Abstract
Key points
Chronic mountain sickness (CMS) is a maladaptation syndrome encountered at high altitude (HA) characterised by severe hypoxaemia that carries a higher risk of stroke and migraine and is associated with increased morbidity and mortality.
We examined if exaggerated oxidative‐inflammatory‐nitrosative stress (OXINOS) and corresponding decrease in vascular nitric oxide bioavailability in patients with CMS (CMS+) is associated with impaired cerebrovascular function and adverse neurological outcome.
Systemic OXINOS was markedly elevated in CMS+ compared to healthy HA (CMS−) and low‐altitude controls.
OXINOS was associated with blunted cerebral perfusion and vasoreactivity to hypercapnia, impaired cognition and, in CMS+, symptoms of depression.
These findings are the first to suggest that a physiological continuum exists for hypoxaemia‐induced systemic OXINOS in HA dwellers that when excessive is associated with accelerated cognitive decline and depression, helping identify those in need of more specialist neurological assessment and targeted support.
Abstract
Chronic mountain sickness (CMS) is a maladaptation syndrome encountered at high altitude (HA) characterised by severe hypoxaemia that carries a higher risk of stroke and migraine and is associated with increased morbidity and mortality. The present cross‐sectional study examined to what extent exaggerated systemic oxidative‐inflammatory‐nitrosative stress (OXINOS), defined by an increase in free radical formation and corresponding decrease in vascular nitric oxide (NO) bioavailability, is associated with impaired cerebrovascular function, accelerated cognitive decline and depression in CMS. Venous blood was obtained from healthy male lowlanders (80 m, n = 17), and age‐ and gender‐matched HA dwellers born and bred in La Paz, Bolivia (3600 m) with (CMS+, n = 23) and without (CMS−, n = 14) CMS. We sampled blood for oxidative (electron paramagnetic resonance spectroscopy, HPLC), nitrosative (ozone‐based chemiluminescence) and inflammatory (fluorescence) biomarkers. We employed transcranial Doppler ultrasound to measure cerebral blood flow (CBF) and reactivity. We utilised psychometric tests and validated questionnaires to assess cognition and depression. Highlanders exhibited elevated systemic OXINOS (P < 0.05 vs. lowlanders) that was especially exaggerated in the more hypoxaemic CMS+ patients (P < 0.05 vs. CMS−). OXINOS was associated with blunted cerebral perfusion and vasoreactivity to hypercapnia, impaired cognition and, in CMS+, symptoms of depression. Collectively, these findings are the first to suggest that a physiological continuum exists for hypoxaemia‐induced OXINOS in HA dwellers that when excessive is associated with accelerated cognitive decline and depression, helping identify those in need of specialist neurological assessment and support.
Keywords: chronic mountain sickness, free radicals, cerebrovascular function, cognition, dementia, depression
Key points
Chronic mountain sickness (CMS) is a maladaptation syndrome encountered at high altitude (HA) characterised by severe hypoxaemia that carries a higher risk of stroke and migraine and is associated with increased morbidity and mortality.
We examined if exaggerated oxidative‐inflammatory‐nitrosative stress (OXINOS) and corresponding decrease in vascular nitric oxide bioavailability in patients with CMS (CMS+) is associated with impaired cerebrovascular function and adverse neurological outcome.
Systemic OXINOS was markedly elevated in CMS+ compared to healthy HA (CMS−) and low‐altitude controls.
OXINOS was associated with blunted cerebral perfusion and vasoreactivity to hypercapnia, impaired cognition and, in CMS+, symptoms of depression.
These findings are the first to suggest that a physiological continuum exists for hypoxaemia‐induced systemic OXINOS in HA dwellers that when excessive is associated with accelerated cognitive decline and depression, helping identify those in need of more specialist neurological assessment and targeted support.
Introduction
The human brain has evolved a much higher rate of obligatory oxygen (O2) consumption because, unlike most other organs, its evolutionary ‘drive for size’ means that the brain is committed to a continually active state, demanding a disproportionate 20% of the body's basal O2 budget in the resting state, more than 10 times that expected from its mass alone (Bailey et al. 2017b). The requirement to process large amounts of O2 over a relatively small tissue mass supports the high rate of ATP formation to fuel the maintenance of ionic equilibria and uptake of neurotransmitters for synaptic transmission (Alle et al. 2009). That cognitive function is impaired by acute hypoxia is thus not surprising and an extensive literature has since documented proportional deficits in executive function, attention, mental speed, language and memory (Virues‐Ortega et al. 2004; Rimoldi et al. 2016; McMorris et al. 2017), including lasting damage to white and grey matter motor architecture (Di Paola et al. 2008).
However, few studies have considered how lifelong exposure to hypoxia affects cognitive function and whether compensatory adaptations to sustain adequate tissue O2 delivery prevent acute impairments from potentially progressing to irreversible dementia. The lack of information is surprising given an estimated 140 million high‐altitude (HA) dwellers permanently live above 2500 m through economic and social necessity (Bailey et al. 2018b) and an evolving body of literature indicating that hypoxaemia is responsible for the higher prevalence of cognitive impairment and dementia observed in patients living at sea level with cardiopulmonary disease (Peers et al. 2009; Bagge et al. 2018).
In the few studies published to date, cognitive function has been shown to be slightly impaired in well‐adapted elderly Andean HA dwellers relative to sea‐level‐based lowlander controls (Yan et al. 2011; Hill et al. 2014; Davis et al. 2015b). In contrast, albeit in the only study conducted to date, the authors observed an inverse relationship between altitude of residence and (age‐adjusted) dementia mortality rate. However, this study examined patients living at considerably lower altitudes (up to 1800 m) in California with no control of potential confounders such as comorbidities and air pollution, highlighting the need for additional studies.
Furthermore, the mechanism underpinning altitude‐induced cognitive impairment is unclear and to what extent further impairments occur in highlanders suffering from chronic mountain sickness (CMS+), a maladaptation syndrome characterised by exaggerated erythrocytosis and more pronounced hypoxaemia (Villafuerte & Corante, 2016), has not been examined. We have previously identified that compared to lowlander (normoxic) controls, systemic oxidative–nitrosative stress (OXNOS), defined by an increase in free radical formation and corresponding decrease in vascular nitric oxide (NO) bioavailability, was permanently elevated in healthy well‐adapted Andeans living at 3600 m without CMS (CMS−). The observation that systemic vascular endothelial function remained intact suggested that physiological concentrations of OXNOS may prove hormetically beneficial for lifelong adaptation to the hypoxia of HA. In contrast, highlanders with CMS (CMS+) exhibited more exaggerated increases in OXNOS and impaired systemic vascular endothelial function, thus implying a potential metabolic basis to HA maladaptation (Bailey et al. 2013c).
In light of these findings, we sought to determine the potential relationships between metabolic (oxidative–inflammatory–nitrosative stress, hereafter OXINOS), haemodynamic (cerebrovascular function) and clinical (cognition and depression) correlates in CMS+ and CMS− in order to provide more integrated mechanistic insight into the potential pathophysiology and consequences of neurological maladaptation to HA. We hypothesised that compared to lowlander controls, systemic OXINOS would be moderately elevated in CMS− in the absence of any impairments in cerebrovascular function, cognition or symptoms of depression. We further hypothesised that the systemic OXINOS response would be further exaggerated in CMS+ subsequent to more pronounced arterial hypoxaemia and associated with impairments in cerebrovascular function, cognition and symptoms of depression. Figure 1 provides a schematic summary of the proposed mechanisms.
Figure 1. Functionally integrated translational hypothesis.
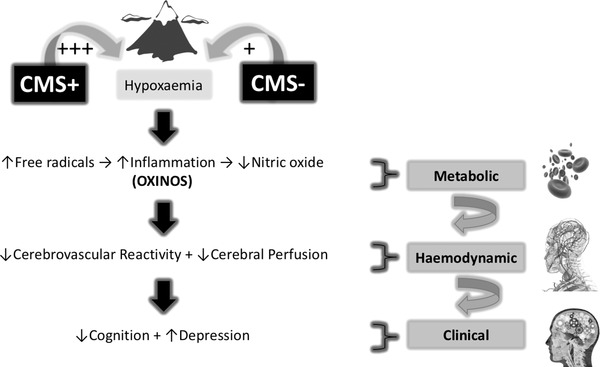
CMS−/CMS+, highlanders without/with chronic mountain sickness; +++, comparatively more hypoxaemic; OXINOS, oxidative–inflammatory–nitrosative stress.
Materials and methods
Ethical approval
The experimental protocol was approved by the Institutional Review Boards for Human Investigation at the University of San Andres, La Paz, Bolivia (CNB #52/04), University of Lausanne, Lasusanne, Switzerland (#89/06, #94/10), and University of Glamorgan, Pontypridd, UK (#4/07), and subsequently registered (clinicaltrials.gov; Identifier: NCT01182792). All participants were informed of the purpose/risks of the experiment and signed an informed consent form, with all procedures adhering to guidelines set forth in the Declaration of Helsinki.
Experimental design
The study was a cross‐sectional population‐based observational study in accordance with the STROBE statement (von Elm et al. 2014). Figure 2 provides a schematic of the experimental design. The Neurovascular Research Laboratory in the UK (∼80 m) was the site of investigation for the lowlanders and the Instituto Boliviano de Biologia de Altura in La Paz, Bolivia (∼3600 m) for the highlanders.
Figure 2. Experimental design.
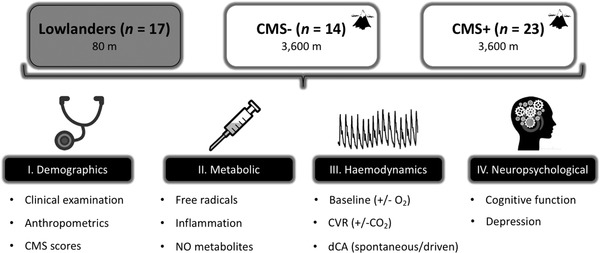
CMS−/CMS+, highlanders without/with chronic mountain sickness; NO, nitric oxide; O2, oxygen; CVR, cerebrovascular reactivity; CO2, carbon dioxide; dCA, dynamic cerebral autoregulation.
Participants
For all participants, inclusion criteria specified that they were born and had lived permanently at their resident altitude and were sedentary (defined as no formal recreational activity outside of everyday living, Bailey et al. 2013b). Exclusion criteria included those with significant developmental delay or learning difficulties, diagnosis of any central neurological disease such as aneurysm, stroke, transient ischaemic attack, epilepsy, multiple sclerosis and psychiatric disorders including any history of traumatic brain injury and hypertension. None of the participants was taking nutritional supplements including over‐the‐counter antioxidant or anti‐inflammatory medications. We specifically chose to exclude females given our inability to control for differences in circulating oestrogen known to affect cerebral blood flow (CBF) and cognition (Yao et al. 2009). Before inclusion into the study, all participants were subject to an extensive clinical examination that consisted of a thorough medical history, chest auscultation and 12‐lead ECG. Participants were subsequently familiarised with the equipment and procedures.
