Abstract
Background
May‐Thurner Syndrome (MTS) is caused by compression of the left common iliac vein between the right common iliac artery and the pelvis. It likely predisposes an individual to lower extremity deep vein thrombosis (DVT) as well as symptoms of unilateral lower extremity swelling and discomfort in the absence of a known history of thrombosis. In the case of MTS‐associated acute thrombosis, there is low‐quality evidence to suggest that endovascular intervention including thrombolysis and endovascular stent placement reduces the risk of recurrent thrombosis. However, the optimal type and duration of antithrombotic therapy after stent placement for left iliofemoral vein stenosis is not known.
Methods
A systematic literature search including studies that evaluated the outcome of endovascular stent occlusion and systemic anticoagulant use in patients with MTS associated DVT was performed. The primary outcome of interest was 12‐month risk of endovascular stent occlusion or recurrent DVT.
Results
A total of five studies encompassing 61 patients were included in our study. All studies were retrospective without a comparator group. A variety of anticoagulants and durations were prescribed. Of the 55 patients evaluable, the 12‐month rate of endovascular stent occlusion or recurrent DVT ranged from 0% to 40%. The 12‐month stent patency rate ranged from 60% to 100%.
Conclusions
The published evidence regarding antithrombotic treatment for patients with MTS who have undergone stent placement for a DVT is limited. Further high‐quality, prospective studies are needed in this setting to inform clinical decision making.
Keywords: antithrombotic, deep vein thrombosis, endovascular stent, iliac venous compression syndrome, May‐Thurner syndrome, stent occlusion
Essentials.
May‐Thurner syndrome (MTS) is an anatomical variant that may be associated with deep vein thromboembolism (DVT).
Optimal antithrombotic management in patients with MTS and DVT who undergo endovascular stenting is unknown.
The available evidence on antithrombotic management in this setting is reviewed and discussed.
Optimal systemic management in this setting remains uncertain and further high‐quality, prospective studies are needed.
1. INTRODUCTION
The development of venous thromboembolism is multifactorial and results from environmental risk factors and patient characteristics that can be acquired, inherited, or unknown. Left iliac venous compression syndrome (IVCS), also known as May‐Thurner Syndrome (MTS), which denotes compression of the left common iliac vein by the right common iliac artery, can cause unilateral lower extremity swelling, discomfort, and is thought to be a risk factor for thrombosis.1 There are no standard diagnostic criteria for MTS diagnosis by imaging2; the degree of measured venous compression may depend on more than one factor, including an individual's volume status.3 Diagnosis requires persistent narrowing of the left iliac vein regardless of positioning2 with MTS anatomy suggested by venous collateral development, hemodynamic flow >2 mmHg across the stenotic segment, or degree of left common iliac vein stenosis (e.g, >50% reduction in the luminal venous diameter).2 The prevalence of MTS is currently unknown with a spectrum of estimates. Autopsy assessments have identified a left iliac “spur,” caused by left iliac vein compression by the right iliac artery, in 14%‐22% of cadavers.1, 4 One study of 77 patients presenting with left lower extremity symptoms, found that nearly 50% had evidence of iliac vein compression.5 Another study screened 50 patients with abdominal complaints but without lower extremity symptoms utilizing computed tomographic scans; 25% of the individuals had hemodynamically significant lesions causing at least 50% stenosis in the left common iliac vein while 66% had at least 25% compression.6 The lack of precise prevalence rates for MTS in the general population and among those with lower extremity symptoms makes it difficult to evaluate the clinical significance of iliac vein compression.
Management of acute thrombosis in the setting of MTS has evolved over the past two decades with the use of endovascular management, angioplasty, and stent placement to address the acute thrombus and vascular stenosis.7, 8 Compared to anticoagulation alone, endovascular angioplasty and stent placement may decrease the risk of recurrent thrombosis and/or severe post‐thrombotic syndrome,5 but its benefits are not well established. This is not a review of the evidence evaluating the impact of endovascular stent placement itself. Rather, this review summarizes the available evidence to support choice of drug, dose, and duration of antithrombotic therapy after stent placement for patients with left IVCS and acute thrombosis.
