Abstract
Melanoma is one of the most fatal cancers, and its incidence is increasing worldwide. Thanks to the better understanding of the molecular mechanisms involved in the pathogenesis of melanoma, recently new targeted agents have been developed. In this article, we review the current state of knowledge of clinical presentation, mechanisms, and management of the most common cutaneous side effects observed during treatment with targeted and immunological therapies approved for advanced melanoma. We include discussion of BRAF/MEK inhibitors and immune-checkpoint inhibitors, notably CTLA-4 and PD-1 inhibitors.
1. Introduction
Melanoma is one of the most fatal cancers, and its incidence is increasing worldwide. Metastatic melanoma has a poor prognosis representing about 90% of skin cancer mortality. In the recent past, the therapy of metastatic melanoma was based only on dacarbazine, an alkylating chemotherapy agent, which has not shown improvement of overall survival [1]. Classical chemotherapy has cytotoxic and antiproliferative effects which give rise to well-known cutaneous adverse events (AEs). More recently, thanks to the better understanding of the molecular mechanisms involved in the pathogenesis of melanoma, new agents have been developed. In particular, inhibitors of the cytoplasmic serine/threonine kinase BRAF and the immune-checkpoint targeted agents, anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), and anti-programmed cell death 1 (PD-1) inhibitors have been approved for the treatment of advanced melanoma. With the introduction of these novel therapies, a new spectrum of skin side effects has emerged. Management of these cutaneous AEs is very important for treatment adherence and patient quality of life. In this article, we review the current state of knowledge of clinical presentation, mechanisms, and management of the most common cutaneous side effects observed during treatment with targeted and immunological therapies approved for advanced melanoma. The main skin adverse events and their management are summarized in Table 1.
Table 1.
Cutaneous side effects observed during targeted therapy (BRAF and MEK inhibitors) and immunotherapy (CTLA-4 and PD-1 inhibitors) and their management.
| Target | Skin toxicity | Management |
|---|---|---|
| Skin rash (maculopapular) | Topical steroids (clobetasol propionate); oral corticosteroids (prednisone); oral antihistamines; emollient agents | |
| BRAF inhibitors (i) Vemurafenib (ii) Dabrafenib | ||
| Photosensitivity | Avoid sun (broad-spectrum sunscreens that cover UVA spectrum, protective clothing) | |
| Palmarplantar hyperkeratosis | Urea cream; avoid friction | |
| Verrucal keratosis | Cryotherapy; monitor for changes suggestive of SCC; acitrein as a chemopreventive drug | |
| Squamous cell carcinoma, alopecia, and hair modifications | Excision, minoxidil 2% | |
| Panniculitis | Nonsteroidal anti-inflammatory drugs; oral steroids (prednisolone) | |
| Melanocytic proliferation | Dermoscopic monitoring; radical surgery for melanomas; education on photoprotection and self-skin examination | |
| BCC | Excision | |
|
| ||
| MEK inhibitors | Acneiform rash (papulo-pustular) | Topical antibiotics (clindamycin, erythromycin); oral antibiotics (doxycycline, monocycline); topical steroids (prednicarbate); oral steroids (prednisone); oral antihistamines; oral isotretinoin |
| (i) Trametinib | ||
| (ii) Cobimetinib | ||
|
| ||
| CTLA-4 inhibitors | Rash (maculopapular, lichenoid eruption), eczema | Medium-to-high potency topical (and sometimes oral) corticosteroids; antihistamines |
| (i) Ipilimumab | ||
|
| ||
| PD-1 inhibitors | Vitiligo, psoriasis, autoimmune blistering disorders | |
| (i) Nivolumab | ||
| (ii) Pembrolizumab | ||
2. Targeting the BRAF/MEK Pathway
Genetic alterations resulting in a constitutive activation of mitogen-activated protein kinase (MAPK) pathway occur in almost all cases of melanoma. The most frequent mutation is the BRAF mutation which has been found in about 50% of melanomas [2–4]. BRAF is a component of the RAS-RAF-MEK-ERK signalling pathway. The constitutive activation of mutated BRAF leads to an uncontrolled signal transduction of the MAPK pathway causing an increased proliferation, reduced apoptosis, and enhanced invasiveness [2, 3]. Since the discovery of BRAF mutations in cutaneous melanoma, nonselective and selective pharmacological agents have been developed to inhibit this target [5] (Figure 1).