Highlanders
We recruited 23 male patients with primary CMS (CMS+) and 14 healthy age‐, gender‐ and education‐ (consecutive years in secondary and University education) matched controls without CMS (CMS−) native to La Paz, Bolivia (Table 1). We scored symptoms of CMS and confirmed clinical diagnosis by an excessive erythrocytosis [haemoglobin (Hb) > 20 g/dL] in the presence of normal pulmonary function and no history of working in the mining industry (Leon‐Velarde et al. 2005). All participants identified themselves as Aymaras and were from similar socio‐economic backgrounds having been born and bred in La Paz with Spanish spoken as their first language.
Table 1.
Demographic data
| Group: | Lowlanders | Highlanders | P values | |||
|---|---|---|---|---|---|---|
| Subgroup: | Controls (n = 17) | CMS− (n = 14) | CMS+ (n = 23) | CMS− vs. controls | CMS+ vs. controls | CMS+ vs. CMS− |
| Clinical | ||||||
| Age (years) | 56 ± 18 | 52 ± 12 | 56 ± 11 | 0.614 (between groups) | ||
| Hb (g/dL) | 15.0 ± 1.3 | 17.2 ± 1.1* | 20.9 ± 1.7*† | <0.001 | <0.001 | <0.001 |
| Hct (%) | 46 ± 4 | 52 ± 4* | 63 ± 6*† | 0.002 | <0.001 | <0.001 |
| (%) | 97 ± 0 | 91 ± 5* | 88 ± 4*† | <0.001 | <0.001 | 0.044 |
| caO2 (mg/dL) | 20.2 ± 1.8 | 21.6 ± 1.8 | 25.5 ± 2.3 | 0.147 | <0.001 | <0.001 |
| CMS score (points) | 0 ± 0 | 2 ± 2 | 8 ± 5*† | 0.369 | <0.001 | <0.001 |
| Anthropometrics | ||||||
| Body mass (kg) | 82.0 ± 13.4 | 71.6 ± 8.9 | 78.2 ± 11.8 | 0.053 (between groups) | ||
| Stature (m) | 1.77 ± 0.06 | 1.63 ± 0.04 | 1.62 ± 0.06 | <0.001 | <0.001 | 1.000 |
| BMI (units) | 26 ± 4 | 27 ± 4 | 30 ± 4* | 1.000 | 0.025 | 0.122 |
| Waist:hip | 0.93 ± 0.08 | 0.95 ± 0.05 | 0.99 ± 0.04* | 1.000 | 0.019 | 0.162 |
| Education | ||||||
| Secondary (n/%) | 16/94 | 13/93 | 20/87 | 0.697 (between groups) | ||
| University (n/%) | 8/47 | 4/29 | 5/22 | 0.225 (between groups) | ||
Values are mean ± SD; CMS−/CMS+, highlanders without/with chronic mountain sickness; Hb, haemoglobin; Hct, haematocrit; , arterial oxyhaemoglobin saturation; caO2, arterial oxygen content; BMI, body mass index. *Different vs. lowlanders (P < 0.05); †different vs. CMS− (P < 0.05). Clinical and anthropometric data were analysed using one‐way ANOVAs and post hoc Bonferonni‐adjusted independent samples t‐tests. Education data were analysed using Pearson chi‐square tests.
Lowlanders
We also recruited 17 age‐ and education‐matched healthy Caucasian males born and bred close to sea‐level (∼80 m) in the UK (Table 1) as a sea‐level (normoxic) comparator.
Educational status
Data were collected on level of full‐time education attained.
Metabolic assessments
Participants were asked to refrain from physical activity, caffeine and alcohol and to follow a low nitrate/nitrite (/) diet 24 h prior to formal experimentation (Woodside et al. 2014) and were subject to a 12 h overnight fast when they attended the laboratory at 08.00 h. We obtained blood samples without stasis following 20 min of seated rest to control for plasma volume shifts.
Chemicals
All chemicals were of the highest available purity from Sigma‐Aldrich (Poole, UK).
Blood sampling
We collected blood from an indwelling cannula located in a forearm antecubital vein into Vacutainers (Becton, Dickinson and Company, Oxford, UK) before centrifugation at 600 g (4°C) for 10 min. We decanted plasma and serum samples into cryogenic vials (Nalgene Labware, Thermo Fisher Scientific Inc., Waltham, MA, USA), that were immediately snap‐frozen in liquid nitrogen (N2) and shipped/stored under N2 gas (Cryopak, Taylor‐Wharton, Theodore, AL, USA) before analysis in the UK. We left samples to defrost at 37°C in the dark for 5 min before batch analysis.
Oxidative stress
Antioxidants
We assayed plasma concentrations of reduced and oxidised glutathione (GSH/GSSG) according to the methods established by N'Guessan et al. (2011) with modifications (Stocker et al. 2017). Intra‐ and inter‐assay coefficients of variation (CVs) were both <5%.
Free radicals
The ascorbate free radical (A•−) was used as a direct measure of systemic free radical formation (Buettner & Jurkiewicz, 1993). We injected 1 mL of plasma into a high‐sensitivity multiple‐bore sample cell (AquaX, Bruker Daltonics Inc., Billerica, MA, USA) housed within a TM110 cavity of an electron paramagnetic resonance spectrometer operating at X‐band (9.87 GHz). We recorded samples by cumulative signal averaging of 10 scans using the following instrument parameters: resolution, 1024 points; microwave power, 20 mW; modulation amplitude, 0.65 G; receiver gain, 2 × 105; time constant, 40.96 ms; sweep rate, 0.14 G/s; sweep width, 6 G; centre field, 3486 G. We filtered spectra identically (moving average, 15 conversion points) using WINEPR software (Version 2.11, Bruker, Karlsruhe, Germany) and determined the double integral of each doublet using specialist software (OriginLab Corps, Northampton, MA, USA). The intra‐ and inter‐assay CVs were both <5%.
Inflammatory stress
Myeloperoxidase (MPO) activity
We employed a high‐throughput, sensitive and homogeneous fluorescence‐based method for detection of MPO chlorination activity using 7‐hydroxy‐2‐oxo‐2H‐chromene‐8‐carbaldehyde oxime as a selective probe for hypochlorous acid as recently described (Stocker et al. 2017). The intra‐ and inter‐assay CVs were both <5%.
Nitrosative stress
We measured plasma NO metabolites using ozone‐based chemiluminescence (OBC) as outlined below (Bailey et al. 2017a).
S‐Nitrosothiols (RSNO)
Plasma (400 μL) was mixed with 5% acidified sulphanilamide and left to incubate in the dark at 21°C for 15 min to remove before injection into tri‐iodide reagent for direct measurement of RSNO.
Nitrite ()
A separate sample (200 μL) was also injected into tri‐iodide reagent for the combined measurement of and RSNO with calculated by subtracting the concentration of RSNO. We performed all calculations using Origin/Peak Analysis software. The intra‐ and inter‐assay CVs were 7% and 10%, respectively.
Total bioactive NO
This was calculated as the sum of RSNO + .
Haemodynamic assessments
We performed all resting measurements following 10 min of seated rest breathing room air at the prevailing barometric pressures in all groups (normoxic normocapnia for lowlanders, hypoxic hypocapnia for highlanders). Measurements were also repeated following the administration of hyperoxia ( = 1.0, ∼10 L/min for 10 min) to the inspired air in the highlanders only (hyperoxic normocapnia).
Cardiopulmonary function
We used finger photoplethysmography (Finometer PRO, Finapres Medical Systems, Amsterdam, the Netherlands) to monitor beat‐to‐beat mean arterial pressure (MAP) using the Model Flow method that incorporates participant sex, age, stature and mass (BeatScope 1.0 software; TNO; TPD Biomedical Instruments) to calculate stroke volume (SV) and cardiac output (). We corrected for vertical displacement of the finger cuff relative to heart level using a reference probe placed on the chest at the fourth intercostal space in the mid‐clavicular line. We measured heart rate (HR) using a lead II electrocardiogram (Dual BioAmp; ADInstruments, Oxford, UK). We sampled end‐tidal partial pressures of oxygen and carbon dioxide (PETO2/CO2) from a leak‐free mask and analysed via capnography (ML 206, ADInstruments Ltd, Oxford, UK). Pulse oximetry (Nonin 9550 Onyx II, Nonin Medical, Inc., Plymouth, MI, USA) monitored arterial oxyhaemoglobin saturation () on the third digit of the right hand.
Cerebrovascular function
Cerebral blood flow
We insonated the M1 segment of the right middle cerebral artery (MCA) at a depth of 40–60 mm using a 2 MHz pulsed trans‐cranial Doppler (TCD) ultrasound system (Multi‐Dop X4, DWL Elektroniche Systeme GmbH, Sipplingen, Germany) to yield MCA velocity (MCAv). A headband device (Spencer Technologies, Nicolet Instruments, Madison, WI, USA) secured the Doppler probe over the trans‐temporal window to achieve optimal insonation position and was maintained in this position for the duration of the study to avoid movement artefacts. We calculated cerebrovascular and total peripheral resistance (CVR and TPR) as MAP/MCAv or and cerebrovascular conductance index (CVCi) as MCAv/MAP. We calculated pulsatility index (PI) as systolic MCAv – diastolic MCAv/MCAv and further normalised the PI relative to the prevailing MAP. We calculated cerebral O2 delivery (CDO2) as the product of (arterial) O2 content [c(a)O2] (1.39 × Hb × /100) and MCAv. In order to normalise for the cerebral vasoconstrictor effects of polycythaemia and hypocapnia in the highlanders, we adjusted absolute CBF values for differences in (elevated) Hct and (lower) PETCO2 using the following equations:
(Bailey et al. 2009b) where the asterisk indicates fixed PETCO2 of 40 mmHg.
Data sampling
We sampled beat‐by‐beat data continuously at 1 kHz using an analog‐to‐digital converter (Powerlab/16SP ML795; ADInstruments) stored on a personal computer for off‐line analysis (Chart version 7.2.2, ADInstruments). We gave chart files a coded number (not named) by an investigator blinded to the study. We ‘time‐aligned’ the MAP and TCD channels given the time delay (1.07 s) associated with MAP signal processing when using the Finometer PRO device.
