Abstract
Purpose
This study evaluated the current status of reproductive disorders and provision of information on oncofertility to female adolescent and young adult (AYA) cancer patients in Japan.
Methods
A national survey of AYA cancer survivors was conducted. Children were <15 years old, and AYAs were 15‐39 years old. Results from the survivors of other than gynecological disease who underwent chemotherapy were analyzed.
Results
Among the survivors, 41.4% were concerned about their reproductive function and infertility, and 36.2% were aware of menstrual cycle abnormalities. Among them, 15.5% (n = 20) of all and 21.2% (n = 17) of the AYA‐onset survivors suffered infertility due to chemo‐ or radiotherapy and gave up childbearing. These rates were significantly higher than those of healthy AYAs. Although 80.8% of AYA‐onset survivors answered that they had received information on reproductive function and infertility, only 55.8% had received information on fertility preservation methods. Furthermore, only 22.4% of all and 42.3% of AYA‐onset survivors had received pretreatment information on fertility preservation methods.
Conclusions
Not a few AYA cancer survivors reported reproductive dysfunction. These findings indicate that information provided on therapy‐related problems before cancer treatment in Japan was insufficient and highlight the need to improve patient decision‐making and support systems for fertility preservation.
Keywords: adolescent, cancer survivors, fertility preservation, reproduction, young adults
1. INTRODUCTION
Total‐body irradiation, irradiation of the gonads, and chemotherapy regimens containing high doses of alkylators can place adolescents and young adults (AYA) with cancer at risk of decreased fertility after the successful completion of cancer treatment. The provision of information on the risk of infertility and possible interventions to maintain reproductive potential at the time of diagnosis are critical for the AYA population. The loss of reproductive potential after cancer treatment negatively impacts the quality of life of these young survivors.1, 2
In the developing field of oncofertility, for which there have been few evidence‐based studies, decision‐making requires the assessment of an individual's risk of fertility loss, and decisions regarding fertility preservation are necessarily made at a time of emotional distress.3 To further advance this field, better information should be provided to patients and their medical teams, and the provision of services should be improved to match technical and scientific advancements.3
Specialized counseling on the topic of reproductive loss and methods of fertility preservation are associated with less regret and a greater quality of life in survivors; however, few patients are exposed to this potential benefit. Women of reproductive age should receive expert counseling and be given the opportunity to make active decisions about fertility preservation.4 Patients should be given complete and detailed information about cancer treatment, subsequent fertility preservation, and ethical issues; thus, it may be preferable to have a specialized team address these issues with AYA patients rather than the patient's primary oncologist.5, 6 A medical network system with specialties beyond those found in the typical hospital setting is needed to effectively and efficiently provide information and support the decisions of patients.7
Because of the small number of available studies on the status of oncofertility information8, we decided to evaluate the oncofertility‐related information that is currently provided to female cancer patients and AYA cancer survivors in Japan. In this study, we evaluated the current status of oncofertility practice in Japan and analyzed some of the results from a large‐scale national survey that we performed.
2. MATERIALS AND METHODS
2.1. Definitions
We defined “children” as individuals of <15 years of age; “adolescents and young adults (AYAs)” as individuals of 15‐39 years of age; “survivors” as individuals who had completed their cancer therapy at least 12 months previously; and “healthy AYAs” as AYAs who had never been diagnosed as having cancer.
2.2. Surveys
For this national survey, we distributed questionnaires to AYA survivors via their doctors and patient advocacy groups from June 2016 to November 2016. The selection of survivors was made with the help of staff cooperating with the survey researchers in facilities participating in this study, the secretariats of most of the cancer‐related associations and societies in Japan, and the community through cancer clinics and patient organizations.
An additional 100 healthy female AYAs registered to a research company were recruited to complete a web‐based questionnaire. The healthy AYAs were divided into four age groups, with 25 people in each age group. Of the 752 questionnaires distributed, 261 were collected (including from men), giving a response rate of 34.7%.
