Abstract
Background
Several previous studies have confirmed that thrombocytosis was related to reduced survival in many solid tumors. However, the prognostic significance of thrombocytosis in endometrial carcinoma (EC) was still controversy. Therefore, we conducted this study to assess the prognostic value of thrombocytosis in EC.
Methods
The database including PubMed, MEDLINE, EMBASE, and Web of Science was searched to explore available literature. Above all, the hazard ratio (HR), odds ratios (OR) with 95% confidence intervals (CIs) was used to investigate the correlation between thrombocytosis and overall survival (OS) and disease-free survival (DFS). Moreover, the association between thrombocytosis and patient clinicopathological characteristics was explored. Publication bias and sensitivity analysis also were conducted in this study.
Results
Overall, 11 studies involving 3439 patients were contained in this study. The results revealed that pretreatment thrombocytosis was significantly related to a decreased OS (pooled HR = 2.99; 95% CI = 2.35–3.8; P < 0.001) and DFS (pooled HR = 2.86; 95% CI = 2.27–3.6; P < 0.001) in patients with EC. Moreover, thrombocytosis was correlated with adverse clinicopathological parameters.
Conclusions
Pretreatment thrombocytosis is an adverse prognostic marker in patients with EC.
Electronic supplementary material
The online version of this article (10.1186/s12885-018-5264-y) contains supplementary material, which is available to authorized users.
Keywords: Thrombocytosis, Prognosis, Endometrial carcinoma
Background
Endometrial carcinoma (EC) remains one of the most common gynecological cancer in developed countries [1]. In China, EC is the third most common female reproductive system malignancy [2]. According to the previous study, the 5-year overall survival (OS) in EC patients with International Federation of Gynecology and Obstetrics (FIGO) stages I-II are 74–91% [3]. However,10–20% early-stages (I-II) and 50–70% advanced-stage (III-IV) patients will recur after primary treatment [4]. Therefore, it is urgently necessary to explore biomarkers that can be used to tailor distinct treatment protocols, identify high-risk recurrence patients, guide postoperative therapy, and determine follow-up protocols.
Previous studies have revealed that clinicopathological parameters such as histologic type and grade, FIGO stage, myometrial invasion, lymph node (LN) metastasis, lymphovascular space invasion (LVSI), tumor size, and the patients’ age has prognostic effect in patients with EC [5, 6]. However, these factors usually obtained postoperation and demonstrated to be insufficient to predict recurrence and estimate survival [5, 7, 8]. Thus, it is necessary to recognize more effective prognostic predictors to identify high-risk patients preoperation.
Thrombocytosis, often defined as platelet count higher than 400 × 109/L, has been observed correlate with prognosis in various malignancies such as lung, renal, gastric, colorectal and hepatocellular cancer [9–13]. In gynecologic malignancies, pretreatment thrombocytosis was associated with decreased survival in ovarian, vulvar and cervical cancers [14–16]. The prognostic role of thrombocytosis in EC also has been reported in several studies, but the conclusions were controversy [17–29]. Therefore, we conducted this meta-analysis to elucidate the prognostic value of pretreatment thrombocytosis in EC.
Methods
Search strategy
We identified relevant studies by searching database including PubMed, MEDLINE, EMBASE, and Web of Science. The search was updated in August 2018. The search strategy was as follows: (((((((“Endometrial Neoplasms”[Mesh]) OR endometrial cancer) OR endometrial carcinoma) OR endometrium cancer) OR endometrium carcinoma)) AND (((((((“Thrombocytosis”[Mesh]) OR thrombocytosis) OR thrombocythemia) OR platelet count) OR blood platelets) OR platelets) OR platelet)) AND (((((“Prognosis”[Mesh]) OR prognosis) OR prognostic) OR survival) OR mortality). This study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Additional file 1) [30].
Selection criteria
The including criteria were as follows: (1) EC was diagnosed by histopathological examination; (2) platelet count was measured preoperation; (3) hazard ratios(HRs) and their 95% confidence intervals (CIs) for platelet count can be obtained; (4) the cut-off value of thrombocytosis was provided.
Exclusion criteria
The exclusion criteria were as follows: (1) letters, meeting abstracts, reviews; (2) articles not written in English; (3) studies with duplicate data; (4) incomplete data for evaluating the HR and its 95%CI. The candidate articles were assessed by two reviewers independently. Any disputes were resolved through their discussion.