Cerebrovascular reactivity to CO2 (CVRCO2)
Following 10 min of breathing room air, the inspirate was rapidly changed to 5% CO2 with 21% O2 and balanced nitrogen for 5 min at the prevailing barometric pressure. Following 5 min of recovery breathing room air, participants hyperventilated at 15 breaths/min for 5 min. From this, we calculated CVRCO2 as the percentage increase/decrease in MCAv from baseline per 1 mmHg increase/decrease in PETCO2 recorded during the final 30 s (average taken) of the hypercapneic/hypocapneic challenge having achieved steady‐state:
From these data, we derived the CVRCO2 range as a useful indication of the cerebral circulation's combined ability to respond to differential changes in CO2. We calculated the CVRCO2 range as the sum of the fractional vasodilatation and vasoconstriction incurred during the respective hypercapnoea and hypocapnia challenges as described:
Dynamic cerebral autoregulatory capacity (dCA)
We used a combination of spontaneous (seated) and driven (repeated squat‐stands) oscillations in blood pressure (BP) and MCAv to assess dCA via transfer function analysis (TFA). Following 10 min of rest in the seated position, we obtained a 5 min segment of BP and MCAv data for spectral analysis of spontaneous oscillations. To increase BP variability and improve the reliability and interpretation of the TFA metrics (Katsogridakis et al. 2013), participants then performed 5 min periods of repeated squat‐stand manoeuvres at randomly assigned frequencies of 0.05 Hz [10 s squat, 10 s standing) and 0.10 Hz (5 s squat, 5 s standing) with 5 min of standing rest between frequencies (Smirl et al. 2015). During these manoeuvres, we instructed participants to maintain normal breathing and to avoid Valsalva. Beat‐to‐beat MAP and MCAv signals were calculated across each cardiac cycle, linearly interpolated and resampled at 2 Hz for TFA (Zhang et al. 1998) in accordance with the recommendations of the Cerebral Autoregulation Research Network (Claassen et al. 2016). Spontaneous MAP and MCAv power spectrum density and the mean value of TFA coherence gain, and phase of the spontaneous oscillations were band averaged across the very‐low‐frequency (VLF: 0.02–0.07 Hz, 50 to 14.3‐second cycles) and low‐frequency (LF: 0.07–0.20 Hz, 14.3 to 5 s cycles) ranges where CA is most operant (Zhang et al. 1998). The TFA coherence, gain and phase of the driven MAP oscillations were sampled at the driven frequencies (0.05 or 0.10 Hz). To ensure we entered robust phase and gain estimates for analysis, we averaged only those gain and phase (positive to eliminate wrap‐around) values where the corresponding coherence was ≥0.5. Accordingly, we interpreted an increase in gain and decrease in phase to reflect impaired dCA indicative of a more pressure‐passive relationship between MAP and MCAv.
Cognitive function
Psychometric tests
We conducted a battery of psychometric tests with three consecutive testing periods to ensure habituation and to avoid the confounding influence of learning effects as previously outlined (Marley et al. 2017):
(1) Learning and memory
Rey Auditory Verbal Learning consists of two lists containing 15 unrelated words. We read List 1 aloud at a rate of 1 word per second. The participant recalled as many of the 15 words in any order. We repeated this four times (total of five recalls, A1–A5). We read List 2 aloud and the participant recalled as many of the 15 words as possible, again in any order (B1). Finally, we asked the participant to recall as many words as possible from List 1 (delayed recall, A6) (Rey, 1958).
(2) Working memory
Repetition of Digits Backwards (RDB) is based on the Repetition of Digits test (Wechsler, 1955) which comprises digits forwards (RDF) and RDB. Both tests consist of seven pairs of random number sequences that the researcher reads aloud at a rate of one per second. RDF required the participant to recall the numbers in the correct sequence until they got the sequence wrong. The sequence began with three numbers and increased by one number every other pair. RDB required the participant to recall the numbers in the reverse order from the sequences they were presented, until they got the sequence wrong. The sequence began with two numbers and increased by one every other pair. We scored the tests by the total number of sequences re‐called. Trail Making Test B (TMT‐B) is based on the Army Individual Test Battery (War Department Adjutant General's Office, 1944) that consists of two parts, A (TMT‐A) and TMT‐B constructed with 25 circles distributed over a sheet of A4 paper. In part A, the circles are numbered 1–25; the participant connected the numbers in ascending order. In part B, the circles include both numbers (1–13) and letters (A–L); again participants connected the circles in ascending pattern, but alternating between number and letter (i.e. 1‐A‐2‐B‐3‐C, etc.). We asked the participant to complete the task as quickly as possible without removing the pen from the paper until completion. We scored the tests as the time taken in seconds to complete each trail.
(3) Attention and information processing
We assessed RDF and TMT‐A as outlined above. The Digit Symbol Substitution Test (DSST) consists of matching nine digits with their corresponding symbols. We gave the participant a piece of A4 paper with nine digits and their corresponding symbol and a grid of 93 digits; under each digit the participant drew as many of the corresponding symbols as possible within 90 s. We scored the test by how many they drew correctly within the alloted time (Wechsler, 1955). The Stroop test (ST) consists of two parts A and B, with 24 words written in different colours on a 4 × 6 grid. Test A required the participants to read aloud the word written as quickly as possible. Test B required the participant to read aloud the colour of the word written and not what the word said as quickly as possible (Stroop, 1935). This test was assessed in the highlander subgroups only and not the lowlanders due to logistical constraints.
(4) Visuo‐motor coordination
The grooved pegboard dexterity test (GPDT) consists of two parts and required the participant to place 25 pegs in a pegboard containing equally sized holes with randomly positioned slots. Participants placed identical pegs with a key on one side that had to be rotated to match the hole before they could be inserted, into the 25 holes on the pegboard working from left to right, front to back. The test consisted of two parts, the first completed using the dominant hand (GPDT‐D) and then repeated using the non‐dominant hand (GPDT‐ND). The test was scored by the time taken to complete in seconds (Klove, 1963).
Higher scores in the RAVLT‐A/B and RDB/RDF tests and lower scores in the TMT‐A/B, GPDT‐D/GPDT‐ND and Stroop A/B tests indicated superior performance.
Questionnaires
The original English questionnaires outlined below were used for the lowlanders whereas the equivalent validated Spanish versions were used for the highlanders.
Montreal Cognitive Assessment (MoCA)
The MoCA measures visuospatial/executive, naming, memory, attention, language, abstraction, delayed recall and orientation, with each section having a designated point value with a maximum score of 30 points (Nasreddine et al. 2005). We scored the test as the number of questions answered correctly and participants with 12 years or less of education had a point added to their total score. We graded MoCA on three levels: normal, 25.2–29.6 points; mild cognitive impairment, 19–25.2 points; and Alzheimer's disease, 11.4–21 points according to established guidelines (Nasreddine et al. 2005). Because MoCA assesses executive function, it is particularly useful for patients with vascular impairment, including vascular dementia (Sheehan, 2012).
Depression
We assessed depressive symptoms using the Becks Depression Inventory (BDI), which has been shown to be a reliable and valid measure of self‐reported depression in both normal and psychiatric populations (Beck et al. 1961). The BDI is a self‐report inventory with 21 items assessing the behavioural and cognitive symptoms of depression. Each item consists of four statements numbered from 0 to 3 with higher numbers indicating more severe depressive symptoms. We scored the questionnaire by the sum of all the responses with a maximum score of 63 points. A score of 0–9 points indicated minimal depression whereas scores of 10–18 points, 19–29 points and 30–63 points indicated mild, moderate and severe depression, respectively.
Statistical analysis
Power calculation
We analysed these data using G* Power 3.1 software. Assuming comparable differences and corresponding effect sizes previously observed in plasma A•− (η² = 0.54) and (η² = 0.67) (Bailey et al. 2013c), our primary end‐outcome variables for OXNOX stress, the present study required a (minimum) sample size of 24–36 participants (8–12 per group) in order to achieve a power of 0.80 at P < 0.05. We chose to further inflate this during recruitment given the potential for incomplete data collection.
Inferential statistics
We analysed these data using using the Statistics Package for Social Scientists (IBM SPSS Statistics Version 24.0). Shapiro‐Wilk W tests (P > 0.05) confirmed that all data sets were normally distributed. We analysed demographic (Table 1), metabolic (Table 2), haemodynamic (Table 3), cognition and depression (Table 5), CVRCO2 (Fig. 2) and TFA (Fig. 3) datasets using one‐way ANOVAs and post hoc Bonferonni‐adjusted independent samples t‐tests if we observed a main effect. We analysed education data (Table 1) using Pearson chi‐square tests. We analysed responses to hyperoxia in the highlanders (Table 4) using two‐way (subgroup × inspirate) ANOVAs and post hoc Bonferonni‐adjusted paired samples (within subgroups) or independent (between subgroups) samples t‐tests if we observed an interaction effect. We determined relationships between metabolic, haemodynamic and clinical variables (Fig. 4) using Pearson product moment correlations. We established significance at P < 0.05 for all two‐tailed tests (with individual P values for all comparisons shown) and data are expressed as mean ± SD.
Table 2.
Metabolic data
| Group: | Lowlanders | Highlanders | P values | |||
|---|---|---|---|---|---|---|
| Subgroup: | Controls (n = 17) | CMS− (n = 14) | CMS+ (n = 23) | CMS− vs. controls | CMS+ vs. controls | CMS+ vs. CMS− |
| Oxidative stress | ||||||
| GSH (μM) | N/A | 549 ± 154 | 412 ± 151† | N/A | N/A | 0.012 |
| GSSG (μM) | N/A | 176 ± 48 | 197 ± 34† | N/A | N/A | 0.018 |
| GSH:GSSG (AU) | N/A | 3.3 ± 1.1 | 2.1 ± 0.9† | N/A | N/A | 0.049 |
| A•− (AU) | 29,450 ± 6,929 | 54,451 ± 20,722* | 59,729 ± 18,133*† | <0.001 | <0.001 | 0.042 |
| Inflammatory stress | ||||||
| MPO (μg/L) | N/A | 609 ± 12 | 894 ± 168† | N/A | N/A | 0.016 |
| Nitrosative stress | ||||||
| (nM) | 249.1 ± 65.1 | 139.9 ± 76.7* | 130.7 ± 83.6* | 0.001 | <0.001 | 1.000 |
| RSNO (nM) | 5.4 ± 3.0 | 5.0 ± 2.7 | 4.7 ± 3.9* | 0.850 (between groups) | ||
| Total bioactive NO (nM) | 254.5 ± 64.2 | 144.9 ± 78.0* | 135.4 ± 83.7* | 0.001 | <0.001 | 1.000 |
Values are mean ± SD; CMS−/CMS+, highlanders without/with chronic mountain sickness; GSH/GSSH, reduced/oxidised glutathione; A•−, ascorbate radical; NO, nitric oxide; , nitrite; RSNO, S‐nitrosothiols; total bioactive NO ( + RSNO); MPO, myeloperoxidase; N/A, not assessed. *Different vs. lowlanders (P < 0.05); †different vs. CMS− (P < 0.05). Data were analysed using independent samples t‐tests (where lowlander data were unavailable) and one‐way ANOVAs with post hoc Bonferonni‐adjusted independent samples t‐tests.
Table 3.