2.3. Questionnaires
The questionnaire consisted of 258 questions on 10 major items and included questions on the topics of disease, influence and support, decision‐making and communication, health and mental well‐being, reproductive function and fertility, education, employment, economic status, self‐management, and background of the respondents. The present report analyzed only the topics of background and reproductive function and fertility of the female AYAs. The specific questions and answers related to reproductive function are shown in Figure 1.
Figure 1.
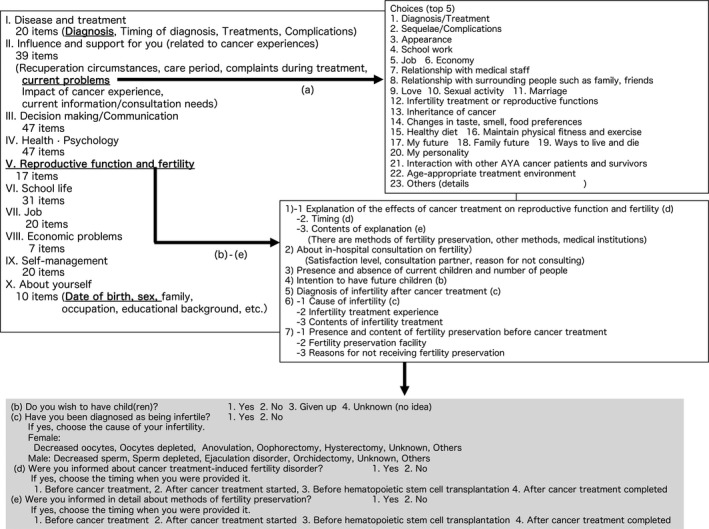
Outline of questionnaire contents. The specific questions and answers related to reproductive function are shown in the gray‐colored box
2.4. Statistical analysis
Fisher's exact test was used in the comparisons of the responses related to the problems of survivors and healthy AYAs. Differences were considered significant at P < 0.01.
2.5. Human/animal rights
This article does not contain any studies with human and animal subjects performed by any of the authors.
2.6. Ethics committee approval
This study was approved by the ethics review board of Gifu University Hospital (approval no. 28‐126). The work was conducted as a joint study by a Working Group tasked to perform a “Study on the ideal way of comprehensive care for adolescents and young adults (AYA) with cancer” (Principal Investigator: Keizo Horibe).
3. RESULTS
3.1. Demographic information of the participants
In total, there were 167 female respondents (mean age ± SD at diagnosis, 19.3 ± 11.9 years; mean age ± SD at survey, 29.2 ± 7.7 years) of whom 115 respondents were nongynecologic cancer survivors who had undergone chemotherapy (mean age ± SD at diagnosis, 16.2 ± 11.5 years; mean age ± SD at survey, 27.6 ± 7.8 years; Table 1). This subgroup included 23 breast cancer survivors and 55 hematologic malignancy survivors; 52 of the survivors were AYAs at the time of primary diagnosis. In many advanced gynecologic cancers, reproductive organs themselves are the targets of surgical treatment, and because the direction of fertility preservation, information provision in other diseases, and psychological care of patients with gynecologic cancers are different, survivors of gynecologic cancer were excluded as subjects of this study.
Table 1.
Characteristics of the participants
| Healthy female AYA | Number |
|---|---|
| Age (y) | |
| 15‐19 | 25 |
| 20‐24 | 25 |
| 25‐29 | 25 |
| 30‐39 | 25 |
| Female AYA survivors | Age at diagnosis/At survey (mean ± SD) |
|---|---|
| Total number (n = 167) | 19.3 ± 11.9 (19)/29.2 ± 7.7 (31) |
| Underwent chemotherapy (n = 130) | |
| Gynecologic cancer as primary disease (n = 28) | |
| Patients with non‐gynecological diseases undergoing chemotherapy | |
| Number of patients (n = 115) | 16.2 ± 11.5 (14)/27.6 ± 7.8 (27) |
| Breast cancer (n = 23) | 31.4 ± 3.4 (32)/35.9 ± 3.1 (37) |
| Hematologic malignancy (n = 55) | 12.0 ± 9.4 (11)/26.2 ± 7.4 (26) |
| AYA at primary diagnosis (n = 52) | 27.2 ± 7.3 (29)/32.3 ± 6.6 (35) |
AYA, adolescent and young adults.
