Abstract
Purpose
A retrospective, cohort study was conducted between 2009 and 2017 in a private infertility center to determine the predictive value of endogenous estrogen (E2) and progesterone (P4) levels in hormone‐replacement frozen embryo replacement (FER) treatment cycles.
Methods
A total of 120 consecutive, infertile patients who became pregnant after FER cycles were analyzed (age: 37.4 ± 4.4 years). Electively vitrified blastocysts were created during natural cycle IVF or mild ovarian stimulation treatments and subsequently transferred through delayed vitrified‐thawed blastocyst transfer cycles supplemented with estrogens and a combination of synthetic progestogens. Serum E2 and progesterone P4 levels were intensively monitored every five days (from the day after embryo transfer until 9w1d of pregnancy) and compared among patients with a subsequent live birth (n = 76) or first‐trimester pregnancy loss (n = 44).
Results
Endogenous placental activity started as early as 5‐6th pregnancy week differing significantly according to pregnancy outcome. For P4, the exponential rise from 6w2d onwards allowed distinguishing between failing and successful conceptions. For P4, lower quartiles of the live birth group did not intersect with upper quartiles of the miscarriage group.
Conclusions
Innovative FER protocols incorporating synthetic progestogens allow the correct measurement of endogenous placental activity and could help to monitor early first‐trimester ART pregnancies.
Keywords: blastocyst transfer, dydrogesterone, fertilization in vitro, placental progesterone, single embryo transfer
1. INTRODUCTION
Frozen embryo replacement (FER) cycles are on the increase worldwide due to the introduction of more efficient cryopreservation methods, the push toward an elective single embryo transfer (SET) policy, as well as the spread of segmented IVF cycles in cases of premature progesterone elevation, preimplantation genetic testing (PGT), or the recently advocated “freeze‐all” strategy1. In Japan, these trends are particularly strong, and according to a national IVF registry, in 2015, frozen‐thawed cycles constituted approximately 70% of fresh treatments, whereas single embryo transfer was the highest in the world at 80%2.
Parallel to these developments, endometrial preparation protocols have evolved little since their first description3. To date, according to several systematic reviews, no endometrial preparation regime or a combination of specific drugs has proved itself superior compared to any other protocol.4, 5 Supplemented estrogen/progesterone‐based protocols are convenient because they require minimal monitoring and could be easily scheduled. However, compared to natural cycles, they are associated with an increased medication cost, potential side effects, and inconveniences of vaginal or intramuscular administration.1 Therefore, less‐investigated synthetic progestogens (especially dydrogesterone) could become a very useful alternative to the more widely used natural progesterone preparations, and they could also allow the precise monitoring of endogenous placental activity.
Thus, the aim of this retrospective review was to examine placental steroid output in FER cycles supplemented with estrogens and a unique combination of synthetic progestogens in a cohort of intensively monitored pregnant patients. This design has also enabled us to precisely time the onset of the luteo‐placental shift during an early first‐trimester pregnancy.
2. MATERIALS AND METHODS
2.1. Study patients
This retrospective review included all consecutive patients (n = 126) from a single center (Kobe Motomachi Yume Clinic, Kobe, Japan) between June 2009 and April 2017 who became pregnant after single vitrified‐warmed blastocyst transfer (SVBT) cycles and satisfied the following inclusion criteria: (a) underwent artificial endometrial preparation with oral estrogens and a combination of synthetic progestogens only (patients with other protocols were not included), (b) completed intensive hormonal monitoring with measurements of their endogenous serum estrogen (E2) and progesterone (P4) levels every five days (from the day after embryo transfer until 9w1d of pregnancy), (c) the first ultrasound examination showed the presence of at least one gestational sac (thus, biochemical pregnancies were not included), and (d) pregnancy outcome was known to be either live birth (n = 76) or a first‐trimester miscarriage (n = 44). Exclusion criteria were as follows: monozygotic twinning (n = 5) or a voluntary termination of pregnancy (n = 1). This retrospective was based on anonymized, retrospective review of data and involved established clinical procedures performed routinely in our center, and its research protocol was approved by a local Institutional Review Board.6
2.2. Patient screening, ovarian stimulation, and laboratory procedures
Before treatment starts, all women had a normal basic fertility workup, including hysterosonography, hysterosalpingography, or laparoscopy in most of them. Unstimulated natural cycle IVF or clomiphene citrate‐based minimal stimulation was used as a mainstream treatment in our clinic. Details of these protocols were described previously.7, 8 Fertilized zygotes were cultured until blastocyst stage and vitrified electively.9 Since the inception of our center, only single embryo transfers (SET) were performed and a universal SET policy was strictly observed. These delayed FERs were usually performed within the 3‐month period following the oocyte retrieval from where embryos originated.