Haemodynamic data
| Group: | Lowlanders | Highlanders | P values | |||
|---|---|---|---|---|---|---|
| Subgroup: | Controls (n = 17) | CMS− (n = 14) | CMS+ (n = 23) | CMS− vs. controls | CMS+ vs. controls | CMS+ vs. CMS− |
| MAP (mmHg) | 79 ± 21 | 80 ± 10 | 87 ± 14 | 0.238 (between groups) | ||
| SBP (mmHg) | 118 ± 30 | 118 ± 18 | 128 ± 22 | 0.309 (between groups) | ||
| DBP (mmHg) | 61 ± 18 | 61 ± 8 | 67 ± 11 | 0.228 (between groups) | ||
| (L/min) | 7.42 ± 1.33 | 6.99 ± 0.80 | 7.24 ± 1.38 | 0.627 (between groups) | ||
| HR (beats/min) | 71 ± 14 | 71 ± 12 | 72 ± 14 | 0.990 (between groups) | ||
| SV (mL) | 105 ± 13 | 101 ± 22 | 103 ± 22 | 0.853 (between groups) | ||
| TPR (mmHg/L/min) | 10.97 ± 2.93 | 11.60 ± 1.73 | 12.41 ± 2.80 | 0.231 (between groups) | ||
| (%) | 97 ± 0 | 91 ± 5* | 88 ± 4*† | <0.001 | <0.001 | 0.044 |
| PETO2 (mmHg) | 107 ± 5 | 54 ± 3* | 51 ± 5* | <0.001 | <0.001 | 0.236 |
| PETCO2 (mmHg) | 39 ± 3 | 34 ± 5* | 33 ± 4* | 0.019 | 0.022 | 1.000 |
| Cerebrovascular | ||||||
| MCAv (cm/s) | 49 ± 12 | 39 ± 12* | 35 ± 12*† | 0.044 | 0.001 | 0.048 |
| MCAv/Hct (cm/s) | 50 ± 11 | 42 ± 13* | 43 ± 14* | 0.039 | 0.037 | 1.000 |
| MCAv/PETCO2 (cm/s) | 51 ± 11 | 48 ± 16 | 44 ± 15* | 1.000 | 0.040 | 1.000 |
| PI (AU) | 1.04 ± 0.18 | 1.15 ± 0.41 | 1.45 ± 0.39*† | 1.000 | 0.002 | 0.048 |
| Normalised PI (AU) | 0.014 ± 0.006 | 0.014 ± 0.005 | 0.017 ± 0.004 | 0.216 (between groups) | ||
| CVR (mmHg/cm/s) | 1.74 ± 0.69 | 2.27 ± 0.80 | 2.89 ± 0.57*† | 0.667 | 0.010 | 0.037 |
| CVCi (cm/s/mmHg) | 0.70 ± 0.39 | 0.49 ± 0.16 | 0.42 ± 0.18*† | 0.088 | 0.003 | 0.010 |
| CDO2 (mL/cm/s) | 983 ± 209 | 850 ± 305* | 889 ± 303* | 0.046 | 0.047 | 1.000 |
Values are mean ± SD; CMS−/CMS+, highlanders without/with chronic mountain sickness; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; , cardiac output; SV, stroke volume; TPR, total peripheral resistance; , arterial oxyhaemoglobin saturation; PETO2/CO2, end‐tidal partial pressure of oxygen/carbon dioxide; MCAv, middle cerebral artery velocity; SMCAv/DMCAv, systolic/diastolic MCAv; MCAv/Hct, MCAv (retrospectively) adjusted for differences in haematocrit (Hct); MCAv/PETCO2, MCAv (retrospectively) adjusted for differences in end‐tidal partial pressure of carbon dioxide, see Methods; AU, arbitrary units; CVR, cerebrovascular resistance; CVCi, cerebrovascular conductance index; CDO2, cerebral oxygen delivery. *Different vs. lowlanders (P < 0.05); **different vs. hypoxia for given highlanders subgroup (P < 0.05); †different vs. CMS− for given inspirate (P < 0.05). Data were analysed using one‐way ANOVAs and post hoc Bonferonni‐adjusted independent samples t‐tests.
Table 5.
Cognition and depression
| Group: | Lowlanders | Highlanders | P values | |||
|---|---|---|---|---|---|---|
| Subgroup: | Controls (n = 17) | CMS− (n = 14) | CMS+ (n = 23) | CMS− vs. controls | CMS+ vs. controls | CMS+ vs. CMS− |
| Learning and memory | ||||||
| Rey Auditory Verbal Learning Test A1–A5 (n) | 48 ± 8 | 34 ± 9* | 30 ± 9* | <0.001 | <0.001 | 0.538 |
| Rey Auditory Verbal Learning Test B1 (n) | 5 ± 1 | 4 ± 1 | 3 ± 1* | 0.335 | <0.001 | 0.116 |
| Rey Auditory Verbal Learning Test A6 (n) | 9 ± 4 | 8 ± 3 | 5 ± 2*† | 0.392 | <0.001 | 0.044 |
| Working memory | ||||||
| Repetition of Digits Backwards (n) | 6 ± 2 | 4 ± 1* | 4 ± 2* | <0.001 | <0.001 | 1.000 |
| Trail Making Test B (s) | 74 ± 31 | 106 ± 43 | 109 ± 51* | 0.137 | 0.044 | 1.000 |
| Attention/information processing | ||||||
| Repetition of Digits Forwards (n) | 8 ± 2 | 4 ± 2* | 4 ± 2* | <0.001 | <0.001 | 1.000 |
| Trail Making Test A (s) | 37 ± 15 | 41 ± 18 | 48 ± 17 | 0.155 (between groups) | ||
| Digit Symbol Substitution Test (n) | 54 ± 12 | 42 ± 12* | 37 ± 13* | 0.034 | <0.001 | 0.555 |
| Stroop Task A (s) | No data | 14 ± 2 | 17 ± 5† | N/A | N/A | 0.048 |
| Stroop Task B (s) | No data | 38 ± 14 | 41 ± 15 | N/A | N/A | 0.555 |
| Montreal Cognitive Assessment (points) | 26 ± 3 | 24 ± 4 | 21 ± 5*† | 0.676 | 0.001 | 0.044 |
| Montreal Cognitive Assessment Score ≤25 points (n/%) | 5/29 | 7/50 | 20/87*† | 0.241 | <0.001 | 0.014 |
| Visuomotor coordination | ||||||
| Grooved Pegboard Dexterity Test‐Dominant (s) | 73 ± 11 | 67 ± 8 | 71 ± 12 | 0.296 (between groups) | ||
| Grooved Pegboard Dexterity Test‐Non Dominant (s) | 83 ± 24 | 72 ± 11 | 81 ± 23 | 0.309 (between groups) | ||
| Depression | ||||||
| Beck's Depression Inventory (points) | 6 ± 5 | 7 ± 10 | 16 ± 13*† | 1.000 | 0.014 | 0.044 |
Values are mean ± SD; CMS−/CMS+, highlanders without/with chronic mountain sickness; n, number correct. *Different vs. lowlanders (P < 0.05); †different vs. CMS− (P < 0.05). Data were analysed using one‐way ANOVAs and post hoc Bonferonni‐adjusted independent samples t‐tests with the exception of MoCA cut‐point (≤25 points) analysed using Pearson chi‐square tests with standardised residuals.
Figure 3. Typical electron paramagnetic resonance (EPR) spectra of the plasma ascorbate radical (A–C) and ozone‐based chemiluminescence detection of bioactive (nitrite + S‐nitrosothiols) nitric oxide metabolites (D–F) at rest in the systemic circulation of a lowlander and highlanders without (CMS−) and with (CMS+) chronic mountain sickness.
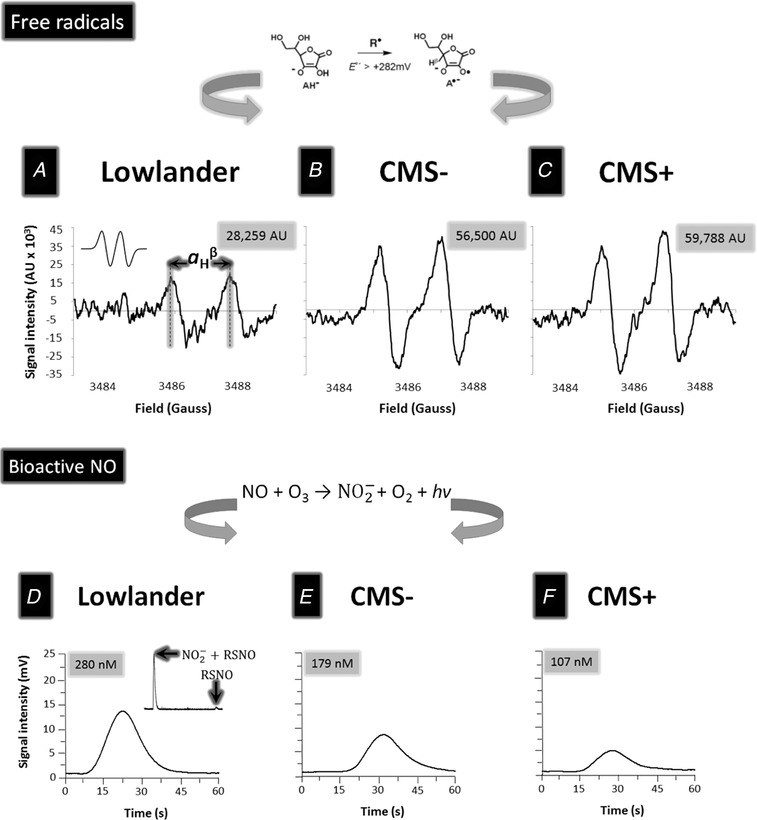
A–C, oxidation of the ascorbate monoanion (AH−) by any free radical (R•) with a one‐electron reduction potential that exceeds +282 mV will yield A•− (schematic illustrated above). The unpaired electron is delocalised over a highly conjugated tri‐carbonyl π‐system, rendering it resonance‐stabilised and thereby facilitating direct detection by EPR spectroscopy. At the current settings, A•− appears as a (filtered) doublet with a hydrogen hyperfine coupling constant (a H β) of ∼1.76 G (see inset to A for simulated spectrum). D–F, filtered traces of bioactive nitric oxide (NO) metabolites (nitrite + S‐nitrosothiols) generated via ozone‐based chemiluminescence involving the reaction of NO with ozone (O3) that yields a photon (hv) and subsequent conversion to a potential difference. Insert top right highlights the composite signals (before and after sulphanilamide incubation) for separate measurement of nitrite () and S‐nitrosothiols (RSNO). Note general elevations in the signal intensity (AU, arbitrary units) of A•− and reciprocal decrease in the circulating concentration of bioactive NO metabolites in the highlanders, especially in the patient with CMS. Spectra were chosen to best reflect the average signal intensities observed in each of the respective groups.
