Abstract
Purpose
To determine whether the presence of intact cumulus cells during the preincubation period for ICSI should be considered as a critical factor in fertilization and embryonic development.
Methods
The cohort of this prospective randomized study was limited to infertile women younger than 39 years of age who underwent controlled ovarian stimulation for ICSI between October 2013 and May 2015 and whose embryos were to be incubated until day 5. Women with estradiol levels of <2000 pmol/L on the day of HCG injection were excluded. Cumulus cells were removed immediately after OPU in Group A and at 120 minutes after OPU in Group B. ICSI was performed with all mature oocytes, and fertilized oocytes were cultured to the blastocyst stage. Maturation, fertilization, blastocyst, good quality blastocyst, pregnancy, live birth, and miscarriage rates were compared.
Results
There were no significant differences in maturation, fertilization, blastocyst, pregnancy, live birth, or miscarriage rates between Groups A and B. However, the percentage of good quality blastocysts was significantly higher in Group B than Group A (52.0% vs 33.1%).
Conclusions
Intact cumulus cells should be maintained during the preincubation period, as they are important to embryonic development after fertilization.
Keywords: cumulus cells removal, cytoplasmic maturation, oocyte denudation, oocyte maturity, preincubation
1. INTRODUCTION
Preincubation of oocytes before insemination in human in vitro fertilization (IVF) was originally introduced by Trounson et al,1 who demonstrated improved fertilization rates and embryo quality by insemination at 5.5 hours after oocyte retrieval. Since then, this concept is now applied in most IVF facilities, as most agree that insemination at 2‐6 hours after oocyte retrieval achieves more favorable outcomes.2, 3, 4 Since oocytes just after retrieval have not yet completely matured, insemination should be delayed to allow enough time to complete the maturation process to optimize fertilization, embryonic development, and clinical outcomes. However, it has also been reported that preincubation before fertilization had no influence on embryonic growth5; thus, efficacy of delayed insemination in IVF remains controversial.
Intracytoplasmic sperm injection (ICSI), first introduced by Palermo et al, is a powerful therapeutic tool for the treatment of severe male factor infertility that is based on the principles of IVF and also involves preincubation of oocytes for 3‐9 hours in most facilities.6, 7, 8, 9, 10 On the contrary, some recent studies also report that there is no influence of preincubation in ICSI procedures on laboratory outcomes including oocyte maturity and embryo development.11, 12 Currently, the necessity of preincubation of oocytes in ICSI is controversial as well as IVF.
Oocytes are normally fertilized soon after ovulation, and the window for optimal fertilization differs among species. If fertilization does not occur within the appropriate period, the unfertilized oocyte remains in the oviduct or culture medium, where it undergoes a time‐dependent deterioration in quality, in a process called “oocyte aging,” which has been reported as a main cause of compromised embryonic development following IVF, ICSI, and parthenogenetic activation.13, 14 Therefore, optimization of oocyte aging should improve laboratory and clinical outcomes in assisted reproductive technology. However, in addition to factors that may adversely affect oocyte aging, extended in vitro culture may impair oocyte competence.14, 15, 16 For example, Wakayama et al17 reported negative effects of temperature change on mouse oocyte aging, while Goud et al18 indicated that the production of reactive oxygen species significantly enhanced oocyte aging. Also, cumulus cells can reportedly accelerate the aging progression of both in vivo‐ and in vitro‐matured oocytes.19, 20, 21 On the contrary, Takahashi et al22 reported that cumulus cells partly prevent poor embryonic development during in vitro aging. Furthermore, several chemicals, including vanadate,23, 24 nitric oxide,25 and trichostatin A,26, 27 are known to regulate oocyte aging.
