Complexation certainly involves the issue of the mechanism(s) of drug release. The answer is thought to be dissociation owing to dilution, protein binding, tissue uptake of drugs, competitive displacement of drugs from the complex, and host elimination [88,89]. These drug/host complexes may alter drug metabolism route, biodistribution, and tubular reabsorption, and thereby the bioavailability of drugs can be changed. In particular, myricetin [90], isotretinoin [91], benznidazole [92], and etodolac exhibit 9.4-, 4.7-, 3.6-, and 2.5-fold increases in bioavailability upon addition of drug/CD derivative complexes for their oral administration [93]. As well as, the CDs are ultimately digested by bacteria in the gastrointestinal tract or colon to monosaccharides or gases including carbon dioxide, hydrogen, and methane [94].
3.2. Ternary Systems
Ternary systems have been designed to improve the shortcomings of the above binary systems. In general, the main property of carbohydrate hosts is the modification of the physicochemical and biological characteristics of poorly soluble drugs by complex formation. Using another factor, ternary complexes could be more efficient for the solubility and bioavailability of drugs with the use of a small amount of carbohydrates. As modifiers, organic acids such as ascorbic acids, citric acids, and tartaric acids are used together with β-CD, and they can modulate the pH under aqueous conditions [95,96]. Accordingly, the solubility, stability, and phase solubility types are simultaneously affected. Besides, arginine, co-solvent, and a suitable water-soluble polymer also provided a synergistic effect for improving drug solubility in the presence of CD [97,98,99]. By adding the third auxiliary substance, lecithin, the solubility, dissolution, and stability of drugs were better than that those of the original drugs and binary systems [100]. Based on the amphiphilic properties of lecithin, the hydrophobic interactions with drugs, hydrogen bonding with HP-β-CD, and the cooperative advantage is greater than the simple statistical contributions of individual components.
3.3. Multinary Systems
3.3.1. CD Amphiphiles
CD can even be conjugated to lipid molecules, and the amphiphile forms a micellar or vesicular system via self-assembly [101,102,103,104]. The resulting structure provides a higher drug loading, stable colloidal system, and controlled drug release. The first amphiphilic CDs were synthesized by Kawabata et al., in 1986, and the alkylsulfinyl β-CD showed interesting molecular assembly properties [105]. Recently, anthraquinoyl-, hexadecyl-, sulfated hexanoyl-modified β-CDs were developed for the delivery of anticancer (paclitaxel, docetaxel), and antiviral (acyclovir) drugs [106,107,108]. Supramolecular amphiphiles are of significant interest as hybrid materials with inclusion capacity and nanoparticulate properties [101].
3.3.2. CD Pendent Polymers
At the same time, CD-containing polymers have been explored for novel drug delivery platforms and are more suitable in medication for parenteral adminstration than the natural β-CD [109,110]. The systems consist of the polymeric, assembled network, and host-guest complexation, allowing the effective loading and controlled release of drugs. Depending on the crosslinkers, soluble or insoluble β-CD polymers are obtained. Hyper-crosslinked CDs were first called nanosponges in 1998 [111], and insoluble polymers have been used as excipients in preparing tablets, suspensions, and capsules for drug carriers with soluble CD polymers [112,113]. For doxorubicin delivery to treat some leukemias and cancers, quaternary ammonium crosslinked β-CD and β-CD-centered amphiphilic polymers were achieved [114,115]. The three-dimensional architecture of CD pendent polymers usually forms hydrogels or nanoparticles, which are potentially superior in the biomedical and pharmaceutical fields.
4. Polysaccharides
Polysaccharides are polymeric carbohydrate molecules composed of monosaccharide units bound together by glycosidic linkages. They vary depending on their sources and structural units, and are easily available, inexpensive, eco-friendly, and biocompatible materials. Based on their primary sequence, they can adopt certain shapes (secondary structures like ribbons and helices) and the ordered specific structure (tertiary structures of multiple ribbons and helices) formed via energetically favorable interactions [116]. Sometimes, quaternary structures have shown higher levels of organization. Using these supramolecular polysaccharides, multi-dimensional carrying systems were prepared to control the drug release in a desirable manner.
4.1. Polysaccharide Drug Conjugates
In 1975, the concept of polymer-drug conjugates was introduced for the first time for the delivery of hydrophobic drugs to their sites of action [117]. The conjugates alter the biodistribution and circulation time of the original drugs. So far, hyaluronic acid [118,119], dextran [120], chitosan [121], heparin [122], alginate [123], pullulan [124], arabinogalactan [125,126], and starch have all been developed as drug conjugation platforms [127]. Hyaluronic acid is an anionic polysaccharide containing alternating disaccharide units of d-glucuronic acid and N-acetyl-d-glucosamine linked by β-1,4 glycosidic linkages. In the structure, the hydroxyl and carboxylic acid groups provide the appropriate sites for the conjugate. Because it has a strong affinity for the cancer cell marker, CD44, the selective adsorption is an additional advantage for the anticancer bioavailability [128]. Dextran is a glucan with mostly α-1,6 glycosidic linkages and has been approved as a plasma expander [129]. Chitosan is a biopolymer of β-(1-4)-linked d-glucosamine and N-acetyl-d-glucosamine, and the reactive amine group is important for chemical modification. The muco-adhesive property and the insolubility at pH 7.4 are considered for the development into advanced materials. Heparin is a highly sulfated glycosaminoglycan and has been used as the anticoagulant. Alginate is a linear copolymer of β-1,4-mannuronate and α-guluronate, and its gel-forming ability via calcium binding is a characteristic property. Pullulan is a neutral polysaccharide composed of maltotrioses linked by α-1,4 and α-1,6 bond types, and starch consists of helical amylose and branched amylopectin. Arabionogalactan is a heteropolymer consisting of arabinose and galactose. Among these, hyaluronic acid, carboxymethyl dextran, and oxidized dextran conjugates have entered clinical trials [118,130,131,132], showing improved adverse reaction profiles, enhanced therapeutic efficacy, and target-oriented properties.
4.2. Supramolecular Architectures for Polysaccharide Drug Carriers
Polysaccharides provide supramolecular architectures derived from cooperative intra- and interchain association by hydrogen bonding, dipole and ionic interactions, and solvation (Figure 3). Supramolecular structures have distinct structural characteristics and functions that are not shown in monosaccharide units. Furthermore, their reactive groups (hydroxyl, amino, and carboxylic acid) can be easily modified, and various polysaccharide derivatives have been synthesized as novel supramolecular architectures. The driving forces for the three-dimensional structures are classified as noncovalent and covalent methods (Table 1). Supramolecules can provide the high encapsulation efficiency and cell internalization, decrease side effects, and protect the liable drugs via supramolecular complexation. As a result, they are designed for controlled drug release to maintain a constant drug concentration for the desired time with minimum side effects.
Figure 3.
Polysaccharide-based advanced materials and supramolecular architectures for biomedical applications. The supramolecular architecture is formed by noncovalent methods such as hydrogen bonding (a); metal coordination (b); ionic interaction (c); hydrophobic interaction (d); and covalent methods using cross-linkers (e).
Table 1.
Polysaccharide-based carriers for the bioavailability enhancement of poorly soluble drugs.