Figure 1.
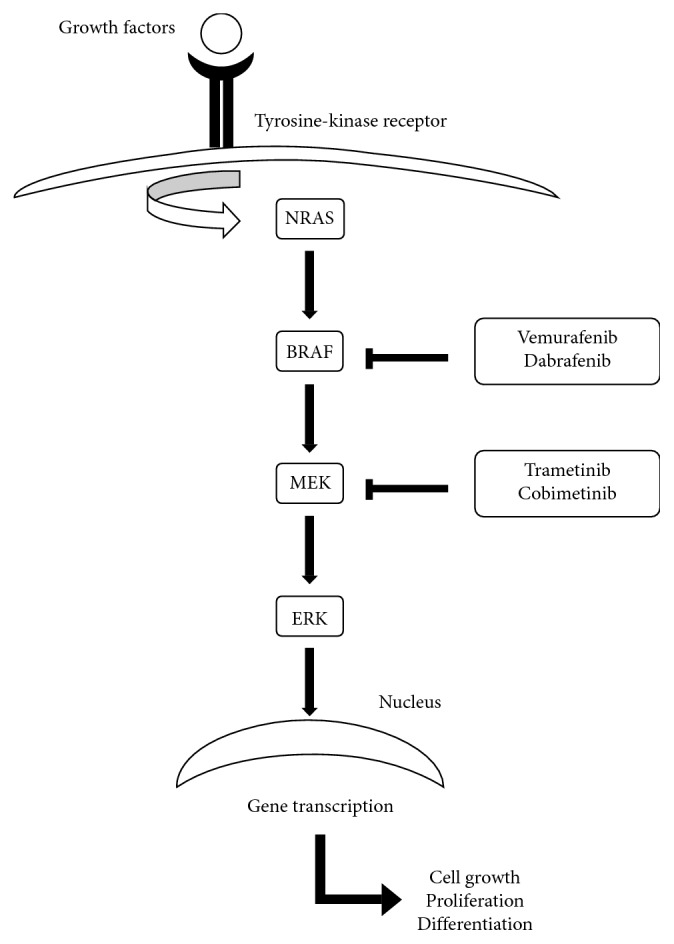
MAPK signalling pathway and its inhibitors.
The first selective BRAF inhibitor approved by FDA and EMA in 2011 for the treatment of patients with metastatic or unresectable melanoma was vemurafenib [2, 6], followed by dabrafenib which was approved by FDA and EMA in 2013 [7]. The adding inhibition of MEK, downstream of BRAF, has been reported to improve the duration of the therapeutic effect of BRAF inhibitors [8]. Two BRAF-MEK inhibitor combinations (vemurafenib-cobimetinib and dabrafenib-trametinib) are currently regarded as treatment options for metastatic or unresectable melanoma [9, 10], and in the near future, a new combination therapy (encorafenib-binimetinib) is expected to emerge as a valuable alternative to established BRAF-MEK combinations [11].
2.1. Cutaneous Side Effects of BRAF-MEK Inhibitors
Cutaneous side effects of BRAF inhibitors are very common and usually occur within days of the undergoing treatment. Skin toxicity induced by BRAF inhibitors is similar to that observed with EGFR inhibitors as BRAF represents a downstream mediator of EGFR signalling which is a critical regulator of epidermal homeostasis [12]. Although skin toxicity induced by BRAF inhibitors may significantly reduce the quality of life of these patients, it is generally managed with dose adjustment and supportive treatments. Furthermore, the combination of a MEK inhibitor with a BRAF inhibitor has been reported to induce less skin toxicity compared to the BRAF inhibitor monotherapy approach [9, 12–17].
Biological results demonstrate that BRAF inhibitors promote paradoxical activation of the MAPK pathway in cells that do not carry BRAF mutation leading to keratinocyte proliferation and hyperproliferative skin lesions which are the most common side effect of BRAF inhibitors [18]. A concurrent inhibition downstream of RAF with a MEK inhibitor preventing the pathway activation results in the significant reduction in cutaneous side effects [19].
The profile of skin toxicity associated with MEK inhibitors differs from that occurring during BRAF inhibitors therapy. Squamoproliferative skin lesions developed in patients treated with BRAF inhibitors have not been reported during treatment with MEK inhibitors. The typical rash (papulopustular or acneiform) observed during MEK inhibitors agents is different from the rash (hyperkeratotic maculopapular) caused by BRAF inhibitors [20].