The focus of the present study is the effect of cumulus cells on the oocyte aging process, as these cells play important roles in oocyte maturation and subsequent embryonic development.28, 29, 30 Although preincubation of oocytes before ICSI is carried out without removal of cumulus cells in some facilities,8, 9, 10, 11 a recent study of the murine cumulus cell‐oocyte complex (COC) reported that prolonged culture of oocytes with intact cumulus cells induces apoptotic changes,19, 20, 21 suggesting that oocyte denudation should be performed as soon as possible after oocyte retrieval. Although some researchers have investigated the optimal timing of cumulus cell removal in ICSI procedures,8, 10, 11, 12, 31 there is yet no consensus. Therefore, the aim of the present study was to determine whether the presence of intact cumulus cells during the preincubation period for ICSI should be considered as a critical factor in fertilization and embryonic development.
2. MATERIALS AND METHODS
2.1. Patients
The study protocol was approved by the Institutional Ethics Committee of the reporting IVF Osaka clinic in Osaka, Japan. The cohort of this prospective randomized study was limited to infertile women younger than 39 years of age who underwent controlled ovarian stimulation for ICSI between October 2013 and May 2015 and whose embryos were to be incubated until day 5. Women with estradiol (E2) levels of <2000 pmol/L on the day of human chorionic gonadotropin (HCG) injection were excluded. The schedule and experimental groups in the present study are described in Figure 1. The participants were randomly divided at the time of HCG injection into two groups according to the timing of cumulus cell removal, which was conducted immediately after oocyte retrieval in Group A (21 patients, 21 cycles) and 120 minutes after retrieval in Group B (33 patients, 33 cycles).
Figure 1.
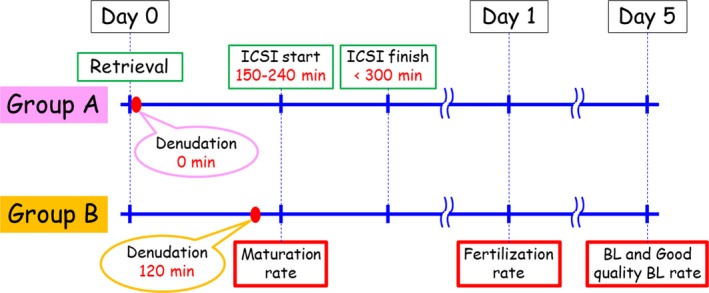
Treatment schedules of the experimental groups in the present study
2.2. Ovarian stimulation
Two ovarian stimulation protocols with the use of gonadotropin‐releasing hormone (GnRH) agonist or the GnRH antagonist were applied in the present study. Intranasal buserelin acetate (Suprecur; Mochida Pharmaceutical Co., Tokyo, Japan) was initiated on day 21 of the previous cycle and continued until the day of HCG trigger to prevent an endogenous surge in luteinizing hormone during the long protocol. Recombinant follicle‐stimulating hormone (rFSH; 150‐300 IU; Gonalef; Merck Serono Co., Tokyo, Japan) was injected from day 3 to 6 of the treatment cycle. Human menopausal gonadotropin (HMG; Ferring Pharmaceuticals Co., Tokyo, Japan) was administered from day 7 until follicle maturation.
During the GnRH antagonist cycles, rFSH injection (150‐300 IU) was initiated on day 3 of the treatment cycle and replaced with HMG on day 7. Administration of the GnRH antagonist (Ganirest; MSD KK, Tokyo Japan) was started on day 7 of the stimulation cycle to inhibit a premature surge in luteinizing hormone.
In both ovarian stimulation protocols, the HMG dose was adjusted according to the follicular response, as determined by serial transvaginal ultrasonography and measurement of serum E2 levels until the day of HCG administration (5000‐10 000 IU, Mochida Pharmaceutical Co.), which was given subcutaneously when there were at least three follicles with diameters of >17 mm or E2 levels >3000 pmol/L. Oocyte retrieval was performed by transvaginal ultrasound‐guided puncture of the ovarian follicles at 36‐38 hours after HCG administration.
