Abstract
Tissue factor (TF) is the cellular receptor for plasma factor (F) VII/FVIIa and triggers blood coagulation. Extracellular vesicles (EVs) are small membrane vesicles that are released from activated cells and tumor cells. Different cell types, including activated monocytes and tumors cells release TF‐positive EVs into the circulation. We developed an assay to measure levels of TF activity in EVs isolated from human plasma samples. We and others have used this assay to demonstrate increased levels of EV TF activity in a variety of diseases associated with thrombosis, including cancer. These studies suggest that EV TF can be used as a biomarker of thrombotic risk. The strength of this laboratory assay is that it is relatively sensitive and specific. However, the limitations of the assay are that it is labor intensive and the coefficient of variation is too high for it to be used as a clinical assay.
Keywords: biomarker, extracellular vesicles, plasma, thrombosis, tissue factor
Essentials.
Measurement of extracellular vesicle tissue factor activity.
Activated monocytes and tumor cells release tissue factor‐positive extracellular vesicles.
Tissue factor activity can be measured in extracellular vesicles isolated from blood.
Levels of extracellular vesicle tissue factor activity in blood are increased in various diseases.
1. INTRODUCTION
Tissue factor (TF) is a transmembrane protein that binds factor (F) VII/VIIa. The TF‐FVIIa complex is the primary initiator of blood coagulation.1 There are two conformational forms of TF that have high (active) and low (encrypted) procoagulant activity.1 Submicron membrane vesicles released from various types of cells have been called microparticles, microvesicles, exosomes, ectosomes, prosteosomes, and matrix vesicles. However, there are no criteria to clearly distinguish, isolate, and identify different (sub) populations of cell‐derived vesicles. Therefore, the term “extracellular vesicles (EVs)” was introduced by International Society of Extracellular Vesicles (ISEV) for all vesicles released by cells.2 Various cell types, including activated monocytes and tumor cells, release TF‐positive EVs into the circulation.
Levels of TF can be measured using antigen and activity assays.3 We and another group developed in‐house assays that measure TF procoagulant activity.4, 5 As expected, activity assays are more sensitive than antigen assays. Indeed, we have shown that our in‐house activity assay has higher sensitivity and specificity compared with a commercial activity assay, commercial ELISAs and flow cytometry using TF‐positive EVs isolated from plasma prepared from untreated and lipopolysaccharide (LPS) treated whole blood from healthy donors.6, 7, 8, 9, 10, 11 Another disadvantage of antigen assays is that they measure all forms of TF, including degraded TF, and cannot distinguish encrypted versus active TF.
In this tutorial, we will describe our in‐house EV TF activity assay.
2. PRE‐ANALYTICAL SETTINGS
As with other assays, pre‐analytical variables can affect the results of the EV TF activity assay.3, 6 The most important pre‐analytical variable is the method used to prepare plasma. Platelet‐poor plasma (PPP) is typically prepared by a centrifugation of whole blood at 1500 to 2000 g for 15 minutes at room temperature. Platelet‐free plasma (PFP) is prepared by centrifugation of whole blood at 2500 g for 15 minutes at room temperature followed by a second centrifugation at 2500 g for 15 minutes at room temperature as described by the International Society on Thrombosis and Haemostasis Scientific and Standardization Committee Collaborative Workshop.12 Published studies describing the measurement of EV TF activity have used both PPP and PFP.13 We and others observed a 52%‐80% reduction in EV TF activity in PFP compared to PPP.6, 13 Therefore, we would expect more variability and higher EV TF activity using PPP compared with PFP.
2.1. Preparation of platelet‐free plasma
Whole blood is drawn into vacutainers containing 3.2% sodium citrate (Becton Dickinson, Franklin Lakes, NJ, USA). The first 2‐3 mL of blood is discarded because it may contain contaminating TF derived from the vessel wall. Vacutainers are gently inverted immediately after blood collection to disperse the anticoagulant. If vacutainers are transported before processing, agitation should be avoided. The time between blood collection and sample processing should be less than 1 hour to avoid activation of monocytes. PFP is prepared by double centrifugation at 2500 g for 15 minutes at room temperature.12 PFP is aliquoted (110 μL) and frozen by placing at −80°C.