2.3. Endometrial preparation protocol and luteal support
Details of endometrial preparation and luteal support in FER protocols are summarized in Figure 1. In cycling patients, oral estrogen tablets 4‐6 mg/d (Julina, Bayer, Japan) were started from the onset (day 2‐3) of a spontaneous menstrual bleeding and continued throughout the endometrial preparation. Endometrial thickness was checked by transvaginal ultrasound at day 10, and if reaching at least 7 mm, oral dydrogesterone tablets 30 mg/d (Duphaston, Mylan EPD, Japan) were started from day 11. Four days afterward (day 14), oral, combined contraceptive tablets (containing 0.05 mg ethinylestradiol, 0.5 mg norgestrel) were also added (Planovar; Aska Pharmaceutical Co., Tokyo, Japan) in order to block the development of any dominant follicle and prevent spontaneous LH surges. Vitrified‐thawed, single blastocyst transfer was scheduled four days later (day 18), and the above drugs were continued until the pregnancy test day. To avoid any effect on endogenous progesterone levels, patients in this study have not used any luteal support containing natural progesterone preparations. β‐hCG levels were tested three times; at first five days following embryo transfer and twice afterward (4w1d and 4w6d) to confirm steadily rising levels and to exclude biochemical pregnancies. Patients in this study have undergone intensive hormonal monitoring with measurement of their endogenous serum E2 and P4 levels every five days (from the day after embryo transfer until 9w1d). A first transvaginal ultrasound examination was performed at around 4‐5 pregnancy weeks. Patients with an ultrasound‐confirmed ongoing pregnancy reaching at least nine pregnancy weeks were discharged to their treating gynecologists for further obstetrical care and were only contacted later when ascertaining the final reproductive outcome.
Figure 1.
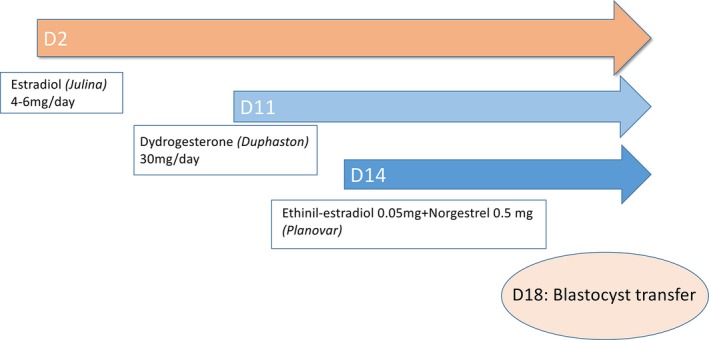
Endometrial preparation protocol and follow‐up
2.4. Hormonal assays
Sera were analyzed for E2 and P4 at the clinic immediately after blood drawing using the iE2 and PROGIII enzyme immunoassay kit according to the manufacturer's instructions (Tosoh Corp., Tokyo, Japan). The synthetic progestogens used (dydrogesterone and norgestrel) did not have any cross‐reaction with the progesterone assay.
2.5. Outcome measures and statistical analysis
Early, first‐trimester miscarriage was defined as pregnancy loss occurring after detection of a gestational sac following an initial scanning at 4‐5 weeks. Live birth was defined as a child born after 22 weeks or weighing at least 500 g. Hormonal levels of the live birth and miscarriage groups were compared using the Mann‐Whitney U test due to non‐normal distribution. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Reproductive outcome and patient age
Out of a total 120 pregnant patients with a confirmed gestational sac, 44 (36%) suffered a first‐trimester pregnancy loss and 76 (64%) achieved a singleton live birth. Patients with a pregnancy loss were significantly older compared to their counterparts who achieved a live birth (40.2 ± 3.2 vs 35.8 ± 4.3 years, P < 0.0001). The endometrial thickness measured during the first phase of estrogen supplementation was slightly thinner in the pregnancy loss group (9.7 ± 1.9 vs 10.4 ± 1.8 mm, P = 0.036).
