Abstract
Purpose
The direct effects of Lepidium meyenii (Maca) on sperm remain unclear. Herein, we examined the direct effect of Maca on in vitro fertilization.
Methods
We examined the fertilization rate in a mouse model and the rate of acrosome reaction in sperm from transgenic mice expressing enhanced green fluorescent protein (EGFP) in a Maca extract‐containing human tubal fluid (HTF) medium. Using human sperm, we assessed acrosome status via fluorescein isothiocyanate‐conjugated peanut agglutinin (FITC‐PNA) staining and performed detailed analysis using a sperm motility analysis system (SMAS).
Results
In the mouse model, the fertilization rate in the Maca extract‐containing HTF was significantly higher than that in the control medium. The acrosome reaction rate in sperm from transgenic mice expressing EGFP was also significantly higher in the Maca extract‐containing HTF than that in the control medium. Similarly, a high acrosome reaction rate, identified via FITC‐PNA staining of human sperm samples, was found in the Maca extract‐containing HTF compared with that in the control medium. Human sperm motility in the Maca extract‐containing HTF was also increased compared with that in the control medium as measured using an SMAS.
Conclusions
Maca improved in vitro fertilization rates by inducing an acrosome reaction and increasing sperm motility.
Keywords: acrosome reaction, in vitro fertilization, Lepidium meyenii, Maca, sperm motility
1. INTRODUCTION
Human fertility rates are declining worldwide, and infertility is a serious and rapidly increasing problem in reproductive medicine and public health. Infertility is defined as failure to achieve clinical pregnancy after ≥12 months of regular unprotected sexual intercourse,1 and its prevalence is ~9%.2 It is known that the cause of infertility exists in the male partner in approximately half of all infertile couples. There are several causes of male infertility, including production of defective spermatozoa, obstruction of the reproductive tract, inflammation, and sexual disorders such as erectile dysfunction and retrograde ejaculation.3 A recent review revealed that the prevalence of male infertility, defined as men reporting an experience of infertility (generally >12 months in duration), varied from 9.0% to 15.8% in surveys of general populations.4 Treatment of male infertility has become a significant issue for urologists and physicians in reproductive medicine. Therefore, several technical innovations in surgical treatment, including microdissection testicular sperm extraction for patients with azoospermia and microsurgery for patients with varicoceles, have been reported.5, 6 However, very few treatment options are available for idiopathic oligoasthenoteratozoospermia (OAT), which is the most common phenotype observed in male infertility. Although more than half of all infertile couples seek medical care, no effective and reliable medicines have been developed or approved for patients with OAT so far.2 To address this problem, several supplements, including vitamins B12, C, and E, and herbal medicines have been used clinically as OAT treatment options and have shown relatively favorable effects on spermatogenesis. Antioxidants such as coenzyme Q10 and l‐carnitine have also been used to reverse oxidative stress‐induced sperm dysfunction, and small clinical trials have shown their efficacy, particularly with respect to sperm motility.7, 8, 9 However, randomized control studies with larger numbers of participants have not yet been performed to show clinical evidence for the recommendation of these antioxidants as OAT treatment options. Thus, in practice, several antioxidants and other substances have been used for treating male infertility based on the clinical experience of the attending physicians.
Among several candidate medications for male infertility, Lepidium meyenii (Maca) has been one of the most popular supplements gaining increased attention as a treatment option. Maca is known as an antioxidant supplement10 with efficacy in several diseases and conditions. It has been reported that Maca may improve hypertension, diabetes mellitus, dyslipidemia, and depression. With respect to sexual and reproductive function in rats, Maca has favorable effects on sexual behavior,11 testicular weight,12 and spermatogenesis, particularly at the initial stages.13 In humans, treatment with Maca is reported to improve sexual desire without a change in the state of mind in healthy adult men.14 A systematic review of three randomized clinical trials and two uncontrolled observational studies has already provided suggestive evidence for the effectiveness of Maca in improving semen quality, although the total number of trials and the total sample size of the included studies prevented concrete conclusions.15 To date, the direct effect of Maca on sperm has not been investigated.
In the present study, we evaluated the direct effect of Maca on the rate of successful in vitro fertilization (IVF) in mice. We also investigated the direct effect of Maca on acrosome reaction in mice and humans and its efficacy in improving human sperm motility.
2. MATERIALS AND METHODS
2.1. Animals
Female ICR mice (10 weeks old) and male BALB/cA mice were purchased from Japan SLC (Shizuoka, Japan). C57BL/6 mice expressing enhanced green fluorescent protein (EGFP) in their acrosomes were bred in the animal experimentation facility of Nagasaki International University.16 The animals were euthanized via cervical dislocation immediately prior to the experiments. All animal experiments conformed to the Guide for the Care and Use of Laboratory Animals (Guide for the Care and Use of Laboratory Animals. 2011) and were approved by the Institutional Committee of Laboratory Animal Experimentation (Nagasaki International University, Nagasaki, Japan). The mice were maintained under controlled temperature (22°C ± 3°C), humidity (60% ± 20%), and light conditions throughout the experiments and were provided with food and water ad libitum.