2.2. Rash
Skin rash has been reported in patients treated both with BRAF and MEK inhibitors. Skin rash is mostly seen in patients under treatment with BRAF inhibitors (64–71% with vemurafenib and up to 18% with dabrafenib) [21]. It usually occurs 2 weeks after initiation of therapy and develops on the face/neck, trunk, and extremities. Many subtypes of rash have been described: macular, maculopapular, papular, and papulopustular. The maculopapular rash, also known as morbilliform rash, is the most common during BRAF inhibitors therapy. It is characterized by macules (flat) and papules (elevated) frequently involving the upper trunk, expanding centripetally and associated with pruritus. Topical steroids (clobetasol propionate), oral corticosteroids (prednisone), oral antihistamines, and emollient agents can be used for treatment. Acneiform rash characterized by an eruption of papules and pustules, typically occurring in face, scalp, upper chest, and back is typically seen during MEK inhibitors therapy occurring in about 14% of patients [18, 22].
Papulopustular rashes can be treated with topical (clindamycin and erythromycin) and oral antibiotics (doxycycline and monocycline), topical (prednicarbate) and oral steroids (prednisone), oral antihistamines, and oral isotretinoin [18, 22].
Occasionally, keratosis pilaris-like eruptions may appear, and they can be managed with mild keratolytics such as urea cream or salicylic acid and topical steroids [19].
It has also been reported a nonspecific rash similar both clinically and histologically to idiopathic Grover's disease. This benign acantholytic dermatosis presents as scattered erythematous papules with or without crusting usually asymptomatic or only slightly pruritic. Lesions involve the trunk, the upper arms, and legs. It can be treated with moisturisers, topical steroids, and oral antihistamines or oral prednisone and acitretin in severe cases [19, 23].
Serious cutaneous rashes such as Stevens–Johnson syndrome and toxic epidermal necrolysis have been rarely reported [21].
2.3. Photosensitivity
Photosensitivity reaction represents one of the main adverse effects related to BRAF inhibitors, and it is experienced more frequently with the use of vemurafenib (22–67%) compared to dabrafenib (3–33%) [21]. Photosensitivity developed within days of drug initiation. Patients reported cutaneous eruptions on sun-exposed skin areas within hours of sun exposure. It is estimated that the cause of the reaction is related to the drug's chemical structure and UVA. Broad-spectrum sunscreens including UVA protection and protective clothing should be mandatory for patients [19, 21].
2.4. Palmarplantar Hyperkeratosis
Localized hyperkeratosis appeared as painful hyperkeratotic areas on points of pressure or friction mostly on the soles and less frequently on the palms [17]. It is observed in up to 60% in patients treated with vemurafenib and in up to 39% in patients treated with dabrafenib [21]. Differently from hand-foot skin reaction seen during chemotherapy, patients on BRAF inhibitors therapy report lesions with less inflammation, dysesthesia, blistering, desquamation, erythema, and ulceration [22, 24]. Plantar hyperkeratosis can be managed with regular use of urea cream and avoiding friction [19].
2.5. Verrucal Keratosis
The term verrucal keratosis is used to describe keratotic lesions as warts, keratoacanthomas, or nonspecific hyperkeratotic papules induced by BRAF inhibitors. These keratotic proliferative lesions develop as single lesions or a diffuse eruption at various anatomical sites, on both sun-damaged and non-sun-damaged skin with a median time to presentation of 11 weeks. Although verrucal keratoses are benign lesions, they should be monitored for changes such as rapid growth, pain, and erythema which are indicative signs of evolution into cutaneous SCC. Cryotherapy can be useful for small lesions. The use of acitrein as a chemopreventive drug has been beneficial [19, 23].
2.6. Squamous Cell Carcinoma
One of the most relevant adverse events reported in patients receiving BRAF inhibitors is the development of cutaneous tumors in the form of well-differentiated and keratoacanthoma-type squamous cell carcinomas. Clinically, these lesions appear as hyperkeratotic crateriform papules in various anatomical sites. SCCs have been reported to occur early during BRAF inhibitors therapy with a median time to onset of approximately 8 weeks. The reported incidence is 4–31% in patients treated with vemurafenib and 6–11% in patients treated with dabrafenib [19]. The treatment is simple surgical resection, and no dose adjustment of the therapy is required [19].