Data extraction and quality assessment
In the light of the guidelines for meta-analysis of observational studies [31], two reviewers independently extracted data from the selected literature. The obtained data were as follows: the first author name, study publication time, country, sample size, FIGO stage, grade, LVSI, histological type, LN metastasis, recurrence, the cut-off value of thrombocytosis, venous thromboembolism (VTE), follow-up time, and primary outcome. The Newcastle-Ottawa Scale (NOS) scoring system was used to assess the quality of selected articles [32]. High-quality studies were defined as NOS score more than six (Additional file 2).
Statistical analysis
The HRs and 95% CIs were used to evaluate the prognostic significance of thrombocytosis on EC. Additionally, the correlation between thrombocytosis and clinicopathological characteristics were analyzed. Heterogeneity analysis was assessed using Chi-square test based on the Q value and I2 statistic value. Using a random-effects model, or using a fixed-effects model was determined by the level of heterogeneity (e.g., P-value < 0.1 and/or I2 > 50%, the random-effects model was used). Besides, we conducted a sensitivity analysis to validate the stability of the pooled outcomes. Begg’s test and Egger’s test was used to assessing the publication bias. The data in our study were analyzed using STATA 14.0 software (Stata Corporation, College Station, TX, USA).
Results
Characteristics of eligible studies
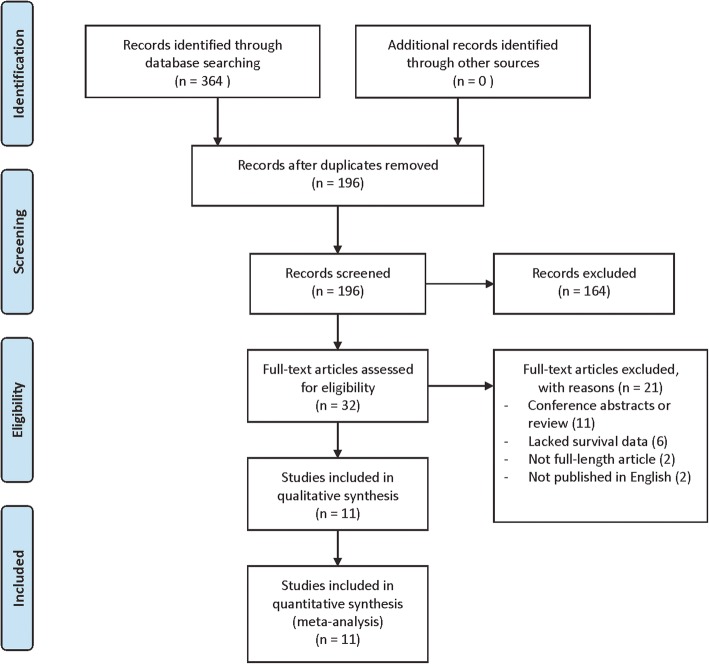
The flow diagram illustrated the search procedure (Fig. 1). After the preliminary search, we identified a total of 364 articles. First, we removed 168 duplicate articles, and another 164 irrelevant items also were excluded. The remaining 32 full-text articles were left for further review. Among them, six studies were excluded due to lack of survival data. Next, we excluded two non-English articles, 11 conference abstracts or reviews, and two not full-length articles. Finally, 11 eligible studies were involved in this meta-analysis. All of the qualified studies were observational retrospective studies. The main characteristics of the eligible studies were summarized in Table 1.
Fig. 1.
The flow diagram of the study selection strategy
Table 1.