3.2. Serum hormonal levels during the first trimester
In both groups, serum hormonal levels were steady until 4w1d for E2 and 5w4d for P4. For P4, they were uniformly very low (<1 ng/mL) indicating the absence of any endogenous placental activity, whereas for E2, physiological levels were observed (200‐300 pg/mL) as expected following oral estrogen supplementation. From 4w6d onwards for E2 and somewhat later at 6w2d for P4, there was an increase for both steroid hormones heralding the onset of placental secretion. This increase was exponential in the live birth group and much more pronounced for P4 than E2. On the other hand, in the miscarriage group, E2 started to plateau and P4 has risen only weakly. For P4, from 6w2d onwards, the lower quartiles (bottom of the box) of the live birth group did not intersect with the upper quartiles (top of the box) of the miscarriage group. For E2, however, such distinction was only observed toward the end of the follow‐up period after 8w3d. Serum E2 and P4 levels until 9w1d are summarized in Tables 1‐2 and depicted as box plots in Figures 2‐3.
Table 1.
Quartiles of serum E2 hormonal levels (pg/ml) according to pregnancy outcome, Q1: first quartile, Q3: third quartile
| 2w5d | 3w3d | 4w1d | 4w6d | 5w4d | 6w2d | 7w0d | 7w5d | 8w3d | 9w1d | |
|---|---|---|---|---|---|---|---|---|---|---|
| Live birth | ||||||||||
| Max | 411 | 332 | 374 | 537 | 826 | 1103 | 1888 | 2049 | 2500 | 2563 |
| Q3 | 266 | 233 | 250 | 339 | 467 | 703 | 873 | 1140 | 1248 | 1390 |
| Median | 213 | 192 | 201 | 302 | 386 | 543 | 680 | 886 | 1029 | 1104 |
| Q1 | 174 | 150 | 170 | 260 | 329 | 426 | 534 | 706 | 796 | 877 |
| Min | 119 | 107 | 112 | 132 | 210 | 263 | 339 | 356 | 548 | 538 |
| Miscarriage | ||||||||||
| Max | 382 | 400 | 314 | 437 | 549 | 750 | 895 | 1085 | 1167 | 918 |
| Q3 | 288 | 236 | 255 | 320 | 392 | 470 | 641 | 725 | 703 | 854 |
| Median | 218 | 204 | 215 | 286 | 352 | 396 | 485 | 559 | 566 | 584 |
| Q1 | 185 | 165 | 176 | 248 | 298 | 334 | 421 | 437 | 478 | 494 |
| Min | 121 | 111 | 82 | 194 | 207 | 21 | 167 | 9 | 421 | 46 |
| P | NS | NS | NS | NS | ** | *** | *** | *** | *** | *** |
Mann‐Whitney U test, NS >0.05, *<0.05, **<0.01, ***<0.001, ****<0.0001.
Table 2.
Quartiles of serum P4 hormonal levels (ng/ml) according to pregnancy outcome, Q1: first quartile, Q3: third quartile
| 2w5d | 3w3d | 4w1d | 4w6d | 5w4d | 6w2d | 7w0d | 7w5d | 8w3d | 9w1d | |
|---|---|---|---|---|---|---|---|---|---|---|
| Live birth | ||||||||||
| Max | 2.7 | 2.3 | 2.4 | 2.2 | 2.0 | 5.2 | 14.7 | 24.2 | 24.4 | 29.3 |
| Q3 | 0.7 | 0.6 | 0.67 | 0.6 | 1.0 | 2.2 | 5.2 | 9.8 | 13.4 | 18.6 |
| Median | 0.6 | 0.5 | 0.5 | 0.6 | 0.8 | 1.72 | 3.7 | 7.4 | 10.9 | 13.9 |
| Q1 | 0.45 | 0.4 | 0.45 | 0.40 | 0.6 | 1.2 | 2.5 | 4.7 | 7.2 | 10.0 |
| Min | 0.2 | 0.3 | 0.3 | 0.3 | 0.4 | 0.6 | 1.10 | 1.9 | 3.4 | 6.7 |
| Miscarriage | ||||||||||
| Max | 2.5 | 1.2 | 1.4 | 1.3 | 1.4 | 2.5 | 5.5 | 8.7 | 12.5 | 15.9 |
| Q3 | 0.6 | 0.6 | 0.5 | 0.6 | 0.7 | 1.2 | 2.3 | 3.7 | 7.9 | 6.9 |
| Median | 0.5 | 0.5 | 0.5 | 0.5 | 0.6 | 0.8 | 1.4 | 2.4 | 4.4 | 5.8 |
| Q1 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.6 | 0.9 | 1.6 | 2.9 | 3.8 |
| Min | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 1.3 | 1.7 |
| P | NS | NS | * | NS | *** | *** | *** | *** | *** | *** |
Mann‐Whitney U test, NS >0.05, *<0.05, **<0.01, ***<0.001, ****<0.0001.