2.2. Preparation of positive and negative controls
Negative control plasma is prepared using whole blood from healthy volunteers immediately after collection. Positive control plasma is prepared from whole blood stimulated with LPS (Sigma Aldrich, MO, USA, 10 μg/mL) for 5 hours at 37°C with agitation. It should be noted that volunteers exhibit a range of responses to LPS.6, 14
3. METHODS
We use a two‐stage assay to measure TF activity. The first stage involves binding of FVIIa to TF and conversion of FX to FXa. The second stage measures the amount of FXa generated in the first stage using a chromogenic substrate. For each reagent, the same lot number should be used for a given study. The positive and negative controls are used to test the activity of the reagents and monitor day‐to‐day variations. Figure 1 represents the flow chart of the methods.
Figure 1.
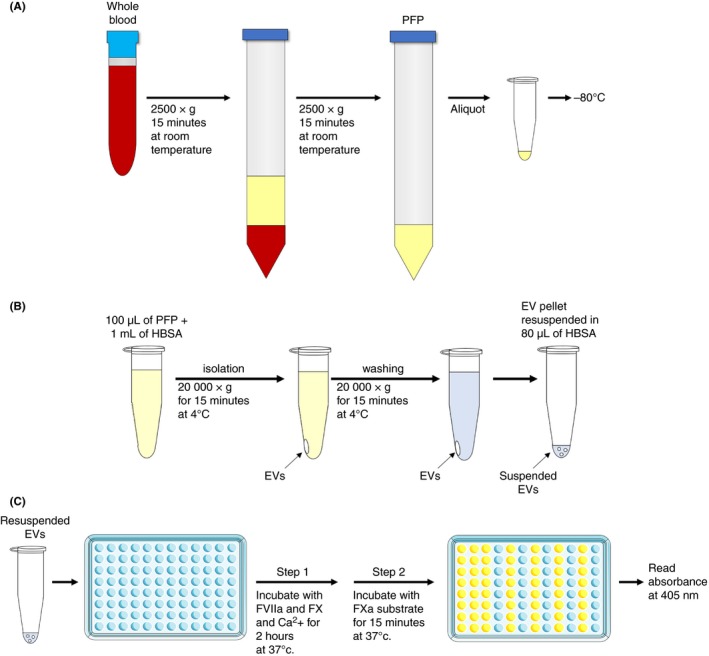
Illustrated summary of extracellular vesicle tissue factor activity assay. (A) Preparation of platelet‐free plasma (PFP). (B) Preparation of extracellular vesicle (EV) samples from PFP. (C) Measurement of EV tissue factor activity assay. Illustration was modified from Servier Medical Art, licensed under Creative Common Attribution 3.0 Unported License. (http://www.servier.fr/servier‐medical‐art)
3.1. Isolation of extracellular vesicles
PFP is thawed for 15 minutes at 37°C. It is important to thaw the samples thoroughly. One milliliter of Hepes buffer saline with bovine serum albumin (HBSA pH 7.4: 137 mmol/L NaCl, 5.38 mmol/L KCl, 5.55 mmol/L Glucose, 10 mmol/L Hepes, and 0.1% BSA) is added to 100 μL of PFP and the EVs are isolated by centrifugation at 20 000 g for 15 minutes at 4°C. After aspirating the supernatant, EVs are washed once by resuspending in 1 mL of HBSA. Samples are centrifugated under the same condition to re‐pellet the EVs. After aspirating the supernatant, EVs are resuspended in 80 μL of HBSA by pipetting (~20 times) and vortexing.