2.3. Timing of cumulus cell removal, maturity verification, and ICSI
The retrieved COC was incubated in fertilization medium (G‐IVFTM; Vitrolife, Gothenburg, Sweden) until cumulus cell removal at 0 minutes (Group A) or 120 minutes (Group B) after oocyte retrieval by brief pipetting with 40 IU of hyaluronidase (Irvine Scientific, Santa Ana, CA, USA). Oocyte maturity was evaluated just before ICSI (not at the time of cumulus cells removal) in both the groups (Figure 1). ICSI was initiated between 2.5 and 4.0 hours after retrieval in both the groups (Figure 1). All ICSI procedures were completed within 5 hours after retrieval (Figure 1). Injected oocytes were cultured in human tubal fluid medium until fertilization verification on the next day.
2.4. Fertilization check and embryo culture
Oocytes with two visible pronuclei at 16‐20 hours after ICSI were considered as normally fertilized and immediately cultured in Continuous Single Culture® medium (Irvine Scientific) until day 5. The degree of blastocyst formation was determined on day 5 of the culture. The grade of blastocyst expansion, inner cell mass, and trophectoderm formation was evaluated based on Gardner's classification system.32 Blastocysts with an expansion grade of >2 with an inner cell mass and trophectoderm grades of A or B, respectively, were defined as being of good quality.
2.5. Embryo transfer and cryopreservation
The embryos which reached blastocysts were transferred into the patient's uterus or cryopreserved. The cryopreserved blastocysts were thawed and transferred in the other cycle. Clinical pregnancy was determined by observing a gestational sac.
2.6. Statistical analysis
Maturation, fertilization, blastocyst, good quality blastocyst, pregnancy, live birth, and miscarriage rates were compared between Groups A and B (Figure 1). The characteristics of patients and laboratory data are expressed as the mean ± standard deviation of the mean and tested for significant differences by the Student's t test. Blastocyst and good quality blastocyst rates were based on embryos cultured to day 5 in each cycle. In addition, laboratory between both the groups were also compared according to each ovarian stimulation protocol, the long and the antagonist protocols.
The analysis of the pregnancy data between the groups was based on single blastocyst transfer cycles and the first embryo transfer cycle after the oocyte retrieval. For comparison of the pregnancy data, Fisher's exact test was used. A probability (P) value of <0.05 was considered statistically significant.
3. RESULTS
Cumulus cells were removed immediately or at 120 minutes after oocyte retrieval in 21 cycles in Group A and 33 cycles in Group B, respectively. The percentages of each ovarian stimulation protocol in Group A and B were similar (Long: 76% vs 73%, Antagonist: 24% vs 23%, respectively). The baseline characteristics of the two groups are shown in Table 1. There were no significant differences in average patient age (35.6 ± 3.0 vs 34.1 ± 3.9 years), average anti‐Müllerian hormone level (4.78 ± 2.49 vs 4.69 ± 2.29 ng/mL), body mass index (21.5 ± 2.3 vs 21.0 ± 2.7, respectively), number of retrieved oocytes (12.8 ± 7.0 vs 12.1 ± 6.3), number of retrieved oocytes in each ovarian stimulation protocol, (Long: 12.1 ± 6.4 vs 10.9 ± 5.5, Antagonist: 14.8 ± 9.0 vs 15.1 ± 7.4), or the period from oocyte retrieval to ICSI completion (257.2 ± 53.7 vs 263.6 ± 44.4 minutes) between Groups A and B.
Table 1.