2. MATERIALS AND METHODS
We performed a systematic review of the literature to examine the utilization of anticoagulants in the setting of venous stent placement for acute deep venous thrombosis associated MTS. A search of PubMed from inception until December 15, 2017 was undertaken regarding terms “left iliac venous” or “left iliac vein” or “May‐Thurner syndrome” or “May‐Thurner Syndrome” and “Stents” or “stent” and English language. A search of Embase was undertaken regarding terms “May‐Thurner or “lower extremity deep vein thrombosis” or “left iliac venous” or “left iliac vein” and “stent” and English language. A search of Scopus was undertaken regarding terms “stent” and “may‐thurner syndrome” or “lower extremity deep vein thrombosis” and English language. A search of Web of Science was undertaken regarding terms “lower extremity deep vein thrombosis” or “may‐thurner syndrome”
2.1. Study selection
Studies were considered potentially eligible for this systematic review if they met all of the following criteria: (a) assessed outcomes of stent patency and/or recurrent thrombosis in individuals diagnosed with an acute DVT and MTS treated with endovascular stenting; (b) followed patients for at least 12 months with stent or lower extremity imaging; (c) at least 75% of the patients were evaluated at 12 months; (d) anticoagulation was described as drug, dose, and duration; and (e) included at least three patients.
From 396 papers, five were eligible for inclusion.9, 10, 11, 12, 13 The other 391 studies were excluded for a variety of reasons, including: small number of patients (114), lack of outcome reporting (30), and published as abstract only (61). Figure 1 provides details about the reasons that most papers were excluded. Some articles were excluded if the title and abstract clearly did not meet all the inclusion criteria, but if there was doubt the full paper was reviewed (see Figure 1).
Figure 1.
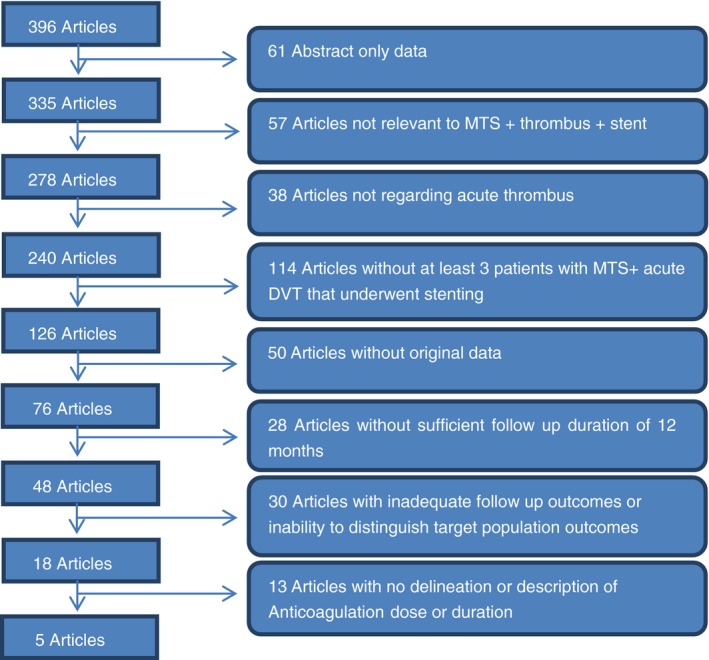
Flowchart of article review and selection
2.2. Data extraction
Key articles characteristics, including author, year of publication, study type, inclusion and exclusion criteria, number of patients included, patient demographics of age and gender, thrombotic risk factors if known, location of thrombosis, endovascular procedure, utilization and removal of inferior vena cava filter, type of endovascular stent, anticoagulant drug and dose, duration of anticoagulant, antiplatelet therapy drug and duration, stent occlusion at 12 months, thrombosis recurrence at 12 months and post‐thrombotic syndrome scores, were extracted and recorded in evidence tables.