Figure 2.
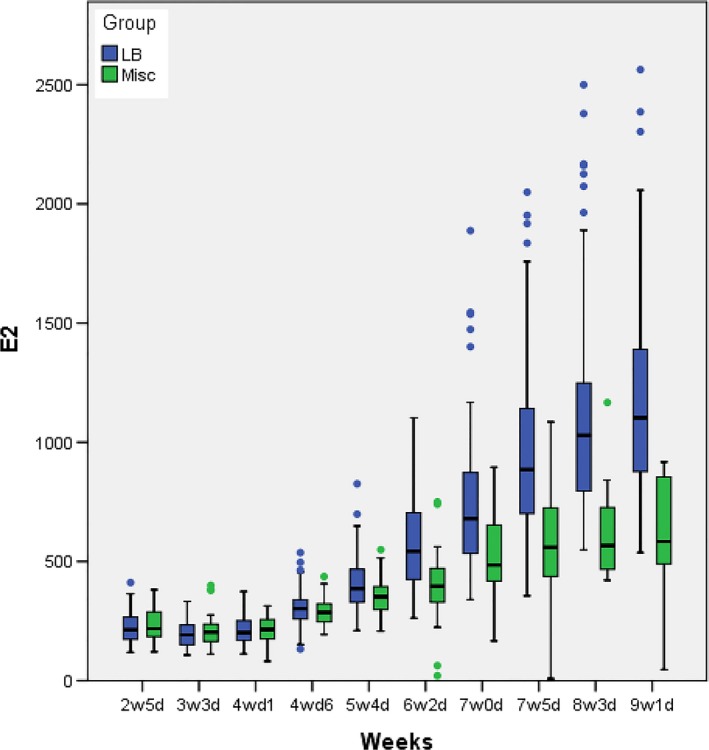
E2 hormonal levels (pg/ml) according to pregnancy outcome (LB: live birth; Misc: miscarriage)
Figure 3.
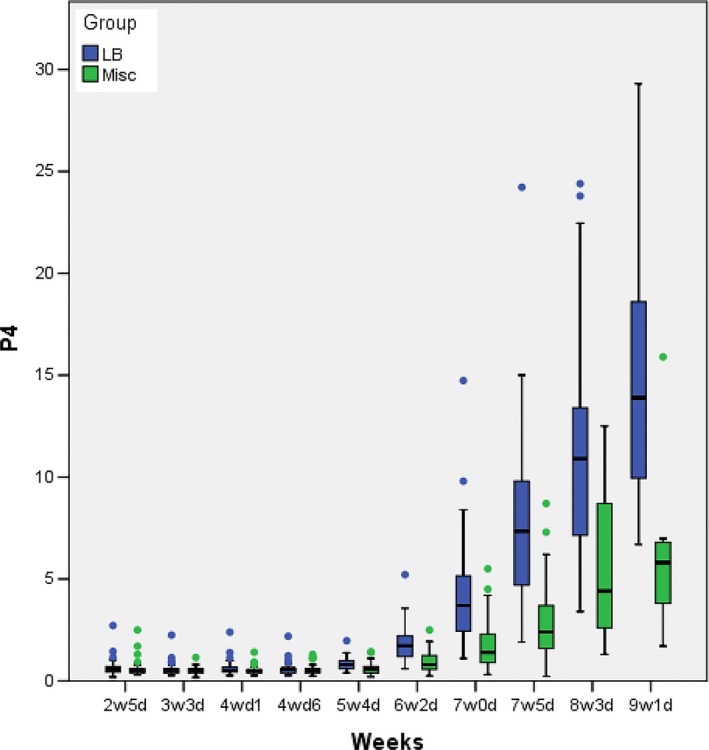
P4 hormonal levels (ng/ml) according to pregnancy outcome (LB: live birth; Misc: miscarriage)
4. DISCUSSION
Our retrospective cohort study suggests that in frozen embryo replacement cycles substituted with estrogens and a combination of synthetic progestogens, the rising endogenous progesterone levels could be used to predict the outcome of an early first‐trimester pregnancy. Moreover, this unique endometrial preparation protocol also provides evidence of an endogenous (luteo)‐placental shift occurring as early as from the 5‐6th pregnancy week.