Characteristics of ICSI cycles according to the timing of oocyte denudation
| Group A | Group B | P value | |
|---|---|---|---|
| No. of patients | 21 | 33 | — |
| No. of treatment cycles | 21 | 33 | — |
| No. of long protocols (%) | 16 (76) | 24 (73) | — |
| No. of antagonist protocols (%) | 5 (24) | 9 (27) | — |
| Age (y) | 35.6 ± 3.0 | 34.1 ± 3.9 | 0.12 |
| AMH (ng/mL) | 4.78 ± 2.49 | 4.69 ± 2.29 | 0.91 |
| BMI | 21.5 ± 2.3 | 21.0 ± 2.7 | 0.51 |
| No. of retrieved oocytes per cycle | 12.8 ± 7.0 | 12.1 ± 6.3 | 0.68 |
| No. of retrieved oocytes per cycle in long protocols | 12.1 ± 6.4 | 10.9 ± 5.5 | 0.51 |
| No. of retrieved oocytes per cycle in antagonist protocols | 14.8 ± 9.0 | 15.1 ± 7.4 | 0.95 |
| Period retrieval to ICSI (min) | 257.2 ± 53.7 | 263.6 ± 44.4 | 0.63 |
AMH, anti‐Mullerian hormone; BMI, body mass index
As shown by the laboratory data in Table 2, there were no significant differences in maturation rates (84.2% ± 12.9% vs 88.1% ± 11.3%), fertilization rates (88.4% ± 10.2% vs 88.0% ± 11.2%), or blastocyst rates (62.8% ± 30.0% vs 73.7% ± 23.3%) between Groups A and B. However, the percentage of good quality blastocysts was significantly higher in Group B than Group A (52.0% ± 30.8% vs 33.1% ± 28.4%, respectively; P = 0.03). The laboratory data in each ovarian stimulation protocols are also included in Table 2. No significant differences between Groups A and B were found in the both ovarian stimulation protocols, except good quality blastocyst rates in the long protocols. The good quality blastocyst rate of Group B was significantly higher than Group A in the long protocols (55.9% ± 31.2% vs 34.2% ± 24.9%; P = 0.03).
Table 2.
Laboratory outcomes of ICSI cycles according to the timing of oocyte denudation
| Group A | Group B | P value | |
|---|---|---|---|
| No. of treatment cycles | 21 | 33 | — |
| No. of long protocols (%) | 16 (76) | 24 (73) | — |
| No. of antagonist protocols (%) | 5 (24) | 9 (27) | — |
| No. of retrieved oocytes | 269 | 398 | — |
| No. of retrieved oocytes per cycle | 12.8 ± 7.0 | 12.1 ± 6.3 | 0.68 |
| No. of retrieved oocytes per cycle in long protocols | 12.1 ± 6.4 | 10.9 ± 5.5 | 0.51 |
| No. of retrieved oocytes per cycle in antagonist protocols | 14.8 ± 9.0 | 15.1 ± 7.4 | 0.95 |
| No. of matured oocytes | 216 | 345 | — |
| Maturation rates per cycle (%) | 84.2 ± 12.9 | 88.1 ± 11.3 | 0.25 |
| Maturation rates per cycle in long protocols (%) | 83.1 ± 13.7 | 89.1 ± 11.8 | 0.15 |
| Maturation rates per cycle in antagonist protocols (%) | 87.6 ± 10.8 | 85.3 ± 10.0 | 0.70 |
| No. of fertilized oocytes | 198 | 305 | — |
| Fertilization rates per cycle (%) | 88.4 ± 10.2 | 88.0 ± 11.2 | 0.98 |
| Fertilization rates per cycle in long protocols (%) | 90.3 ± 8.2 | 86.8 ± 11.5 | 0.29 |
| Fertilization rates per cycle in antagonist protocols (%) | 82.1 ± 14.2 | 92.3 ± 10.6 | 0.15 |
| No. of embryos cultured to day 5 | 116 | 221 | — |
| No. of blastocysts | 69 | 164 | — |
| Blastocyst rates per cycle (%) | 62.8 ± 30.0 | 73.7 ± 23.3 | 0.14 |
| Blastocyst rates per cycle in long protocols (%) | 62.9 ± 29.5 | 77.7 ± 19.2 | 0.06 |
| Blastocyst rates per cycle in antagonist protocols (%) | 62.4 ± 35.3 | 63.2 ± 30.5 | 0.96 |
| No. of good quality blastocysts | 37 | 106 | — |
| Good quality blastocyst rates per cycle (%) | 33.1 ± 28.4 | 52.0 ± 30.8 | 0.03* |
| Good quality blastocyst rates per cycle in long protocols (%) | 34.2 ± 24.9 | 55.9 ± 31.2 | 0.03* |
| Good quality blastocyst rates per cycle in antagonist protocols (%) | 29.4 ± 41.2 | 40.1 ± 28.3 | 0.59 |
Significant difference by student's t test
The clinical data are shown in Table 3. No significant differences between Groups A and B were confirmed in pregnancy rates (57.1% vs 78.9%), live birth rates (50.0% vs 63.2%), or miscarriage rates (12.5% vs 20.0%, respectively).