Table 4.
Responses to hyperoxia in highlanders
| Subgroup: | CMS− (n = 14) | CMS+ (n = 23) | P values | ||||
|---|---|---|---|---|---|---|---|
| Inspirate: | Hypoxia | Hyperoxia | Hypoxia | Hyperoxia | Subgroup | Inspirate | Interaction |
| Cardiopulmonary | |||||||
| MAP (mmHg) | 80 ± 10 | 83 ± 8 | 87 ± 14 | 87 ± 16 | 0.188 | 0.500 | 0.405 |
| SBP (mmHg) | 118 ± 18 | 121 ± 13 | 128 ± 22 | 125 ± 24 | 0.284 | 0.885 | 0.267 |
| DBP (mmHg) | 61 ± 8 | 64 ± 7 | 67 ± 11 | 68 ± 13 | 0.167 | 0.241 | 0.426 |
| (L/min) | 6.99 ± 0.80 | 6.11 ± 0.77 | 7.24 ± 1.38 | 6.17 ± 1.22 | 0.663 | <0.001 | 0.540 |
| HR (beats/min) | 71 ± 12 | 68 ± 11 | 72 ± 14 | 67 ± 14 | 0.993 | <0.001 | 0.456 |
| SV (mL) | 101 ± 22 | 92 ± 21 | 103 ± 22 | 95 ± 28 | 0.746 | 0.001 | 0.863 |
| TPR (mmHg/L/min) | 11.60 ± 1.73 | 13.81 ± 2.00 | 12.41 ± 2.80 | 14.55 ± 3.99 | 0.385 | <0.001 | 0.948 |
| (%) | 91 ± 5 | 97 ± 1** | 88 ± 4† | 97 ± 1** | 0.043 | <0.001 | 0.043 |
| PETO2 (mmHg) | 54 ± 3 | 374 ± 81 | 51 ± 5 | 380 ± 51 | 0.887 | <0.001 | 0.699 |
| PETCO2 (mmHg) | 34 ± 5 | 35 ± 6 | 33 ± 4 | 39 ± 4 | 0.007 | 0.385 | 0.847 |
| Cerebrovascular | |||||||
| MCAv (cm/s) | 39 ± 12 | 39 ± 9 | 35 ± 12† | 34 ± 7† | 0.052 | 0.756 | 0.029 |
| MCAv/Hct (cm/s) | 42 ± 13 | 43 ± 11 | 43 ± 14 | 42 ± 8 | 0.939 | 0.759 | 0.626 |
| MCAv/PETCO2 (cm/s) | 48 ± 16 | 48 ± 14 | 44 ± 15 | 36 ± 11**† | 0.045 | 0.009 | 0.011 |
| PI (AU) | 1.15 ± 0.41 | 0.99 ± 0.18 | 1.45 ± 0.39† | 1.21 ± 0.27** | 0.013 | 0.001 | 0.041 |
| Normalised PI (AU) | 0.014 ± 0.005 | 0.012 ± 0.003 | 0.017 ± 0.004 | 0.015 ± 0.006 | 0.068 | 0.010 | 0.803 |
| CVR (mmHg/cm/s) | 2.27 ± 0.80 | 2.23 ± 0.53 | 2.89 ± 0.57 | 2.67 ± 0.74 | 0.054 | 0.518 | 0.640 |
| CVCi (cm/s/mmHg) | 0.49 ± 0.16 | 0.47 ± 0.11 | 0.42 ± 0.18 | 0.40 ± 0.12 | 0.119 | 0.504 | 0.861 |
| CDO2 (mL/cm/s) | 850 ± 305 | 909 ± 243 | 889 ± 303 | 951 ± 193 | 0.626 | 0.083 | 0.981 |
Values are mean ± SD; CMS−/CMS+, highlanders without/with chronic mountain sickness; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; , cardiac output; SV, stroke volume; TPR, total peripheral resistance; , arterial oxyhaemoglobin saturation; PETO2/CO2, end‐tidal partial pressure of oxygen/carbon dioxide; MCAv, middle cerebral artery velocity; SMCAv/DMCAv, systolic/diastolic MCAv; MCAv/Hct, MCAv (retrospectively) adjusted for differences in haematocrit (Hct); MCAv/PETCO2, MCAv (retrospectively) adjusted for differences in end‐tidal partial pressure of carbon dioxide, see Methods; AU, arbitrary units; CVR, cerebrovascular resistance; CVCi, cerebrovascular conductance index; CDO2, cerebral oxygen delivery. **Different vs. hypoxia for given subgroup (P < 0.05); †different vs. CMS− for given inspirate (P < 0.05). Data were analysed using two‐way (subgroup × inspirate) ANOVAs and post hoc Bonferonni‐adjusted paired (within subgroups) or independent (between subgroups) samples t‐tests.
Figure 4. Cerebrovascular reactivity to carbon dioxide (CVRCO2).
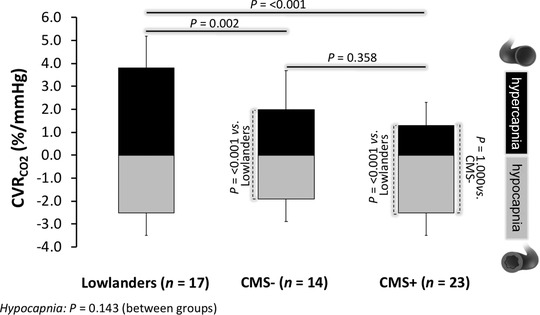
Values are mean ± SD; CMS−/CMS+, highlanders without/with chronic mountain sickness; *different vs. lowlanders for respective challenge (P < 0.05). Individual hypocapnia and hypercapnia datasets were analysed using one‐way ANOVAs and post hoc Bonferonni‐adjusted independent samples t‐tests.
Results
Anthropometric data
All groups were of a comparable age and body mass whereas the highlanders were generally shorter with a corresponding elevation in body mass index (BMI) in CMS+ (Table 1).
Metabolic data
Oxygenation and erythrocytosis
As anticipated, highlanders presented with arterial hypoxaemia with the most pronounced erythrocytosis observed in CMS+ (Table 1).
Oxidative stress
We observed lower GSH:GSSG in CMS+ due to the combination of lower GSH and higher GSSG (Table 2). We observed a reciprocal elevation in A•– that was generally more pronounced in highlanders and further exaggerated in CMS+ (Table 2). Figure 3 A–C provides typical examples of electron paramagnetic resonance (EPR) doublets observed exhibiting hydrogen hyperfine coupling constants (a H) of ∼1.8 G (g = 2.0052).
Inflammatory stress
MPO activity was higher in CMS+ (Table 2).
Nitrosative stress
Plasma and RSNO were consistently lower in highlanders (Table 2) but not different between CMS+ and CMS−. Figure 3 D–F provides typical examples of OBC traces observed.
Haemodynamic data
Cardiopulmonary
As anticipated, highlanders were more hypocapnic whereas we observed no between‐group/subgroup differences in MAP, SBP, DBP, , HR, SV or TPR (Table 3). Hyperoxia and the corresponding normalisation of decreased in the highlanders due to the combined decrease in both HR and SV and as a consequence was associated with elevated TPR (Table 4).
Cerebrovascular
Cerebral perfusion was generally lower in highlanders and was associated with a corresponding elevation in CVR and PI and reciprocal decrease in CVCi and CDO2 that was most marked in CMS+ (Table 3). While hyperoxia failed to normalise perfusion, it tended to restore CDO2 subsequent to an elevation in caO2 (Table 4). The lower cerebral perfusion still persisted in CMS+ when CBF was (retrospectively) adjusted for the independent vasoconstrictor effect of hypocapnoea but not polycythaemia (Table 4). Highlanders exhibited a lower CVRCO2 range due primarily to a blunted response to hypercapnoea (Fig. 4) whereas we observed no differences between CMS+ and CMS−. Figure 5 illustrates typical waveform data for a representative patient with CMS+ and corresponding spectral analysis for both spontaneous (resting) and driven (squat‐stand manoeuvres) oscillations in MAP and MCAv. Spontaneous and driven TFA metrics are illustrated in Fig. 6. During spontaneous TFA with moderate coherence values (Fig. 6 A), LF phase was higher in CMS+ (Fig. 6 B) with no (between‐group) differences observed in gain at either frequency (Fig. 6 C). The driven protocols, although not altering PETCO2 (P < 0.05 vs. spontaneous, data not shown), resulted in markedly amplified oscillations culminating in an augmented signal‐to‐noise ratio as confirmed by the 106–238‐fold (0.05 Hz) and 665–1320‐fold (0.10 Hz) increase in BP spectral power and 133–374‐fold (0.05 Hz) and 424–1039‐fold (0.10 Hz), increase in MCAv spectral power (compared to spontaneous VLF/LF measures at the respective frequency of interest) resulting in TFA coherence values exceeding 0.95 AU in all cases (Fig. 6 D). Despite no differences in phase (Fig. 6 E), we observed consistently lower (35–40%) point estimates of VLF and LF gain in the highlanders with no differences observed between CMS+ and CMS− (Fig. 6 F).
Figure 5. Typical waveforms and spectral analysis of haemodynamic responses observed during spontaneous (A) and repeated squat‐stand manoeuvres (B, C) in a representative patient with chronic mountain sickness.
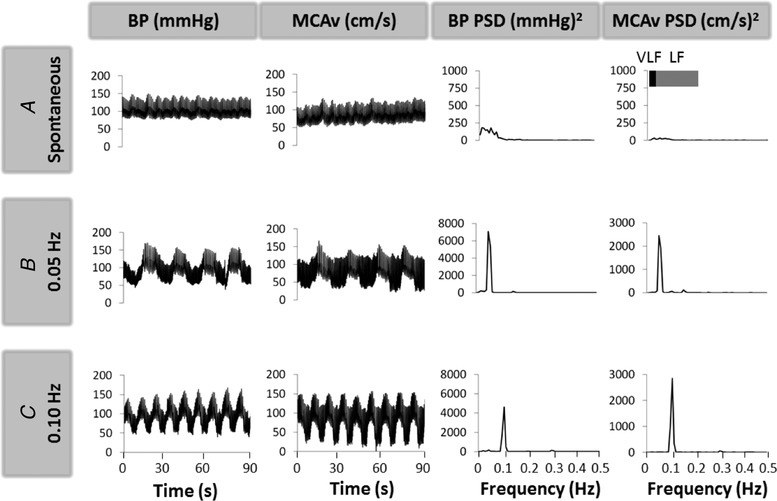
BP, blood pressure; MCAv, middle cerebral artery velocity; PSD, power spectral density; V/LF, very/low frequency. Note the markedly amplified and coherent oscillations in MAP and MCAv during the repeated squat‐stand manoeuvres compared to resting (spontaneous) measures, leading to improved estimation of transfer function of dynamic cerebral autoregulation at the frequency of interest.