3.2. Measurement of extracellular vesicle tissue factor activity
For each assay, 40 μL of EV sample is added to wells of a 96‐well plate. Eleven microliter of an inhibitory mouse anti‐human TF antibody (clone: HTF‐1, BD Biosciences, San Jose, CA) (36.4 μg/mL [final concentration: 7.8 μg/mL]) is added to one sample while another sample receives 11 μL of a control mouse IgG (Sigma Aldrich). Plates are incubated for 15 minutes at room temperature. During the incubation time, TF standards (0, 0.32, 0.63, 1.25, 2.5, 5, 10, and 20 pg/mL) are prepared using re‐lipidated recombinant TF (Dade Innovin, Siemens, Munich, Germany). The TF concentration of Dade Innovin is not provided and must be measured using the Imubind TF‐ELISA (Sekisui Diagnostics, Lexington, MA, USA).15 Fifty microliters of each TF standard is added to each well of a 96‐well plate. Standards should be made in duplicate. After the 15 minutes incubation, 50 μL of a mixture of human FVIIa (4.8 nmol/L [final concentration: 2.4 nmol/L], Enzyme Research Laboratories, South Bend, IN, USA) and human FX (146.4 nmol/L [final concentration: 73.2 nmol/L], Enzyme Research Laboratories) in HBSA with 10 mmol/L (final concentration 5 mmol/L) calcium chloride (pH 7.4) is added to each well of the 96‐well plate and the plates sealed with an adhesive plate sealing film and incubated for 2 hours at 37°C. After the incubation, FXa generation is stopped by adding 25 μL of HBSA with 25 mmol/L (final concentration: 5 mmol/L) EDTA (pH 7.4) and incubating the samples for 5 minutes at room temperature. Next, 25 μL of chromogenic substrate (Pefachrome FXa 8595, 4 mmol/L [final concentration: 0.67 mmol/L], Enzyme Research laboratory) in water is added to each well, the 96‐well plate is sealed with an adhesive plate sealing film, wrapped in aluminum foil to keep dark and incubated for 15 minutes at 37°C. Finally, the absorbance at 405 nm is measured using a plate reader (SpectraMax M5, Molecular Device, San Jose, CA). FXa generation of each well is converted to TF activity using the standard curve of Innovin. TF‐dependent FXa generation is calculated using the following formula: TF‐dependent FXa generation (EV TF activity [pg/mL]) = Total FXa generation (control IgG well) – TF independent FXa generation (HTF‐1 well). Based on previous studies, we proposed four response classification of EV TF activity for PPP: zero (0 to <0.5 pg/mL); weak (0.5 to <1.0 pg/mL), moderate (1 to <2.0 pg/mL), and strong (>2.0 pg/mL).13
4. DISCUSSION
The advantages of our EV TF activity assay are its higher sensitivity and specificity compared with antigen assays. The disadvantages of the assays are that it is time consuming, labor intensive and has a high interassay variability. It takes ~4 to 5 hours for a well‐trained operator to run the assay. Furthermore, the assay does not separate TF‐positive EVs from other TF‐positive material in the plasma. In addition, the mean coefficient of variation (CV) for the positive control in seven independent studies was 24%6 (Y. Hisada and N. Mackman, unpubl. data). Therefore, our in‐house EV TF activity assay is too variable for use as a clinical assay.
There are two in‐house EV TF activity assays that were developed in parallel. We developed an endpoint assay and the Osanto group developed a kinetic assay.4, 5 The endpoint assay is presented pg/mL of TF whereas the kinetic assay is presented fM Xa/min. The two assays gave similar results in 54 plasma samples from pancreatic cancer patients (r = 0.61, P < 0.001).16 The conversion between the assays can be made using the following equation: y = 0.0009x + 0.0831 where y = pg/mL, x=fM Xa/min).13