2.2. Preparation of Maca (Lepidium meyenii)
Maca was collected, dried, and powdered in Japan and was obtained from Shokubunka.co.jp, Tokyo, Japan.^ Twenty milligrams of the air‐dried Maca powder was added to 1 mL of dimethyl sulfoxide (DMSO; D2650; Sigma–Aldrich Japan, Tokyo, Japan), and the supernatant of this Maca solution was added to human tubal fluid (HTF) medium (LifeGlobal Group, Guilford, CT, USA).
2.3. IVF in mice
Female mice were superovulated via intraperitoneal injection of 5 IU pregnant mare serum gonadotropin (Asuka Inc, Tokyo, Japan), followed by a 5 IU human chorionic gonadotropin (Asuka Inc) injection 46‐48 hours later, and then euthanized via cervical dislocation 14‐16 hours later, immediately prior to the start of the experiment. Ovaries with oviducts were transferred to 30‐mm‐diameter dishes filled with paraffin oil (Nacalai Tesque, Kyoto, Japan). Cumulus–oocyte complexes were obtained from the ampullae of uterine tubes and transferred under a stereomicroscope to the dishes, each containing a 200‐µL drop of HTF medium covered with paraffin oil. Two to four cumulus–oocyte masses were transferred to each 200‐µL drop of HTF medium covered with paraffin oil for insemination. Approximately, 10‐month‐old Balb/c mice were euthanized via cervical dislocation immediately prior to the start of the experiments. Mature caudal epididymal sperm cells were collected for this examination. Mature caudal epididymal sperm cells (~8 × 106) from each mouse were incubated in 200 µL of HTF medium without bovine serum albumin (BSA), covered with paraffin oil. After 5 minutes, each sperm suspension was transferred to conditioned medium for preincubation. The control conditioned medium for sperm preincubation was HTF medium containing 0.4% BSA. Subsequently, 25‐µL aliquots of the sperm suspensions in HTF without BSA were transferred to 25 µL of conditioned medium samples containing twice the concentration of Maca extract and were placed at 37°C in a humidified incubator under 5% CO2/95% air (motile sperm concentration, ~10,000 per mL). After 50 minutes, 2‐4 µL aliquots of sperm from each conditioned medium sample were used for insemination (final motile sperm concentration, 150 per mL). Motile sperm swimming at the periphery of each drop were used for insemination, as described previously.17 A sperm suspension cultured in conditioned medium was transferred to the insemination drop. At 24 hours after insemination, the fertilization rate was determined as the proportion of two‐cell‐stage embryos among all oocytes.
2.4. Acrosome reaction in mouse sperm
To examine the acrosome reaction rate, sperm from transgenic mice expressing EGFP16 were incubated in each conditioned medium sample for 3 hours and spotted onto glass slides with 5% glycerol. The acrosome status was observed under a fluorescence microscope following propidium iodide staining.
2.5. Acrosome reaction in human sperm
Human semen samples were obtained from fertile male volunteers via masturbation after 2‐3 days of abstinence, after they provided their informed consent. The semen was covered with 2 mL of HTF and incubated at 37°C for 1 hours. Then, 25‐µL aliquots of swim‐up sperm from the semen in the HTF were transferred to 25 µL of the control conditioned medium or Maca extract‐containing conditioned medium and placed at 37°C in a humidified incubator under 5% CO2/95% air for 4 hours. The acrosome status was observed as previously described.18 The human sperm samples were spotted onto silane‐coated Superfrost glass microslides (Matsunami Glass Ind. Ltd., Osaka, Japan), treated with 70% methanol on ice for 10 min, and stained with fluorescein isothiocyanate‐conjugated peanut agglutinin (FITC‐PNA; L7381; Sigma–Aldrich, Japan).
2.6. Human sperm motility
Sperm motility was analyzed using a sperm motility analysis system (SMAS; SOLNET, Tokyo, Japan).19 Sperm in conditioned medium were spotted onto a 20‐μm Leja counting chamber (standard Count Analysis Chamber 20 µm; Nieuw‐Vennep, The Netherlands) for the analysis of sperm motility. The SMAS consists of a high‐resolution digital scanning camera, personal computer with a digital frame grabber and image‐processing software, and computer monitor. The system records images at a rate of 1 frame/s (60 Hz) and can analyze ~200 spermatozoa simultaneously in real time. A previous study showed that the results obtained using the SMAS strongly correlated with those obtained from manual microscopic sperm analysis based on the World Health Organization Laboratory Manual.19 The procedures were approved by the regional ethics committee of Juntendo University Urayasu Hospital.