Figure 6. Transfer function analysis of the cerebral pressure–flow relationship during spontaneous (A–C) and driven (D–F) oscillations in blood pressure and middle cerebral artery velocity.

Values are mean ± SD; CMS−/CMS+, highlanders without/with chronic mountain sickness; *different vs. lowlanders for the given frequency (P < 0.05). Individual frequency data sets were analysed using using one‐way ANOVAs and post hoc Bonferonni‐adjusted independent samples t‐tests.
Clinical data
Cognitive function
We observed consistently lower performances on RAVLT, RDB, RDF, TMT‐B and DSST in the highlanders, with the lowest performance recorded for RAVLT‐A6 and Stroop Task‐A in CMS+ (Table 5). We observed comparable MoCA scores between CMS− and lowlanders and consistently lower scores in CMS+, with 87% of the group (20/23 participants) scoring ≤25 points (Table 5).
Depression
Likewise, we observed comparable BDI scores between CMS− and lowlanders and consistently lower scores in CMS+ (Table 5).
Correlations
We observed inverse relationships between A•– and the following variables: , total bioactive NO, MCAv and CVRCO2Hyper (Fig. 7 A–D). We observed positive relationships between total bioactive NO and the following variables: CDO2 and MoCA (Fig. 7 E, F) and some aspects of cognitive function, namely performance on the RAVLT‐A1‐A5 (r = 0.438, P = 0.001), RAVLT‐B1 (r = 0.358, P = 0.008), RAVLT‐A6 (r = 0.270, P = 0.048), RDF (r = 0.536, P < 0.001), RDB (r = 0.565, P < 0.001) and DSST (r = 0.266, P = 0.052).
Figure 7. Relationships between metabolic, haemodynamic and clinical correlates in lowlanders and highlanders with and without chronic mountain sickness (CMS).
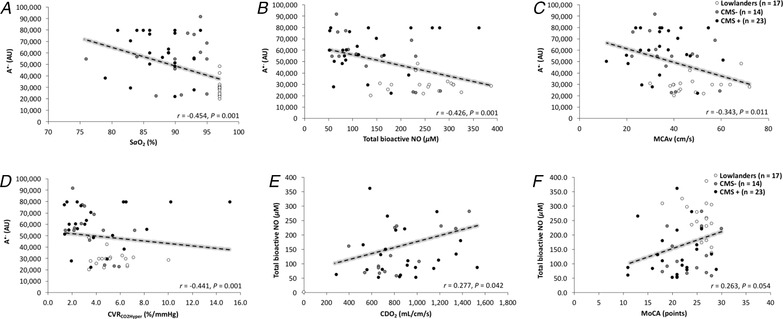
A, ascorbate radical; , arterial oxyhaemoglobin saturation; NO, nitric oxide; MCAv, middle cerebral artery blood flow velocity; CDO2, cerebral oxygen delivery; CVRCO2Hyper, cerebrovascular reactivity to carbon dioxide in the hypercapnic range; MoCA, Montreal Cognitive Assessment. Data were analysed using a Pearson product moment correlation.
Discussion
More than 150 million HA dwellers are permanently exposed to hypoxaemia, a problem predisposing lowlanders suffering from cardiopulmonary disease to cognitive dysfunction and dementia. Here, we show for the first time that systemic OXINOS was permanently elevated in healthy well‐adapted (CMS−) highlanders and accompanied by a proportional decrease in cerebral perfusion and blunted reactivity to hypercapnia. These alterations were associated with a mild decrease in cognitive performance, with learning/memory and attention/information processing the domains being most affected, but without any clinical evidence for depression. Second and in stark contrast, the sustained elevation in systemic OXINOS was further exaggerated in maladapted (CMS+) highlanders and accompanied by more pronounced impairments in cerebrovascular function, cognition and clinical symptoms of depression. Collectively, these findings are the first to suggest that a physiological continuum may exist for hypoxaemia‐induced systemic OXINOS that when excessive is associated with accelerated cognitive decline and depression, helping identify those in need of more specialist neurological follow‐up and targeted support.
Metabolic function
Because the concentration of ascorbate in human plasma is orders of magnitude greater than any oxidising free radical combined with the low one‐electron reduction potential for the A•−/ascorbate monanion (AH−) couple (Eo = 282 mV) (Williams & Yandell, 1982), any oxidising species (R•) generated within the systemic circulation will result in the one‐electron oxidation of ascorbate to form the distinctive EPR‐detectable A•− doublet (AH− + R• → A•− + R‐H, Fig. 3 A–C) (Buettner, 1993). Thus, the elevations observed in plasma A•− provide direct evidence that systemic free radical formation was permanently elevated in the highlanders, approximately double that observed in the lowlanders, with the highest concentrations recorded in the comparatively more hypoxaemic CMS+ patients.
These observations combined with the inverse relationships consistently observed between A•− and suggest that hypoxaemia may have been the upstream stimulus for oxidative catalysis, thus confirming our original hypothesis proposed in Fig. 1. Our findings agree with an evolving body of literature indicating that hypoxaemia catalyses not only systemic but also local cerebral free radical formation (Bailey et al. 2009c, 2018a) classically attributed to mitochondrial superoxide (O2 •−) release by complex III of the electron transport chain, notwithstanding additional contributions from other (i.e. extra‐mitochondrial) sources (Waypa et al. 2010). Systemic free radical formation coincided with the selective oxidation of GSH, the most abundant intracellular thiol found in the brain, which probably reflects an attempt to constrain OXINOS through the targeted scavenging of reactive oxygen, nitrogen and carbon‐centred species (Rae & Williams, 2017).
Oxidative stress coincided with both inflammatory and nitrosative stress as confirmed by the combined, sustained elevation in MPO and lower and RSNO, respectively, culminating in OXINOS that was most pronounced in CMS+. These findings extend our previous observations of a permanent and graded elevation in systemic OXNOS stress ranging from moderate in CMS− to severe in CMS+ (Bailey et al. 2013c). Because plasma is a source of NO with conversion catalysed by deoxyhaemoglobin‐mediated reduction and acidic disproportionation (Gladwin et al. 2000; Cosby et al. 2003) we interpreted the more exaggerated decrease in CMS+ to be one of the contributory factors responsible for the observed impairment in systemic vascular function in the form of blunted flow‐mediated dilatation and increased arterial stiffness (Bailey et al. 2013c). To further extend these observations, we sought to determine if the same concept applied more locally to the hypoxic cerebrovasculature, including to what extent exaggerated OXINOS associates with the neurological deficits underpinning CMS.
Haemodynamic function
Consistent with previous studies (Jansen & Basnyat, 2011), highlanders, in particular CMS+, exhibited ∼30% lower cerebral perfusion, notwithstanding the interpretive constraints associated with TCD ultrasound (Liu et al. 2017) and corresponding decrease in CDO2 that persisted even following correction for the independent vasoconstrictor effects of polycythaemia‐induced alteration in blood rheology and hypocapnia. The decrease in cerebral perfusion coincided with an impaired ability of the cerebrovasculature to respond to vasodilator stimuli, notably hyperoxia and hypercapnia, the latter being consistently more pronounced in CMS+. The consistent relationships observed between total bioactive NO, MCAv and CVRCO2Hyper, although not disassociating cause from effect, support a potential contributory role for OXINOS and corresponding decrease in vascular NO availability, consistent with previous studies that have highlighted endothelial‐derived NO as an important, albeit not exclusive, mediator of cerebral (hyper)perfusion (Lavi et al. 2003). Interestingly, OXINOS appeared to exert more of an effect on the cerebral than systemic circulation given the lack of difference in MAP and TPR in CMS+.
Furthermore, we have previously identified that NO plays an important role in the maintenance of cerebral vasomotor tone and blood–brain barrier integrity by dynamically buffering changes in CBF in response to spontaneous changes in MAP (Bailey et al. 2009a, c , 2011) justifying a complementary examination of the pressure–flow coupling dynamic. Contrary to our original expectations based on the published literature (Jansen et al. 2000), the elevated LF phase observed in CMS+ during spontaneous oscillations indicated improved pressure–flow coupling potentially related to the elevated CVR or hypocapnia as observed subsequent to changes in the respiratory chemoreflex (Ogoh et al. 2010) or, equally, to increased sympathetic tone (Lundby et al. 2018). The phase and gain findings associated with the elevated coherence during the driven oscillations revealed a similar mechanism, namely that gain was lower in the highlanders with the lowest values observed in CMS+. Lower gain is consistent with findings in Himalayan Sherpas (Smirl et al. 2014) and may reflect an improved ability to buffer perfusion during rapid alterations in BP to protect against vasogenic oedema in the face of exaggerated OXINOS. However, it would seem unlikely that this constitutes a functionally neuroprotective adaptation given that our participants, in particular CMS+, exhibited clinical signs of neurodegeneration (see below).
Clinical function
Researchers have suggested that the Andean model of HA living, defined by cerebral hypoperfusion and polycythaemia, is phenotypically maladaptive given that it carries a higher risk of stroke and migraine and is associated with increased morbidity and earlier mortality (Virues‐Ortega et al. 2009, Jansen & Basnyat, 2011). In support of this, Bolivians born and bred at the same altitude and location to that used in the present study exhibited slower psychomotor speed in attention and digit symbol coding tasks that persisted across the lifespan, reflecting a ‘speed–accuracy’ trade‐off such that slower may be surer (Hill et al. 2014). The present findings further extend the albeit limited literature identifying impairments in learning/memory and attention/information processing that were especially pronounced in CMS+.
Thus, it would appear that the highlander's brain, especially in CMS+, is unable to compensate for its hypoperfusion‐induced CDO2 constraint. Any global or local cerebral O2 deficit has the potential to impact cognition potentially due to impaired neurotransmitter release, which animal studies suggest is related to depressed monoamine synthesis (Freeman & Gibson, 1988), elevated glutamate excitotoxicity (Hota et al. 2008) and perturbations in choline acetyltranserase/acetyl cholinesterase expression (Guerra‐Narbona et al. 2013).
The observation that clinical symptoms of depression were absent in CMS− and only apparent in CMS+, in whom cognitive/haemodynamic impairments were most pronounced, suggests that a physiological continuum for hypoxaemia‐induced systemic OXINOS may potentially exist that when surpassed may prove maladaptive and contribute to the neurological complications associated with CMS. This hypothesis is not unreasonable given that hypoxia and OXINOS contribute to the pathophysiology of dementia (Sun et al. 2006; Wojsiat et al. 2018) and depression (Salim, 2014) in lowlander patient groups and extends our previous observations, albeit confined to the systemic vascular circulation (Bailey et al. 2013c). Alternatively, if systemic OXINOS/hypoxaemia are less pronounced it may simply take more time to develop clinical symptoms. Furthermore, our measurements obtained at rest may have underestimated the true magnitude of hypoxic stress encountered given that hypoxaemia in CMS+ is further compounded by physical activity (Stuber et al. 2010; Pratali et al. 2012) and sleep‐disordered breathing (Rexhaj et al. 2016).