| Supramolecular Forces | Used Polysaccharides | Architecture Types | Drugs | References | |
|---|---|---|---|---|---|
| Non-covalent bond | Hydrogen bond | Hydrolyzed xyloglucan | Hydrogel | Ondansetron Indomethacin Mytomycin C | [133,134,135,136] |
| Hydroxypropyl Methylcellulose | Hydrogel | Indomethacin | [137] | ||
| Metal coordination | Alginate-calcium ion | Nanoparticle | Rifampicin, Doxorubicin | [138,139] | |
| N-succinyl chitosan-alginate | Hydrogel | Nifedipine | [140] | ||
| Alginate-calcium carbonate | Hydrogel | Ibuprofen | [141] | ||
| Ionic interaction | Chitosan-tripolyphosphate | Nanoparticle | Ciprofloxacin | [142] | |
| Chitosan-tripolyphosphate-hydroxypropylcyclodextrin | Nanoparticle | Furosemide, Triclosan | [143] | ||
| Chitosan-tripolyphosphate-dextran sulfate | Microsphere | Ibuprofen | [144] | ||
| Chitosan-dextran sulfate | Nanoparticle | Amphotericin B | [145] | ||
| Chitosan-glycyrrhetic acid | Nanoparticle | Glycyrrhetic acid | [146] | ||
| Chitosan-β-glycerophosphate | Hydrogel | Paclitaxel | [147] | ||
| Carrageenan Dextran sulfate | Nanosphere | Ciprofloxacin | [148] | ||
| Hydrophobic interaction | Ceramide modified hyaluronic acid | Nanoparticle | Docetaxel, Doxorubicin | [149,150] | |
| Deoxycholic acid modified hyaluronic acid | Nanoparticle | Paclitaxel | [151] | ||
| Histidine modified hyaluronic acid | Nanoparticle | Doxorubicin | [152] | ||
| Pullulan acetate | Nanoparticle | Silymarin | [153] | ||
| Cholesterol modified chitosan | Nanoparticle | Epirubicin | [154] | ||
| Deoxycholic acid-modified chitosan | Nanoparticle | Adriamycin, Doxorubicin | [155,156] | ||
| 5β-cholanic acid modified chitosan | Nanoparticle | Paclitaxel, Camptothecin | [157,158] | ||
| Stearic acid-g-chitosan | Nanosphere | Doxorubicin | [159,160] | ||
| N-acetyl histidine-conjugated glycol chitosan | Nanoparticle | Paclitaxel | [161] | ||
| Cholic acid modified dextran | Nanosphere | Indomethacin | [162] | ||
| CD polymer-dextran polymer | Nanogel | Benzophenone, Tamoxifen | [163] | ||
| Acetylated chondroitin sulfate | Nanogel | Doxorubicin | [164] | ||
| Covalent bond | Cross-linker or Copolymer | Chitosan (glutaraldehyde, sulphuric acid) | Microsphere | Diclofenac, Docetaxol Clozapine | [165,166,167] |
| Polyacrylamide-g-chitosan copolymer | Microsphere | Nifedipine | [168] | ||
| Chitosan-Pluronic copolymer | Nanoparticle | Indometacin, Doxorubicin | [169,170] | ||
| Pullulan-g-poly(l-lactide) copolymers | Hydrogel | Doxorubicin | [171] | ||
| Poly(dl-lactide-co-glycolide)-grafted pullulan | Nanosphere | Adriamycin | [172] | ||
| Cellulose-graft-poly(l-lactide) copolymers | Nanosphere | Paclitaxel | [173] | ||
| Dextran-b-poly(DL-lactide-coglycolide) copolymer | Nanosphere | Doxorubicin, Amphotericin B | [174,175] | ||
| Poly[lactic-co-(glycolic acid)]-grafted hyaluronic acid copolymer | Nanoparticle | Doxorubicin | [176] | ||
| Dextran-b-poly(ε-caprolactone) | Nanoparticle | Doxorubicin | [177] | ||
| Chondroitin sulfate-Pluronic copolymer | Nanoparticle | Doxorubicin | [178] | ||
| Starch (epichlorohydrin) | Microsphere | Ampicillin | [179] | ||
| Hyaluronic acid (1,3-diaminopropane) | Hydrogel | Ibuprofen | [180] | ||
Among non-covalent bonds, hydrogen bonding is basically involved to make the supramolecular association of polysaccharides. With the same β-1,4-d-glucan backbone, xyloglucan and cellulose have ribbon-like shapes, and the modified structures can form hydrogel structures based on non-covalent interactions [133,134,135,136,137]. Carboxyl (–COOH) groups in alginate are able to interact with divalent metals, and the resulting structures have been used for isoniazid, doxorubicin, nifedipine, and ibuprofen delivery [138,139,140,141]. On the other hand, cationic chitosan has been reported to form ionic self-assemblies by Coulombic interaction with various anionic molecules. The ionic interaction produces self-assembled delivery systems with well-defined shapes and dimensions (nano/micro-particles, hydrogels and films, Table 1. The other factor can be hydrophobic interactions caused by various hydrophobically modified polysaccharides, where the hydrophobic groups range from acetyl groups to lipid molecules [149,153,154,159,164]. The hydrophobized carrier might effectively adsorb and deliver the target guest via supramolecular chemistry [181]. Cross-linkers such as glutaraldehyde [165], epichlorohydrin [179], and 1,3-diamino-propane [180] can produce irreversible supramolecular architectures, which are also prepared from co-polymer structures with polyacrylamide [168], polylactic acid [171], and poly-ε-caprolactone [177]. Throughout physical and chemical forces, various carbohydrate architectures are observed such as microspheres, nanoparticles, films and hydrogels, which properties and advantages for the representative drug delivery system are discussed in the following subsections.
4.2.1. Nano-Particles (Spheres)
Nanoparticle entrapping drugs can penetrate cells and tissue gaps, and be delivered to the target organ with their nanosized dimensions. Particularly, polysaccharide-based nanoparticles control release profiles based on the biodegradability and stimuli (pH or temperature)-responsive structural changes [182,183]. The major problems such as the cytotoxicity and degradation products of nanoparticles can also be solved based on their biocompatibility and biodegradability. The hydrophilic groups (hydroxyl, amino, and carboxyl groups) in polysaccharides provide bio-adhesive properties forming non-covalent bonds with biological tissues. Furthermore, some polysaccharides have recognition ability on specific cell types, allowing targeted delivery. These materials have the additional potential to combine diagnosis and therapy, and can be applied to nanomedicine. In this respect, polysaccharide (mostly dextran or chitosan)-coated magnetic nanoparticles have been attractive for drug delivery owing to their improved drug absorption, their prolonged blood residence time and target-specific delivery [184].
4.2.2. Microspheres
Polysaccharide-based microspheres can encapsulate many types of drugs, localize delivery of drug, and are easily administered through a syringe needle [185]. They have been commonly developed for treating many respiratory diseases, and the microsphere size is decisive for the release rate of drugs. The drug-polysaccharide supramolecular interactions and porosity may be other important factors. For pulmonary delivery, large particles may deposit in the respiratory tract before the target site, whereas small particles can aggregate before reaching the desired location. Depending on the polysaccharide species or molecular weight, the average particle sizes are varied by changing the solution viscosity [186]. The preparation methods for microsphere using polysaccharides are solvent extraction [187], spray-drying [188], emulsion-crosslinking [166,179,189] and precipitation [190]. The conventional preparation methods have also been modified for property improvement. In the spray drying method, good sphericity and a narrow size distributions could be obtained, however the burst effect and fast drug release need to be solved. Using the w/o/w emulsion-spray drying method, famotidine was released for several hours with a sustained type from chitosan microsphere, and wetting agents could be utilized to increase the release rate [191].
4.2.3. Membrane (Film)
Membranes are mainly designed to control the release rate in transdermal delivery of drugs, and the applied materials can be used as tablet coatings, patches and wound dressings. The bio-adhesive and film-forming properties of polysaccharides are helpful for layer-by-layer type supramolecular structures [192]. Multilayers for the controlled delivery of drugs have advantages in terms of loading multiple components into interlayers and releasing the drugs sequentially layer-by-layer [193]. Although these films are regarded as more appropriate for hydrophilic drugs than hydrophobic ones [194,195], chitosan films were fabricated and used for local delivery of paclitaxel, and the biodegradation by lysozyme was used to control the drug release [196].
4.2.4. Hydrogels
Hydrogels are water-swollen and hydrophilic membranes with pseudo-plastic properties that absorb a high amount of water to maintain their shape. Water can penetrate into the interstitial spaces of the three-dimensional polysaccharide network, providing hydrogel-like artificial tissues [197]. The highly porous structure allows loading drugs into the gel matrix and subsequent drug release [198]. Furthermore, the physical properties are elastically active and have low interfacial tension in biological fluids. The drug release from hydrogels can be controlled by swelling, diffusion, and degradation [199]. As well as, the charge distribution and chemical modification in polysaccharide structures contributes to it along with the surface state or porosity. In many cases, since polysaccharides are resistant to degradation by gastric and intestinal bacteria and susceptible to digestion by colonic microbial flora, they have been studied as colon-specific drug delivery systems [200].
5. Conclusions
Supramolecular complexation of carbohydrates and poorly soluble drugs efficiently enhances the bioavailability of drugs. In the structure of carbohydrates, –OH and –CH groups provide the sites for hydrogen bonding and hydrophobic interactions, respectively. Furthermore, various modified carbohydrates can expand the scope of available drugs as well as the architecture. With monosaccharides, the targeted delivery of drugs was mainly considered. Typical binary complexes using linear and cyclic oligosaccharides were developed for poorly soluble drugs as two-dimensional systems, and the aqueous drug solubility was clearly enhanced by the binary system. In the intermediate version, tertiary complexes with another factor could reinforce the effect. Further, multiple complexes and polysaccharide-based supramolecules were recently studied as three-dimensional drug delivery systems. So far, various combinatorial hybrid materials of carbohydrates have been successfully developed for advanced drug carrier systems. Therefore, novel materials based on carbohydrates will be promising for advanced applications in the biomedical and pharmaceutical fields. Further, these works will improve micro/nano fabrication technology, bio-mimetics, and tissue engineering based on the supramolecular carbohydrate architecture.
Acknowledgments
This paper was supported by the KU Research Professor Program of Konkuk University. This research was also supported by grants from the National Research Foundation of Korea, funded by the Korean Government (NRF-2011-619-E0002), the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (2015M3A9B8031831), and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A02037375).