3. RESULTS
The five included articles were all retrospective and describe outcomes of 61 patients with MTS and thrombosis who underwent endovascular stent placement and a subsequent antithrombotic treatment (Table 1). The majority of the patients included in this review were female, which is consistent with prior reports of MTS‐associated DVT (Table 2).
Table 1.
Characteristics of included studies
| Author | Year | Study type | Inclusion criteria | Exclusion criteria | # Patients | Acute DVT management procedure | Angioplasty | IVC filter |
|---|---|---|---|---|---|---|---|---|
| Goldman | 2017 | Retrospective | Pediatric patients who underwent endovascular therapy for lower‐extremity venous obstruction | Stent placement prior to initial presentation to target institution, lack of postintervention follow up | 6 | Catheter directed thrombolysis or pharmocomechanical thrombolysis | Y | No |
| Husmann | 2007 | Retrospective | First episode DVT, symptoms <7 days | Not reported | 11 | Surgical Thrombectomy and Thrombolysis and AV fistula formation | Y | No |
| Kim | 2017 | Retrospective | Iliac vein–involved DVT, symptom onset <30 days, patients who underwent PMT with/without stent insertion. | Patients who have any risk for thrombolysis or anticoagulation. Patients who have hematologic disorder affecting the coagulation cascade. Pregnancy. Caused by tumor obstruction | 25a | Pharmocomechanical Thrombectomy ± Thrombolysis | N | 11/25 received IVC (removed 4 weeks after procedure) |
| Matsuda | 2014 | Retrospective | Individuals with continued left lower extremity symptoms due to venous stenosis or stasis detected by venography after thrombolytic therapy or thrombectomy for acute DVT | <40 years old, rich collateral through pelvic plexus, large vessel diameter | 13 | Catheter‐directed thrombolysis | N | Yes, all retrieved after procedure |
| Roy | 2017 | Retrospective | DVT in the setting of MTS, managed with PMT as the primary mode of intervention, in combination with angioplasty and stent placement | Not reported | 6 | Pharmocomechanical thrombolysis + stent, or angioplasty + stent | Y | No |
AV, arteriovenous; DVT, deep vein thrombosis; IVC, iliac vein compression; MTS, May‐Thurner syndrome; PMT, pharmocomechanical thrombolysis.
One patient with right iliac vein compression between tortuous right iliac artery and psoas muscle included.
Table 2.
Characteristics of included studies regarding utilization endovascular techniques
| Article (year) | Number of study participants | Median age (years) | Female | Male | Endovascular procedure | AV fistula formation | Stent type | Additional thrombolysis required after intervention | Time to occlusion by 12 months | Eventsa within 12 months | Note |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Goldman (2017) | 6 | 17 | 3 | 3 | Variable | No | Variable | 2/6 | 1 and 11 | 2 | One stent occlusion associated with anticoagulation nonadherence |
| Husman (2007) | 11 | 34 | 9 | 2 | TT | 7/11 | Wall‐stent | No | 0.25 and 3 | 2 | |
| Kim (2017) | 25 | 61 | 19 | 5 | PMT | No | Unknown | No | “Acute”b | 1 | |
| Matsuda (2014) | 13 | 63 | 7 | 6 | CDT | No | Variable | 4/13 | 0.5 and 1 | 2 | One stent occlusion associated with anticoagulation nonadherence |
| Roy (2017) | 6 | 48 | 5c | 1 | PMT | No | Luminexx | No | 0 |
CDT, catheter directed thrombolysis; PMT, pharmocomechanical thrombolysis; TT, surgical thrombectomy + thrombolysis.
“In‐stent thrombosis” or recurrent venous thromboembolism.
“Acute” but no specific time to occlusion noted.
It is unclear the genders of the six patients who underwent stent placement in the Roy study. Seven patients identified, (six female, one male), but one did not have stent placement. That patient not included in this systematic review.