The unique treatment approaches used in our study were derived from decades of experience in a Japanese center that has routinely used the above innovative protocols including minimal ovarian stimulation, elective embryo vitrification, and delayed vitrified‐thawed blastocyst transfers.8 In fact, our group (Kato Ladies Clinic, Tokyo and its branches) has already employed these approaches on a large‐scale basis, before embryo vitrification and the “freeze‐all” strategy was increasingly advocated in the Western reproductive literature.10 In the same manner, our endometrial preparation protocol is also quite unique in that it includes a combination of synthetic progestogens instead of the elsewhere more widely used (vaginally or intramuscularly administered) natural progesterone preparations.
So far, according to numerous reviews, no endometrial preparation regime (natural or supplemented cycle) or a combination of specific drugs has proved itself superior compared to other alternative FER protocols.4, 5 In our study, we have used dydrogesterone which is an orally administered synthetic progestogen that has been successfully used for luteal‐phase support in stimulated IVF cycles for many decades.11, 12, 13, 14, 15, 16 Due to its unique molecular structure, it has a more selective binding capacity to the natural progesterone receptor; therefore, much lower doses are required compared to micronized progesterone.17 It is cheap, has an excellent safety profile, and is associated with increased patient satisfaction due to the lack of the side effects of vaginal (irritation, discharge, bleeding, interference with sexual life) or intramuscular administration (local inflammation, abscesses, pain, anaphylactic reaction). In a recent systematic review and meta‐analysis involving eight randomized clinical trials, dydrogesterone was found to be as effective as vaginal progesterone for luteal‐phase support in stimulated IVF cycles. This was also corroborated by a more recent, large, multicentric phase III trial involving 1301 randomized subjects confirming the noninferiority of dydrogesterone (30 mg/d) to micronized vaginal progesterone (600 mg/d) in the setting of conventional IVF treatment cycles.18 However, up to day, there are no similar studies involving dydrogesterone in the setting of FER cycles.
Apart from the use of synthetic progestogens, our endometrial preparation protocol also has other distinctive features setting it apart from various other HRT‐based supplemented protocols described in the literature. When sufficient endometrial development has been detected by ultrasound scanning after 10 days of a full dose of oral estrogen administration, dydrogesterone was started from day 11 onwards. This is to mimic the slight progesterone rise (1‐3 ng/mL) which occurs in a natural cycle (12 hours to 3 days prior to ovulation) and is not thought to influence the window of implantation. Following this from day 14 onwards, an oral contraceptive pill was also added (also containing additional synthetic progestogen) and day‐5 blastocyst transfer was scheduled four days later on day 18. This is in line with the established recommendation of transferring blastocysts after 4 to 5 full days of natural progesterone supplementation.1 Although the addition of a contraceptive pill might seem unorthodox, it could be also beneficial by avoiding escape ovulations which are thought to occur in 1.9%‐7.4% of supplemented cycles without pituitary suppression. Contraceptive pill cotreatment was used in our clinic group's FER protocol for decades without observing any significant adverse effects.19, 20
Our study findings are comparable with a small US series from the 1990s, in which the onset of placental steroid production was studied in nine oocyte recipient patients.21 Although—unlike in our study—those patients were substituted with a conventional estrogen‐progesterone protocol, the authors have still managed to detect a significant increase in secreted steroid levels beginning from the 6‐7th pregnancy week onwards and estimated the onset of placental secretion at the 5th pregnancy week. This was approximately 3 weeks earlier than thought previously, based on early experimental studies of luteo‐placental shift in humans.22, 23 In our study, an unequivocal endogenous progesterone increase has appeared from the 6w2d onwards and continued exponentially in case of a successful ongoing pregnancy.