Table 3.
Clinical outcomes of single blastocyst transfersa
| Group A | Group B | P value | |
|---|---|---|---|
| No. of embryo transfersa | 14 | 19 | — |
| No. of embryo transfers in fresh cycles | 5 | 8 | — |
| No. of embryo transfers in thawing cycles | 9 | 11 | — |
| No. of pregnanciesb | 8 | 15 | — |
| No. of pregnancies in fresh cycles | 3 | 5 | — |
| No. of pregnancies in thawing cycles | 5 | 10 | — |
| Pregnancy rates per embryo transfers (%) | 57.1 | 78.9 | 0.17 |
| No. of live birthsb | 7 | 12 | — |
| No. of live births in fresh cycles | 2 | 4 | — |
| No. of live births in thawing cycles | 5 | 8 | — |
| Live birth rates per embryo transfers (%) | 50.0 | 63.2 | 0.34 |
| No. of miscarriagesb | 1 | 3 | — |
| No. of miscarriages in fresh cycles | 1 | 1 | — |
| No. of miscarriages in thawing cycles | 0 | 2 | — |
| Miscarriage rates per pregnancies (%) | 12.5 | 20.0 | 0.64 |
Data are based on only the first embryo transfer cycle.
Total number of fresh and thawing cycles.
4. DISCUSSION
As previously stated, if fertilization does not occur within the appropriate period, unfertilized oocytes remain in the oviduct or culture medium and undergo oocyte aging, which impairs oocyte competence.14, 16 In our facility, the percentage of blastocysts gradually worsened in a time‐dependent manner over a 5‐hour period before ICSI (data not shown). Hence, all ICSI procedures in the present study were started at 2.5‐4.0 hours after oocyte retrieval and completed within 5 hours to eliminate the risk of oocyte aging caused by extended in vitro culture. As shown in Table 1, the periods from oocyte retrieval to ICSI completion in Groups A and B were similar (257.2 and 263.6 minutes, respectively). Therefore, it is possible to compare the effect of the timing of cumulus cell removal on embryonic development capacity regardless of the effect of oocyte aging.
Although many studies have attempted to determine an optimal time for cumulus cell removal in ICSI procedures,8, 9, 10, 11, 12, 31 there is currently no consensus. According to a recent report on the culture of murine COCs, long‐term culture of oocytes with intact cumulus cells induces apoptotic changes.19, 20, 21 Therefore, oocyte denudation should be completed as soon as possible after oocyte retrieval. In our laboratory, cumulus cells are denuded immediately to 120 minutes after oocyte retrieval and the ICSI procedure is started between 2.5 and 4.0 hours after retrieval. In this study, the denudation time was set 0 minutes after retrieval (Group A), the shortest timing of cumulus cell removal in our normal clinical practice, or 120 minutes (Group B), the longest.