2.7. Statistical analysis
Data were expressed as average ± SEM and compared between the groups using the Mann–Whitney U test. The threshold for significance was P < 0.05. All statistical analyses were conducted using IBM SPSS version 24.0.
3. RESULTS
3.1. Success rate of IVF in mice
The fertilization ability of sperm from wild‐type C57BL/6 mice was higher when using the standard medium. When sperm from aged BALB/cA mice (age >48 weeks) were used,20 IVF was difficult and the fertilization ability of the sperm varied between mice. We tested whether the fertilization rate increased because of the addition of Maca extract. The fertilization rates in HTF medium and Maca extract‐containing medium are shown in Figure 1. The fertilization rate in HTF medium containing Maca extract at a concentration of 4% (w/v) with 1% DMSO was significantly higher (33.4% ± 7.6%) than that in HTF medium without Maca extract (14.3% ± 4.6%; P < 0.05). The fertilization rates in HTF medium with Maca extract at concentrations of 2% and 8% (w/v) were also higher (23.3% ± 5.9% and 20.7% ± 9.6%, respectively) than that in HTF medium without Maca extract. However, these differences were not statistically significant.
Figure 1.
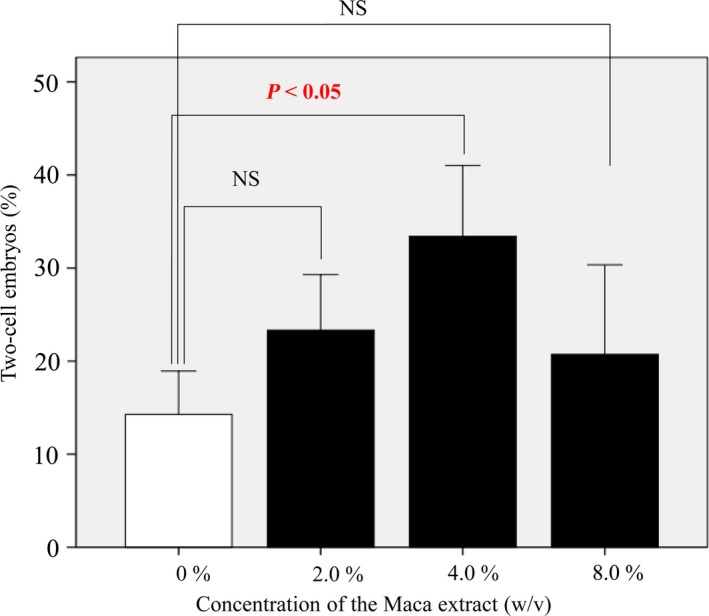
Rate of successful fertilization determined as the proportion of two‐cell‐stage embryos among all mouse oocytes in the experiment. The fertilization rate in medium containing Maca extract at a concentration of 4% (w/v) with 1% DMSO was significantly higher (33.4% ± 7.6%) than that in HTF medium without Maca extract (14.3% ± 4.6%; P < 0.05). The fertilization rates in medium containing Maca extract at concentrations of 2% and 8% (w/v) were also higher (23.3% ± 5.9% and 20.7% ± 9.6%, respectively) than those in HTF medium without Maca extract, but these differences were not statistically significant. DMSO, dimethyl sulfoxide; HTM, human tubal fluid
3.2. Acrosome reaction rate in mouse sperm
We examined the sperm acrosome reaction rate in Maca extract‐containing HTF medium using mouse sperm expressing GFP in their acrosomes. The rates of acrosome‐reacted sperm in HTF medium containing Maca extract at concentrations of 4.0%, 8.0%, and 16.0% (w/v) with 1% DMSO were significantly higher (68% ± 3.1%, 71% ± 1.2%, and 71% ± 2.9%, respectively) than those in medium without Maca (44% ± 5.1%; P < 0.05, Figure 2). The rate of acrosome‐reacted sperm in HTF medium containing Maca extract at a concentration of 2.0% (w/v) with 1% DMSO was also higher (59% ± 5.5%) than that in HTF medium without Maca extract. However, this difference was not statistically significant.
Figure 2.