As for potential endocrine markers that could predict early pregnancy failure, progesterone currently seems to be the most promising one.24 A recent large prospective cohort study from Singapore identified a level of <11 ng/mL (35 nmol/L) that could identify women during their first trimester (4‐12 weeks) who are later likely to suffer a spontaneous miscarriage.25 Although this threshold is comparable with median P4 levels in our live birth group (around 10 ng/mL at 8‐9 weeks), it must be emphasized that our P4 levels should be inherently lower because of the absence of any internal corpus luteum production. The clinical utility of a specific threshold valid for synthetic progesterone‐supplemented FER cycles could only be evaluated in a larger, prospectively gathered dataset. Furthermore, if such a threshold is confirmed in a future interventional study, the effect of increased progesterone supplementation could be evaluated in FER cycles with low P4 levels.
The main limitation of our study is due to its retrospective nature and a relatively limited number of intensively monitored patients, even if all consecutive cases fulfilling inclusion criteria were analyzed thus excluding any potential selection bias. Also, other hormonal markers of pregnancy viability such as hCG were not evaluated as part of an intensive hormonal monitoring; therefore, it was impossible to determine their prognostic value or compare them to E2 and P4 levels.
In conclusion, our retrospective study has shown that (a) endometrial preparation protocols including synthetic progestogens allow the precise measurement of an endogenous progesterone rise of placental origin, (b) different patterns of placental E2 and P4 secretion were observed between the live birth and miscarriage groups, (c) the above could predict the outcome of an early first‐trimester pregnancy. Furthermore, the use of oral synthetic progestogens (especially dydrogesterone) could represent an efficient and convenient alternative to the more widely used natural progesterone preparations.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human rights statements and informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for the retrospective review of their anonymized data. Approval by Ethics Committee: This retrospective study was based on anonymized, retrospective review of data and involved established clinical procedures performed routinely in our center, and its research protocol was approved by a local Institutional Review Board. Animal studies: This article does not contain any studies with animal subjects performed by the any of the authors.
Kawachiya S, Bodri D, Hirosawa T, Yao Serna J, Kuwahara A, Irahara M. Endogenous progesterone levels could predict reproductive outcome in frozen embryo replacement cycles supplemented with synthetic progestogens: A retrospective cohort study. Reprod Med Biol. 2019;18:91–96. 10.1002/rmb2.12254
Presented at the 2017 ART World Congress, New York, 25‐26 October 2017.
REFERENCES
- 1. Mackens S, Santos‐Ribeiro S, van de Vijver A, et al. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod. 2017;32(11):2234‐2242. [DOI] [PubMed] [Google Scholar]
- 2. Saito H, Jwa SC, Kuwahara A, et al. Assisted reproductive technology in Japan: a summary report for 2015 by The Ethics Committee of The Japan Society of Obstetrics and Gynecology. Reprod Med Biol. 2018;17(1):20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lutjen P, Trounson A, Leeton J, Findlay J, Wood C, Renou P. The establishment and maintenance of pregnancy using in vitro fertilization and embryo donation in a patient with primary ovarian failure. Nature. 1984;307(5947):174‐175. [DOI] [PubMed] [Google Scholar]
- 4. Yarali H, Polat M, Mumusoglu S, Yarali I, Bozdag G. Preparation of endometrium for frozen embryo replacement cycles: a systematic review and meta‐analysis. J Assist Reprod Genet. 2016;33(10):1287‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. 2010;20(1):CD006359. [DOI] [PubMed] [Google Scholar]