3.1. Endovascular procedure
A variety of methods of acute thrombus management were utilized in the included studies. Of the five included articles, one utilized catheter directed thrombolysis (CDT),13 two utilized pharmocomechanical thrombolysis (PMT),9, 10 one used either CDT or PMT,12 and one utilized surgical thrombectomy with arterio‐venous fistula formation and thrombolysis (TT)11 as initial management of acute DVT (Table 2). In‐stent occlusion or thrombosis was seen in one patient treated with PMT, one patient treated with a combination of CDT + PMT, two patients treated with TT, and three patients treated with CDT alone.
3.2. Thrombotic risk factors
Three of the five articles reported hypercoagulable testing or thrombotic risk factors. The use of thrombophilia testing (and the clinical impact of its results) was inconsistent and incompletely reported among the five studies (Table 3). Regarding stent outcomes, patients with and without thrombophilia were found to have stent occlusion within 12 months of intervention. Two of the six patients with thrombophilia (30%), two of the 19 patients found to be negative for thrombophilia (10%), and three of the 36 with an unknown thrombophilia status (8%) experienced stent occlusion of thrombosis recurrence within 12 months of stenting. The available data do not permit any conclusions about the impact (or lack thereof) that thrombophilia testing should have on decisions about antithrombotic therapy after stent placement.
Table 3.
Thrombosis risk factors and associated outcomes
| Article (year) | Demographics | Risk factors for thrombosis | Anticoagulant therapy | Stent outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of study participants | Age, mean (years) | Female to male ratio | Hypercoagulable testing | Transient provoking factor | Chronic provoking factor | Unknown | Anticoagulant | INR goal | Duration (months) | Off A/C at 6 months | Time to occlusion in by 12 months | # Patients with occlusion or VTE recurrence within 12 months | |
| Goldman (2017) | 6 | 17 | 3:3 | 4/6 hypercoagulable testing positiveb | 2 | 0 | 4 | Variable but described (Fondaparinux, Enoxaparin, Warfarin) | Variable but delineated (6—lifelong) | 1/6 | 1, 11 | 2 | |
| Husman (2007) | 11 | 34 | 9:2 | Not identified | 11 | Warfarin | 2‐3 | 6 | Yes | 0.25 and 3 | 2 | ||
| Kim (2017) | 25 | 61 | 19:5 | Not identifieda | 11 | 14 | Warfarin | 2‐3 | 6 | Yes | 1 | ||
| Matsuda (2014) | 13 | 63 | 7:6 | 2/13 hypercoagulable testing positiveb | 6 | 6 | 1 | Warfarin | 1.5‐2.5 | Variable but delineated (6—lifelong) | 1/13 | 0.5 and 1 | 2 |
| Roy (2017) | 6 | 48 | 5:1b | Tested, but none identified | 1b | 5 | Warfarin | 2‐3 | “Long‐term” | N | 0 | ||
A/C, anticoagulation; DVT, deep vein thrombosis; INR, international normalized ratio; VTE, venous thromboembolism.
One patient with recurrence diagnosed with antiphospholipid antibody syndrome.
“Patients with hematologic disorders affecting the coagulation cascade” were excluded but these disorders were not defined.
One patient with recurrence was diagnosed with protein C deficiency.
It is unclear the genders of the six patients who underwent stent placement in the Roy study. Seven patients identified, (six female, one male), but one did not have stent placement, unclear which gender. That patient not included in this systematic review.
Patient recovering from a traumatic subdural hematoma requiring surgical intervention and rehabilitative services when DVT developed.
3.3. Antithrombotic therapy
Of the five studies, four utilized warfarin as the anticoagulant for all patients studied,9, 10, 11, 13 with goal international normalized ratio (INR) 2‐3 for three studies, and 1.5‐2.5 for the remaining article (Table 4). A total of five stent occlusions or recurrent thrombosis occurred within these four studies.
Table 4.