Author Contributions
Author E. Cho prepared the first draft of the manuscript and S. Jung edited the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Takagi T., Ramachandran C., Bermejo M., Yamashita S., Yu L.X., Amidon G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006;3:631–643. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- 2.Lipinski C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods. 2000;44:235–249. doi: 10.1016/S1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto H. Novel Approaches for oral delivery of poorly soluble drugs; Oral Delivery of Poorly Soluble Actives—From Drug Discovery to Marketed Products, Proceeding of Symposia on the Oral Delivery of Poorly Soluble Actives; Tokyo, Japan. 6 June 2003; Morristown, NJ, USA: Capsugel® Library; [Google Scholar]
- 4.Kawabata Y., Wada K., Nakatani M., Yamada S., Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011;420:1–10. doi: 10.1016/j.ijpharm.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Jensen K.T., Blaabjerg L.I., Lenz E., Bohr A., Grohganz H., Kleinebudde P., Rades T., Löbmann K. Preparation and characterization of spray-dried co-amorphous drug-amino acid salts. J. Pharm. Pharmacol. 2015 doi: 10.1111/jphp.12458. [DOI] [PubMed] [Google Scholar]
- 6.Lehn J.-M. Supramolecular chemistry—Scope and perspectives: Molecules—Supermolecules—Molecular devices. J. Incl. Phenom. 1988;6:351–396. doi: 10.1007/BF00658981. [DOI] [Google Scholar]
- 7.Timsit Y., Moras D. DNA self-fitting: The double helix directs the geometry of its supramolecular assembly. EMBO J. 1994;13:2737–2746. doi: 10.1002/j.1460-2075.1994.tb06567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uhlenheuer D.A., Petkau K., Brunsveld L. Combining supramolecular chemistry with biology. Chem. Soc. Rev. 2010;39:2817–2826. doi: 10.1039/b820283b. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y., Sakai F., Su L., Liu Y., Wei K., Chen G., Jiang M. Progressive macromolecular self-assembly: From biomimetic chemistry to bio-inspired materials. Adv. Mater. 2013;25:5215–5256. doi: 10.1002/adma.201302215. [DOI] [PubMed] [Google Scholar]
- 10.Lehn J.M. Supramolecular Chemistry. Volume 1 Wiley-VCH; Weinheim, Germany: 1995. [Google Scholar]
- 11.Lehn J.M. Perspectives in supramolecular chemistry—From molecular recognition towards molecular information processing and self-organization. Angew. Chem. Int. Ed. Engl. 1990;29:1304–1319. doi: 10.1002/anie.199013041. [DOI] [Google Scholar]
- 12.Schneider H.J. Binding mechanisms in supramolecular complexes. Angew. Chem. Int. Ed. 2009;48:3924–3977. doi: 10.1002/anie.200802947. [DOI] [PubMed] [Google Scholar]
- 13.Ariga K., Kunitake T. Molecular recognition at air-water and related interfaces: Complementary hydrogen bonding and multisite interaction. Acc. Chem. Res. 1998;31:371–378. doi: 10.1021/ar970014i. [DOI] [Google Scholar]
- 14.Weis W.I., Drickamer K. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 1996;65:441–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- 15.Carçabal P., Jockusch R.A., Hünig I., Snoek L.C., Kroemer R.T., Davis B.G., Gamblin D.P., Compagnon I., Oomens J., Simons J.P. Hydrogen bonding and cooperativity in isolated and hydrated sugars: Mannose, galactose, glucose, and lactose. J. Am. Chem. Soc. 2005;127:11414–11425. doi: 10.1021/ja0518575. [DOI] [PubMed] [Google Scholar]
- 16.Shimoda K., Kubota N. Chemo-enzymatic synthesis of ester-linked docetaxel-monosaccharide conjugates as water-soluble prodrugs. Molecules. 2011;16:6769–6777. doi: 10.3390/molecules16086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas M., Rivault F., Tranoy-Opalinski I., Roche J., Gesson J.-P., Papot S. Synthesis and biological evaluation of the suberoylanilide hydroxamic acid (SAHA) β-glucuronide and β-galactoside for application in selective prodrug chemotherapy. Bioorg. Med. Chem. Lett. 2007;17:983–986. doi: 10.1016/j.bmcl.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 18.Giorgioni G., Ruggieri S., di Stefano A., Sozio P., Cinque B., di Marzio L., Santoni G., Claudi F. Glycosyl and polyalcoholic prodrugs of lonidamine. Bioorg. Med. Chem. Lett. 2008;18:2445–2450. doi: 10.1016/j.bmcl.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 19.Houba P., Boven E., van der Meulen-Muileman I., Leenders R., Scheeren J., Pinedo H., Haisma H. A novel doxorubicin-glucuronide prodrug DOX-GA3 for tumour-selective chemotherapy: Distribution and efficacy in experimental human ovarian cancer. Br. J. Cancer. 2001;84:550–557. doi: 10.1054/bjoc.2000.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown R.S., Wahl R.L. Overexpression of glut-1 glucose transporter in human breast cancer an immunohistochemical study. Cancer. 1993;72:2979–2985. doi: 10.1002/1097-0142(19931115)72:10<2979::AID-CNCR2820721020>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Fernández C., Nieto O., Fontenla J.A., Rivas E., de Ceballos M.L., Fernández-Mayoralas A. Synthesis of glycosyl derivatives as dopamine prodrugs: Interaction with glucose carrier GLUT-1. Org. Biomol. Chem. 2003;1:767–771. doi: 10.1039/b212066f. [DOI] [PubMed] [Google Scholar]
- 22.Fernández C., Nieto O., Rivas E., Montenegro G., Fontenla J.A., Fernández-Mayoralas A. Synthesis and biological studies of glycosyl dopamine derivatives as potential antiparkinsonian agents. Carbohydr. Res. 2000;327:353–365. doi: 10.1016/S0008-6215(00)00073-2. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez M.C., Cudic M. Optimization of physicochemical and pharmacological properties of Peptide drugs by glycosylation. In: Cudic R., editor. Peptide Modifications to Increase Metabolic Stability and Activity. Humana Press; New York City, NY, USA: 2013. pp. 107–136. [DOI] [PubMed] [Google Scholar]
- 24.Polt R., Porreca F., Szabo L.Z., Bilsky E.J., Davis P., Abbruscato T.J., Davis T.P., Harvath R., Yamamura H.I., Hruby V.J. Glycopeptide enkephalin analogues produce analgesia in mice: Evidence for penetration of the blood-brain barrier. Proc. Natl. Acad. Sci. USA. 1994;91:7114–7118. doi: 10.1073/pnas.91.15.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahr A., Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007;4:403–416. doi: 10.1517/17425247.4.4.403. [DOI] [PubMed] [Google Scholar]
- 26.Douroumis D., Fahr A. Drug Delivery Strategies for Poorly Water-Soluble Drugs. John Wiley & Sons; Weinheim, Germany: 2012. [Google Scholar]
- 27.Sahoo S.K., Jain T.K., Reddy M.K., Labhasetwar V. NanoBioTechnology. Humana Press; New York City, NY, USA: 2008. Nano-sized carriers for drug delivery; pp. 329–348. [Google Scholar]
- 28.Jain K., Kesharwani P., Gupta U., Jain N.K. A review of glycosylated carriers for drug delivery. Biomaterials. 2012;33:4166–4186. doi: 10.1016/j.biomaterials.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 29.Monsigny M., Roche A.-C., Midoux P., Mayer R. Glycoconjugates as carriers for specific delivery of therapeutic drugs and genes. Adv. Drug Deliv. Rev. 1994;14:1–24. doi: 10.1016/0169-409X(94)90003-5. [DOI] [Google Scholar]
- 30.Agrawal P., Gupta U., Jain N. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials. 2007;28:3349–3359. doi: 10.1016/j.biomaterials.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Jain S., Vyas S. Mannosylated niosomes as adjuvant-carrier system for oral mucosal immunization. J. Liposome Res. 2006;16:331–345. doi: 10.1080/08982100600992302. [DOI] [PubMed] [Google Scholar]
- 32.Stewart A., Pichon C., Meunier L., Midoux P., Monsigny M., Roche A. Enhanced biological activity of antisense oligonucleotides complexed with glycosylated poly-l-lysine. Mol. Pharmacol. 1996;50:1487–1494. [PubMed] [Google Scholar]
- 33.Cramer F. Einschlussverbindungen. Springer; Berlin, Germany: 1954. [Google Scholar]
- 34.Loftsson T., Duchene D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007;329:1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 35.Schardinger F. Bildung kristallisierter polysaccharide (dextrine) aus stärkekleister durch microben. Zentr. Bakt. Parasit. Abt. II. 1911;29:188–197. [Google Scholar]
- 36.Freudenberg K., Cramer F., Plieninger H. Verfahren zur Herstellung von Einschlussverbindungen Physiologisch Wirksamer Organischer Verbindungen. DE895769C. German Patent. 1953 Nov 5;