2.7. Other Dermatological Side Effects
Several changes affecting the hair follicle have been observed in patients during BRAF inhibitors treatment including alopecia, slower and thinner scalp hair growth, and structural changes in shape (from straight to curly) and in color (turn gray) of hair. These hair abnormalities are temporary, and they could spontaneously regress without therapy modifications. Although alopecia is a reversible effect, it has a strong impact on patient quality of life and may also lead to voluntary treatment disruption. Minoxidil solution until 3–6 months after therapy termination can be considered as a treatment for alopecia [19, 22].
Panniculitis on the lower extremities has been described with both vemurafenib and dabrafenib. It occurs as a painful, erythematous to livid, subcutaneous nodules, often accompanied by fever, chills, and arthralgia. Panniculitis responds to regular nonsteroidal anti-inflammatory drugs, and oral steroids (prednisolone) can also be considered for treatment [19, 22].
Although melanocytic proliferations are less frequent than squamoproliferative lesions, changes in preexisting nevi, new melanocytic nevi, and new primary melanomas have been reported in patients receiving BRAF inhibitors. The melanocytic proliferation under BRAF inhibitors treatment seems to be due to paradoxical activation of wild-type BRAF cells. However, it cannot be excluded that the onset of a new primary melanoma is related to the increased risk of developing a second primary melanoma among patients with a personal history of melanoma, rather than therapy. Therefore, a dermoscopic careful monitoring should be included in the follow-up of these patients [22].
Rare cases of basal cell carcinoma have been also described in patients under BRAF inhibitors treatment [21].
3. Targeting Immune Checkpoints
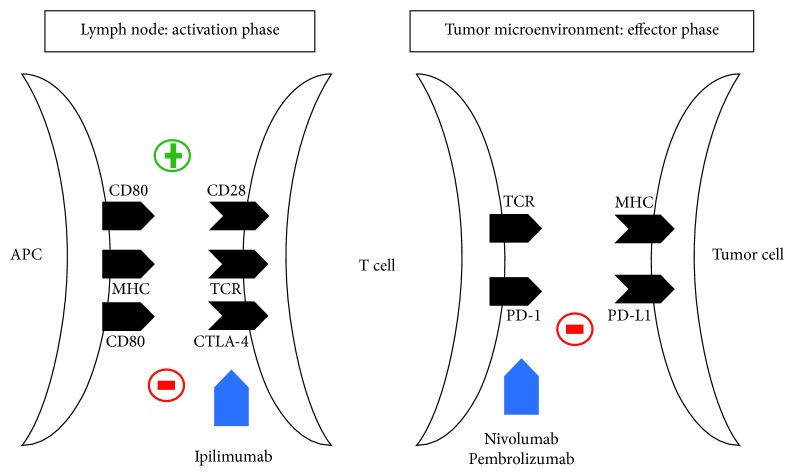
Immunotherapy through immune-checkpoint inhibitors is an established treatment for advanced melanoma. The main immune-checkpoint-targeted molecules are CTLA-4 and PD-1, which are expressed on activated T-cells and are involved in the inhibition of the immune system. CTLA-4 is involved in the interaction between T-cells and antigen presenting cells (activation phase in lymphatic organs), whereas PD-1 mediates the interaction between T-cells and tumor cells (effector phase in peripheral tissues) [25] (Figure 2).
Figure 2.
Immune-checkpoint inhibition of CTLA-4 and PD-1 pathways by antitumor immunotherapy.
Currently, there are three monoclonal antibodies approved for the treatment of patients affected by metastatic melanoma: ipilimumab, which is an anti-CTLA-4 antibody, approved by FDA and EMA in 2011, and pembrolizumab and nivolumab, which are both anti-PD-1 antibodies, approved by FDA in 2014 and EMA in 2015. In 2016, FDA and EMA also approved the combination therapy of ipilimumab and nivolumab for the treatment of unresectable or metastatic melanoma [26].
Ipilimumab blocks the interaction of CTLA-4 with its ligands, CD80/CD86, allowing T-cell activation through reestablishment of CD28 to CD80/CD86 between the T-cell and antigen presenting cell. The inhibition of CTLA-4 signalling, through the activation of cytotoxic T-lymphocytes against cancer cells and the general increase in T-cell responsiveness, enhances the patient's antitumor immune response [27].