Antithrombotic therapy and thrombosis or occlusion outcomes
| Article | Demographics | Anticoagulant therapy | Antiplatelet therapy | Stent outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of study participants | Mean age | Anticoagulant | INR goal | Duration (m) | Off A/C at 6 months | Antiplatelet therapy | Duration of antiplatelet therapy (m) | Occlusion is acute phase | Occlusion in midterm (12 m) phase | # Patients with occlusion or VTE recurrence within 12 months | |
| Goldman (2017) | 6 | 17 | Variable but described (Fondaparinux, Enoxaparin, Warfarin) | Variable but delineated (6—lifelong) | 1/6 | No | No | 2 | 2 | ||
| Husman (2007) | 11 | 34 | Warfarin | 2‐3 | 6 | Yes | N | 1 | 1 | 2 | |
| Kim (2017) | 25 | 61 | Warfarin | 2‐3 | 6 | Yes | Aspirin and clopidogrel | 12 | 1 | No | 1 |
| Matsuda (2014) | 13 | 63 | Warfarin | 1.5‐2.5 | Variable but delineated (6—lifelong) | No | 1 | 1 | 2 | ||
| Roy (2017) | 6a | 48 | Warfarin | 2‐3 | “Long‐term” | N | No | No | 0 | ||
A/C, anticoagulation; INR, international normalized ratio; m, months; VTE, venous thromboembolism.
It is unclear the genders of the 6 patients who underwent stent placement in the Roy study. 7 patients identified, (6 female, 1 male), but 1 did not have stent placement, unclear which gender. That patient not included in this systematic review.
The only study that utilized a spectrum of anticoagulants (Goldman, N = 6) including fondaparinux (N = 1), warfarin (N = 1), or a combination of enoxaparin and warfarin (N = 4) therapy also utilized a variable duration of therapy. The two occlusions that occurred within this study occurred in one patient at 1 month while on warfarin therapy and in another patient at 11 months associated with warfarin noncompliance.
For the 11 patients with only 6 months of warfarin antithrombotic therapy the 12‐month stent patency rate was 78% (7 of 9 evaluable at 12 months).11 For the 50 patients treated with more than 6 months of antithrombotic therapy (variable length of systemic anticoagulation or anticoagulation followed by dual‐antiplatelet therapy) the 12‐month stent patency rate was 89% (41 of 46 evaluable at 12 months). For the 25 patients treated with variable anticoagulation or long‐term anticoagulation without antiplatelet therapy, the 12‐month a stent patency rate (17 of 21 evaluable at 12 months) was 81%.
Thus, stent thrombosis or occlusion occurred in 10%‐20% of patients post‐stent placement regardless of antithrombotic management, with 12.7% stent thrombosis or occlusion overall (7 of 55 evaluable at 12 months).
3.4. Post‐thrombotic syndrome
Patients were assessed for post thrombotic syndrome (PTS) in all five included studies. Goldman et al., Husman et al., Kim et al., and Roy et al. all used Villalta scoring (VS) to categorize PTS severity. In the Goldman et al. study, the two individuals who experienced in‐stent thrombosis during follow‐up were the only patients with any PTS symptomology defined as a VS score of 3 and a Modified Villalta score of 1. In contrast, the Husman et al. study reported one patient with PTS symptoms (VS = 3) with no in‐stent thrombosis or recurrent thrombosis. Two studies did not describe if the PTS symptoms were seen in patients with in‐stent thrombosis or recurrent thrombosis (Kim et al. and Matsuda et al.). The last study, Roy et al., reported no stent occlusions and no PTS symptoms at 12 months.
3.5. Follow‐up
As it is as least typical for patients to be anticoagulated for a minimum of 6 months after stent placement, we sought a follow‐up period of at least 12 months to evaluate the risk of stent occlusion following antithrombotic discontinuation, in the cases of discontinuation. The percent of stented patients evaluable for stent occlusion or recurrent thrombosis at 12 months ranged from 77% to 100%. Of the 55 patients evaluable, the 12‐month rate of endovascular stent occlusion or recurrent DVT ranged from 0% to 40%. Stent patency based on those evaluable at 12 months ranged from 60% to 100% (see Table 5).
Table 5.