Main characteristics of the eligible studies included in the meta-analysis
| Author | Year | Country | Patients number | Age (years) | FIGO Stage | Tumor Grade | Tumor type | cut-off value (× 109/L) | Incidence of thrombocytosis (%) | Follow-up time (month) | Primary Outcome | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abu-Zaid [24] | 2017 | Saudi Arabia | 162 | 59 | I–IV | 1–3 | endometrioid | 400 | 8.6 | NR | OS, DFS | 6 |
| Andersen [27] | 2017 | Denmark | 218 | 18–80 | I–IV | 1–3 | mixed | 400 | 11.5 | NR | OS, DSS | 6 |
| Njølstad [28] | 2013 | Norway | 512 | 28–93 | I–IV | 1–3 | mixed | 390 | 12.3 | 55(0–97) | DFS | 6 |
| Kizer [22] | 2015 | USA | 318 | 62 | I–IV | 1–3 | mixed | 400 | 16.7 | NR | DFS, DSS | 6 |
| Nakamura [23] | 2016 | Japan | 108 | 60 | I–IV | 1–3 | mixed | 350 | 11.02 | NR | OS, PFS | 6 |
| Takahashi [26] | 2017 | Japan | 508 | 58 | I–IV | 1–3 | mixed | 400 | 7 | NR | OS | 6 |
| Heng [20] | 2014 | Thailand | 238 | 28–88 | I–IV | 1–3 | mixed | 400 | 18.1 | 59.6(1–98) | OS, DFS | 7 |
| Matsuo [19] | 2013 | USA | 516 | 52 | I–IV | 1–3 | mixed | 400 | 15.1 | 43.7 | OS, DFS | 8 |
| Gorelick [18] | 2009 | USA | 77 | 65 | I–IV | 1–3 | mixed | 400 | 18.2 | NR | OS | 6 |
| Lerner [17] | 2007 | USA | 68 | NR | III-IV | NR | serous | 400 | 12 | NR | OS | 6 |
| Moeini [25] | 2017 | USA | 714 | 53.1 | I–IV | 1–3 | mixed | 400 | 24.8 | 28.8 | OS, DFS | 8 |
Abbreviations: NOS Newcastle Ottawa Scale, NR not reported, FIGO International Federation of Gynecology and Obstetrics, OS overall survival, PFS progression-free survival, DFS disease-free survival, DSS disease-specific survival
The prognostic impact of thrombocytosis on overall survival
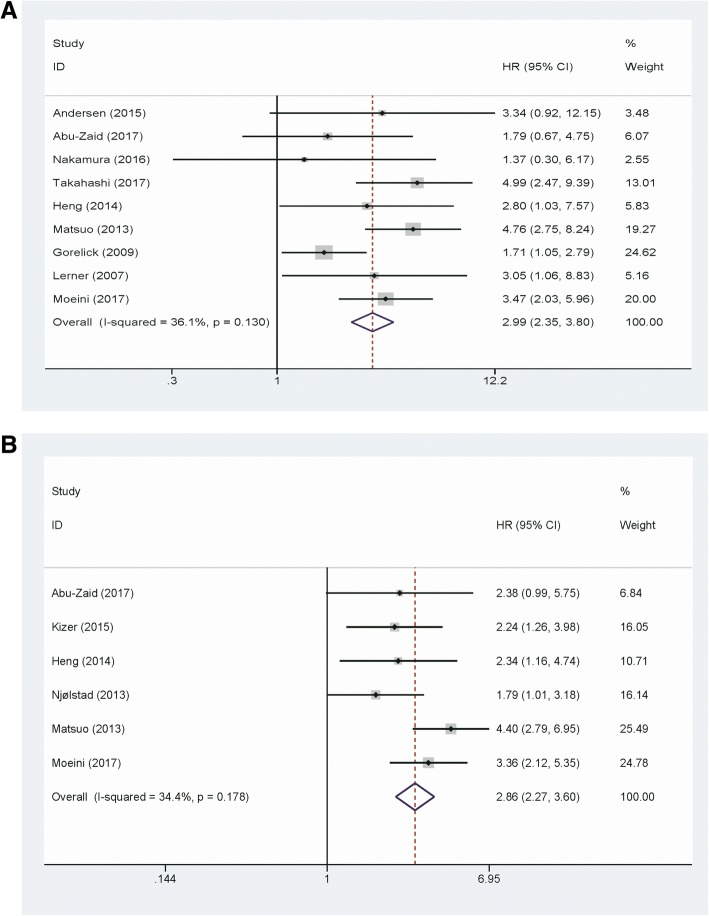
A total of nine studies including 2609 EC patients provided the overall survival data for analysis. The results revealed that elevated platelet count correlated with poor OS in EC patients (pooled HR = 2.99; 95% CI = 2.35–3.8; P < 0.001) (Fig. 2a). Subgroup analysis was conducted to further investigate the prognostic role of thrombocytosis on OS in patients with EC. We observed significant results in subgroup analysis based on the study region (Asian vs. Non-Asian), sample size (< 200 vs. ≥ 200), FIGO stage (I-IV vs. III-IV), and analysis method (Multivariate vs. Univariate). In subgroup analysis according to the cut-off value of platelet count, a significant result was observed in the platelet count = 400 × 109/L group (HR = 3.05, 95% CI = 2.39–3.89, fixed effects). However, platelet count = 350 × 109/L group did not predict poor prognosis for EC (HR = 1.37,95% CI = 0.3–6.17, fixed effects) (Table 2).