Pembrolizumab and nivolumab inhibit the interaction between PD-1 and PD-L1 enhancing antitumor responses, delaying tumor growth and facilitating tumor rejection [28, 29].
3.1. Cutaneous Side Effects of CTLA-4 and PD-1 Inhibitors
The alteration of the immune system induced by CTLA-4 and PD-1 inhibitors results in the development of various autoimmune manifestations referred as immune-related adverse events (irAEs). Dermatologic toxicity is very common, and it is potentially mediated by a shared antigen coexpressed by the dermoepidermal junction and tumor cells. Rash and pruritus are the most reported cutaneous side effects both in patients under treatment with ipilimumab and in patients treated with PD-1 inhibitors [30–34].
Cutaneous adverse events occurring during anti-PD-1 therapy are usually less severe and develop later than those seen with anti-CTLA-4 therapy (4–10 months compared with 3–6 weeks) [35].
Other common dermatologic adverse effects are lichenoid eruption, eczema, and vitiligo which are all mediated by lymphocyte damage confirming the immune-related mechanism.
Cutaneous side effects are mostly low grade and usually can be managed with symptomatic treatment that includes medium-to-high potency topical corticosteroids and oral antihistamines, while therapy interruption is rarely necessary [36].
Interestingly, skin immune-related events may be useful as visible clinical parameters of antimelanoma immunity and clinical response to checkpoint inhibitors. The development of vitiligo seems to be an indicator of antimelanoma immunity and improved survival [37].
3.2. Rash
Rash has been frequently reported in patients under treatment with immune-checkpoint inhibitors with an incidence of 15–40% [25]. The most common cutaneous toxicity of ipilimumab is a morbilliform rash which is characterized by erythematous macules and papules that typically involves the trunk and extremities sparing head, palms, and soles and is often associated with generalized pruritus [35]. It has a wide variable time to onset ranging from 3 weeks to 2 years after therapy initiation [38].
Other common subtypes of rash include lichenoid eruption and prurigo nodularis.
Lichenoid eruption has also been described in association with PD-1 inhibitor therapy. It predominantly appears on the chest and back as multiple erythematous and sometimes violaceous papules and plaques. Sometimes, lichenoid oral lesions may appear [35].
3.3. Eczema
Eczematous eruption is an adverse effect typically seen with anti-PD-1 therapy (up to 17% of patients) [39]. It occurs most commonly on the back and lower or upper limbs, less frequently on the face, chest, and abdomen. The lesions varied from classic eczema (ill-defined erythematous scaly lesions) to multiple nummular papules and are associated to pruritus in most cases [39].
3.4. Vitiligo
It is well known that the development of vitiligo in patients affected by advanced melanoma (stages III and IV) is associated with tumor regression and prolonged survival [40, 41]. Furthermore, it has been described that patients affected by vitiligo have a decreased risk of developing melanoma during life [42]. Vitiligo-like hypopigmentation has been frequently reported in patients treated with immune-checkpoint inhibitors with an incidence of 2–11% [25]. Vitiligo-like hypopigmentation lesions may appear on various areas of the body with a typical clinical presentation [39]. A recent meta-analysis suggests that vitiligo is associated with a better prognosis and it may be a marker of clinical response to immunotherapy [37].
3.5. Psoriasis
Some cases of psoriasis exacerbation have been reported in patients under treatment with nivolumab [43, 44]. T-cells, especially Th1 and Th17, play pivotal roles in pathogenesis of psoriasis. Anti-PD-1 agents upregulate Th1 and Th17 cells which induce the release of IL-17 and IL-22 leading to inflammation and keratinocyte proliferation. This mechanism supports the potentially exacerbating role of PD-1 inhibitors in psoriasis.
3.6. Autoimmune Blistering Diseases
Several cases of bullous pemphigoid and bullous pemphigoid-like skin lesions have been described in patients under treatment with anti-PD-1 agents [45–48], whereas rare cases of bullous skin lesions have been reported after anti-CTLA-4 antibodies [49, 50]. Systemic corticosteroids are the first-line treatment.
Bullous pemphigoid has also been reported as a paraneoplastic manifestation of melanoma. The exact mechanism of the paraneoplastic phenomenon remains poorly understood, but it has been described that BP180 is expressed in melanoma cells and not in normal melanocytes [51]. While most cases of bullous pemphigoid have been reported in patients treated with anti-PD-1 agents, it is possible that the use of immune-checkpoint inhibitors induces a loss of self-tolerance responsible of the appearance of bullous skin lesions.