Stent and recurrent thrombosis outcomes
| Article (year) | Acute DVT management procedure | A/C drug after treatment | A/C duration | Antiplatelet therapy after treatment | # MTS patients with acute DVT treated with stent placement | # Of pts evaluable at 12 months | # Of events by 12 monthsa | # Of patients events free at 12 months | Stent patency and event free at 12 months (of those evaluable at 12 months) |
|---|---|---|---|---|---|---|---|---|---|
| Goldman (2017) | Catheter directed thrombolysis or pharmocomechanical thrombolysis | Variable, but delineated for each patient | Variable | No | 6 | 5/6 | 2 | 3 | 3/5, 60% |
| Husman (2007) | Surgical thrombectomy and Thrombolysis and AV fistula formation | Warfarin | 6 months | No | 11 | 9/11 | 2 | 7 | 7/9, 78% |
| Kim (2017) | Pharmocomechanical thrombolysis | Warfarin | 6 months | Asprin and clopidogrel prescribed for 1 year, after warfarin | 25b | 25/25 | 1 | 24 | 24/25, 96% |
| Matsuda (2014) | Catheter‐directed thrombolysis | Warfarin | Variable | No | 13 | 10/13 | 2 | 8 | 8/10, 80% |
| Roy (2017) | Pharmocomechanical thrombolysis + stent, or angioplasty + stent | Warfarin | “Long‐term” | No | 6 | 6 | 0 | 6 | 6/6, 100% |
A/C, anticoagulation; AV, arteriovenous; DVT, deep vein thrombosis; MTS, May‐Thurner syndrome.
Events defined as in‐stent thrombosis, recurrent thrombosis, stent occlusion.
1/25 had right‐sided May‐Thurner syndrome.
4. DISCUSSION
The optimal management of May‐Thurner Syndrome associated thrombosis following venous stenting is not well established. We evaluated the available relevant literature to assess antithrombotic management in the post‐venous‐stent setting in MTS patients in order to determine the impact of anticoagulant choices on the risk of stent occlusion or thrombosis recurrence at 12 months. Our search identified five studies that met our inclusion criteria, but were limited by the fact that the antithrombotic management described in the included studies was variable and none of the published studies prospectively compared more than one management strategy. Therefore, there is no standard type, dose, or duration of antithrombotic management after endovascular stenting for left IVCS‐associated thrombosis.
The lack of consensus for MTS‐thrombosis management encompasses both acute and chronic therapeutic considerations and this includes endovascular management. Weighing the risks and benefits of endovascular stent placement in MTS thrombosis was not within the scope of our systematic review. The literature on that topic can be reviewed elsewhere but includes the Mickley et al. study of 30 patients with MTS and acute iliac venous thrombosis5 treated with surgical intervention including transfemoral venous thrombectomy and construction of a temporary inguinal arteriovenous fistula followed by at least 12 months of warfarin anticoagulation with or without the additional of angioplasty and endovascular stent placement. More recently, Meng et al.,14 reported management of acute thrombosis in 74 MTS patients prospectively randomized to thrombolysis alone (N = 29) or thrombolysis in combination with angioplasty and endovascular stent placement (N = 45) with all patients anticoagulated with warfarin adjusted to a goal INR 2.0‐2.5 for a minimum of 6 months. Follow‐up ranged from 6 to 24 months, and stent occlusion was assessed by imaging in 90% of participants (67 of 74) revealing stent occlusion of 65% in the thrombolysis alone group (19 of 27) and 11% in the study group (5 of 45). This study could not be utilized in our systematic review because patients were not followed for a minimum of 12 months and the duration of anticoagulation was not clearly delineated. While suggesting that endovascular stenting may provide a role in the management of some cases of MTS, the benefit of stent placement is not well established. We did not find any evidence to address pertinent questions regarding stenting such as the role for stenting in individuals without a thrombosis.
Left iliac vein compression syndrome is a nonpathologic variant in some individuals, affecting possibly 25% of the adult population, most of whom have never experienced thrombosis.6 Population data like these suggest that the presence of IVCS is unlikely to be the sole contributor to thrombosis formation in MTS. Addressing IVCS stenosis with angioplasty or stent placement therefore may not be sufficient treatment in all patients with this anatomic anomaly. Since a foreign intravascular device can be prothrombotic, some anticoagulation therapy is almost certainly necessary, but the optimal type and duration is unclear.