Fig. 2.
a Forest plot to assess the association between thrombocytosis and overall survival. b Forest plot to assess the association between thrombocytosis and disease-free survival
Table 2.
Summary of subgroup analysis
| Categories | Number of studies | Number of patients | Model | HR (95% CI) | I2 (%) | P h | Z | P |
|---|---|---|---|---|---|---|---|---|
| Study region | ||||||||
| Asian | 4 | 1016 | Fixed | 3.12 (0.54–5.20) | 31 | 0.23 | 4.85 | < 0.001 |
| Non-Asian | 5 | 1593 | Random | 3.03 (1.96–4.69) | 51 | 0.09 | 4.98 | < 0.001 |
| Sample size | ||||||||
| < 200 | 4 | 415 | Fixed | 1.84 (1.25–2.71) | 0 | 0.78 | 3.07 | 0.002 |
| ≥ 200 | 5 | 2194 | Fixed | 4.04 (2.98–5.50) | 0 | 0.8 | 8.92 | < 0.001 |
| FIGO stage | ||||||||
| I–IV | 8 | 2541 | Fixed | 2.98 (2.33–3.82) | 44 | 0.08 | 8.66 | < 0.001 |
| III–IV | 1 | 68 | Fixed | 3.05 (1.06–8.80) | NA | NA | 2.06 | 0.04 |
| Cut-off value (×109/L) | ||||||||
| 350 | 1 | 108 | Fixed | 1.37 (0.30–6.17) | NA | NA | 0.41 | 0.68 |
| 400 | 8 | 2501 | Fixed | 3.05 (2.39–3.89) | 39 | 0.12 | 8.95 | < 0.001 |
| Analysis method | ||||||||
| Multivariate | 5 | 1053 | Fixed | 2.47 (1.78–4.42) | 43 | 0.13 | 5.44 | < 0.001 |
| Univariate | 4 | 1556 | Fixed | 3.76 (2.63–5.37) | 0 | 0.46 | 7.25 | < 0.001 |
Random-effects model was used when P-value for heterogeneity test < 0.1; otherwise, fixed-effects model was used
Abbreviations: FIGO International Federation of Gynecology and Obstetrics, HR hazard ratio, CI confidence interval, Ph, P-value for heterogeneity based on Q test, P P-value for statistical significance based on Z test, NA Not applicable
The prognostic impact of thrombocytosis on disease-free survival
Six studies contain 2460 patients were included to evaluate the correlation between thrombocytosis and DFS in EC patients. The result showed that thrombocytosis predicted a worse DFS in the fixed effects model (pooled HR = 2.86; 95% CI = 2.27–3.6; P < 0.001) (Fig. 2b).
Correlation between thrombocytosis and clinicopathological characteristics in patients with EC
We further analyzed the correlation between thrombocytosis and clinicopathologic characteristics (Table 3). Thrombocytosis was positively related to FIGO stage (odds ratio (OR) = 3.24; 95% CI =1.78–5.88; P < 0.001), tumor grade (OR = 1.89; 95% CI =1.3–2.76; P = 0.001), LN metastasis (OR = 3.13; 95% CI =1.71–5.75; P < 0.001), LVSI (OR = 1.98, 95% CI =1.34–2.94; P = 0.001), cancer recurrence (OR = 8.57; 95% CI =3.71–19.83; P < 0.001), and VTE (OR = 6.45; 95% CI =4.06–10.24; P < 0.001). However, thrombocytosis was not associated with histologic type of EC (OR = 1.45; 95% CI = 0.76–2.77; P = 0.265).
Table 3.