We observed a similar profile for performance on MoCA with consistently lower scores recorded in CMS+. Although originally developed as a measure of global cognitive function (Nasreddine et al. 2005), MoCA is frequently used as a clinical screening tool for the dementias (Ballard et al. 2013) with a cut‐off score of 25 points or lower widely used as the threshold for detecting mild cognitive impairment and possible dementia (Davis et al. 2015a). Thus, with 87% of the CMS+ group fulfilling these clinical criteria, our findings may help identify those HA dwellers most ‘at risk’ and in need of more specialist neurological assessment to diagnose an emerging dementia syndrome. Clinical diagnosis is inherently complex, depending on the triad of cognitive function, patient report and informant history, notwithstanding complementary assessments of cerebrospinal fluid, blood‐borne and structural biomarkers to exclude inflammatory, infective and malignancy‐related causes of dementia (Burns & Iliffe, 2009; Robinson et al. 2015).
Experimental limitations
We need to consider several limitations when interpreting the present findings. First, the OXINOS assays we used, despite taking advantage of the most direct analytical techniques currently available, ultimately rely on ex vivo detection of relatively stable reactants confined to circulating plasma/red blood cells formed downstream of the primary source/reaction pathway that we assume reflects dynamic events in vivo (Bailey et al. 2009a). Given that these metabolites, especially GSH:GSSG, partition heterogenously across different tissues, our conclusions only apply to what we observed in circulating blood. In addition, there remains the inevitable translational challenge when attempting to determine the clinical outcomes associated with elevated OXINOS given our current inability to differentiate between physiologically adaptive and pathologically maladaptive concentration thresholds (Bailey et al. 2013c). Furthermore, we encourage interventional studies incorporating targeted antioxidant prophylaxis to disassociate cause from effect and confirm the mechanisms proposed herein. Second, we need to be cautious when interpreting the perfusion data in HA dwellers given that we relied on differences in MCAv as an indirect surrogate of global CBF that fails to take into account the antagonistic dilatory/constricting effects caused by prevailing hypoxia/hypocapnia (Wilson et al. 2011). Finally, future researchers need to consider more specialist follow‐up neurological assessments to complement the current approaches taken to determine the prevalence of dementia in the most vulnerable CMS+ patient subgroup.
Conclusions
Notwithstanding the experimental limitations as outlined, these findings indicate that a chronic state of disequilibrium potentially exists between free radical formation and antioxidant defence in highlanders, causing systemic OXINOS to be permanently elevated and especially exaggerated in more hypoxaemic CMS+ patients. OXINOS was associated with blunted perfusion and reactivity to hypercapnia, impaired cognition and, in CMS+, symptoms of depression. Collectively, these findings are the first to suggest that a physiological continuum may exist for hypoxaemia‐induced systemic OXINOS that when excessive is associated with accelerated cognitive decline and depression, helping identify those HA dwellers, especially CMS+, who may require more specialist neurological follow‐up and targeted support. Future investigators need to consider the potential neuroprotective benefits of targeted antioxidant prophylaxis and cognitive training in this patient population. Finally, because arterial hypoxaemia is a hallmark feature of circulatory diseases, including those that affect the brain, the current findings in Aymaras may provide complementary insight into the pathophysiology and treatment of patients suffering from early‐onset neurodegeneration at sea‐level.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
DMB, US and CS conceived and designed the research. DMB, US and CS obtained funding. All authors contributed to data collection and analysis. All authors interpreted the results of the experiments. DMB drafted the manuscript and revisions thereof. All authors edited and revised the manuscript(s) and approved the final version submitted for publication. All authors agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Supported by a Royal Society Wolfson Research Fellowship (#WM170007) to DMB and grants from the Swiss National Science Foundation, Cloëtta Foundation, Eagle Foundation, Leenaards Foundation and Placide Nicod Foundation to US and CS.
Acknowledgements
We thank Mrs Catherine Romero and staff of the Instituto Boliviano de Biologia de Altura (La Paz, Bolivia) for technical support. ADInstruments provided exceptional technical input/support during the period of data logging. Finally, we appreciate the participants and patients’ enthusiasm and commitment to this study. Our study is dedicated to the memory of the late Dr Christopher K Willie (University of British Columbia Okanagan, Kelowna, Canada) whose memory remains a constant source of inspiration.
Biography
Damian M. Bailey embarked on a PhD in human physiology while working as a research physiologist at the British Olympic Medical Centre in collaboration with Oxford University. Following training at the Universities of California San Diego and Colorado Health Sciences Center, he returned to the University of South Wales where he is currently Professor of Physiology & Biochemistry and a Royal Society Wolfson Research Fellow leading the Neurovascular Research Laboratory. His research programme takes an integrated translational approach to investigate how free radicals and associated reactive oxygen/nitrogen species control oxygen delivery to the brain across the clinical spectrum of human health and disease.

Edited by: Michael Hogan & Emma Hart
This is an Editor's Choice article from the 15 January 2019 issue.
References
- War Department Adjutant General's Office (1944). Army Individual Test Battery. Manual of Directions and Scoring. War Department Adjutant General's Office, Washington, DC. [Google Scholar]
- Alle H, Roth A & Geiger JR (2009). Energy‐efficient action potentials in hippocampal mossy fibers. Science 325, 1405–1408. [DOI] [PubMed] [Google Scholar]
- Bagge CN, Henderson VW, Laursen HB, Adelborg K, Olsen M & Madsen NL (2018). Risk of dementia in adults with congenital heart disease: population‐based cohort study. Circulation 137, 1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, Evans KA, James PE, McEneny J, Young IS, Fall L, Gutowski M, Kewley E, McCord JM, Moller K & Ainslie PN (2009a). Altered free radical metabolism in acute mountain sickness: implications for dynamic cerebral autoregulation and blood–brain barrier function. J Physiol 587, 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, Evans KA, McEneny J, Young IS, Hullin DA, James PE, Ogoh S, Ainslie PN, Lucchesi C, Rockenbauer A, Culcasi M & Pietri S (2011). Exercise‐induced oxidative‐nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood‐brain barrier leakage. Exp Physiol 96, 1196–1207. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Jones DW, Sinnott A, Brugniaux JV, New KJ, Hodson D, Marley CJ, Smirl JD, Ogoh S & Ainslie PN (2013a). Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin Sci 124, 177–189. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S& Ainslie PN (2013b). Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44, 3235–3238. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Rasmussen P, Evans KA, Bohm AM, Zaar M, Nielsen HB, Brassard P, Nordsborg NB, Homann PH, Raven PB, McEneny J, Young IS, McCord JM & Secher NH (2018a). Hypoxia compounds exercise‐induced free radical formation in humans; partitioning contributions from the cerebral and femoral circulation. Free Radic Biol Med 124, 104–113. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Rasmussen P, Overgaard M, Evans KA, Bohm AM, Seifert T, Brassard P, Zaar M, Nielsen HB, Raven PB & Secher NH (2017a). Nitrite and S‐nitrosohemoglobin exchange across the human cerebral and femoral circulation: relationship to basal and exercise blood flow responses to hypoxia. Circulation 135, 166–176. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Rimoldi SF, Rexhaj E, Pratali L, Salinas Salmon C, Villena M, McEneny J, Young IS, Nicod P, Allemann Y, Scherrer U & Sartori C (2013c). Oxidative‐nitrosative stress and systemic vascular function in highlanders with and without exaggerated hypoxemia. Chest 143, 444–451. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Stacey BS & Gumbleton M (2018b). A systematic review and meta‐analysis reveals altered drug pharmacokinetics in humans during acute exposure to terrestrial high altitude‐clinical justification for dose adjustment? High Alt Med Biol 19, 141–148. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Taudorf S, Berg RMG, Jensen LT, Lundby C, Evans KA, James PE, Pedersen BK & Moller K (2009b). Transcerebral exchange kinetics of nitrite and calcitonin gene‐related peptide in acute mountain sickness: evidence against trigeminovascular activation? Stroke 40, 2205–2208. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Taudorf S, Berg RMG, Lundby C, McEneny J, Young IS, Evans KA, James PE, Shore A, Hullin DA, McCord JM, Pedersen BK & Moller K (2009c). Increased cerebral output of free radicals during hypoxia: implications for acute mountain sickness? Am J Physiol Regul Integr Comp Physiol 297, R1283‐1292. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Willie CK, Hoiland RL, Bain AR, MacLeod DB, Santoro MA, DeMasi DK, Andrijanic A, Mijacika T, Barak OF, Dujic Z & Ainslie PN (2017b). Surviving without oxygen: how low can the human brain go? High Alt Med Biol 18, 73–79. [DOI] [PubMed] [Google Scholar]
- Ballard C, Burns A, Corbett A, Livingston G & Rasmussen J (2013). Helping You Assess Cognition: A Practical Toolkit For Clinicians. Alzheimer's Society, London. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J & Erbaugh J (1961). An inventory for measuring depression. Arch Gen Psych 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Buettner GR (1993). The pecking order of free radicals and antioxidants: lipid peroxidation, α;‐tocopherol, and ascorbate. Arch Biochem Biophys 300, 535–543. [DOI] [PubMed] [Google Scholar]
- Buettner GR & Jurkiewicz BA (1993). Ascorbate free radical as a marker of oxidative stress: an EPR study. Free Radic Biol Med 14, 49–55. [DOI] [PubMed] [Google Scholar]
- Burns A & Iliffe S(2009). Dementia. BMJ 338, b75. [DOI] [PubMed] [Google Scholar]
- Claassen JA, Meel‐van den Abeelen AS, Simpson DM & Panerai RB; International Cerebral Autoregulation Research Network (2016). Transfer function analysis of dynamic cerebral autoregulation: A white paper from the International Cerebral Autoregulation Research Network. J Cereb Blood Flow Metab 36, 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim‐Shapiro DB, Schechter AN, Cannon RO & Gladwin MT (2003). Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9, 1498–1505. [DOI] [PubMed] [Google Scholar]