3.2. Primary nongynecological diseases of AYA survivors undergoing chemotherapy
The primary diseases of the AYA survivors who had undergone chemotherapy are shown Figure 2. In total, 20% were survivors of breast cancer, 47% were survivors of hematological disease, and 30% were survivors of other types of cancer. When the survivor population was limited only to the AYA‐onset survivors, 45% were survivors of breast cancer, 33% were survivors of hematological disease, and 22% were survivors of other types of cancer.
Figure 2.
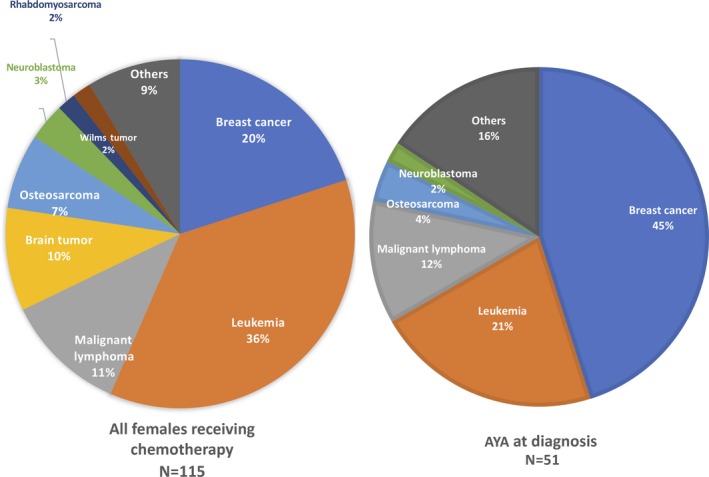
Primary nongynecological diseases of adolescent and young adult (AYA) cancer survivors who had received chemotherapy. Left panel: all survivors, right panel: AYA at the diagnosis
3.3. Reproductive problems and irregular menstruation or amenorrhea as delayed effects
Table 2 summarizes the survivors who listed reproductive function as one of their top five “current problems” and the survivors who were aware of menstrual cycle abnormalities as a late effect of cancer therapy. Among them, 41.4% of the overall survivors, 69.6% of the breast cancer survivors, and 34.8% of the nonbreast cancer survivors were concerned about infertility and their reproductive function. The rates among AYA survivors were significantly higher in comparison to those of healthy AYAs. Among the survivors, 36.2% of the overall survivors, 30.4% of the breast cancer survivors, and 38.0% of the nonbreast cancer survivors were aware of menstrual cycle abnormalities. This suggests that disorders of ovulation were the main cause of reproductive dysfunction among the AYA survivors.
Table 2.
Patients with reproductive problems and irregular menstruation or amenorrhea as delayed effects
| Healthy AYA age groups |
Respondents with infertility or problems with reproductive function No. (%) |
|---|---|
| 15‐39 y (n = 100) | 3 (3.0)—(a) |
| 30‐39 y (n = 25) | 3 (12.0)—(b) |
| AYA survivors who underwent chemotherapy | ||
|---|---|---|
| Number of respondents with infertility or problems with reproductive function | Irregular menstruation or amenorrhea as late effect | |
| All, n = 115 | 48 (41.4%) vs (b) P < 0.01 | 42 (36.2%) |
| Excluding breast cancer, n = 92 | 32 (34.8%) vs (b) P < 0.05 | 35 (38.0%) |
| Breast cancer, n = 23 | 16 (69.6%) vs (b) P < 0.01 | 7 (30.4%) (excludes eight survivors with amenorrhea induced by hormone therapy) |
| Hematologic malignancy, n = 55 | 19 (34.5%) vs (b) 0.05 < P<0.10, vs (a) P < 0.01 | 23 (41.8%) |
| Excluding breast cancer and hematologic malignancy, n = 37 | 13 (35.1%) vs (b) 0.05 < P<0.10, vs (a) P < 0.01 | 12 (32.4%) |
| AYA at primary diagnosis, n = 52 | 27 (51.9%) vs (b) P < 0.01, vs (a) P < 0.01 | 19 (36.5%) |
AYA, adolescent and young adults.