We and others have used the endpoint and kinetic assays EV TF activity assays to show increased levels of EV TF activity are detected in patients with various types of disease, including cancer, endotoxemia, liver injury, cirrhosis, urinary tract infection, and influenza A infection.13, 17, 18, 19, 20, 21 In cancer, most of the TF‐positive EVs appear to be derived from tumors whereas in endotoxemia and other diseases most of the TF‐positive EVs are probably derived from activated monocytes. Pancreatic cancer has the highest EV TF activity (16‐ to 26‐fold higher than healthy controls) among different types of cancer.13 Importantly, increased levels of EV TF activity were associated with venous thromboembolism in patients with pancreatic cancer but not in other types of cancer including brain, colorectal, ovarian, and lung cancer.13, 16, 22, 23 Interestingly, patients with cirrhosis and acute liver injury have 17‐fold and 38‐fold increased levels of EV TF activity compared with healthy controls, respectively.13 Increased levels of EV TF were detected in patients with endotoxemia (nine‐fold) and urinary tract infection (six‐fold).13
We recently compared our in‐house EV TF activity assay with a commercial EV TF activity assay kit that captures TF‐positive EVs onto plates using a non‐inhibitory anti‐human TF monoclonal antibody (clone: 10H10) (Zymuphen MP‐TF, Hyphen BioMed, Neuville‐sur‐Oise, France).10 We found that our in‐house activity assay had higher sensitivity and specificity, and less background compared with the Zymuphen MP‐TF.10 The higher background of Zymuphen MP‐TF may be due to the use of a higher concentration of FVIIa (12 nmol/L) compared to our assay (2.4 nmol/L). Indeed, a previous study showed that high doses of FVIIa can activate FX in the absence of TF.24 In addition, the Zymuphen MP‐TF does not use an anti‐TF antibody to distinguish TF‐dependent versus TF‐independent FXa generation and this probably explain the lower specificity. Finally, the lower sensitivity may be due to using capture versus pelleting to isolate the TF‐positive EVs and the use of a small amount of sample (20 μL). However, a major advantage of the Zymuphen MP‐TF assay is all steps are performed in one well.
We and others continue to try to improve the sensitivity of the EV TF activity assay. A recent study reported that the sensitivity of EV TF activity assay could be significantly improved (a) using a different anti‐TF antibody (clone: SBTF1), (b) increasing the speed and time of centrifugation (24 000 g for 1 hour, twice), (c) using FVII (10 nmol/L) instead of FVIIa (10 nmol/L) (to reduce TF‐independent FXa generation), (d) increasing the FX concentration (760 nmol/L), and (e) increasing the volume of plasma (up to 1000 μL).25 We confirmed that (a) an increased time of centrifugation (1 hour), (b) use of FVII, and (c) an increased volume of plasma (250 μL) all independently increased the sensitivity of EV TF activity with a slight increasing of background (Y. Hisada and N. Mackman, unpubl. data). Indeed, we observed a 2‐ to 11‐fold increase in sensitivity when we compare our current assay with the new assay. Interestingly, the greatest increases in EV TF activity were observed in samples with lower levels of TF suggesting that we may have reached the upper limit of the assay with samples with higher activity. Another possible improvement may be using magnetic beads to isolate TF‐positive EVs from a larger volume of plasma because this can increase sensitivity and specificity, and reduce the time of isolating EVs and washing EVs.
In conclusion, our EV TF activity assays is currently the best way to detect EV TF in plasma. However, we and others are working on new assays that are less time consuming, have increased sensitivity and specificity and have a lower CV.
RELATIONSHIP DISCLOSURE
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
Y. Hisada and N. Mackman wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (Y.H. T32 HL007149) and the John C. Parker Professorship (N.M.). We would like to thank R. Lacroix and F. Dignat‐George for sharing their methods for improving the EV TF activity assay. We thank S. Grover and A. Sachetto for helpful comments and Jian‐Guo Wang for establishing the assay.