3.7. Other Dermatological Side Effects
Less common cutaneous adverse events afflicting patients during immunotherapy include xerosis, photosensitivity reactions, pyoderma gangrenosum-like ulcerations, Sweet syndrome, cutaneous sarcoidosis, alopecia, actinic keratosis, squamous cell carcinomas, seborrheic keratosis, toxic epidermal necrolysis, and severe drug rash with systemic symptoms and eosinophilia [26, 35].
4. Conclusions
Targeted therapies have a crucial role in patients with advanced melanoma. They have significant benefits in the prognosis, but they are frequently associated with cutaneous side effects that may affect quality of life of patients. Cutaneous adverse events are usually low grade and manageable. Understanding and managing skin toxicity could improve the quality of life and prevent the interruption of the tumor therapy leading to a better clinical outcome. Although current targeted therapies have been shown to reduce melanoma mortality, advanced melanoma is still a significant therapeutic challenge to clinicians. New therapeutic approaches are currently being developed, and they will probably include combination therapies as the association of BRAF inhibitors and immune-checkpoint inhibitors. New potential side effects will likely emerge introducing these novel combination therapies.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Garbe C., Peris K., Hauschild A., et al. Diagnosis and treatment of melanoma: european consensus-based interdisciplinary guideline-Update 2012. European Journal of Cancer. 2012;48(15):2375–2390. doi: 10.1016/j.ejca.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Ascierto P. A., Kirkwood J. M., Grob J. J., et al. The role of BRAF V600 mutation in melanoma. Journal of Translational Medicine. 2012;10(1):p. 85. doi: 10.1186/1479-5876-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhomen N., Marais R. BRAF signaling and targeted therapies in melanoma. Hematology/Oncology Clinics of North America. 2009;23(3):529–545. doi: 10.1016/j.hoc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Davies H., Bignell G. R., Cox C., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.Ji Z., Flaherty K. T., Tsao H. Targeting the RAS pathway in melanoma. Trends in Molecular Medicine. 2012;18(1):27–35. doi: 10.1016/j.molmed.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman P. B., Hauschild A., Robert C., et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New England Journal of Medicine. 2011;364(26):2507–2516. doi: 10.1056/nejmoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauschild A., Grob J.-J., Demidov L. V., et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. The Lancet. 2012;380(9839):358–365. doi: 10.1016/s0140-6736(12)60868-x. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty K. T., Infante J. R., Daud A., et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. New England Journal of Medicine. 2012;367(18):1694–1703. doi: 10.1056/nejmoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dummer R., Ascierto P. A., Gogas H. J., et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. The Lancet Oncology. 2018;19(5):603–615. doi: 10.1016/s1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 10.Dummer R., Hauschild A., Guggenheim M., Keilholz U., Pentheroudakis G. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2012;23(S7):vii86–vii91. doi: 10.1093/annonc/mds229. [DOI] [PubMed] [Google Scholar]
- 11.Koelblinger P., Thuerigen O., Dummer R. Development of encorafenib for BRAF-mutated advanced melanoma. Current Opinion in Oncology. 2018;30(2):125–133. doi: 10.1097/CCO.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long G. V., Stroyakovskiy D., Gogas H., et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. New England Journal of Medicine. 2014;371(20):1877–1888. doi: 10.1056/nejmoa1406037. [DOI] [PubMed] [Google Scholar]
- 13.Grob J. J., Amonkar M. M., Karaszewska B., et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. The Lancet Oncology. 2015;16(13):1389–1398. doi: 10.1016/s1470-2045(15)00087-x. [DOI] [PubMed] [Google Scholar]
- 14.Sanlorenzo M., Choudhry A., Vujic I., et al. Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma. Journal of the American Academy of Dermatology. 2014;71(6):1102.e1–1109.e1. doi: 10.1016/j.jaad.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattei P. L., Alora-Palli M. B., Kraft S., Lawrence D. P., Flaherty K. T., Kimball A. B. Cutaneous effects of BRAF inhibitor therapy: a case series. Annals of Oncology. 2012;24(2):530–537. doi: 10.1093/annonc/mds292. [DOI] [PubMed] [Google Scholar]