3.4. Infertility and hope for childbearing after cancer therapy
Twenty overall survivors (Table 3), 3 breast cancer survivors, and 17 nonbreast cancer survivors were diagnosed as being infertile, and in 17, 2, and 15 of these patients, respectively, the infertility was caused by chemotherapy or radiotherapy. Among the survivors, 15.5% of overall survivors, 15.2% of the breast cancer survivors, 17.4% of the nonbreast cancer survivors, and 21.2% of the AYA‐onset survivors responded that “I want a child but gave up.” These rates were significantly higher in comparison to those of the healthy AYAs (Table 3).
Table 3.
Patients with infertility and hope for childbearing after cancer therapy
|
Healthy female AYA desiring childbearing Yes/No/Gave up/Unknown |
|
|---|---|
| Age (y) | |
| 15‐19 | 19/2/0/4 |
| 20‐24 | 18/2/0/5 |
| 25‐29 | 19/1/0/5 |
| 30‐39 | 8/10/0/7 |
| Recognition of AYA survivors who underwent chemotherapy | |||
|---|---|---|---|
| Diagnosed as infertile (Unknown/No answer) | Chemotherapy or irradiation as cause of infertility |
Desire childbearing Yes/No/Gave up/Unknown/No answer |
|
| All (n = 115) | 20 (43/12) | 17 |
57/8/18/17/15 Gave up =15.5% |
| Excluding breast cancer (n = 92) | 17 (31/12) | 15 |
47/4/14/12 Gave up =15.2% |
| Breast cancer (n = 23) | 3 (12/0) | 2 |
10/4/4/5 Gave up =17.4% |
| Hematologic malignancy (n = 55) | 11 (20/7) | 10 |
24/2/12/7 Gave up =21.8% |
| Excluding breast cancer and hematologic malignancy (n = 37) | 6 (11/5) | 5 | 23/2/2/5 Gave up =5.4% |
| AYA at primary diagnosis (n = 52) | 10 (19/1) | 9 |
24/8/11/7 Gave up =21.2% |
AYA, adolescent and young adults.
3.5. Provision of oncofertility information
Among the survivors, 56.0% of the overall survivors, 48.6% of the survivors of other than hematological disease or breast cancer, 47.8% of the survivors of other than breast cancer, and 80.8% of the AYA‐onset survivors responded that “I received information on infertility and reproductive dysfunction as a side effect of cancer treatment.” However, the rate of patients who responded that they received this information was very high (91.3%) in the breast cancer survivors; thus, a disparity due to the type of disease and clinical specialty was recognized.
In contrast, 30.2% of the overall survivors and 78.3% of the breast cancer survivors indicated that they were provided with information on infertility and reproductive dysfunction before the start of treatment. However, 22.4% of the overall survivors and 60.9% of the breast cancer survivors indicated that they had been told that “there are methods to preserve fertility” before they underwent cancer treatment (Table 4).
Table 4.
Patients receiving oncofertility information
| AYA survivors who underwent chemotherapy | ||
|---|---|---|
| No. of patients provided information on fertility damage/methods to preserve fertility (%) | No. of patients provided information on fertility damage/methods to preserve fertility before cancer therapy (%) | |
| Total number (n = 115) | 65/37 (56.0/31.9) | 35/26 (30.2/22.4) |
| Excluding breast cancer (n = 92) | 44/20 (47.8/21.7) | 17/12 (18.5/13.0) |
| Breast cancer (n = 23) | 21/17 (91.3/73.9) | 18/14 (78.3/60.9) |
| Hematological diseases (n = 55) | 26/10 (47.3/18.2) | 9/6 (16.4/10.9) |
| Excluding breast cancer and hematological diseases (n = 37) | 18/10 (48.6/2.7) | 8/6 (21.6/16.2) |
| AYA at primary diagnosis (n = 52) | 42/29 (80.8/55.8) | 29/22 (55.8/42.3) |
AYA, adolescent and young adults.