Hisada Y, Mackman N. Measurement of tissue factor activity in extracellular vesicles from human plasma samples. Res Pract Thromb Haemost. 2019;3:44–48. 10.1002/rth2.12165
REFERENCES
- 1. Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–25. [DOI] [PubMed] [Google Scholar]
- 2. Coumans FAW, Brisson AR, Buzas EI, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120:1632–48. [DOI] [PubMed] [Google Scholar]
- 3. Key NS, Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Semin Thromb Hemost. 2010;36:865–75. [DOI] [PubMed] [Google Scholar]
- 4. Khorana AA, Francis CW, Menzies KE, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle‐associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. [DOI] [PubMed] [Google Scholar]
- 6. Lee RD, Barcel DA, Williams JC, et al. Pre‐analytical and analytical variables affecting the measurement of plasma‐derived microparticle tissue factor activity. Thromb Res. 2012;129:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Claussen C, Rausch AV, Lezius S, et al. Microvesicle‐associated tissue factor procoagulant activity for the preoperative diagnosis of ovarian cancer. Thromb Res. 2016;141:39–48. [DOI] [PubMed] [Google Scholar]
- 8. Parhami‐Seren B, Butenas S, Krudysz‐Amblo J, Mann KG. Immunologic quantitation of tissue factors. J Thromb Haemost. 2006;4:1747–55. [DOI] [PubMed] [Google Scholar]
- 9. Ay C, Mackman N. Tissue factor: catch me if you can!. J Clin Oncol. 2017;35:1128–30. [DOI] [PubMed] [Google Scholar]
- 10. Tatsumi K, Antoniak S, Monroe DM 3rd, Khorana AA, Mackman N; Subcommittee on Hemostasis and Malignancy of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis . Evaluation of a new commercial assay to measure microparticle tissue factor activity in plasma: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1932–4. [DOI] [PubMed] [Google Scholar]
- 11. Bogdanov VY, Cimmino G, Tardos JG, Tunstead JR, Badimon JJ. Assessment of plasma tissue factor activity in patients presenting with coronary artery disease: limitations of a commercial assay. J Thromb Haemost. 2009;7:894–7. [DOI] [PubMed] [Google Scholar]
- 12. Lacroix R, Judicone C, Mooberry M, Boucekine M, Key NS, Dignat‐George F; The ISTH SSC Workshop . Standardization of pre‐analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2013;11:1190–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hisada Y, Alexander W, Kasthuri R, et al. Measurement of microparticle tissue factor activity in clinical samples: a summary of two tissue factor‐dependent FXa generation assays. Thromb Res. 2016;139:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egorina EM, Sovershaev MA, Bjorkoy G, et al. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25:1493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duckers C, Simioni P, Spiezia L, et al. Residual platelet factor V ensures thrombin generation in patients with severe congenital factor V deficiency and mild bleeding symptoms. Blood. 2010;115:879–86. [DOI] [PubMed] [Google Scholar]
- 16. Thaler J, Ay C, Mackman N, et al. Microparticle‐associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–70. [DOI] [PubMed] [Google Scholar]
- 17. Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle‐associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost. 2009;7:1421–3. [DOI] [PubMed] [Google Scholar]
- 18. Auwerda JJ, Yuana Y, Osanto S, et al. Microparticle‐associated tissue factor activity and venous thrombosis in multiple myeloma. Thromb Haemost. 2011;105:14–20. [DOI] [PubMed] [Google Scholar]
- 19. Woei AJFJ, De Kruif MD, Garcia Rodriguez P, Osanto S, Bertina RM. Microparticles expressing tissue factor are concurrently released with markers of inflammation and coagulation during human endotoxemia. J Thromb Haemost. 2012;10:1185–8. [DOI] [PubMed] [Google Scholar]
- 20. Woei AJFJ, van der Starre WE, Tesselaar ME, et al. Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections. Thromb Res. 2014;133:799–803. [DOI] [PubMed] [Google Scholar]
- 21. Woei AJFJ, Tesselaar ME, Garcia Rodriguez P, Romijn FP, Bertina RM, Osanto S. Tissue factor‐bearing microparticles and CA19.9: two players in pancreatic cancer‐associated thrombosis? Br J Cancer. 2016;115:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen JG, Prendergast E, Geddings JE, et al. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol Oncol. 2017;146:146–52. [DOI] [PubMed] [Google Scholar]
- 23. Gezelius E, Kristensen AF, Bendahl PO, et al. Coagulation biomarkers and prediction of venous thromboembolism and survival in small cell lung cancer: A sub‐study of RASTEN ‐ a randomized trial with low molecular weight heparin. PLoS ONE. 2018;13:e0207387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bom VJ, Bertina RM. The contributions of Ca2+, phospholipids and tissue‐factor apoprotein to the activation of human blood‐coagulation factor X by activated factor VII. Biochem J. 1990;265:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vallier L, Bouriche T, Bonifay A, et al. New specific and highly sensitive procoagulant test to measure tissue factor activity on microparticles. Res Pract Thromb Haemost. 2017;1:1087. [Google Scholar]