- 16.Carlos G., Anforth R., Clements A., et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatology. 2015;151(10):1103–1109. doi: 10.1001/jamadermatol.2015.1745. [DOI] [PubMed] [Google Scholar]
- 17.Russo I., Zorzetto L., Frigo A. C., Sileni V. C., Alaibac M. A comparative study of the cutaneous side effects between BRAF monotherapy and BRAF/MEK inhibitor combination therapy in patients with advanced melanoma: a single-centre experience. European Journal of Dermatology. 2017;27(5) doi: 10.1684/ejd.2017.3069. [DOI] [PubMed] [Google Scholar]
- 18.Manousaridis I., Mavridou S., Goerdt S., Leverkus M., Utikal J. Cutaneous side effects of inhibitors of the RAS/RAF/MEK/ERK signalling pathway and their management. Journal of the European Academy of Dermatology and Venereology. 2018;27(1):11–18. doi: 10.1111/j.1468-3083.2012.04546.x. [DOI] [PubMed] [Google Scholar]
- 19.Anforth R., Fernandez-Peñas P., Long G. V. Cutaneous toxicities of RAF inhibitors. The Lancet Oncology. 2013;14(1):e11–e18. doi: 10.1016/s1470-2045(12)70413-8. [DOI] [PubMed] [Google Scholar]
- 20.Lugowska I., Koseła-Paterczyk H., Kozak K., Rutkowski P. Trametinib: a MEK inhibitor for management of metastatic melanoma. OncoTargets and Therapy. 2015;8:2251–2259. doi: 10.2147/OTT.S72951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gençler B., Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatology Research and Practice. 2016;2016:6. doi: 10.1155/2016/5361569.5361569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandalà M., Massi D., De Giorgi V. Cutaneous toxicities of BRAF inhibitors: clinical and pathological challenges and call to action. Critical Reviews in Oncology/Hematology. 2013;88(2):318–337. doi: 10.1016/j.critrevonc.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Anforth R. M., Blumetti T. C. M. P., Kefford R. F., et al. Cutaneous manifestations of dabrafenib (GSK2118436): a selective inhibitor of mutant BRAF in patients with metastatic melanoma. British Journal of Dermatology. 2012;167(5):1153–1160. doi: 10.1111/j.1365-2133.2012.11155.x. [DOI] [PubMed] [Google Scholar]
- 24.Brumana M. B., Sileni V. C., Pigozzo J., Zarian H., Alaibac M. Skin complications in a patient under treatment with vemurafenib: case-based review. Clinical Dermatology. 2013;1(3) [Google Scholar]
- 25.Kähler K. C., Hassel J. C., Heinzerling L., et al. Nebenwirkungsmanagement bei Immun-Checkpoint-Blockade durch CTLA-4- und PD1-Antikörper beim metastasierten Melanom. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2016;14(7):662–683. doi: 10.1111/ddg.13047_g. [DOI] [PubMed] [Google Scholar]
- 26.Sosa A., Lopez Cadena E., Simon Olive C., Karachaliou N., Rosell R. Clinical assessment of immune-related adverse events. Therapeutic Advances in Medical Oncology. 2018;10 doi: 10.1177/1758835918764628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olszanski A. J. Current and future roles of targeted therapy and immunotherapy in advanced melanoma. Journal of Managed Care Pharmacy. 2014;20(4):346–356. doi: 10.18553/jmcp.2014.20.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topalian S. L., Hodi F. S., Brahmer J. R., et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New England Journal of Medicine. 2012;366(26):2443–2454. doi: 10.1056/nejmoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamalis A., Garcha M., Jagdeo J. Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Archives of Dermatological Research. 2014;306(6):511–519. doi: 10.1007/s00403-014-1457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robert C., Thomas L., Bondarenko I., et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New England Journal of Medicine. 2011;364(26):2517–2526. doi: 10.1056/nejmoa1104621. [DOI] [PubMed] [Google Scholar]
- 31.Lomax A. J., Lim J., Cheng R, et al. Immune toxicity with checkpoint inhibition for metastatic melanoma: case series and clinical management. Journal of Skin Cancer. 2018;2018:13. doi: 10.1155/2018/9602540.9602540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber J. S., Yang J. C., Atkins M. B., Disis M. L. Toxicities of immunotherapy for the practitioner. Journal of Clinical Oncology. 2015;33(18):2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert C., Schachter J., Long G. V., et al. Pembrolizumab versus ipilimumab in advanced melanoma. New England Journal of Medicine. 2015;372(26):2521–2532. doi: 10.1056/nejmoa1503093. [DOI] [PubMed] [Google Scholar]