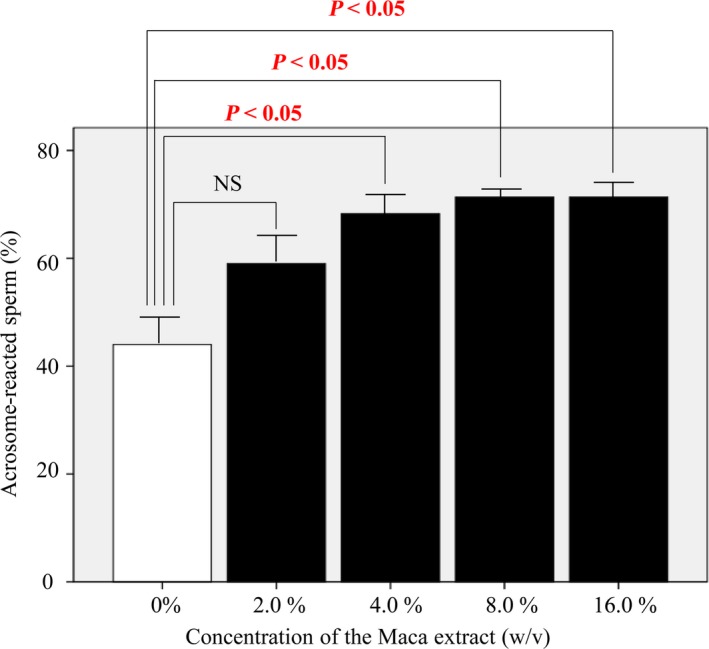
The rate of acrosome reaction in mouse sperm. The rates of acrosome‐reacted sperm in HTF medium containing Maca extract at concentrations of 4.0%, 8.0%, and 16.0% (w/v) with 1% DMSO were significantly higher (68% ± 3.1%, 71% ± 1.2%, and 71% ± 2.9%, respectively) than those in medium without Maca extract (44% ± 5.1%; P < 0.05). The rate of acrosome‐reacted sperm in HTF medium containing Maca extract at a concentration of 2.0% (w/v) with 1% DMSO was also higher (59% ± 5.5%) than that in HTF medium without Maca extract, but this difference was not statistically significant. HTM, human tubal fluid; DMSO, dimethyl sulfoxide
3.3. Acrosome reaction rate in human sperm
To investigate the effect of Maca on human sperm, the acrosome reaction in Maca extract‐containing HTF medium was assessed using FITC‐PNA staining. The rate of acrosome‐reacted sperm in HTF medium containing Maca extract at a concentration of 1.0% (w/v) with 1% DMSO was significantly higher (69% ± 7.3%) than that in medium without Maca (23% ± 5.4%; P < 0.01, Figure 3). The rates of acrosome‐reacted sperm in HTF medium containing Maca extract at concentrations of 0.5% and 2.0% (w/v) with 1% DMSO were also higher (39.6% ± 3.2% and 39.8% ± 5.8%, respectively) than those in HTF medium without Maca extract, but these differences were not statistically significant.
Figure 3.
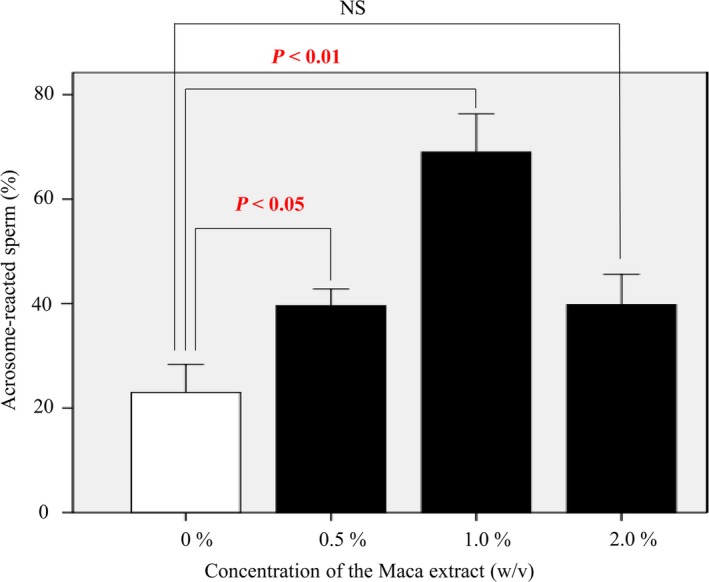
The rate of acrosome reaction in human sperm. The rate of acrosome‐reacted sperm in HTF medium containing Maca extract at a concentration of 1.0% (w/v) with 1% DMSO was significantly higher (69% ± 7.3%) than that in medium without Maca extract (23% ± 5.4%; P < 0.01). The rates of acrosome‐reacted sperm in HTF medium containing Maca extract at concentrations of 0.5% and 2.0% (w/v) with 1% DMSO were also higher (39.6% ± 3.2% and 39.8% ± 5.8%, respectively) than those in HTF medium without Maca extract, but these differences were not statistically significant. HTM, human tubal fluid; DMSO, dimethyl sulfoxide
3.4. Human sperm motility and amplitude of lateral head displacement (ALH)
The motility of human sperm in Maca extract‐containing HTF medium was analyzed in detail using SMAS (Table 1). The percentage of motile sperm in the medium containing Maca extract was significantly higher (82.1% ± 5.1%) than that in the control medium containing 1% DMSO (53.9% ± 9.5%; P < 0.05, Figure 4). ALH in the medium containing Maca extract was also higher (2.9 ± 0.3 μm) than that in the control medium (2.4 ± 0.2 μm). However, this difference was not statistically significant.
Table 1.