Analysis of the association between thrombocytosis and clinicopathological parameters of endometrial carcinoma
| Clinicopathological characteristics | Number of studies | Model | OR (95% CI) | I2 (%) | P h | Z | P |
|---|---|---|---|---|---|---|---|
| FIGO stage (III-IV vs. I-II) | 5 | Random | 3.24 (1.78–5.88) | 58.1 | 0.049 | 3.86 | < 0.001 |
| Grade (2–3 vs.1) | 6 | Fixed | 1.89 (1.30–2.76) | 19.4 | 0.287 | 3.32 | 0.001 |
| LVSI (yes vs. no) | 5 | Fixed | 1.98 (1.34–2.94) | 0 | 0.603 | 3.4 | 0.001 |
| Histological type (non-endometrioid vs. endometrioid) | 5 | Random | 1.45 (0.76–2.77) | 60.6 | 0.038 | 1.11 | 0.265 |
| LN metastasis (yes vs. no) | 2 | Fixed | 3.13 (1.71–5.75) | 0 | 0.416 | 3.69 | < 0.001 |
| Recurrence (yes vs. no) | 2 | Fixed | 8.57 (3.71–19.83) | 0 | 0.458 | 5.02 | < 0.001 |
| VTE (yes vs. no) | 2 | Fixed | 6.45 (4.06–10.24) | 0 | 0.807 | 7.91 | < 0.001 |
Random-effects model was used when P-value for heterogeneity test < 0.1; otherwise, fixed-effects model was used
Abbreviations: FIGO International Federation of Gynecology and Obstetrics, LVSI lymphovascular space invasion, LN lymph node, VTE venous thromboembolism, OR odds ratio, CI confidence interval, Ph, P-value for heterogeneity based on Q test, P P-value for statistical significance based on Z test
Publication bias and sensitivity analysis
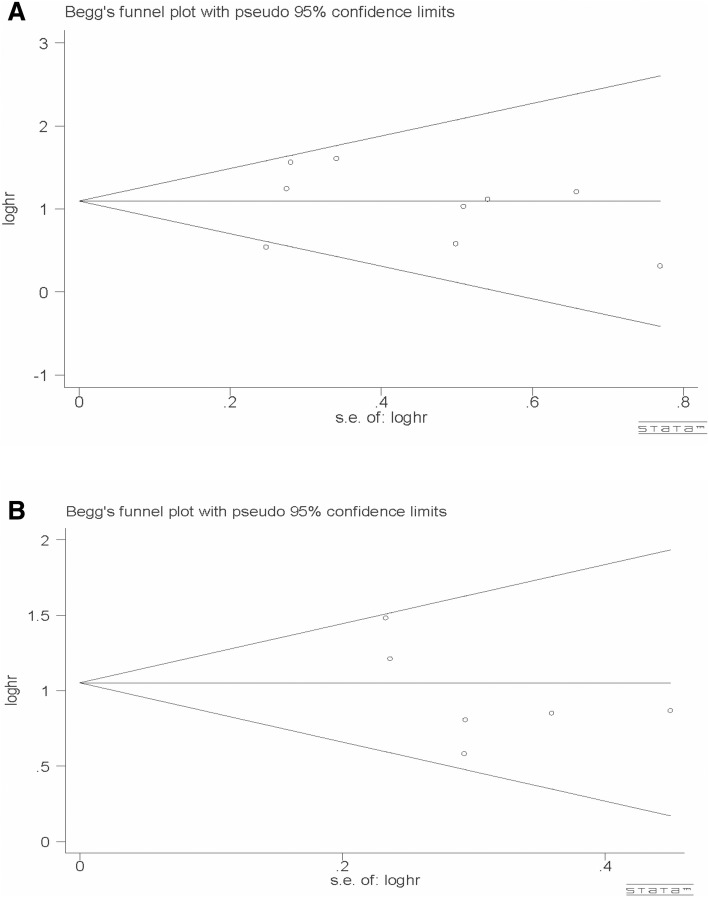
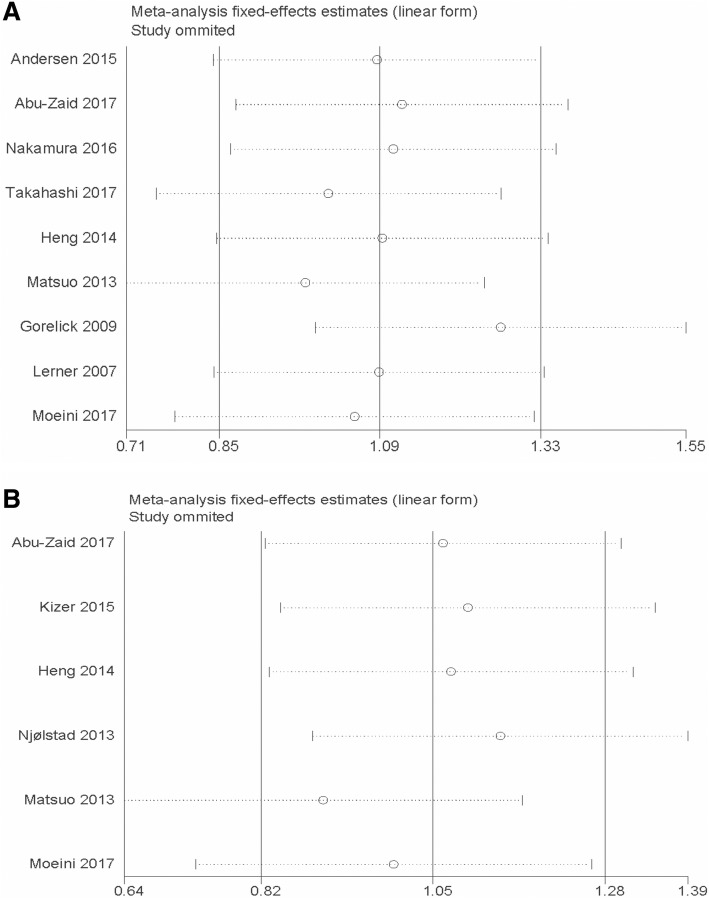
There was no apparent publication bias for OS (P = 0.917 for Begg’s test and P = 0.740 for Egger’s test) and DFS (P = 0.707 for Begg’s test and P = 0.192 for Egger’s test) (Fig. 3) in our study. Additionally, a sensitivity analysis was carried out by sequentially omitting eligible studies. The results confirmed the stability and reliability of the outcomes (Fig. 4).