4. DISCUSSION
Few studies have been reported on the provision of oncofertility information8. Young patients with breast cancer are now much more frequently provided with information on treatment‐related infertility than in the past; however, only 56% of the breast cancer patients under 40 years of age were provided with information on fertility‐related issues.9, 10 In the present study, we used an approach similar to that of previous studies.9, 10 Our findings essentially showed good agreement with those studies and extend their observations in several aspects, ie, that 41.4% of the survivors and 36.2% of the entire study population were concerned about infertility and reproductive function and were aware of menstrual cycle abnormalities suggesting that ovulation disorder is the main cause of reproductive dysfunction in AYA survivors. Twenty of our overall survivors were diagnosed as being infertile, and in 17 this was caused by chemotherapy or radiotherapy. Among the survivors, 15.5% of the overall survivors and 21.2% of the AYA‐onset survivors answered that “I want a child but gave up,” which was significantly higher than the rate in healthy AYAs. Although 80.8% of the AYA‐onset survivors answered that they were provided with information on reproductive function and infertility, only 55.8% of them were provided with information on fertility preservation methods. Furthermore, only 22.4% of the overall survivors and 42.3% of the AYA‐onset survivors were provided with information on fertility preservation methods before the start of treatment. Considering the main age group of the patients and the cancer treatments undergone, breast cancer and hematopoietic malignancy are the most common cancer types requiring fertility preservation. Also, there are major differences in the primary disease between childhood‐onset and AYA‐onset cancer. Therefore, Table 1 shows the initial age and current age of all, breast cancer, hematopoietic malignancy, and AYA‐onset survivors. Overall, the number of respondents with pediatric onset in this survey was large, and the age at onset was low. In contrast, in the breast cancer patients, the age at onset and current age were higher than the those in the other groups.
In addition, in Tables 2, 3, 4, we show the results of our analysis of current problems and complications related to reproductive function, giving up on having children, and information provision. In the survivors, the frequency of complications related to reproductive function and other problems was high, as was the proportion of those who gave up on having children. Interestingly, this tendency was similar for patients with breast cancer and hematopoietic malignancy.
The present study revealed the actual situation of the pretreatment provision of information. It showed that detailed information was provided at a high rate to breast cancer patients before treatment, but pretreatment provision of information to patients with other diseases including hematopoietic malignancy was not adequate.
According to our study, many AYA survivors who received chemotherapy had problems with infertility and reproductive function that were thought to be associated with ovulation disorders, and the rate of survivors who gave up on bearing children was significantly higher in comparison to that of healthy AYAs. In a US population‐based study, Shnorhavorian et al11 found that >70% of AYA cancer patients reported being told that treatment might affect their fertility. In Japan, however, although the provision of information on issues such as gonadal dysfunction and infertility is improving, the present results revealed large disparities according to the specific disease type and clinical specialty of the treating physician. In addition, although breast cancer survivors were frequently provided with information on infertility and reproductive dysfunction, it was still insufficient with respect to the timing and content of the information, with fewer patients reporting that they were provided with information on methods of fertility preservation before cancer treatment. This may be due to a lack of awareness or because the provision of information on oncofertility is outside the physician's field of expertise.
In Japan, the first live birth following ovarian tissue freezing/autotransplantation was reported in 2015,12 and nationwide medical cooperation is being developed under the leadership of the Japan Society for Fertility Preservation.7, 13, 14 These circumstances may highlight the importance of providing cancer patients with support in relation to decision‐making on fertility preservation before treatment.15 Many councilors have mentioned the necessity of interdisciplinary communication among health care providers.6, 7, 16 In oncofertility practice, it is critical that appropriate information is provided at the appropriate time and that the self‐determination of the patients is supported through counseling.