Results of sperm motility analysis using semen from healthy men in medium with or without Maca extract (N = 5)
| With Maca extract | Without Maca extract | P value | |
|---|---|---|---|
| Concentration (×10⁶) | 95.5 ± 54.1 | 60.5 ± 20.6 | NS |
| Total sperm count (×10⁶) | 190.9 ± 108.1 | 120.6 ± 41.5 | NS |
| Sperm motility (%) | 82.1 ± 5.1 | 53.9 ± 9.5 | <0.05 |
| Straight‐line velocity (μm/s) | 32.1 ± 6.4 | 34.7 ± 4.4 | NS |
| Curvilinear velocity (μm/s) | 86.3 ± 11.3 | 94.6 ± 8.6 | NS |
| Linearity | 0.36 ± 0.03 | 0.36 ± 0.02 | NS |
| Amplitude of lateral head displacement (μm) | 2.9 ± 0.3 | 2.4 ± 0.2 | NS |
| Beat‐cross frequency (Hz) | 13.4 ± 0.5 | 11.9 ± 0.9 | NS |
Figure 4.
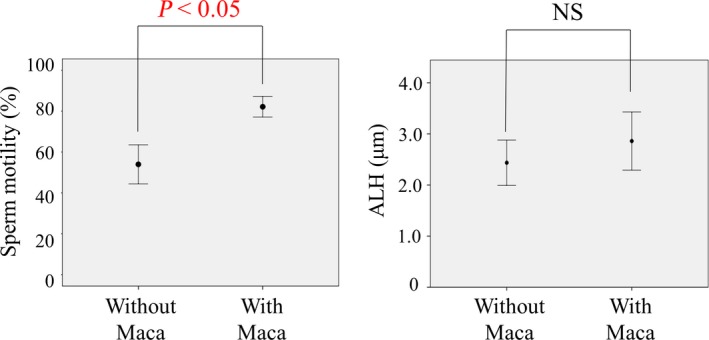
Sperm motility and amplitude of lateral head displacement in medium with or without Maca extract. Sperm motility was analyzed using a sperm motility analysis system. The percentage of sperm motility in the Maca extract‐containing medium was significantly higher (82.1% ± 5.1%) than that in the control medium (53.9% ± 9.5%; P < 0.05). The amplitude of lateral head displacement in the Maca extract‐containing medium was also higher (2.9 ± 0.3 μm) than that in the control medium (2.4 ± 0.2 μm), but this difference was not statistically significant
4. DISCUSSION
Maca belongs to the plant family Brassicaceae and is native to Peru. It has traditionally been used as folk medicine and is considered a food supplement. It was first cultivated at least 2000 years ago in the Andes Mountains of Peru at an altitude of 4000‐4500 m. There are numerous substances in the tubers of Maca; several typical components include amino acids, alkaloids (macaines), fatty acids (linoleic, palmitic, oleic acid, etc), tannins, saponins, and several microelements (Cu, Su, Mn, Al, etc).10 Because Maca is rich in these substances, it has significant potential to treat several diseases and disorders. Maca has been thought to improve sexual dysfunction and male infertility since long, even before it was reported that consumption of Maca extract increased serum testosterone concentration by enhancing the steroidogenic ability of Leydig cells and improving sexual performance parameters in an animal model.11, 21 Furthermore, a previous study in humans showed that the administration of Maca improves seminal volume, sperm count per ejaculum sample, the number of motile sperms, and sperm motility despite the fact that it does not affect the levels of relevant serum hormones, including serum luteinizing hormone, follicle‐stimulating hormone (FSH), prolactin, testosterone, or estradiol.12 This indicates that improved semen quality observed because of the administration of Maca might be caused by enhanced bioavailable testosterone or testosterone receptors and an improved response of Sertoli cells to FSH.22, 23 A systematic review recently supported the effectiveness of Maca in improving semen quality in humans.15 Maca has currently gained increased attention in reproductive medicine, particularly in male infertility, because no reliable and effective medical treatment has been established for this condition so far. However, the underlying mechanism of Maca in male infertility, particularly the direct effect of Maca on sperm, has not been elucidated.
In the present study, we first investigated whether the addition of Maca to culture medium improved the rate of successful IVF. As expected, in mice, the rate of fertilization in medium containing Maca was significantly increased compared with that in the control medium. Our results indicated that HTF medium containing Maca extract at a concentration of 4% (w/v) was the most suitable for IVF in mice. Second, we evaluated the influence of Maca on acrosome reaction as another direct effect. In mouse samples, we showed that the rate of acrosome‐reacted sperm in HTF medium containing Maca extract was significantly higher than that in medium without Maca extract. Furthermore, this tendency was also found in human sperm, although the effective concentration of Maca was different from that in mice. Finally, we showed that Maca was clearly beneficial for sperm motility in humans. On the basis of our findings, we speculate that adding Maca to the medium used during IVF may increase its success rate by improving acrosome reaction and sperm motility.