- Davis DH, Creavin ST, Yip JL, Noel‐Storr AH, Brayne C & Cullum S(2015a). Montreal Cognitive Assessment for the diagnosis of Alzheimer's disease and other dementias. Cochrane Database Syst Rev CD010775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JE, Wagner DR, Garvin N, Moilanen D, Thorington J & Schall C (2015b). Cognitive and psychomotor responses to high‐altitude exposure in sea level and high‐altitude residents of Ecuador. J Physiol Anthropol 34, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola M, Bozzali M, Fadda L, Musicco M, Sabatini U & Caltagirone C (2008). Reduced oxygen due to high‐altitude exposure relates to atrophy in motor‐function brain areas. Eur J Neurol 15, 1050–1057. [DOI] [PubMed] [Google Scholar]
- Freeman GB & Gibson GE (1988). Dopamine, acetylcholine, and glutamate interactions in aging. Behavioral and neurochemical correlates. Ann N Y Acad Sci 515, 191–202. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Shelhamer JH, Schechter AN, Pease‐Fye ME, Waclawiw MA, Panza JA, Ognibene FP & Cannon RO, III (2000). Role of circulating nitrite and S‐nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A 97, 11482–11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra‐Narbona R, Delgado‐Garcia JM & Lopez‐Ramos JC (2013). Altitude acclimatization improves submaximal cognitive performance in mice and involves an imbalance of the cholinergic system. J Appl Physiol 114, 1705–1716. [DOI] [PubMed] [Google Scholar]
- Hill CM, Dimitriou D, Baya A, Webster R, Gavlak‐Dingle J, Lesperance V, Heathcote K & Bucks RS (2014). Cognitive performance in high‐altitude Andean residents compared with low‐altitude populations: from childhood to older age. Neuropsychology 28, 752–760. [DOI] [PubMed] [Google Scholar]
- Hota SK, Barhwal K, Singh SB, Sairam M & Ilavazhagan G (2008). NR1 and GluR2 expression mediates excitotoxicity in chronic hypobaric hypoxia. J Neurosci Res 86, 1142–1152. [DOI] [PubMed] [Google Scholar]
- Jansen GF & Basnyat B (2011). Brain blood flow in Andean and Himalayan high‐altitude populations: evidence of different traits for the same environmental constraint. J Cereb Blood Flow Metab 31, 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen GFA, Krins A, Basnyat B, Bosch A & Odoom JA (2000). Cerebral autoregulation in subjects adapted and not adapted to high altitude. Stroke 31, 2314–2318. [DOI] [PubMed] [Google Scholar]
- Katsogridakis E, Bush G, Fan L, Birch AA, Simpson DM, Allen R, Potter JF & Panerai RB. (2013). Detection of impaired cerebral autoregulation improves by increasing arterial blood pressure variability. J Cereb Blood Flow Metab 33, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H (1963). Clinical meuropsychology. Med Clin N Am 47, 1647–1658. [PubMed] [Google Scholar]
- Lavi S, Egbarya R, Lavi R & Jacob G (2003). Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation 107, 1901–1905. [DOI] [PubMed] [Google Scholar]
- Leon‐Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta‐Castillo G & Zubieta‐Calleja G (2005). Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 6, 147–157. [DOI] [PubMed] [Google Scholar]
- Liu W, Liu J, Lou X, Zheng D, Wu B, Wang DJ & Ma L. (2017). A longitudinal study of cerebral blood flow under hypoxia at high altitude using 3D pseudo‐continuous arterial spin labeling. Sci Rep 7, 43246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Calbet J, van Hall G, Saltin B & Sander M. (2018). Sustained sympathetic activity in altitude acclimatizing lowlanders and high‐altitude natives. Scand J Med Sci Sports 28, 854–861. [DOI] [PubMed] [Google Scholar]
- Marley CJ, Sinnott A, Hall JE, Morris‐Stiff G, Woodsford PV, Lewis MH & Bailey DM (2017). Failure to account for practice effects leads to clinical misinterpretation of cognitive outcome following carotid endarterectomy. Physiol Rep 5, p11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris T, Hale BJ, Barwood M, Costello J & Corbett J (2017). Effect of acute hypoxia on cognition: A systematic review and meta‐regression analysis. Neurosci Biobehav Rev 74, 225–232. [DOI] [PubMed] [Google Scholar]
- N'Guessan P, Pouyet L, Gosset G, Hamlaoui S, Seillier M, Cano CE, Seux M, Stocker P, Culcasi M, Iovanna JL, Dusetti NJ, Pietri S& Carrier A (2011). Absence of tumor suppressor tumor protein 53‐induced nuclear protein 1 (TP53INP1) sensitizes mouse thymocytes and embryonic fibroblasts to redox‐driven apoptosis. Antioxid Redox Signal 15, 1639–1653. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL & Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatric Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Nakahara H, Ainslie PN & Miyamoto T (2010). The effect of oxygen on dynamic cerebral autoregulation: critical role of hypocapnia. J Appl Physiol 108, 538–543. [DOI] [PubMed] [Google Scholar]
- Peers C, Dallas ML, Boycott HE, Scragg JL, Pearson HA & Boyle JP (2009). Hypoxia and neurodegeneration. Ann N Y Acad Sci 1177, 169–177. [DOI] [PubMed] [Google Scholar]
- Pratali L, Rimoldi SF, Rexhaj E, Hutter D, Faita F, Salmon CS, Villena M, Sicari R, Picano E, Allemann Y, Scherrer U & Sartori C (2012). Exercise induces rapid interstitial lung water accumulation in patients with chronic mountain sickness. Chest 141, 953–958. [DOI] [PubMed] [Google Scholar]
- Rae CD & Williams SR (2017). Glutathione in the human brain: review of its roles and measurement by magnetic resonance spectroscopy. Ann Biochem 529, 127–143. [DOI] [PubMed] [Google Scholar]
- Rexhaj E, Rimoldi SF, Pratali L, Brenner R, Andries D, Soria R, Salinas C, Villena M, Romero C, Allemann Y, Lovis A, Heinzer R, Sartori C & Scherrer U (2016). Sleep‐disordered breathing and vascular function in patients with chronic mountain sickness and healthy high‐altitude dwellers. Chest 149, 991–998. [DOI] [PubMed] [Google Scholar]
- Rey A. (1958). L'examen clinique en psychologie. (The clinical examination in psychology). Presses Universitaries de France, Oxford. [Google Scholar]
- Rimoldi SF, Rexhaj E, Duplain H, Urben S, Billieux J, Allemann Y, Romero C, Ayaviri A, Salinas C, Villena M, Scherrer U & Sartori C (2016). Acute and chronic altitude‐induced cognitive dysfunction in children and adolescents. J Pediatr 169, 238–243. [DOI] [PubMed] [Google Scholar]
- Robinson L, Tang E & Taylor JP (2015). Dementia: timely diagnosis and early intervention. BMJ 350, h3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S (2014). Oxidative stress and psychological disorders. Curr Neuropharmacol 12, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinghaus JW (2001). Cerebral circulation at altitude In High Altitude, an Exploration of Human Adaptation, ed. Hornbein TF. & Schoene RB, pp. 343–375. Marcel Dekker, New York. [Google Scholar]
- Sheehan B (2012). Assessment scales in dementia. Therap Adv Neurol Disord 5, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirl JD, Hoffman K, Tzeng YC, Hansen A & Ainslie PN (2015). Methodological comparison of active‐ and passive‐driven oscillations in blood pressure; implications for the assessment of cerebral pressure‐flow relationships. J Appl Physiol 119, 487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirl JD, Lucas SJ, Lewis NC, duManoir GR, Smith KJ, Bakker A, Basnyat AS & Ainslie PN (2014). Cerebral pressure‐flow relationship in lowlanders and natives at high altitude. J Cereb Blood Flow Metab 34, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker P, Cassien M, Vidal N, Thetiot‐Laurent S& Pietri S (2017). A fluorescent homogeneous assay for myeloperoxidase measurement in biological samples. A positive correlation between myeloperoxidase‐generated HOCl level and oxidative status in STZ‐diabetic rats. Talanta 170, 119–127. [DOI] [PubMed] [Google Scholar]
- Stroop JS (1935). Studies of the interference in serial verbal reactions. J Exp Psychol 18, 643–662. [Google Scholar]
- Stuber T, Sartori C, Schwab M, Jayet PY, Rimoldi SF, Garcin S, Thalmann S, Spielvogel H, Salmon CS, Villena M, Scherrer U & Allemann Y (2010). Exaggerated pulmonary hypertension during mild exercise in chronic mountain sickness. Chest 137, 388–392. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE & Song W (2006). Hypoxia facilitates Alzheimer's disease pathogenesis by up‐regulating BACE1 gene expression. Proc Natl Acad Sci U S A 103, 18727–18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte FC & Corante N (2016). Chronic mountain sickness: clinical aspects, etiology, management, and treatment. High Alt Med Biol 17, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virues‐Ortega J, Buela‐Casal G, Garrido E & Alcazar B (2004). Neuropsychological functioning associated with high‐altitude exposure. Neuropsychol Rev 14, 197–224. [DOI] [PubMed] [Google Scholar]
- Virues‐Ortega J, Hogan AM, Baya‐Botti A, Kirkham FJ, Baldeweg T, Mahillo‐Fernandez I, de Pedro‐Cuesta J & Bucks RS; Bolivian Children Living at Altitude Project (2009). Survival and mortality in older adults living at high altitude in Bolivia: a preliminary report. J Am Geriatr Soc 57, 1955–1956. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP & Initiative S(2014). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 12, 1495–1499. [DOI] [PubMed] [Google Scholar]
- Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D & Schumacker PT (2010). Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 106, 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1955). Manual For The Wechsler Adult Intelligence Scale. Psychological Corp., Oxford. [Google Scholar]
- Williams NH & Yandell JK (1982). Outer‐sphere electron‐transfer reactions of ascorbic anions. Austr J Chem 35, 1133–1144. [Google Scholar]
- Wilson MH, Edsell ME, Davagnanam I, Hirani SP, Martin DS, Levett DZ, Thornton JS, Golay X, Strycharczuk L, Newman SP, Montgomery HE, Grocott MP, Imray CH & Caudwell Xtreme Everest Research G (2011). Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia‐an ultrasound and MRI study. J Cereb Blood Flow Metab 31, 2019–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojsiat J, Zoltowska KM, Laskowska‐Kaszub K & Wojda U (2018). Oxidant/antioxidant imbalance in alzheimer's disease: therapeutic and diagnostic prospects. Oxid Med Cell Longev 2018, 6435861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside JD, Gutowski M, Fall L, James PE, McEneny J, Young IS, Ogoh S & Bailey DM (2014). Systemic oxidative‐nitrosative‐inflammatory stress during acute exercise in hypoxia; implications for microvascular oxygenation and aerobic capacity. Exp Physiol 99, 1648–1662. [DOI] [PubMed] [Google Scholar]
- Yan X, Zhang J, Gong Q & Weng X (2011). Adaptive influence of long term high altitude residence on spatial working memory: an fMRI study. Brain Cogn 77, 53–59. [DOI] [PubMed] [Google Scholar]
- Yao WJ, Pan HA, Wang ST, Yang YK, Yu CY & Lin HD (2009). Frontal cerebral blood flow changes after hormone replacement therapy in depressed postmenopausal women. J Cereb Blood Flow Metab 29, 1885–1890. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Giller CA & Levine BD (1998). Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274, H233–241. [DOI] [PubMed] [Google Scholar]