This review specifically evaluated the evidence informing antithrombotic use in the post‐stent setting of MTS patients with a thrombosis. The optimal duration of anticoagulation or antiplatelet therapy following stent placement is not known.15 Our review demonstrates that 12‐month stent patency rate was high, ranging from 60% to 96%, with the variety of antithrombotic management described. As these were all retrospective studies with small sample sizes, the decisions regarding specific antithrombotic therapy decisions in the review may be due to varying risks of recurrence. Thus, patients treated with longer‐term anticoagulation may have been deemed to be at higher risk of recurrent thrombosis. This illustrates a need for prospective randomized studies with well‐delineated baseline thrombosis risk factor assessment. In agreement with others who have published on this topic,16 we suggest assessing patients with MTS and acute thrombosis for risk factors associated with VTE recurrences and weighing the likelihood of future VTE against the risk of antithrombotic‐related major bleeding. In the presence of major transient risk factors, such as surgery or estrogen exposure, treatment with anticoagulation for a finite duration of 6 months after stent placement (with or without subsequent antiplatelet therapy) is reasonable. In the absence of a major transient risk factor (other than the narrowed left iliac vein), the decision about whether to extend anticoagulation beyond 12 months is more challenging. In our practice, for a young patient with an otherwise unprovoked DVT, we consider discontinuing anticoagulation after 6‐12 months of therapy, especially if there is little or no evidence of venous insufficiency.
This review and its conclusions are significantly limited by the paucity of data informing the use of antithrombotic therapy following stent placement in patients with MTS presenting with acute DVT. Our search strategy required 12‐month follow‐up which likely excluded studies with some information on anticoagulant use in the post‐stent setting because many studies used “at least” or “a minimum of 6 months” of anticoagulation. We accepted a lower number of studies included in order to examine the outcomes of patients after the traditional 6 months of anticoagulation to compare outcomes between those who continued or discontinued anticoagulation after 6 months. Our inclusion criteria excluded some studies that did not clearly define anticoagulant dosing and duration; the goal of this review was to assess the impact of antithrombotic agents on stent outcomes. Other studies that would not have been captured by our search strategy would include those that did not incorporate our search terms, conference proceedings, non‐English language studies, papers not included in the electronic databases utilized for our search, or those unable to be obtained in full‐text form for review. All five of the included studies are retrospective. The 61 patients included in our analysis were managed with divergent antithrombotic therapy. Thrombosis risk factors (including the results of thrombophilia testing) was reported in only some of the included studies. Unfortunately, no study reported on bleeding complications during antithrombotic therapy. Key questions remain unanswered for patients with MTS‐associated thrombosis: Are stents beneficial? Are direct oral anticoagulants effective to prevent stent thrombosis? How long should systemic anticoagulation (or antiplatelet therapy) be continued after stent placement for MTS? Future studies should be prospective, with well described baseline thrombosis risk factor assessment as well as controlled duration of anticoagulation and follow‐up after stenting. Of course the studies that could provide the strongest information in this setting would be randomized, controlled trials with two groups treated with different therapeutic strategies.
In summary, patients with MTS diagnosed with left iliac vein thrombosis are often treated with endovascular intervention and stent placement. Such patients almost certainly require antithrombotic therapy in the post‐stent setting. However, the optimal duration, type and intensity of anticoagulation following stent placement for MTS is unknown.
RELATIONSHIP DISCLOSURE
L.J. Padrnos and D. Garcia report no financial or nonfinancial disclosures.
AUTHOR CONTRIBUTIONS
L.J. Padrnos reviewed the literature search and takes full responsibility for the inclusion of articles. L.J. Padrnos wrote the first draft of the manuscript. L.J. Padrnos and D. Garcia contributed to the study design, data analysis and interpretation, and writing of the manuscript.