Several studies have reported improvement in sperm quality using pharmaceutical agents such as caffeine, pentoxifylline, theophylline, Chinese herbal medicine, and myo‐inositol, in vivo and/or in vitro. Caffeine, pentoxifylline, and theophylline are considered to be inhibitors of phosphodiesterase and have been primarily used in vitro in humans. Caffeine is a methylxanthine alkaloid that causes an increase in intracellular cyclic adenosine monophosphate (cAMP) and is reported to induce the acrosome reaction in boar and human sperm in vitro.24, 25 Furthermore, caffeine may induce sperm hyperactivation in the course of promoting activation of calcium ion‐permeable cation channels in the plasma membrane of sperm.26 Pentoxifylline, a methylxanthine derivative, decreases blood viscosity because of platelet inhibition and is primarily used for symptomatic relief of intermittent thrombosis.27 In contrast to caffeine, pentoxifylline significantly increases sperm viability in infertile men without improving sperm motility.28 Apart from its lack of effect on increasing the acrosome reaction rate and sperm motility, pentoxifylline treatment also does not increase sperm responsiveness to the acrosome reaction induced by stimulation with follicular fluid and the ionophore A23187.23 However, pentoxifylline significantly enhances hyperactivated sperm motility and tight binding to the homologous zona pellucida.29 Although caffeine and pentoxifylline have equal inhibitory effects on sperm phosphodiesterase, their effects are different. Myo‐inositol is one of the most biologically important agents and acts in concert with group B vitamins. Administration of myo‐inositol improves spermatozoa concentration in patients with oligoasthenospermia but does not significantly improve sperm motility.30 Myo‐inositol increases the number of spermatozoa with high mitochondrial membrane potential (MMP) in vitro and decreases the number of those with low MMP in patients with oligoasthenospermia.31 MMP is considered a marker of apoptosis because viable cells show high MMP and apoptotic cells show low MMP. Fertile men have spermatozoa with high MMP, whereas infertile men have spermatozoa with low MMP. In herbal medicine, the preparation “Hochuekkito” has also been used clinically to treat male infertility in Japan. Although common oral consumption of Hochuekkito may be expected in the future, it has already been suggested to have a direct protective effect on sperm in infertile men.32
The exact mechanisms of fertilization processes, including those of the acrosome reaction and hyperactivation, remain unknown. The acrosome reaction (ie, exocytosis of the sperm vesicle) is a prerequisite for fertilization. We showed that culture medium containing Maca extract improved the fertilization rate in mice and that it was not dose related (Figure 1). There are no reports about the Maca specific toxicities; however, it is expected that excess amounts of chemical substances can cause negative effects. We also showed that the medium containing Maca extract increased the rate of acrosome‐reacted sperm in mouse and human samples (Figures 2 and 3). These results support the improvement in infertility due to Maca extract via induction of the acrosome reaction. There is difference in suitable concentration of Maca for acrosome reaction between mouse and human sperm. Optimal medium varies among species. It is possible that the quantity of the molecules inside the sperm on which Maca acts and the influence of Maca on the molecules may vary depending on species. In fact, it is known from an IVF experimental model that albumin has an influence on acrosome reaction, and that albumin draws fat from the membrane of sperm and by doing so gradually facilitates the induction of acrosome reaction and promotes the sperm to mature and become active. The amounts of albumin to be added to the medium for conducting efficient IVF are slightly different between mice and humans. Furthermore, the albumin from different lots can cause a large change on the acrosome reactions and the fertility rate. In this experiment as well, the complicated conditions, as stated above, are considered to be the reasons why the suitable Maca concentration was different between mice and humans.
Hyperactivation may support sperm in detachment from the endosalpingeal epithelium, enhancing their ability to easily swim through viscoelastic substances. In addition, hyperactivation assists sperm in penetrating the zona pellucida.33 An association between ALH and sperm hyperactivation has been reported.34 ALH in the present study tended to be higher in the Maca extract‐containing medium than in the control medium, although this difference was not statistically significant (Figure 4). In Table 1, there is difference between sperm concentration with and without Maca extract though not statistically significant. We consider that this tendency was not due to Maca, but rather, it was because the substances from the sperm might have contaminated the medium and possibly influenced the results. These results suggest that Maca extract can induce hyperactivation in humans. Maca is a type of alkaloid similar to caffeine. It is possible that Maca induces the acrosome reaction and hyperactivation because of an increase in cAMP and the number of spermatozoa with high MMP. Furthermore, it has recently been suggested that some hormones, including progesterone, melatonin, and serotonin, enhance hyperactivation through specific membrane receptors and that 17β‐estradiol suppresses such enhancement by progesterone and melatonin via a membrane estrogen receptor.35 Because plant extracts can have several functions, including estrogenic actions, depending on the flavonoids present, it is possible that Maca also has a specific estrogenic action. If so, such an action may also be associated with our findings in the present study. It was recently shown that the rate of IVF in mice was improved by adding an aqueous extract of licorice to the artificial insemination culture medium. Furthermore, it was reported that isoliquiritigenin and formononetin were the active molecules in licorice that contributed to the improved IVF rate.36 Similarly, the identification of molecules in Maca that contribute to the improvement in IVF has begun to attract increased attention.