- 34.Topalian S. L., Sznol M., McDermott D. F., et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of Clinical Oncology. 2014;32(10):1020–1030. doi: 10.1200/jco.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins L. K., Chapman M. S., Carter J. B., Samie F. H. Cutaneous adverse effects of the immune checkpoint inhibitors. Current Problems in Cancer. 2017;41(2):125–128. doi: 10.1016/j.currproblcancer.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Sibaud V. Dermatologic reactions to immune checkpoint inhibitors. American Journal of Clinical Dermatology. 2017;19(3):345–361. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- 37.Teulings H., Limpens J., Jansen S. N., et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. Journal of Clinical Oncology. 2018;33(7):773–781. doi: 10.1200/jco.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 38.Ransohoff J. D., Kwong B. Y. Cutaneous adverse events of targeted therapies for hematolymphoid malignancies. Clinical Lymphoma, Myeloma and Leukemia. 2017;17(12):834–851. doi: 10.1016/j.clml.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Hwang S. J. E., Carlos G., Wakade D., et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD-1) therapy in patients with metastatic melanoma: a single-institution cohort. Journal of the American Academy of Dermatology. 2015;74(3):455.e1–461.e1. doi: 10.1016/j.jaad.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Bystryn J. C., Rigel D., Friedman R. J., Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Archives of Dermatology. 1987;123(8):1053–1055. doi: 10.1001/archderm.1987.01660320095019. [DOI] [PubMed] [Google Scholar]
- 41.Quaglino P., Marenco F., Osella-Abate S., et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Annals of Oncology. 2009;21(2):409–414. doi: 10.1093/annonc/mdp325. [DOI] [PubMed] [Google Scholar]
- 42.Teulings H. E., Overkamp M., Ceylan E., et al. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: a survey among 1307 patients and their partners. British Journal of Dermatology. 2012;168(1):162–171. doi: 10.1111/bjd.12111. [DOI] [PubMed] [Google Scholar]
- 43.Kato Y., Otsuka A., Miyachi Y., Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. Journal of the European Academy of Dermatology and Venereology. 2015;30(10):e89–e91. doi: 10.1111/jdv.13336. [DOI] [PubMed] [Google Scholar]
- 44.Matsumura N., Ohtsuka M., Kikuchi N., Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Dermato Venereologica. 2016;96(2):259–260. doi: 10.2340/00015555-2212. [DOI] [PubMed] [Google Scholar]
- 45.Jour G., Glitza I. C., Ellis R. M., et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. Journal of Cutaneous Pathology. 2016;43(8):688–696. doi: 10.1111/cup.12717. [DOI] [PubMed] [Google Scholar]
- 46.Hwang S. J. E., Carlos G., Chou S., Wakade D., Carlino M. S., Fernandez-Penas P. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Research. 2016;26(4):413–416. doi: 10.1097/cmr.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 47.Carlos G., Anforth R., Chou S., Clements A., Fernandez-Peñas P. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma Research. 2015;25(3):265–268. doi: 10.1097/cmr.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 48.Naidoo J., Schindler K., Querfeld C., et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunology Research. 2016;4(5):383–389. doi: 10.1158/2326-6066.cir-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavalcante L., Lutzky J., Amin A. Ipilimumab was safe and effective in two patients with metastatic melanoma and end-stage renal disease. Cancer Management and Research. 2015;7:47–50. doi: 10.2147/cmar.s73389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuwatsuka Y., Iwanaga A., Kuwatsuka S., et al. Bullous pemphigoid induced by ipilimumab in a patient with metastatic malignant melanoma after unsuccessful treatment with nivolumab. Journal of Dermatology. 2017;45(1):e21–e22. doi: 10.1111/1346-8138.14043. [DOI] [PubMed] [Google Scholar]
- 51.Krenacs T., Kiszner G., Stelkovics E., et al. Collagen XVII is expressed in malignant but not in benign melanocytic tumors and it can mediate antibody induced melanoma apoptosis. Histochemistry and Cell Biology. 2012;138(4):653–667. doi: 10.1007/s00418-012-0981-9. [DOI] [PubMed] [Google Scholar]