- 37.Salústio P., Cabral-Marques H., Costa P., Pinto J. Comparison of ibuprofen release from minitablets and capsules containing ibuprofen: β-Cyclodextrin complex. Eur. J. Pharm. Biopharm. 2011;78:58–66. doi: 10.1016/j.ejpb.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Cartledge J., Midgley J., Youle M., Gazzard B. Itraconazole cyclodextrin solution—Effective treatment for HIV-related candidosis unresponsive to other azole therapy. J. Antimicrob. Chemother. 1994;33:1071–1073. doi: 10.1093/jac/33.5.1071. [DOI] [PubMed] [Google Scholar]
- 39.Ficarra R., Ficarra P., di Bella M., Raneri D., Tommasini S., Calabro M., Villari A., Coppolino S. Study of the inclusion complex of atenolol with β-cyclodextrins. J. Pharm. Biomed. Anal. 2000;23:231–236. doi: 10.1016/S0731-7085(00)00274-0. [DOI] [PubMed] [Google Scholar]
- 40.Manca M.L., Zaru M., Ennas G., Valenti D., Sinico C., Loy G., Fadda A.M. Diclofenac-β-cyclodextrin binary systems: Physicochemical characterization and in vitro dissolution and diffusion studies. AAPS PharmSciTech. 2005;6:E464–E472. doi: 10.1208/pt060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naidoo K.J., Chen J.Y.-J., Jansson J.L., Widmalm G., Maliniak A. Molecular properties related to the anomalous solubility of β-cyclodextrin. J. Phys. Chem. B. 2004;108:4236–4238. doi: 10.1021/jp037704q. [DOI] [Google Scholar]
- 42.Brewster M.E., Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Davis M.E., Brewster M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 44.Gould S., Scott R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005;43:1451–1459. doi: 10.1016/j.fct.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Irie T., Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997;86:147–162. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- 46.Cserháti T. Interaction of some anticancer drugs with carboxymethyl-β-cyclodextrin. Int. J. Pharm. 1995;124:205–211. doi: 10.1016/0378-5173(95)00080-3. [DOI] [Google Scholar]
- 47.Liu Y., Chen Y. Cooperative binding and multiple recognition by bridged bis (β-cyclodextrin)s with functional linkers. Acc. Chem. Res. 2006;39:681–691. doi: 10.1021/ar0502275. [DOI] [PubMed] [Google Scholar]
- 48.Lu Z., Lu C., Ren X., Meng Q. New metallocene-bridged cyclodextrin dimer: A stable derivative of the antitumor drug titanocene dichloride and its potent cytotoxity against human breast cancer (MCF-7) cells. J. Organomet. Chem. 2006;691:5895–5899. doi: 10.1016/j.jorganchem.2006.09.052. [DOI] [Google Scholar]
- 49.Liu Y., Chen G.-S., Li L., Zhang H.-Y., Cao D.-X., Yuan Y.-J. Inclusion complexation and solubilization of paclitaxel by bridged bis (β-cyclodextrin)s containing a tetraethylenepentaamino spacer. J. Med. Chem. 2003;46:4634–4637. doi: 10.1021/jm034148f. [DOI] [PubMed] [Google Scholar]
- 50.French D. The schardinger dextrins. Adv. Carbohydr. Chem. 1957;12:189–260. doi: 10.1016/s0096-5332(08)60209-x. [DOI] [PubMed] [Google Scholar]
- 51.Fujiwara T., Tanaka N., Kobayashi S. Structure of. DELTA.-cyclodextrin 13.75 H2O. Chem. Lett. 1990:739–742. doi: 10.1246/cl.1990.739. [DOI] [Google Scholar]
- 52.Miyazawa I., Ueda H., Nagase H., Endo T., Kobayashi S., Nagai T. Physicochemical properties and inclusion complex formation of δ-cyclodextrin. Eur. J. Pharm. Sci. 1995;3:153–162. doi: 10.1016/0928-0987(95)00006-Y. [DOI] [Google Scholar]
- 53.Jacob J., Geβler K., Hoffmann D., Sanbe H., Koizumi K., Smith S.M., Takaha T., Saenger W. Band-flip and kink as novel structural motifs in α-(1→4)-d-glucose oligosaccharides. Crystal structures of cyclodeca-and cyclotetradecaamylose. Carbohydr. Res. 1999;322:228–246. doi: 10.1016/S0008-6215(99)00216-5. [DOI] [Google Scholar]
- 54.Nimz O., Geßler K., Usón I., Saenger W. An orthorhombic crystal form of cyclohexaicosaose, CA26···32.59 H2O: Comparison with the triclinic form. Carbohydr. Res. 2001;336:141–153. doi: 10.1016/S0008-6215(01)00249-X. [DOI] [PubMed] [Google Scholar]
- 55.Larsen K.L. Large cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002;43:1–13. doi: 10.1023/A:1020494503684. [DOI] [Google Scholar]
- 56.Koizumi K., Okada Y., Horiyama S., Utamura T., Higashiura T., Ikeda M. Clathrate Compounds, Molecular Inclusion Phenomena, and Cyclodextrins. Springer; New York City, NY, USA: 1984. Preparation of cyclosophoraose-A and its complex-forming ability; pp. 891–899. [Google Scholar]
- 57.Lee S., Kwon C., Choi Y., Seo D.-H., Kim H.-W., Jung S. Inclusion complexation of a family of cyclosophoraoses with indomethacin. J. Microbiol. Biotechnol. 2001;11:463–468. [Google Scholar]
- 58.Lee S., Seo D.-H., Kim H.-W., Jung S. Investigation of inclusion complexation of paclitaxel by cyclohenicosakis-(1→2)-(β-d-glucopyranosyl), by cyclic-(1→2)-β-d-glucans (cyclosophoraoses), and by cyclomaltoheptaoses (β-cyclodextrins) Carbohydr. Res. 2001;334:119–126. doi: 10.1016/S0008-6215(01)00178-1. [DOI] [PubMed] [Google Scholar]
- 59.Lee S., Seo D.-H., Park H.-L., Choi Y., Jung S. Solubility enhancement of a hydrophobic flavonoid, luteolin by the complexation with cyclosophoraoses isolated from Rhizobium meliloti. Antonie Leeuwenhoek. 2003;84:201–207. doi: 10.1023/A:1026075215921. [DOI] [PubMed] [Google Scholar]
- 60.Kim H., Jeong K., Lee S., Jung S. Molecular dynamics simulation of cyclosophoroheptadecaose (Cys-A) J. Comput. Aided Mol. Des. 2002;16:601–610. doi: 10.1023/A:1021923815450. [DOI] [PubMed] [Google Scholar]
- 61.Mimura M., Kitamura S., Gotoh S., Takeo K., Urakawa H., Kajiwara K. Conformation of cyclic and linear (1→2)-β-d-glucans in aqueous solution. Carbohydr. Res. 1996;289:25–37. doi: 10.1016/0008-6215(96)00134-6. [DOI] [PubMed] [Google Scholar]
- 62.Lee S., Park H., Seo D., Choi Y., Jung S. Synthesis and characterization of carboxymethylated cyclosophoraose, and its inclusion complexation behavior. Carbohydr. Res. 2004;339:519–527. doi: 10.1016/j.carres.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Park H., Jung S. Separation of some chiral flavonoids by microbial cyclosophoraoses and their sulfated derivatives in micellar electrokinetic chromatography. Electrophoresis. 2005;26:3833–3838. doi: 10.1002/elps.200500194. [DOI] [PubMed] [Google Scholar]
- 64.Kim H., Choi J.M., Choi Y., Tahir M.N., Yang Y.-H., Cho E., Jung S. Enhanced solubility of galangin based on the complexation with methylated microbial cyclosophoraoses. J. Incl. Phenom. Macrocycl. Chem. 2014;79:291–300. doi: 10.1007/s10847-013-0351-9. [DOI] [Google Scholar]
- 65.Piao J., Jang A., Choi Y., Tahir M.N., Kim Y., Park S., Cho E., Jung S. Solubility enhancement of α-naphthoflavone by synthesized hydroxypropyl cyclic-(1→2)-β-d-glucans (cyclosophoroases) Carbohydr. Polym. 2014;101:733–740. doi: 10.1016/j.carbpol.2013.09.104. [DOI] [PubMed] [Google Scholar]
- 66.Jeong D., Choi J.M., Choi Y., Jeong K., Cho E., Jung S. Complexation of fisetin with novel cyclosophoroase dimer to improve solubility and bioavailability. Carbohydr. Polym. 2013;97:196–202. doi: 10.1016/j.carbpol.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 67.Eggens I., Fenderson B., Toyokuni T., Dean B., Stroud M., Hakomori S.-I. Specific interaction between Lex and Lex determinants. A possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J. Biol. Chem. 1989;264:9476–9484. [PubMed] [Google Scholar]
- 68.Vyas N.K. Atomic features of protein-carbohydrate interactions. Curr. Opin. Struct. Biol. 1991;1:732–740. doi: 10.1016/0959-440X(91)90172-P. [DOI] [Google Scholar]
- 69.Aoyama Y., Otsuki J.-I., Nagai Y., Kobayashi K., Toi H. Host-guest complexation of oligosaccharides: Interaction of maltodextrins with hydrophobic fluorescence probes in water. Tetrahedron Lett. 1992;33:3775–3778. doi: 10.1016/0040-4039(92)80022-C. [DOI] [Google Scholar]
- 70.Kim H., Kim K., Choi J.M., Tahir M.N., Cho E., Choi Y., Lee I.-S., Jung S. Solubilization of pyrimethamine, antibacterial drug, by low-molecular-weight succinoglycan dimers isolated from Shinorhizobium meliloti. Bull. Korean Chem. Soc. 2012;33 doi: 10.5012/bkcs.2012.33.8.2731. [DOI] [Google Scholar]