There are many reports about past experiments regarding the effectiveness of oral intake of Maca. In this study, it became clear that some substances in Maca affect the ejaculated sperm. It is speculated that some of the substances, which can affect the ejaculated sperm, might have affected the activity of the ejaculated sperm through the body fluid even when Maca was taken orally. Additionally, because the quality of IVF medium was improved, there is a possibility that Maca can lead to desirable results for women when it is taken orally.
We showed that Lepidium meyenii (Maca) added to culture medium may improve the IVF rate of mouse sperm. We also showed that Maca is beneficial in inducing an acrosome reaction in mouse and human sperm and an increase in motility in human sperm. Our study provides evidence that Maca has a direct effect on sperm in improving fertilization rates.
ETHICAL APPROVAL
Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revision. Informed consent or substitute for it was obtained from all patients for being included in the study. Additionally, the protocol for the research was approved by the Institutional Review Board of the Juntendo University Urayasu Hospital, Chiba, Japan. All institutional and national guidelines for the care and use of laboratory animals were followed.
CONFLICT OF INTEREST
None of the authors declare competing financial interests.
ACKNOWLEDGEMENTS
This work was partly supported by facilities of the Nagasaki International University. Lepidium meyenii (Maca) for the study were provided by Shokubunka.co.jp, Tokyo, Japan.^ The study was not supported by any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Aoki Y, Tsujimura A, Nagashima Y, et al. Effect of Lepidium meyenii on in vitro fertilization via improvement in acrosome reaction and motility of mouse and human sperm. Reprod Med Biol. 2019;18:57–64. 10.1002/rmb2.12251
[Correction added on 26 November 2018, after first online publication: The text 'Shokubunka Co. Ltd., Saitama, Japan' has been corrected to 'Shokubunka.co.jp, Tokyo, Japan'. The changes are marked by the symbol ^.]
REFERENCES
- 1. Zegers‐Hochschild F, Adamson GD, de Mouzon J, et al. International committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520‐1524. [DOI] [PubMed] [Google Scholar]
- 2. Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment‐seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506‐1512. [DOI] [PubMed] [Google Scholar]
- 3. de Kretser DM. Male infertility. Lancet. 1997;349:787‐790. [DOI] [PubMed] [Google Scholar]
- 4. Barratt C, Björndahl L, De Jonge CJ, et al. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance‐challenges and future research opportunities. Hum Reprod Update. 2017;23:660‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606‐609. [DOI] [PubMed] [Google Scholar]
- 6. Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131‐135. [DOI] [PubMed] [Google Scholar]
- 7. Jarow JP. Use of carnitine therapy in selected cases of male factor infertility: a double‐blind crossover trial. J Urol. 2003;170:677. [PubMed] [Google Scholar]
- 8. Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009;182:237‐248. [DOI] [PubMed] [Google Scholar]
- 9. Balercia G, Buldreghini E, Vignini A, et al. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo‐controlled, double‐blind randomized trial. Fertil Steril. 2009;91:1785‐1792. [DOI] [PubMed] [Google Scholar]
- 10. Večeřa R, Orolin J, Škottová N et al. The influence of Maca (Lepidium meyenii) on antioxidant status, lipid and glucose metabolism in rat. Plant Foods Hum Nutr. 2007;62:59‐63. [DOI] [PubMed] [Google Scholar]
- 11. Cicero AF, Bandieri E, Arletti R. Lepidium meyenii Walp. improves sexual behaviour in male rats independently from its action on spontaneous locomotor activity. J Ethnopharmacol. 2001;75: 225–229. [DOI] [PubMed] [Google Scholar]
- 12. Gonzales GF, Cordova A, Gonzales C, Chung A, Vega K, Villena A. Lepidium meyenii (Maca) improved semen parameters in adult men. Asian J Androl. 2001;3:301–303. [PubMed] [Google Scholar]
- 13. Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548–573. [DOI] [PubMed] [Google Scholar]
- 14. Gonzales GF, Córdova A, Vega K et al. Effect of Lepidium meyenii (MACA) on sexual desire and its absent relationship with serum testosterone levels in adult healthy men. Andrologia. 2002;34:367–372. [DOI] [PubMed] [Google Scholar]
- 15. Lee MS, Lee HW, You S, Ha KT. The use of Maca (Lepidium meyenii) to improve semen quality: A systematic review. Maturitas. 2016;92:64–69. [DOI] [PubMed] [Google Scholar]