In some articles reporting the optimal timing of cumulus removal, only the duration of preincubation was investigated and ICSI was conducted just after cumulus cell removal.8, 10, 11 Thus, the necessity of maintaining intact cumulus cells during the preincubation period remains uncertain. Van de Velde et al12 concluded that the presence of intact cumulus cells in the preincubation period did not affect the outcome of ICSI by investigating survival, fertilization, and embryonic development until day 2. However, the investigation period of that study may have been insufficient because there was a significant difference in embryonic development at day 5 in the present study. Hassan reported that preincubation with intact cumulus cells improved blastocyst development, which is compatible with the results of the present study.30 In addition, that study reported the beneficial effects of intact cumulus cells during the preincubation period on maturation and fertilization. The differences in the results between the previous and the present studies might be due to the lack of an exact definition of the timing of oocyte retrieval after HCG trigger. Furthermore, whereas they reported only laboratory data, the present study includes the pregnancy outcomes, which is one of the most suitable indicators of the embryo quality. Although there were no statistical differences in the present pregnancy data between the groups because of the small number (Table 3), the pregnancy and the live birth rates in Group B were better than those in Group A.
Based on the outcomes of previous murine studies on oocyte aging accelerated by cumulus cells,19, 20, 21 we hypothesized that the oocytes denuded immediately after oocyte retrieval (Group A) would have higher capability of embryonic development. However, the results of the present study showed that the percentage of good quality blastocyst was significantly higher in Group B than Group A (Table 2), which may have resulted from the difference in the site and stage of the retrieved oocytes. In the cited murine studies,19, 20, 21 oocytes were collected from the oviduct ampulla after ovulation, while oocytes were aspirated from the antral follicles before ovulation in the present study, which demonstrated that preincubation should be conducted with intact cumulus cells because these cells play important roles in in vitro maturation and subsequent development via gap junctional communications.30 The endogenous surge in luteinizing hormone or exogenous HCG injection decreases cellular communications in COCs through the closure of gap junctions. However, there are no gap junctions between oocytes and cumulus cells during the preincubation period. Therefore, the maintenance of cumulus cells during the preincubation period might be beneficial to oocyte maturation via unknown underlying pathways other than gap junctions.
It is known that in vitro COCs are sensitive to oxidative stress, and reactive oxygen species (ROS) accelerate the oocyte aging. Therefore, scavengers of ROS can slow down oocyte aging progression.33, 34 Cumulus cells of porcine35 and hamster36 generate glutathione, which is known as a scavenger of ROS. Furthermore, Takahashi et al22 also reported that the level of ROS increased in in vitro aged oocytes, and cumulus cells prevent oxidative stress of the oocytes by preventing ROS generation. In the present study, the oocytes of Group B might be prevented from aging progression by the function of glutathione. As the result, the embryonic developments in Group B might be better than that in Group A (Table 2). It awaits further studies on analysis of the components in the culture media after the preincubation of the COC, including ROS to reveal the mechanism.
In summary, the results of the present study showed that the presence of intact cumulus cells during the preincubation period is one of the important factors in embryonic development after fertilization. However, intact cumulus cells should be maintained during the preincubation period for ICSI.
DISCLOSURES
Conflict of interest: Satoshi Mizuno, Yuko Ishikawa, Hiroshi Matsumoto, Manabu Sato, Mamoru Ida, Aisaku Fukuda, and Yoshiharu Morimoto declare that they have no conflict of interest. Human rights and informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration in 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study. Animal rights: This article does not contain any studies with animal subjects performed by any of the authors. Approval by Ethics Committee: The protocol for the research project has been approved by the Institutional Ethics Committee of the IVF Osaka clinic. Clinical trial registry: This study is not a clinical trial.