- 71.Choi J.M., Kim H., Cho E., Choi Y., Jung S. Solubilization of haloperidol by acyclic succinoglycan oligosaccharides. Carbohydr. Polym. 2012;89:564–570. doi: 10.1016/j.carbpol.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 72.Cho E., Choi J.M., Jung S. Solubility enhancement of isoflavonoids by complexation with acyclic hexadecasaccharides, succinoglycan dimers isolated from Sinorhizobium meliloti. J. Incl. Phenom. Macrocycl. Chem. 2013;76:133–141. doi: 10.1007/s10847-012-0182-0. [DOI] [Google Scholar]
- 73.Kim K., Cho E., Choi J.M., Kim H., Jang A., Choi Y., Yu J.-H., Jung S. Intermolecular complexation of low-molecular-weight succinoglycans directs solubility enhancement of pindolol. Carbohydr. Polym. 2014;106:101–108. doi: 10.1016/j.carbpol.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 74.Ma D., Hettiarachchi G., Nguyen D., Zhang B., Wittenberg J.B., Zavalij P.Y., Briken V., Isaacs L. Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals. Nat. Chem. 2012;4:503–510. doi: 10.1038/nchem.1326. [DOI] [PubMed] [Google Scholar]
- 75.Moyano J., Ginés J., Arias M., Rabasco A. Study of the dissolution characteristics of oxazepam via complexation with β-cyclodextrin. Int. J. Pharm. 1995;114:95–102. doi: 10.1016/0378-5173(94)00220-Y. [DOI] [Google Scholar]
- 76.Pose-Vilarnovo B., Perdomo-López I., Echezarreta-López M., Schroth-Pardo P., Estrada E., Torres-Labandeira J.J. Improvement of water solubility of sulfamethizole through its complexation with β-and hydroxypropyl-β-cyclodextrin: Characterization of the interaction in solution and in solid state. Eur. J. Pharm. Sci. 2001;13:325–331. doi: 10.1016/S0928-0987(01)00131-2. [DOI] [PubMed] [Google Scholar]
- 77.Yáñez C., Cañete-Rosales P., Castillo J.P., Catalán N., Undabeytia T., Morillo E. Cyclodextrin inclusion complex to improve physicochemical properties of herbicide bentazon: Exploring better formulations. PLoS ONE. 2012;7:e41072. doi: 10.1371/journal.pone.0041072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moyano J., Arias M., Gines J., Perez J., Rabasco A. Dissolution Behavior of Oxazepam in Presence of Cyclodextrins: Evaluation of Oxazepam-Dimeb Binary Systemxs. Drug Dev. Ind. Pharm. 1997;23:379–385. doi: 10.3109/03639049709146140. [DOI] [Google Scholar]
- 79.Schneider H.-J., Hacket F., Rüdiger V., Ikeda H. NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 1998;98:1755–1786. doi: 10.1021/cr970019t. [DOI] [PubMed] [Google Scholar]
- 80.Ishizuka Y., Fujiwara M., Kanazawa K., Nemoto T., Fujita K.-I., Nakanishi H. Three-dimensional structure of the inclusion complex between phloridzin and β-cyclodextrin. Carbohydr. Res. 2002;337:1737–1743. doi: 10.1016/S0008-6215(02)00279-3. [DOI] [PubMed] [Google Scholar]
- 81.Pînzaru I., Hadaruga D., Hadaruga N., Corpa L., Grozescu I., Peter F. Hepatoprotective flavonoid bioconjugate/β-cyclodextrin nanoparticles: DSC-molecular modeling correlation. Dig. J. Nanomater. Biostruct. 2011;6:1605–1617. [Google Scholar]
- 82.Bilensoy E., Doğan L., Şen M., Hıncal A. Complexation behavior of antiestrogen drug tamoxifen citrate with natural and modified β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2007;57:651–655. doi: 10.1007/s10847-006-9268-x. [DOI] [Google Scholar]
- 83.Sinha V., Anitha R., Ghosh S., Nanda A., Kumria R. Complexation of celecoxib with β-cyclodextrin: Characterization of the interaction in solution and in solid state. J. Pharm. Sci. 2005;94:676–687. doi: 10.1002/jps.20287. [DOI] [PubMed] [Google Scholar]
- 84.Al Omari M.M., Zughul M.B., Davies J.E.D., Badwan A.A. Sildenafil/cyclodextrin complexation: Stability constants, thermodynamics, and guest-host interactions probed by 1 H-NMR and molecular modeling studies. J. Pharm. Biomed. Anal. 2006;41:857–865. doi: 10.1016/j.jpba.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 85.Higuchi T., Connors A. Advances in Analytical Chemistry and Instrumentation. Jonh Wiley & Sons; Weinheim, Germany: 1965. Phase-solubility techniques. [Google Scholar]
- 86.Loftsson T., Magnúsdóttir A., Másson M., Sigurjónsdóttir J.F. Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 2002;91:2307–2316. doi: 10.1002/jps.10226. [DOI] [PubMed] [Google Scholar]
- 87.Connors K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997;97:1325–1358. doi: 10.1021/cr960371r. [DOI] [PubMed] [Google Scholar]
- 88.Stella V.J., Rao V.M., Zannou E.A., Zia V. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Deliv. Rev. 1999;36:3–16. doi: 10.1016/S0169-409X(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 89.Loftsson T., Moya-Ortega M.D., Alvarez-Lorenzo C., Concheiro A. Pharmacokinetics of cyclodextrins and drugs after oral and parenteral administration of drug/cyclodextrin complexes. J. Pharm. Pharmacol. 2015 doi: 10.1111/jphp.12427. [DOI] [PubMed] [Google Scholar]
- 90.Yao Y., Xie Y., Hong C., Li G., Shen H., Ji G. Development of a myricetin/hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization, and evaluation. Carbohydr. Polym. 2014;110:329–337. doi: 10.1016/j.carbpol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 91.Lin H.-S., Leong W.W.Y., Yang J.A., Lee P., Chan S.Y., Ho P.C. Biopharmaceutics of 13-cis-retinoic acid (isotretinoin) formulated with modified β-cyclodextrins. Int. J. Pharm. 2007;341:238–245. doi: 10.1016/j.ijpharm.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 92.Leonardi D., Bombardiere M., Salomon C. Effects of benznidazole: Cyclodextrin complexes on the drug bioavailability upon oral administration to rats. Int. J. Biol. Macromol. 2013;62:543–548. doi: 10.1016/j.ijbiomac.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 93.Sinha V.R. In vivo bioavailability and therapeutic assessment of host-guest inclusion phenomena for the hydrophobic molecule etodolac: Pharmacodynamic and pharmacokinetic evaluation. Sci. Pharm. 2010;78:103–115. doi: 10.3797/scipharm.0909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurkov S.V., Loftsson T. Cyclodextrins. Int. J. Pharm. 2013;453:167–180. doi: 10.1016/j.ijpharm.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 95.Pokharkar V., Khanna A., Venkatpurwar V., Dhar S., Mandpe L. Ternary complexation of carvedilol, β-cyclodextrin and citric acid for mouth-dissolving tablet formulation. Acta Pharm. 2009;59:121–132. doi: 10.2478/v10007-009-0001-3. [DOI] [PubMed] [Google Scholar]
- 96.Dua K., Ramana M., Singh Sara U., Himaja M., Agrawal A., Garg V., Pabreja K. Investigation of enhancement of solubility of norfloxacin β-cyclodextrin in presence of acidic solubilizing additives. Curr. Drug Deliv. 2007;4:21–25. doi: 10.2174/156720107779314776. [DOI] [PubMed] [Google Scholar]
- 97.Mura P., Maestrelli F., Cirri M. Ternary systems of naproxen with hydroxypropyl-β-cyclodextrin and aminoacids. Int. J. Pharm. 2003;260:293–302. doi: 10.1016/S0378-5173(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 98.Li P., Zhao L., Yalkowsky S.H. Combined effect of cosolvent and cyclodextrin on solubilization of nonpolar drugs. J. Pharm. Sci. 1999;88:1107–1111. doi: 10.1021/js990159d. [DOI] [PubMed] [Google Scholar]
- 99.Loftsson T., Másson M. The effects of water-soluble polymers on cyclodextrins and cyclodextrin solubilization of drugs. J. Drug Deliv. Sci. Technol. 2004;14:35–43. doi: 10.1016/S1773-2247(04)50003-5. [DOI] [Google Scholar]
- 100.Wang D., Li H., Gu J., Guo T., Yang S., Guo Z., Zhang X., Zhu W., Zhang J. Ternary system of dihydroartemisinin with hydroxypropyl-β-cyclodextrin and lecithin: Simultaneous enhancement of drug solubility and stability in aqueous solutions. J. Pharm. Biomed. Anal. 2013;83:141–148. doi: 10.1016/j.jpba.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 101.Sallas F., Darcy R. Amphiphilic cyclodextrins-advances in synthesis and supramolecular chemistry. Eur. J. Org. Chem. 2008;2008:957–969. doi: 10.1002/ejoc.200700933. [DOI] [Google Scholar]
- 102.Zhang J., Ma P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013;65:1215–1233. doi: 10.1016/j.addr.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Auzely-Velty R., Djedaini-Pilard F., Desert S., Perly B., Zemb T. Micellization of hydrophobically modified cyclodextrins. 1. Micellar structure. Langmuir. 2000;16:3727–3734. doi: 10.1021/la991361z. [DOI] [Google Scholar]