Fig. 3.
Funnel plot of publication bias for overall survival (a) and disease-free survival (b)
Fig. 4.
Sensitivity analysis of the association between thrombocytosis and overall survival (a) and disease-free survival (b)
Discussion
The critical role of platelets in inflammatory and immune responses has been confirmed [33]. Several studies have indicated that elevated platelets play crucial roles in promoting cancer growth and metastasis [14]. The interactions between platelets and cancer cells activate TGFβ/Smad and NF-κB pathways, subsequently inducing the occurrence of epithelial-mesenchymal transition and promoting cancer metastasis [34, 35]. Platelets promote cancer metastasis also depending on the activating of YAP1 signaling through the RhoA / MYPT-PP1 pathway [36]. Additionally, activated platelets lead to the release of tumor growth factors and chemokines, such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and CXCL5 and CXCL7, which stimulate cancer growth and metastasis [37, 38]. Furthermore, platelets protect cancer cells from the immune clearance by natural killer cells, which also accelerates cancer metastasis [38]. Thus, there exists a complex cross-talk between platelets and cancers.
The elevated platelet counts more than 400 × 109/L often defined as thrombocytosis. Thrombocytosis has been proved to correlate with adverse prognosis in many malignancies. The incidence rate of pretreatment thrombocytosis was range from 7 to 24.8% in the included studies. Our results demonstrated that pretreatment thrombocytosis predicts a worse OS and DFS in patients with EC. In subgroup analysis, we showed that the elevated platelet count also reveals a decreased OS in patients with EC except in subgroup analysis by platelet count cut-off value. Using 350 × 109/L as platelet count cut-off value did not predict a poor prognosis.
Moreover, we investigated the association between thrombocytosis and clinicopathological characteristics in EC patients. According to our findings, pretreatment thrombocytosis was significantly associated with advanced FIGO stage, tumor grade, LVSI, LN metastasis, recurrence, and VTE. However, pretreatment thrombocytosis was not related to the histologic types.
Our meta-analysis also has some flaws. First, EC patients mostly accompanied by menorrhagia or abnormal uterine bleeding, which always leads to anemia [5]. Patients with thrombocytosis commonly coexist with iron deficiency anemia [39]. The included studies did not classify whether anemia-associated thrombocytosis or paraneoplastic thrombocytosis was related to prognosis. That may lead to potential confounding. Second, all of the included studies were retrospective studies, which may cause selection biases. Third, the cut-off values for definition of thrombocytosis differed in the included studies. Most of the studies used 400 × 109/L as cut-off value of platelet count to diagnose thrombocytosis. However, one of the studies used 350 × 109/L as the platelet count cut-off value [24]. The distinct cut-off value may lead to apparent heterogeneity between studies. Thus, establishing a consistent platelet count cutoff value to diagnose thrombocytosis is necessary. Last but not least, several factors such as patients’ age, tumor size, adjuvant therapy, which will affect platelet count did not include in our analysis. Therefore, more studies are needed to verify our findings.
Conclusions
In conclusion, this systematic review demonstrated that pretreatment thrombocytosis is correlated with poor survival outcome and adverse clinicopathological parameters in EC, and thrombocytosis is a potential prognosis predictor for EC.