- 6. Akabayashi A, Slingsby BT, Nagao N, Kai I, Sato H. An eight‐year follow‐up national study of medical school and general hospital ethics committees in Japan. BMC Med Ethics. 2007;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bodri D, Kawachiya S, Kondo M, Kato R, Matsumoto T. Oocyte retrieval timing based on spontaneous luteinizing hormone surge during natural cycle in vitro fertilization treatment. Fertil Steril. 2014;101(4):1001‐1007.e2. [DOI] [PubMed] [Google Scholar]
- 8. Kato K, Takehara Y, Segawa T, et al. Minimal ovarian stimulation combined with elective single embryo transfer policy: age‐specific results of a large, single‐centre, Japanese cohort. Reprod Biol Endocrinol. 2012;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73‐80. [DOI] [PubMed] [Google Scholar]
- 10. Devroey P, Polyzos NP, Blockeel C. An OHSS‐Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26(10):2593‐2597. [DOI] [PubMed] [Google Scholar]
- 11. Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, Ghosh S. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: results of a randomised study. J Steroid Biochem Mol Biol. 2005;97(5):416‐420. [DOI] [PubMed] [Google Scholar]
- 12. Ganesh A, Chakravorty N, Mukherjee R, Goswami S, Chaudhury K, Chakravarty B. Comparison of oral dydrogestrone with progesterone gel and micronized progesterone for luteal support in 1,373 women undergoing in vitro fertilization: a randomized clinical study. Fertil Steril. 2011;95(6):1961‐1965. [DOI] [PubMed] [Google Scholar]
- 13. Patki A, Pawar VC. Modulating fertility outcome in assisted reproductive technologies by the use of dydrogesterone. Gynecol Endocrinol. 2007;23(Suppl 1):68‐72. [DOI] [PubMed] [Google Scholar]
- 14. Saharkhiz N, Zamaniyan M, Salehpour S, et al. A comparative study of dydrogesterone and micronized progesterone for luteal phase support during in vitro fertilization (IVF) cycles. Gynecol Endocrinol. 2016;32(3):213‐217. [DOI] [PubMed] [Google Scholar]
- 15. Salehpour S, Tamimi M, Saharkhiz N. Comparison of oral dydrogesterone with suppository vaginal progesterone for luteal‐phase support in in vitro fertilization (IVF): A randomized clinical trial. Iranian J Reprod Med. 2013;11(11):913‐918. [PMC free article] [PubMed] [Google Scholar]
- 16. Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2015;186:49‐53. [DOI] [PubMed] [Google Scholar]
- 17. Schindler AE. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas. 2009;65(Suppl 1):S3‐S11. [DOI] [PubMed] [Google Scholar]
- 18. Tournaye H, Sukhikh GT, Kahler E, Griesinger G. A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum Reprod. 2017;32(5):1019‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dal Prato L, Borini A, Cattoli M, Bonu MA, Sciajno R, Flamigni C. Endometrial preparation for frozen‐thawed embryo transfer with or without pretreatment with gonadotropin‐releasing hormone agonist. Fertil Steril. 2002;77(5):956‐960. [DOI] [PubMed] [Google Scholar]
- 20. van de Vijver A, Drakopoulos P, Polyzos NP, et al. Vitrified‐warmed blastocyst transfer on the 5th or 7th day of progesterone supplementation in an artificial cycle: a randomised controlled trial. Gynecol Endocrinol. 2017;33(10):783‐786. [DOI] [PubMed] [Google Scholar]
- 21. Scott R, Navot D, Liu HC, Rosenwaks Z. A human in vivo model for the luteoplacental shift. Fertil Steril. 1991;56(3):481‐484. [DOI] [PubMed] [Google Scholar]
- 22. Csapo AI, Pulkkinen MO, Wiest WG. Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am J Obstet Gynecol. 1973;115(6):759‐765. [DOI] [PubMed] [Google Scholar]
- 23. Csapo AI, Pulkkinen MO, Ruttner B, Sauvage JP, Wiest, WG. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am J Obstet Gynecol. 1972;112(8):1061‐1067. [DOI] [PubMed] [Google Scholar]
- 24. Senapati S, Sammel MD, Butts SF, Takacs P, Chung K, Barnhart KT. Predicting first trimester pregnancy outcome: derivation of a multiple marker test. Fertil Steril. 2016;106(7):1725–1732.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lek SM, Ku CW, Allen JC Jr, et al. Validation of serum progesterone <35nmol/L as a predictor of miscarriage among women with threatened miscarriage. BMC Pregnancy Childbirth. 2017;17(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]