- 104.Donohue R., Mazzaglia A., Ravoo B.J., Darcy R. Cationic β-cyclodextrin bilayer vesicles. Chem. Commun. 2002:2864–2865. doi: 10.1039/b207238f. [DOI] [PubMed] [Google Scholar]
- 105.Kawabata Y., Matsumoto M., Tanaka M., Takahashi H., Irinatsu Y., Tamura S., Tagaki W., Nakahara H., Fukuda K. Formation and deposition of monolayers of amphiphilic. β-cyclodextrin derivatives. Chem. Lett. 1986:1933–1934. doi: 10.1246/cl.1986.1933. [DOI] [Google Scholar]
- 106.Quaglia F., Ostacolo L., Mazzaglia A., Villari V., Zaccaria D., Sciortino M.T. The intracellular effects of non-ionic amphiphilic cyclodextrin nanoparticles in the delivery of anticancer drugs. Biomaterials. 2009;30:374–382. doi: 10.1016/j.biomaterials.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 107.Sun T., Guo Q., Zhang C., Hao J., Xing P., Su J., Li S., Hao A., Liu G. Self-assembled vesicles prepared from amphiphilic cyclodextrins as drug carriers. Langmuir. 2012;28:8625–8636. doi: 10.1021/la301497t. [DOI] [PubMed] [Google Scholar]
- 108.Perret F., Duffour M., Chevalier Y., Parrot-Lopez H. Design, synthesis, and in vitro evaluation of new amphiphilic cyclodextrin-based nanoparticles for the incorporation and controlled release of acyclovir. Eur. J. Pharm. Biopharm. 2013;83:25–32. doi: 10.1016/j.ejpb.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 109.Van de Manakker F., Vermonden T., van Nostrum C.F., Hennink W.E. Cyclodextrin-based polymeric materials: Synthesis, properties, and pharmaceutical/biomedical applications. Biomacromolecules. 2009;10:3157–3175. doi: 10.1021/bm901065f. [DOI] [PubMed] [Google Scholar]
- 110.Szeman J., Fenyvesi E., Szejtli J., Ueda H., Machida Y., Nagai T. Water soluble cyclodextrin polymers: Their interaction with drugs. In: Atwood J.L., Davies J.E., editors. Proceedings of the Fourth International Symposium on Inclusion Phenomena and the Third International Symposium on Cyclodextrins; Lancaster, UK. 20–25 July 1986; New York City, NY, USA: Springer; 1987. pp. 319–323. [Google Scholar]
- 111.Ma M., Li D. Cyclodextrin Polymer Separation Materials. WO1998022197A9. 1998 May 28;
- 112.Trotta F., Zanetti M., Cavalli R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012;8:2091–2099. doi: 10.3762/bjoc.8.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou J., Ritter H. Cyclodextrin functionalized polymers as drug delivery systems. Polym. Chem. 2010;1:1552–1559. doi: 10.1039/c0py00219d. [DOI] [Google Scholar]
- 114.Gil E.S., Li J., Xiao H., Lowe T.L. Quaternary ammonium β-cyclodextrin nanoparticles for enhancing doxorubicin permeability across the in vitro blood-brain barrier. Biomacromolecules. 2009;10:505–516. doi: 10.1021/bm801026k. [DOI] [PubMed] [Google Scholar]
- 115.Qiu L.Y., Wang R.J., Zheng C., Jin Y., Jin L.Q. β-cyclodextrin-centered star-shaped amphiphilic polymers for doxorubicin delivery. Nanomed. Nanotechnol. Biol. Med. 2010;5:193–208. doi: 10.2217/nnm.09.108. [DOI] [PubMed] [Google Scholar]
- 116.Posocco B., Dreussi E., de Santa J., Toffoli G., Abrami M., Musiani F., Grassi M., Farra R., Tonon F., Grassi G. Polysaccharides for the delivery of antitumor drugs. Materials. 2015;8:2569–2615. doi: 10.3390/ma8052569. [DOI] [Google Scholar]
- 117.Ringsdorf H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Polym. Symp. 1975;51:135–153. doi: 10.1002/polc.5070510111. [DOI] [Google Scholar]
- 118.Bassi P., Volpe A., D’Agostino D., Palermo G., Renier D., Franchini S., Rosato A., Racioppi M. Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder: Results of a phase I study. J. Urol. 2011;185:445–449. doi: 10.1016/j.juro.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 119.Mero A., Campisi M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers. 2014;6:346–369. doi: 10.3390/polym6020346. [DOI] [Google Scholar]
- 120.Yousefpour P., Atyabi F., Farahani E.V., Sakhtianchi R., Dinarvand R. Polyanionic carbohydrate doxorubicin-dextran nanocomplex as a delivery system for anticancer drugs: In vitro analysis and evaluations. Int. J. Nanomed. 2011;6:1487–1496. doi: 10.2147/IJN.S18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yousefpour P., Atyabi F., Vasheghani-Farahani E., Movahedi A.-A.M., Dinarvand R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-Her2 trastuzumab. Int. J. Nanomed. 2011;6:1977–1990. doi: 10.2147/IJN.S21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y., Xin D., Liu K., Zhu M., Xiang J. Heparin-paclitaxel conjugates as drug delivery system: Synthesis, self-assembly property, drug release, and antitumor activity. Bioconjug. Chem. 2009;20:2214–2221. doi: 10.1021/bc8003809. [DOI] [PubMed] [Google Scholar]
- 123.Al-Shamkhani A., Duncan R. Synthesis, controlled release properties and antitumour activity of alginate-cis-aconityl-daunomycin conjugates. Int. J. Pharm. 1995;122:107–119. doi: 10.1016/0378-5173(95)00055-N. [DOI] [Google Scholar]
- 124.Zhang H., Li F., Yi J., Gu C., Fan L., Qiao Y., Tao Y., Cheng C., Wu H. Folate-decorated maleilated pullulan-doxorubicin conjugate for active tumor-targeted drug delivery. Eur. J. Pharm. Sci. 2011;42:517–526. doi: 10.1016/j.ejps.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 125.Elgart A., Farber S., Domb A.J., Polacheck I., Hoffman A. Polysaccharide pharmacokinetics: Amphotericin B arabinogalactan conjugate—A drug delivery system or a new pharmaceutical entity? Biomacromolecules. 2010;11:1972–1977. doi: 10.1021/bm100298r. [DOI] [PubMed] [Google Scholar]
- 126.Pinhassi R.I., Assaraf Y.G., Farber S., Stark M., Ickowicz D., Drori S., Domb A.J., Livney Y.D. Arabinogalactan-folic acid-drug conjugate for targeted delivery and target-activated release of anticancer drugs to folate receptor-overexpressing cells. Biomacromolecules. 2009;11:294–303. doi: 10.1021/bm900853z. [DOI] [PubMed] [Google Scholar]
- 127.Goodarzi N., Varshochian R., Kamalinia G., Atyabi F., Dinarvand R. A review of polysaccharide cytotoxic drug conjugates for cancer therapy. Carbohydr. Polym. 2013;92:1280–1293. doi: 10.1016/j.carbpol.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 128.Ahrens T., Assmann V., Fieber C., Termeer C.C., Herrlich P., Hofmann M., Simon J.C. CD44 is the principal mediator of hyaluronic-acid-induced melanoma cell proliferation. J. Investig. Dermatol. 2001;116:93–101. doi: 10.1046/j.1523-1747.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 129.Lamke L.-O., Liljedahl S.-O. Plasma volume changes after infusion of various plasma expanders. Resuscitation. 1976;5:93–102. doi: 10.1016/0300-9572(76)90029-0. [DOI] [PubMed] [Google Scholar]
- 130.Soepenberg O., de Jonge M.J., Sparreboom A., de Bruin P., Eskens F.A., de Heus G., Wanders J., Cheverton P., Ducharme M.P., Verweij J. Phase I and pharmacokinetic study of DE-310 in patients with advanced solid tumors. Clin. Cancer Res. 2005;11:703–711. doi: 10.1158/1078-0432.CCR-04-1758. [DOI] [PubMed] [Google Scholar]
- 131.Veltkamp S.A., Witteveen E.O., Capriati A., Crea A., Animati F., Voogel-Fuchs M., van den Heuvel I.J., Beijnen J.H., Voest E.E., Schellens J.H. Clinical and pharmacologic study of the novel prodrug delimotecan (MEN 4901/T-0128) in patients with solid tumors. Clin. Cancer Res. 2008;14:7535–7544. doi: 10.1158/1078-0432.CCR-08-0438. [DOI] [PubMed] [Google Scholar]
- 132.Danhauser-Riedl S., Hausmann E., Schick H.-D., Bender R., Dietzfelbinger H., Rastetter J., Hanauske A.-R. Phase I clinical and pharmacokinetic trial of dextran conjugated doxorubicin (AD-70, DOX-OXD) Investig. New Drugs. 1993;11:187–195. doi: 10.1007/BF00874153. [DOI] [PubMed] [Google Scholar]
- 133.Mahajan H.S., Tyagi V., Lohiya G., Nerkar P. Thermally reversible xyloglucan gels as vehicles for nasal drug delivery. Drug Deliv. 2012;19:270–276. doi: 10.3109/10717544.2012.704095. [DOI] [PubMed] [Google Scholar]
- 134.Miyazaki S., Suisha F., Kawasaki N., Shirakawa M., Yamatoya K., Attwood D. Thermally reversible xyloglucan gels as vehicles for rectal drug delivery. J. Control. Release. 1998;56:75–83. doi: 10.1016/S0168-3659(98)00079-0. [DOI] [PubMed] [Google Scholar]
- 135.Suisha F., Kawasaki N., Miyazaki S., Shirakawa M., Yamatoya K., Sasaki M., Attwood D. Xyloglucan gels as sustained release vehicles for the intraperitoneal administration of mitomycin C. Int. J. Pharm. 1998;172:27–32. doi: 10.1016/S0378-5173(98)00157-4. [DOI] [Google Scholar]