Mizuno S, Ishikawa Y, Matsumoto H, et al. The timing of cumulus cell removal for intracytoplasmic sperm injection influences the capability of embryonic development. Reprod Med Biol. 2019;18:111–117. 10.1002/rmb2.12257
REFERENCES
- 1. Trounson AO, Mohr LR, Wood C, Leeton JF. Effect of delayed insemination on in‐vitro fertilization, culture and transfer of human embryos. J Replod Fertil. 1982;64:285‐294. [DOI] [PubMed] [Google Scholar]
- 2. Veeck LL, Wortham JW Jr, Witmyer J, et al. Maturation and fertilization of morphologically immature human oocytes in a program of in vitro fertilization. Fertil Steril. 1983;39:594‐602. [DOI] [PubMed] [Google Scholar]
- 3. Marrs RP, Saito H, Yee B, Sato F, Brown J. Effect of variation of in vitro culture techniques upon oocyte fertilization and embryo development in human in vitro fertilization procedures. Fertil Steril. 1984;41:519‐523. [DOI] [PubMed] [Google Scholar]
- 4. Khan I, Staessen C, Van den Abbeel E, et al. Time of insemination and its effect on in‐vitro fertilization, cleavage and pregnancy rates in GnRH agonist/HMG‐stimulated cycles. Hum Reprod. 1989;4:921‐926. [DOI] [PubMed] [Google Scholar]
- 5. Fisch B, Kaplan‐Kraicer R, Amit S, Ovadia J, Tadir Y. The effect of preinsemination interval upon fertilization human oocytes in vitro. Hum Reprod. 1989;4:954‐956. [DOI] [PubMed] [Google Scholar]
- 6. Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17‐18. [DOI] [PubMed] [Google Scholar]
- 7. Van Steirteghem AC, Nagy Z, Joris H, et al. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod. 1993;8:1061‐1066. [DOI] [PubMed] [Google Scholar]
- 8. Rienzi L, Ubaldi F, Anniballo R, Cerulo G, Greco E. Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13:1014‐1019. [DOI] [PubMed] [Google Scholar]
- 9. Plachot M, Belaisch‐Allart J, Mayenga JM, Chouraqui A, Tesquier L, Serkine AM. Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility. Hum Reprod. 2002;17:362‐369. [DOI] [PubMed] [Google Scholar]
- 10. Ho JY, Chen MJ, Yi YC, Guu HF, Ho ES. The effect of preincubation period of oocytes on nuclear maturity, fertilization rate, embryo quality, and pregnancy outcome in IVF and ICSI. J Assist Reprod Genet. 2003;20;358‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yanagida K, Yazawa H, Katayose H, Suzuki K, Hoshi K, Sato A. Influence of oocyte preincubation time on fertilization after intracytoplasmic sperm injection. Hum Reprod. 1998;13:2223‐2226. [DOI] [PubMed] [Google Scholar]
- 12. Van de Velde H, Joris H, Nagy ZP, Van Steirteghem AC. Effect of timing of oocyte denudation and micro‐injection on survival, fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3160‐3164. [DOI] [PubMed] [Google Scholar]
- 13. Lacham‐Kaplan O, Trounson A. Reduced developmental competence of immature, in‐vitro matured and postovulatory aged mouse oocytes following IVF and ICSI. Reprod Biol Endocrinol. 2008;6(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mizutani E, Jiang JY, Mizuno S, et al. Determination of optimal conditions for parthenogenetic activation and subsequent development of rat oocytes in vitro. J Reprod Dev. 2004;50:139‐146. [DOI] [PubMed] [Google Scholar]
- 15. Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573‐585. [DOI] [PubMed] [Google Scholar]
- 16. Bianchi S, Macchiarelli G, Micara G, et al. Ultrastructural markers of quality are impaired in human metaphase II aged oocytes: a comparison between reproductive and in vitro aging. J Assist Reprod Genet. 2015;32:1343‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wakayama S, Thuan NV, Kishigami S, et al. Production of offspring from one‐day‐old oocytes stored at room temperature. J Reprod Dev. 2004;50:627‐637. [DOI] [PubMed] [Google Scholar]