- 16. Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. [DOI] [PubMed] [Google Scholar]
- 17. Takeo T, Hoshii T, Kondo Y, et al. Methyl‐beta‐cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod. 2008;78:546–551. [DOI] [PubMed] [Google Scholar]
- 18. Kitamura K, Tanaka H, Nishimune Y. Haprin, a novel haploid germ cell‐specific RING finger protein involved in the acrosome reaction. J Biol Chem. 2003;278:44417–44423. [DOI] [PubMed] [Google Scholar]
- 19. Komori K, Tsujimura A, Ishijima S, et al. Comparative study of sperm motility analysis system and conventional microscopic semen analysis. Reprod Med Biol. 2006;5:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vasudevan K, Sztein JM. Treatment of sperm with extracellular adenosine 5′‐triphosphate improves the in vitro fertility rate of inbred and genetically modified mice with low fertility. Theriogenology. 2011;76:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohta Y, Yoshida K, Kamiya S, et al. Feeding hydroalcoholic extract powder of Lepidium meyenii (Maca) increases serum testosterone concentration and enhances steroidogenic ability of Leydig cells in male rats. Andrologia. 2016;48:347–354. [DOI] [PubMed] [Google Scholar]
- 22. Gonzales GF, Ruiz A, Gonzales C, Villegas L, Cordova A. Effect of Lepidium meyenii (Maca) roots on spermatogenesis of male rats. Asian J Androl. 2001;3:231–233. [PubMed] [Google Scholar]
- 23. Gonzales GF, Córdova A, Vega K, Chung A, Villena A, Góñez C. Effect of Lepidium meyenii (Maca), a root with aphrodisiac and fertility‐enhancing properties, on serum reproductive hormone levels in adult healthy men. J Endocrinol. 2003;176:163–168. [DOI] [PubMed] [Google Scholar]
- 24. Tesarik J, Mendoza C, Carreras A. Effects of phosphodiesterase inhibitors caffeine and pentoxifylline on spontaneous and stimulus‐induced acrosome reactions in human sperm. Fertil Steril. 1992;58:1185–1190. [DOI] [PubMed] [Google Scholar]
- 25. Funahashi H, Nagai T. Regulation of in vitro penetration of frozen‐thawed boar spermatozoa by caffeine and adenosine. Mol Reprod Dev. 2001;58:424–431. [DOI] [PubMed] [Google Scholar]
- 26. Ho HC, Suarez SS. An inositol 1,4,5‐trisphosphate receptor‐gated intracellular Ca(2+) store is involved in regulating sperm hyperactivated motility. Biol Reprod. 2001;65:1606–1615. [DOI] [PubMed] [Google Scholar]
- 27. Magnusson M, Gunnarsson M, Berntorp E, Björkman S, Höglund P. Effects of pentoxifylline and its metabolites on platelet aggregation in whole blood from healthy humans. Eur J Pharmacol. 2008;581:290–295. [DOI] [PubMed] [Google Scholar]
- 28. Ghasemzadeh A, Karkon‐Shayan F, Yousefzadeh S, Naghavi‐Behzad M, Hamdi K. Study of pentoxifylline effects on motility and viability of spermatozoa from infertile asthenozoospermic males. Niger Med J. 2016;57:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nassar A, Mahony M, Morshedi M, Lin MH, Srisombut C, Oehninger S. Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil Steril. 1999;71:919–923. [DOI] [PubMed] [Google Scholar]
- 30. Gulino FA, Leonardi E, Marilli I et al. Effect of treatment with myo‐inositol on semen parameters of patients undergoing an IVF cycle: in vivo study. Gynecol Endocrinol. 2016;32:65–68. [DOI] [PubMed] [Google Scholar]
- 31. Condorelli RA, La Vignera S, Di Bari F, Unfer V, Calogero AE. Effects of myoinositol on sperm mitochondrial function in‐vitro. Eur Rev Med Pharmacol Sci. 2011;15:129–134. [PubMed] [Google Scholar]
- 32. Yamanaka M, Kitamura M, Kishikawa H et al. Direct effects of Chinese herbal medicine “hochuekkito” on sperm movement. Nihon Hinyokika Gakkai Zasshi. 1998; 89: 641–646. [Article in Japanese]. [DOI] [PubMed] [Google Scholar]
- 33. Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12:23–37. [DOI] [PubMed] [Google Scholar]
- 34. Chan PJ, Tredway DR, Henig I, Prough SG. Cyclic CMP (cytidine 3′,5′‐monophosphate) suppresses changes in human sperm amplitude of lateral head displacement and hyperactivation. Experientia. 1990;46:734–736. [DOI] [PubMed] [Google Scholar]
- 35. Fujinoki M, Takei GL, Kon H. Non‐genomic regulation and disruption of spermatozoal in vitro hyperactivation by oviductal hormones. J Physiol Sci. 2016;66:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tung NH, Shoyama Y, Wada M, Tanaka H. Two activators of in vitro fertilization in mice from licorice. Biochem Biophys Res Commun. 2015;467:447–450. [DOI] [PubMed] [Google Scholar]