Additional files
The PRISMA 2009 checklist. (DOC 66 kb)
The Newcastle-Ottawa quality assessment scale. (DOCX 16 kb)
Acknowledgments
Not Applicable.
Funding
No funding was obtained for this study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CI
confidence interval
- DFS
disease-free survival
- EC
endometrial carcinoma
- FIGO
International Federation of Gynecology and Obstetrics
- HR
hazard ratio
- LN
lymph node
- LVSI
lymphovascular space invasion
- OR
odds ratio
- OS
overall survival
- VTE
venous thromboembolism
Authors’ contributions
D.N. conceived the analysis, performed literature search, and wrote the manuscript; E.Y. contributed to the analysis; Z.Y.L. conceived the analysis, contributed to the analysis. All authors approved the final version of the manuscript.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dan Nie, Email: niedan@swmu.edu.cn.
E. Yang, Email: 2273473821@qq.com
Zhengyu Li, Phone: +86 18982151025, Email: zhengyuli@scu.edu.cn.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15(7):e268–e278. doi: 10.1016/S1470-2045(13)70591-6. [DOI] [PubMed] [Google Scholar]
- 4.Lewin SN, Herzog TJ, Barrena MNI, et al. Comparative performance of the 2009 international federation of gynecology and obstetrics' staging system for uterine corpus cancer. Obstet Gynecol. 2010;116(5):1141–1149. doi: 10.1097/AOG.0b013e3181f39849. [DOI] [PubMed] [Google Scholar]
- 5.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387(10023):1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 6.Nie D, Zhang L, Guo Q, Mao X. High mobility group protein A2 overexpression indicates poor prognosis for cancer patients: a meta-analysis. Oncotarget. 2018;9:1237–1247. doi: 10.18632/oncotarget.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012;13(8):e353–e361. doi: 10.1016/S1470-2045(12)70213-9. [DOI] [PubMed] [Google Scholar]
- 8.Tejerizo-García A, Jiménez-López JS, Muñoz-González JL, et al. Overall survival and disease-free survival in endometrial cancer: prognostic factors in 276 patients. Onco Targets Ther. 2013;9:1305–1313. doi: 10.2147/OTT.S51532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Ran Y. Prognostic role of elevated platelet count in patients with lung cancer: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(4):5379–5387. [PMC free article] [PubMed] [Google Scholar]
- 10.Bensalah K, Leray E, Fergelot P, et al. Prognostic value of thrombocytosis in renal cell carcinoma. J Urol. 2006;175(3 Pt 1):859–863. doi: 10.1016/S0022-5347(05)00526-4. [DOI] [PubMed] [Google Scholar]
- 11.Wang YH, Kang JK, Zhi YF, et al. The pretreatment thrombocytosis as one of prognostic factors for gastric cancer: a systematic review and meta-analysis. Int J Surg. 2018;53:304–311. doi: 10.1016/j.ijsu.2018.03.084. [DOI] [PubMed] [Google Scholar]
- 12.Josa V, Krzystanek M, Eklund AC, Salamon F, Zarand A, Szallasi Z, Baranyai Z. Relationship of postoperative thrombocytosis and survival of patients with colorectal cancer. Int J Surg. 2015;18:1–6. doi: 10.1016/j.ijsu.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Pang Q, Qu K, Zhang JY, et al. The prognostic value of platelet count in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94(37):e1431. doi: 10.1097/MD.0000000000001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavie O, Comerci G, Daras V, Bolger BS, Lopes A, Monaghan JM. Thrombocytosis in women with vulvar carcinoma. Gynecol Oncol. 1999;72(1):82–86. doi: 10.1006/gyno.1998.5225. [DOI] [PubMed] [Google Scholar]
- 16.Zhao K, Deng H, Qin Y, Liao W, Liang W. Prognostic significance of pretreatment plasma fibrinogen and platelet levels in patients with early-stage cervical cancer. Gynecol Obstet Investig. 2015;79(1):25–33. doi: 10.1159/000365477. [DOI] [PubMed] [Google Scholar]
- 17.Gucer F, Moser F, Tamussino K, Reich O, Haas J, Arikan G, Petru E, Winter R. Thrombocytosis as a prognostic factor in endometrial carcinoma. Gynecol Oncol. 1998;70:210–214. doi: 10.1006/gyno.1998.5078. [DOI] [PubMed] [Google Scholar]