- 136.Kawasaki N., Ohkura R., Miyazaki S., Uno Y., Sugimoto S., Attwood D. Thermally reversible xyloglucan gels as vehicles for oral drug delivery. Int. J. Pharm. 1999;181:227–234. doi: 10.1016/S0378-5173(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 137.Joshi S.C. Sol-Gel behavior of hydroxypropyl methylcellulose (hpmc) in ionic media including drug release. Materials. 2011;4:1861–1905. doi: 10.3390/ma4101861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zahoor A., Sharma S., Khuller G. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents. 2005;26:298–303. doi: 10.1016/j.ijantimicag.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 139.Rajaonarivony M., Vauthier C., Couarraze G., Puisieux F., Couvreur P. Development of a new drug carrier made from alginate. J. Pharm. Sci. 1993;82:912–917. doi: 10.1002/jps.2600820909. [DOI] [PubMed] [Google Scholar]
- 140.Dai Y.N., Li P., Zhang J.P., Wang A.Q., Wei Q. A novel pH sensitive N-succinyl chitosan/alginate hydrogel bead for nifedipine delivery. Biopharm. Drug Dispos. 2008;29:173–184. doi: 10.1002/bdd.590. [DOI] [PubMed] [Google Scholar]
- 141.Wang C., Liu H., Gao Q., Liu X., Tong Z. Alginate-calcium carbonate porous microparticle hybrid hydrogels with versatile drug loading capabilities and variable mechanical strengths. Carbohydr. Polym. 2008;71:476–480. doi: 10.1016/j.carbpol.2007.06.018. [DOI] [Google Scholar]
- 142.Jain D., Banerjee R. Comparison of ciprofloxacin hydrochloride-loaded protein, lipid, and chitosan nanoparticles for drug delivery. J. Biomed. Mater. Res. B Appl. Biomater. 2008;86:105–112. doi: 10.1002/jbm.b.30994. [DOI] [PubMed] [Google Scholar]
- 143.Maestrelli F., Garcia-Fuentes M., Mura P., Alonso M.J. A new drug nanocarrier consisting of chitosan and hydoxypropylcyclodextrin. Eur. J. Pharm. Biopharm. 2006;63:79–86. doi: 10.1016/j.ejpb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 144.Lin W.-C., Yu D.-G., Yang M.-C. pH-sensitive polyelectrolyte complex gel microspheres composed of chitosan/sodium tripolyphosphate/dextran sulfate: Swelling kinetics and drug delivery properties. Colloids Surf. B Biointerfaces. 2005;44:143–151. doi: 10.1016/j.colsurfb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 145.Tiyaboonchai W., Limpeanchob N. Formulation and characterization of amphotericin B-chitosan-dextran sulfate nanoparticles. Int. J. Pharm. 2007;329:142–149. doi: 10.1016/j.ijpharm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 146.Zheng Y., Wu Y., Yang W., Wang C., Fu S., Shen X. Preparation, characterization, and drug release in vitro of chitosan-glycyrrhetic acid nanoparticles. J. Pharm. Sci. 2006;95:181–191. doi: 10.1002/jps.20399. [DOI] [PubMed] [Google Scholar]
- 147.Ruel-Gariépy E., Shive M., Bichara A., Berrada M., le Garrec D., Chenite A., Leroux J.-C. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2004;57:53–63. doi: 10.1016/S0939-6411(03)00095-X. [DOI] [PubMed] [Google Scholar]
- 148.Cheow W.S., Kiew T.Y., Hadinoto K. Amorphous nanodrugs prepared by complexation with polysaccharides: Carrageenan versus dextran sulfate. Carbohydr. Polym. 2015;117:549–558. doi: 10.1016/j.carbpol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 149.Cho H.-J., Yoon H.Y., Koo H., Ko S.-H., Shim J.-S., Lee J.-H., Kim K., Kwon I.C., Kim D.-D. Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic® for tumor-targeted delivery of docetaxel. Biomaterials. 2011;32:7181–7190. doi: 10.1016/j.biomaterials.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 150.Cho H.-J., Yoon I.-S., Yoon H.Y., Koo H., Jin Y.-J., Ko S.-H., Shim J.-S., Kim K., Kwon I.C., Kim D.-D. Polyethylene glycol-conjugated hyaluronic acid-ceramide self-assembled nanoparticles for targeted delivery of doxorubicin. Biomaterials. 2012;33:1190–1200. doi: 10.1016/j.biomaterials.2011.10.064. [DOI] [PubMed] [Google Scholar]
- 151.Li J., Huo M., Wang J., Zhou J., Mohammad J.M., Zhang Y., Zhu Q., Waddad A.Y., Zhang Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials. 2012;33:2310–2320. doi: 10.1016/j.biomaterials.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 152.Wu J.-L., Liu C.-G., Wang X.-L., Huang Z.-H. Preparation and characterization of nanoparticles based on histidine-hyaluronic acid conjugates as doxorubicin carriers. J. Mater. Sci. Mater. Med. 2012;23:1921–1929. doi: 10.1007/s10856-012-4665-8. [DOI] [PubMed] [Google Scholar]
- 153.Kumar B.S., Kumar M.G., Suguna L., Sastry T., Mandal A. Pullulan acetate nanoparticles based delivery system for hydrophobic drug. Int. J. Pharma Biol. Sci. 2012;3:24–32. [Google Scholar]
- 154.Wang Y.-S., Liu L.-R., Jiang Q., Zhang Q.-Q. Self-aggregated nanoparticles of cholesterol-modified chitosan conjugate as a novel carrier of epirubicin. Eur. Polym. J. 2007;43:43–51. doi: 10.1016/j.eurpolymj.2006.09.007. [DOI] [Google Scholar]
- 155.Lee K., Kim J.-H., Kwon I., Jeong S. Self-aggregates of deoxycholic acid-modified chitosan as a novel carrier of adriamycin. Colloid Polym. Sci. 2000;278:1216–1219. doi: 10.1007/s003960000389. [DOI] [Google Scholar]
- 156.Jin Y.-H., Hu H.-Y., Qiao M.-X., Zhu J., Qi J.-W., Hu C.-J., Zhang Q., Chen D.-W. pH-sensitive chitosan-derived nanoparticles as doxorubicin carriers for effective anti-tumor activity: Preparation and in vitro evaluation. Colloids Surf. B Biointerfaces. 2012;94:184–191. doi: 10.1016/j.colsurfb.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 157.Kim J.-H., Kim Y.-S., Kim S., Park J.H., Kim K., Choi K., Chung H., Jeong S.Y., Park R.-W., Kim I.-S. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J. Control. Release. 2006;111:228–234. doi: 10.1016/j.jconrel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 158.Min K.H., Park K., Kim Y.-S., Bae S.M., Lee S., Jo H.G., Park R.-W., Kim I.-S., Jeong S.Y., Kim K. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J. Control. Release. 2008;127:208–218. doi: 10.1016/j.jconrel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 159.Yuan H., Lu L.-J., Du Y.-Z., Hu F.-Q. Stearic acid-g-chitosan polymeric micelle for oral drug delivery: In vitro transport and in vivo absorption. Mol. Pharm. 2010;8:225–238. doi: 10.1021/mp100289v. [DOI] [PubMed] [Google Scholar]
- 160.Xie Y.-T., Du Y.-Z., Yuan H., Hu F.-Q. Brain-targeting study of stearic acid-grafted chitosan micelle drug-delivery system. Int. J. Nanomed. 2012;7:3235–3244. doi: 10.2147/IJN.S32701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park J.S., Han T.H., Lee K.Y., Han S.S., Hwang J.J., Moon D.H., Kim S.Y., Cho Y.W. N-acetyl histidine-conjugated glycol chitosan self-assembled nanoparticles for intracytoplasmic delivery of drugs: Endocytosis, exocytosis and drug release. J. Control. Release. 2006;115:37–45. doi: 10.1016/j.jconrel.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 162.Xu Q., Yuan X., Chang J. Self-aggregates of cholic acid hydrazide-dextran conjugates as drug carriers. J. Appl. Polym. Sci. 2005;95:487–493. doi: 10.1002/app.21198. [DOI] [Google Scholar]
- 163.Daoud-Mahammed S., Couvreur P., Bouchemal K., Chéron M., Lebas G., Amiel C., Gref R. Cyclodextrin and polysaccharide-based nanogels: Entrapment of two hydrophobic molecules, benzophenone and tamoxifen. Biomacromolecules. 2009;10:547–554. doi: 10.1021/bm801206f. [DOI] [PubMed] [Google Scholar]
- 164.Park W., Park S.-J., Na K. Potential of self-organizing nanogel with acetylated chondroitin sulfate as an anti-cancer drug carrier. Colloids Surf. B Biointerfaces. 2010;79:501–508. doi: 10.1016/j.colsurfb.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 165.Kumbar S., Kulkarni A., Aminabhavi T. Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: Effect of crosslinking agent. J. Microencapsul. 2002;19:173–180. doi: 10.1080/02652040110065422. [DOI] [PubMed] [Google Scholar]