Padrnos LJ, Garcia D. May‐Thurner syndrome and thrombosis: A systematic review of antithrombotic use after endovascular stent placement. Res Pract Thromb Haemost. 2019;3:70–78. 10.1002/rth2.12156
REFERENCES
- 1. Cockett FB, Thomas ML, Negus D. Iliac vein compression—Its relation to iliofemoral thrombosis and the post‐thrombotic syndrome. Br Med J. 1967;2(5543):14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brinegar KN, Sheth RA, Khademhosseini A, Bautista J, Oklu R. Iliac vein compression syndrome: clinical, imaging and pathologic findings. World J Radiol. 2015;7(11):375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDermott S, Oliveira G, Ergül E, Brazeau N, Wicky S, Oklu R. May‐Thurner syndrome: can it be diagnosed by a sinMR venography study? Diagn Interv Radiol. 2013;19(1):44–8. [DOI] [PubMed] [Google Scholar]
- 4. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8(5):419–27. [DOI] [PubMed] [Google Scholar]
- 5. Mickley V, Schwagierek R, Rilinger N, Görich J, Sunder‐Plassmann L. Left iliac venous thrombosis caused by venous spur: treatment with thrombectomy and stent implantation. J Vasc Surg. 1998;28:492–7. [DOI] [PubMed] [Google Scholar]
- 6. Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937–43. [DOI] [PubMed] [Google Scholar]
- 7. Binkert CA, Schoch E, Stuckmann G, et al. Treatment of pelvic venous spur (May‐Thurner syndrome) with self‐expanding metallic endoprostheses. Cardiovasc Intervent Radiol. 1998;21:22–6. [DOI] [PubMed] [Google Scholar]
- 8. Heniford BT, Senler SO, Olsofka JM, Carrillo EH, Bergamini TM. May‐Thurner syndrome: management by endovascular surgical techniques. Ann Vasc Surg. 1998;12(5):482–6. [DOI] [PubMed] [Google Scholar]
- 9. Roy M, Sasson M, Rosales‐Velderrain A, Moon S, Grove M, King T. Pharmocomechanical thrombolysis for deep vein thrombosis in May‐Thurner syndrome. Innovations. 2017;12:466–71. [DOI] [PubMed] [Google Scholar]
- 10. Kim IS, Jo WM, Chung HH, Lee SH. Comparison of clinical outcomes of pharmacomechanical thrombectomy in iliac vein thrombosis with and without May‐Thurner syndrome. Int Angiol. 2017;37(1):12–8. [DOI] [PubMed] [Google Scholar]
- 11. Husmann MJ, Heller G, Kalka C, et al. Stenting of common iliac vein obstructions combined with regional thrombolysis and thrombectomy in acute deep vein thrombosis. Eur J Vasc Endovasc Surg. 2007;34:87–91. [DOI] [PubMed] [Google Scholar]
- 12. Goldman RE, Arendt VA, Kothary N, et al. Endovascular management of May‐Thurner syndrome in adolescents: a single center experience. J Vasc Interv Radiol. 2017;28(1):71–7. [DOI] [PubMed] [Google Scholar]
- 13. Matsuda A, Yamada N, Ogihara Y, et al. Early and long‐term outcomes of venous stent implantation for iliac venous stenosis after catheter‐directed thrombolysis for acute deep vein thrombosis. Circ J. 2014;78(5):1234–9. [DOI] [PubMed] [Google Scholar]
- 14. Meng QY, Li XQ, Jiang K, et al. Stenting of iliac vein obstruction following catheter‐directed thrombolysis in lower extremity deep vein thrombosis. Chin Med J. 2013;126(18):3519–22. [PubMed] [Google Scholar]
- 15. Freisinger E. Endovascular therapy of ilio‐caval venous obstructions—a ray of hope on the horizon? Vasa. 2016;45(6):508. [DOI] [PubMed] [Google Scholar]
- 16. Carroll S, Moll S. Inferior vena cava filters, May‐Thurner syndrome, and vein stents. Circulation. 2016;133(6):e383–7. [DOI] [PubMed] [Google Scholar]