- 18.Lerner DL, Walsh CS, Cass I, Karlan BY, Li AJ. The prognostic significance of thrombocytosis in uterine papillary serous carcinomas. Gynecol Oncol. 2007;104:91–94. doi: 10.1016/j.ygyno.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Gorelick C, Andikyan V, Mack M, Lee YC, Abulafia O. Prognostic significance of preoperative thrombocytosis in patients with endometrial carcinoma in an inner-city population. Int J Gynecol Cancer. 2009;19(8):1384–1389. doi: 10.1111/IGC.0b013e3181a47d47. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo K, Wu E, Yessaian A, Lin Y, Pham H, Muderspach L, Liebman H, Morrow C, Roman L. Predictive model of venous thromboembolism in endometrial cancer. Gynecologic OncologyGynecol Oncol. 2013;130:e56–56e57. doi: 10.1016/j.ygyno.2013.04.195. [DOI] [PubMed] [Google Scholar]
- 21.Heng S, Benjapibal M. Preoperative thrombocytosis and poor prognostic factors in endometrial cancer. Asian Pac J Cancer Prev. 2014;15(23):10231–10236. doi: 10.7314/APJCP.2014.15.23.10231. [DOI] [PubMed] [Google Scholar]
- 22.Kaloglu S, Guraslan H, Tekirdag AI, Dagdeviren H, Kaya C. Relation of preoperative thrombocytosis between tumor stage and grade in patients with endometrial Cancer. Eurasian J Med. 2014;46(3):164–168. doi: 10.5152/eajm.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kizer NT, Hatem H, Nugent EK, Zhou G, Moore K, Heller P, Mutch DG, Thaker PH. Chemotherapy response rates among patients with endometrial Cancer who have elevated serum platelets. Int J Gynecol Cancer. 2015;25:1015–1022. doi: 10.1097/IGC.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura K, Nakayama K, Ishikawa M, et al. High pretreatment plasma D-dimer levels are related to shorter overall survival in endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. 2016;201:89–93. doi: 10.1016/j.ejogrb.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 25.Abu-Zaid A, Alsabban M, Abuzaid M, AlOmar O, Salem H, Al-Badawi IA. Preoperative thrombocytosis as a prognostic factor in endometrioid-type endometrial carcinoma. Ann Saudi Med. 2017;37(5):393–400. doi: 10.5144/0256-4947.2017.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moeini A, Machida H, Takiuchi T, Blake EA, Hom MS, Miki T, Matsuo O, Matsuo K. Association of Nonalcoholic Fatty Liver Disease and Venous Thromboembolism in women with endometrial Cancer. Clin Appl Thromb Hemost. 2017;23:1018–1027. doi: 10.1177/1076029616665925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi R, Mabuchi S, Kuroda H, et al. The significance of pretreatment thrombocytosis and its association with neutrophilia in patients with surgically treated endometrial Cancer. Int J Gynecol Cancer. 2017;27(7):1399–1407. doi: 10.1097/IGC.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 28.Andersen CL, Eskelund CW, Siersma VD, Felding P, Lind B, Palmblad J, Bjerrum OW, Friis S, Hasselbalch HC, de Fine Olivarius N. Is thrombocytosis a valid indicator of advanced stage and high mortality of gynecological cancer. Gynecol Oncol. 2015;139:312–318. doi: 10.1016/j.ygyno.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Njølstad TS, Engerud H, Werner HM, Salvesen HB, Trovik J. Preoperative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomas. Gynecol Oncol. 2013;131:410–415. doi: 10.1016/j.ygyno.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 32.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 33.Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 34.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho MS, Bottsford-Miller J, Vasquez HG, et al. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120(24):4869–4872. doi: 10.1182/blood-2012-06-438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haemmerle M, Taylor ML, Gutschner T, et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun. 2017;8(1):310. doi: 10.1038/s41467-017-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N. Platelets in cancer metastasis: to help the "villain" to do evil. Int J Cancer. 2016;138(9):2078–2087. doi: 10.1002/ijc.29847. [DOI] [PubMed] [Google Scholar]
- 38.Tesfamariam B. Involvement of platelets in tumor cell metastasis. Pharmacol Ther. 2016;157:112–119. doi: 10.1016/j.pharmthera.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Akan H, Güven N, Aydogdu I, Arat M, Beksaç M, Dalva K. Thrombopoietic cytokines in patients with iron deficiency anemia with or without thrombocytosis. Acta Haematol. 2000;103:152–156. doi: 10.1159/000041038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The PRISMA 2009 checklist. (DOC 66 kb)
The Newcastle-Ottawa quality assessment scale. (DOCX 16 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.