- 166.Wang H., Xu Y., Zhou X. Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: In vitro and in vivo evaluation. Int. J. Mol. Sci. 2014;15:3519–3532. doi: 10.3390/ijms15033519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Agnihotri S.A., Aminabhavi T.M. Controlled release of clozapine through chitosan microparticles prepared by a novel method. J. Control. Release. 2004;96:245–259. doi: 10.1016/j.jconrel.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 168.Kumbar S.G., Aminabhavi T.M. Synthesis and characterization of modified chitosan microspheres: Effect of the grafting ratio on the controlled release of nifedipine through microspheres. J. Appl. Polym. Sci. 2003;89:2940–2949. doi: 10.1002/app.12386. [DOI] [Google Scholar]
- 169.Park K.M., Bae J.W., Joung Y.K., Shin J.W., Park K.D. Nanoaggregate of thermosensitive chitosan-Pluronic for sustained release of hydrophobic drug. Colloids Surf. B Biointerfaces. 2008;63:1–6. doi: 10.1016/j.colsurfb.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 170.Manaspon C., Viravaidya-Pasuwat K., Pimpha N. Preparation of folate-conjugated pluronic F127/chitosan core-shell nanoparticles encapsulating doxorubicin for breast cancer treatment. J. Nanomater. 2012;2012 doi: 10.1155/2012/593878. [DOI] [Google Scholar]
- 171.Seo S., Lee C.-S., Jung Y.-S., Na K. Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly (l-lactide) copolymers. Carbohydr. Polym. 2012;87:1105–1111. doi: 10.1016/j.carbpol.2011.08.061. [DOI] [Google Scholar]
- 172.Jeong Y.-I., Na H.-S., Oh J.-S., Choi K.-C., Song C.-E., Lee H.-C. Adriamycin release from self-assembling nanospheres of poly (DL-lactide-co-glycolide)-grafted pullulan. Int. J. Pharm. 2006;322:154–160. doi: 10.1016/j.ijpharm.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 173.Guo Y., Wang X., Shu X., Shen Z., Sun R.-C. Self-assembly and paclitaxel loading capacity of cellulose-graft-poly (lactide) nanomicelles. J. Agric. Food Chem. 2012;60:3900–3908. doi: 10.1021/jf3001873. [DOI] [PubMed] [Google Scholar]
- 174.Jeong Y.-I., Kim D.H., Chung C.-W., Yoo J.-J., Choi K.H., Kim C.H., Ha S.H., Kang D.H. Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly (DL-lactide-co-glycolide) copolymer. Int. J. Nanomed. 2011;6:1415–1427. doi: 10.2147/IJN.S19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bang J.-Y., Song C.-E., Kim C., Park W.-D., Cho K.-R., Kim P.-I., Lee S.-R., Chung W.-T., Choi K.-C. Cytotoxicity of amphotericin B-incorporated polymeric micelles composed of poly (DL-lactide-co-glycolide)/dextran graft copolymer. Arch. Pharm. Res. 2008;31:1463–1469. doi: 10.1007/s12272-001-2131-0. [DOI] [PubMed] [Google Scholar]
- 176.Lee H., Ahn C.H., Park T.G. Poly [lactic-co-(glycolic acid)]-Grafted Hyaluronic Acid Copolymer Micelle Nanoparticles for Target-Specific Delivery of Doxorubicin. Macromol. Biosci. 2009;9:336–342. doi: 10.1002/mabi.200800229. [DOI] [PubMed] [Google Scholar]
- 177.Li B., Wang Q., Wang X., Wang C., Jiang X. Preparation, drug release and cellular uptake of doxorubicin-loaded dextran-b-poly(ε-caprolactone) nanoparticles. Carbohydr. Polym. 2013;93:430–437. doi: 10.1016/j.carbpol.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 178.Huang S.-J., Sun S.-L., Feng T.-H., Sung K.-H., Lui W.-L., Wang L.-F. Folate-mediated chondroitin sulfate-Pluronic® 127 nanogels as a drug carrier. Eur. J. Pharm. Sci. 2009;38:64–73. doi: 10.1016/j.ejps.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 179.Mundargi R.C., Shelke N.B., Rokhade A.P., Patil S.A., Aminabhavi T.M. Formulation and in vitro evaluation of novel starch-based tableted microspheres for controlled release of ampicillin. Carbohydr. Polym. 2008;71:42–53. doi: 10.1016/j.carbpol.2007.05.013. [DOI] [Google Scholar]
- 180.Barbucci R., Fini M., Giavaresi G., Torricelli P., Giardino R., Lamponi S., Leone G. Hyaluronic acid hydrogel added with ibuprofen-lysine for the local treatment of chondral lesions in the knee: In vitro and in vivo investigations. J. Biomed. Mater. Res. B Appl. Biomater. 2005;75:42–48. doi: 10.1002/jbm.b.30234. [DOI] [PubMed] [Google Scholar]
- 181.Cho E., Tahir M.N., Choi J.M., Kim H., Yu J.-H., Jung S. Novel magnetic nanoparticles coated by benzene-and β-cyclodextrin-bearing dextran, and the sorption of polycyclic aromatic hydrocarbon. Carbohydr. Polym. 2015;133:221–228. doi: 10.1016/j.carbpol.2015.06.089. [DOI] [PubMed] [Google Scholar]
- 182.Saravanakumar G., Jo D.-G., Park J.H. Polysaccharide-based nanoparticles: A versatile platform for drug delivery and biomedical imaging. Curr. Med. Chem. 2012;19:3212–3229. doi: 10.2174/092986712800784658. [DOI] [PubMed] [Google Scholar]
- 183.Liu Z., Jiao Y., Wang Y., Zhou C., Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008;60:1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 184.Lemarchand C., Gref R., Couvreur P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004;58:327–341. doi: 10.1016/j.ejpb.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 185.Varde N.K., Pack D.W. Microspheres for controlled release drug delivery. Expert Opin. Biol. Ther. 2004;4:35–51. doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- 186.Baimark Y., Srisuwan Y. Preparation of polysaccharide-based microspheres by a water-in-oil emulsion solvent diffusion method for drug carriers. Int. J. Polym. Sci. 2013;2013:761870. doi: 10.1155/2013/761870. [DOI] [Google Scholar]
- 187.Xu J., Li S., Tostado C., Lan W., Luo G. Preparation of monodispersed chitosan microspheres and in situ encapsulation of BSA in a co-axial microfluidic device. Biomed. Microdevices. 2009;11:243–249. doi: 10.1007/s10544-008-9230-3. [DOI] [PubMed] [Google Scholar]
- 188.He P., Davis S.S., Illum L. Chitosan microspheres prepared by spray drying. Int. J. Pharm. 1999;187:53–65. doi: 10.1016/S0378-5173(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 189.Li B.-Z., Wang L.-J., Li D., Bhandari B., Li S.-J., Lan Y., Chen X.D., Mao Z.-H. Fabrication of starch-based microparticles by an emulsification-crosslinking method. J. Food Eng. 2009;92:250–254. doi: 10.1016/j.jfoodeng.2008.08.011. [DOI] [Google Scholar]
- 190.Mellors R., Benzeval I., Eisenthal R., Hubble J. Preparation of self-assembled microspheres and their potential for drug delivery. Pharm. Dev. Technol. 2010;15:105–111. doi: 10.3109/10837450903036163. [DOI] [PubMed] [Google Scholar]
- 191.Davis S., Illum L. Sustained release chitosan microspheres prepared by novel spray drying methods. J. Microencapsul. 1999;16:343–355. doi: 10.1080/026520499289068. [DOI] [PubMed] [Google Scholar]
- 192.Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008;57:397–430. doi: 10.1002/pi.2378. [DOI] [Google Scholar]
- 193.Wood K.C., Chuang H.F., Batten R.D., Lynn D.M., Hammond P.T. Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films. Proc. Natl. Acad. Sci. USA. 2006;103:10207–10212. doi: 10.1073/pnas.0602884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.He W., Du Q., Cao D.-Y., Xiang B., Fan L.-F. In vitro and in vivo studies of pectin/ethylcellulose-film-coated pellets of 5-fluorouracil for colonic targeting. J. Pharm. Pharmacol. 2008;60:35–44. doi: 10.1211/jpp.60.1.0005. [DOI] [PubMed] [Google Scholar]
- 195.Macleod G.S., Collett J.H., Fell J.T. The potential use of mixed films of pectin, chitosan and HPMC for bimodal drug release. J. Control. Release. 1999;58:303–310. doi: 10.1016/S0168-3659(98)00168-0. [DOI] [PubMed] [Google Scholar]
- 196.Dhanikula A.B., Panchagnula R. Development and characterization of biodegradable chitosan films for local delivery of paclitaxel. AAPS J. 2004;6:88–99. doi: 10.1208/aapsj060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Coviello T., Matricardi P., Marianecci C., Alhaique F. Polysaccharide hydrogels for modified release formulations. J. Control. Release. 2007;119:5–24. doi: 10.1016/j.jconrel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 198.Eiselt P., Yeh J., Latvala R.K., Shea L.D., Mooney D.J. Porous carriers for biomedical applications based on alginate hydrogels. Biomaterials. 2000;21:1921–1927. doi: 10.1016/S0142-9612(00)00033-8. [DOI] [PubMed] [Google Scholar]
- 199.Ashley G.W., Henise J., Reid R., Santi D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA. 2013;110:2318–2323. doi: 10.1073/pnas.1215498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vandamme T.F., Lenourry A., Charrueau C., Chaumeil J. The use of polysaccharides to target drugs to the colon. Carbohydr. Polym. 2002;48:219–231. doi: 10.1016/S0144-8617(01)00263-6. [DOI] [Google Scholar]