Lastly, a considerable number of AYA survivors had trouble with reproductive dysfunction as a delayed complication of treatment, and many had given up on having children. At present, the content of the information on these problems that is provided to patients in Japan is not sufficient. Many of the subjects in this study are survivors of childhood‐onset cancer, and in many cases, more than 10 years have passed since their treatment. Therefore, even though it has become clear that many of them wanted information on reproductive function and its preservation and that their present condition is forcing them to give up on having children, a relation between information provision and having children could not be found. This study highlights the need for improvement in support systems to enhance the patient's decision‐making and preservation of fertility. Further development of interdisciplinary medical collaboration is necessary. Because this study analyzed only questions related to the preservation of reproductive function from a very wide range of questions posed to the AYA cancer survivors, further information on the reproductive function of these survivors and their actual status regarding fertility preservation could not be examined in great detail. As a next step, we would like to focus on the points that could not be clearly elucidated in the present study through specialized research on fertility and reproductive function.
DISCLOSURES
Conflict of interest: K. Horibe received research funding from Pfizer Inc All of the other authors declare no conflicts of interest. Human and animal rights: This study was approved by the ethics review board of Gifu University Hospital (approval no. 28‐126). This article does not contain any study with animal participants that have been performed by any of the authors.
ACKNOWLEDGEMENTS
This project was supported in part by a Health and Labour Sciences Research Grant (Research for Promotion of Cancer Control Program: H27‐Ippan‐005).
Furui T, Takai Y, Kimura F, et al. Fertility preservation in adolescent and young adult cancer patients: From a part of a national survey on oncofertility in Japan. Reprod Med Biol. 2019;18:97–104. 10.1002/rmb2.12256
REFERENCES
- 1. Loprinzi CL, Wolf SL, Barton DL, Laack NN. Symptom management in premenopausal patients with breast cancer. Lancet Oncol. 2008;9(10):993‐1001. [DOI] [PubMed] [Google Scholar]
- 2. Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life‐threatening diseases. Hum Reprod Update. 2009;15(5):587‐597. [DOI] [PubMed] [Google Scholar]
- 3. Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015;3(7):556‐567. [DOI] [PubMed] [Google Scholar]
- 4. Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118(6):1710‐1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917‐2931. [DOI] [PubMed] [Google Scholar]
- 6. Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500‐2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furui T, Takenaka M, Makino H, Terazawa K, Yamamoto A, Morishige KI. An evaluation of the Gifu Model in a trial for a new regional oncofertility network in Japan, focusing on its necessity and effects. Reprod Med Biol. 2016;15:107‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen L, Hamer J, Helwig C, et al. Formal evaluation of PYNK: breast cancer program for young women‐the patient perspective. Curr Oncol. 2016;23(2):e102‐e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kubo A, Koido K, Sawada M, et al. [Survey on oncologists‐provided information on treatment‐related infertility to breast cancer patients]. Gan To Kagaku Ryoho. 2012;39(3):399‐403. In Japanese. [PubMed] [Google Scholar]
- 10. Shimizu C, Bando H, Kato T, Mizota Y, Yamamoto S, Fujiwara Y. Physicians' knowledge, attitude, and behavior regarding fertility issues for young breast cancer patients: a national survey for breast care specialists. Breast Cancer. 2013;20(3):230‐240. [DOI] [PubMed] [Google Scholar]
- 11. Shnorhavorian M, Harlan LC, Smith AW, et al. Fertility preservation knowledge, counseling, and actions among adolescent and young adult patients with cancer: a population‐based study. Cancer. 2015;121(19):3499‐3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki N. Ovarian tissue cryopreservation using vitrification and/or in vitro activated technology. Hum Reprod. 2015;30(11):2461‐2462. [DOI] [PubMed] [Google Scholar]
- 13. Ataman LM, Rodrigues JK, Marinho RM, et al. Creating a global community of practice for oncofertility. J Global Oncol. 2016;2:83‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woodruff TK. The Oncofertility Consortium–addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7(8):466‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ito Y, Shiraishi E, Kato A, et al. The utility of decision trees in oncofertility care in Japan. J Adolesc Young Adult Oncol. 2017;6(1):186‐189. [DOI] [PubMed] [Google Scholar]
- 16. Miyoshi Y, Yorifuji T, Horikawa R, et al. Gonadal function, fertility, and reproductive medicine in childhood and adolescent cancer patients: a national survey of Japanese pediatric endocrinologists. Clin Pediatr Endocrinol. 2016;25(2):45‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]