- 18. Goud AP, Goud PT, Diamond MP, Gonik B, Abu‐Soud HM. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med. 2008;44:1295‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miao YL, Liu XY, Qiao TW, Miao DQ, Luo MJ, Tan JH. Cumulus cells accelerate aging of mouse oocytes. Biol Reprod. 2005;73:1025‐1031. [DOI] [PubMed] [Google Scholar]
- 20. Qiao TW, Liu N, Miao DQ, et al. Cumulus cells accelerate aging of mouse oocytes by secreting a soluble factor(s). Mol Reprod Dev. 2008;75:521‐528. [DOI] [PubMed] [Google Scholar]
- 21. Zhu J, Zhang J, Li H, et al. Cumulus cells accelerate oocyte aging by releasing soluble Fas ligand in mice. Sci Rep. 2015;3:8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takahashi T, Igarashi H, Kawagoe J, Amita M, Hara S, Kurachi H. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol. Reprod. 2009;80:493‐502. [DOI] [PubMed] [Google Scholar]
- 23. Kikuchi K, Naito K, Noguchi J, et al. Maturation/M‐phase promoting factor: a regulator of aging in porcine oocytes. Biol Reprod. 2000;63:715‐722. [DOI] [PubMed] [Google Scholar]
- 24. Kikuchi K, Naito K, Noguchi J, Kaneko H, Tojo H. Maturation/M‐phase promoting factor regulates aging of porcine oocytes matured in vitro. Cloning Stem Cells. 2002;4:211‐222. [DOI] [PubMed] [Google Scholar]
- 25. Goud AP, Goud PT, Diamond MP, Abu‐Soud HM. Nitric oxide delays oocyte aging. Biochemistry. 2005;44:11361‐11368. [DOI] [PubMed] [Google Scholar]
- 26. Huang JC, Yan LY, Lei ZL, et al. Changes in histone acetylation during postovulatory aging of mouse oocyte. Biol Reprod. 2007;77:666‐670. [DOI] [PubMed] [Google Scholar]
- 27. Jeseta M, Petr J, Krejcova T, Chmelikova E, Jilek F. In vitro aging of pig oocytes: effects of the histone deacetylase inhibitor trichostatin A. Zygote. 2008;16:145‐152. [DOI] [PubMed] [Google Scholar]
- 28. Vanderhyden BC, Armstrong DT. Role of cumulus cells and serum on the in vitro maturation, fertilization, and subsequent development of rat oocytes. Biol Reprod. 1989;40:720‐728. [DOI] [PubMed] [Google Scholar]
- 29. Ka HH, Sawai K, Wang WH, Im KS, Niwa K. Amino acids in maturation medium and presence of cumulus cells at fertilization promote male pronuclear formation in porcine oocytes matured and penetrated in vitro. Biol Reprod. 1997;57:1478‐1483. [DOI] [PubMed] [Google Scholar]
- 30. Mori T, Amano T, Shimizu H. Roles of gap junctional communication of cumulus cells in cytoplasmic maturation of porcine oocytes cultured in vitro. Biol Reprod. 2000;62:913‐919. [DOI] [PubMed] [Google Scholar]
- 31. Hassan HA. Cumulus cell contribution to cytoplasmic maturation and oocyte developmental competence in vitro. J Assist Reprod Genet. 2001;18:539‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts In: Jansen R, Mortimer D, ed. Towards Reproductive Certainty: Fertility and Genetics Beyond 1999: The Plenary Proceedings of the 11th World Congress on In Vitro Fertilization & Human Reproductive Genetics. Pearl River, NY: Parthenon; 1999:378–388. [Google Scholar]
- 33. Boerian ML, de Boer P. First cell cycle of zygotes of the mouse derived from oocytes aged postovulation in vivo and fertilized in vivo. Mol Reprod Dev. 1990;25:155‐163. [DOI] [PubMed] [Google Scholar]
- 34. Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre‐implantation embryo and its surroundings. Hum Reprod Update. 2001;7:157‐189. [DOI] [PubMed] [Google Scholar]
- 35. Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod. 2000;63:805‐810. [DOI] [PubMed] [Google Scholar]
- 36. Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre‐implantation stage embryos. Mol Reprod Dev. 2003;64:106‐112. [DOI] [PubMed] [Google Scholar]


