Abstract
Contamination by chemicals from the environment is a major global food safety issue, posing a serious threat to human health. These chemicals belong to many groups, including metals/metalloids, polycyclic aromatic hydrocarbons (PAHs), persistent organic pollutants (POPs), perfluorinated compounds (PFCs), pharmaceutical and personal care products (PPCPs), radioactive elements, electronic waste, plastics, and nanoparticles. Some of these occur naturally in the environment, whilst others are produced from anthropogenic sources. They may contaminate our food—crops, livestock, and seafood—and drinking water and exert adverse effects on our health. It is important to perform assessments of the associated potential risks. Monitoring contamination levels, enactment of control measures including remediation, and consideration of sociopolitical implications are vital to provide safer food globally.
1. Introduction
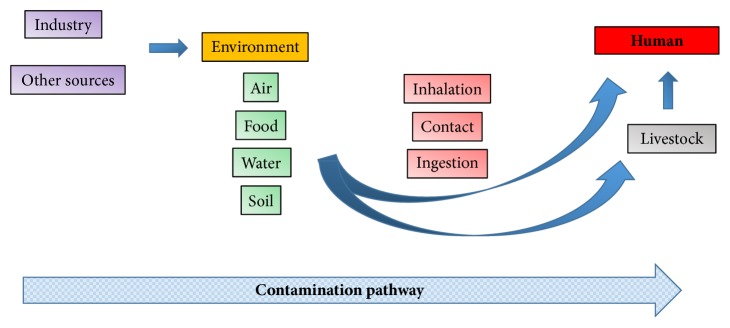
Chemical contamination is a global food safety issue. There are many potentially toxic substances in the environment which may contaminate foods consumed by people. They include inorganic and organic substances and may originate from a wide range of sources (Figure 1 shows the pathway of contaminants through the environment). This review is restricted to chemical contamination of foods and does not address biological or physical hazards.
Figure 1.
Sources of environmental contaminants in human foods.
In certain instances, the source of contaminants may be the environment. This is the case for metals such as lead and mercury, dioxins, and polychlorinated biphenyls (PCBs). Agricultural use of pesticides may lead to food contamination. Similarly, drugs used in both people and animals may contaminate waterways and pose a health risk to consumers. Additionally, food packaging methods may be a source of contamination, so-called “migrants” leaching from packing materials. These contaminants may cause acute or chronic toxic effects. Toxicity may relate to the route of exposure and dose, and personal characteristics such as age and health condition may affect the individual's susceptibility.
Due to the nature of contamination, some food products may be more contaminated than others. This may be due to several factors such as varying exposure to pesticides, differences in plant uptake mechanisms from the environment, or contaminants from food packaging [1, 2]. Dietary make-up will affect an individual's exposure to these contaminants. For example, nursing neonates have a high intake of contaminants that are excreted in breast milk [3]. Exposure at different life stages may result in different toxic effects as well. For example, prenatal exposure to persistent organic pollutants has been linked to an increase in childhood obesity and increased blood pressure [4].
For many food items—including vegetables, fish, and other seafood—human health risk assessment data is available after analysis of available foods [5–7]. Urban farms and gardens may pose additional risks due to contaminants such as metals [8, 9]. Furthermore, drinking water may become contaminated [10, 11]. Xenoestrogenic compounds have even been detected in rainwater [12]. Water contamination may also result in pollution of marine biota, affecting suitability for consumption of seafood [13]. Consequently individuals with high consumption of seafood will intake higher levels of such contaminants. Occupational exposure will not be discussed in detail in this review, but workers may have increased risk of exposure to certain contaminants, for example, car repair workers with lead on their hands which they ingest after hand-to-mouth contact [14].
For most contaminants, there is no completely safe dose level. However, for many, acceptable levels have been calculated—levels below which signs of toxicity should not be evident. Toxic effects seen depend on the contaminant in question, the dose received, and the individual. For example, many contaminants have been linked to an increased risk of cancer. Skin cancer has been associated with long-term exposure to drinking water contaminated by arsenic, gastric cancer with lead contamination, and liver cancer with consumption of grain contaminated by mercury [15–17]. Our understanding of the health risks from combined exposures to more than one contaminant and the means by which we can assess such interactions is lacking [18].
Monitoring programmes are in place both nationally and globally to monitor such contamination in order to assess food safety. However, it is important to note that such monitoring cannot completely preclude supply of contaminated food to consumers. The role of such programmes is to check that food and water contamination levels are below those deemed “unsafe.” To this end, many governmental and nongovernmental organizations strive through risk assessments to ascertain what levels of contamination are acceptable for products destined for human consumption. In addition, national and international policies are in place to reduce contamination. For example, under the Stockholm Convention on Persistent Organic Pollutants, production and use of such substances are eliminated or restricted. This international treaty came into force in 2004 and currently has 152 signatories from 182 parties [19]. The Codex Alimentarius describes international food standards, setting permitted maximum levels (ML) for contaminants in foods based on risk assessment and scientific evidence [20]. The Codex Committee on Contaminants in Food (CCCF) is a global forum, but it can be difficult to compromise national legislations and harmonize global standards. Lists of contaminants also undergo risk assessment by the Joint FAO/WHO Expert Committee on Food Additives [21, 22]. Recommendations are made for standards such as provisional maximum tolerable daily intake (PMTDI) or provisional tolerable weekly intake (PTWI). These are usually calculated based on chronic toxicity data, and thus it may also be useful to consider acute reference doses (ARfDs). The Codex Committee on Pesticide Residues (CCPR) has established maximum residue limits (MRLs) for over 5,000 pesticide residues [23]. This committee also considers reports from the FAO/WHO Meeting on Pesticide Residues (JMPR), which estimates MRLs and acceptable daily intakes (ADIs) for people [24].
2. Sources of Contaminants from the Environment to Food and Water
It is useful to consider the sources of contaminants in order to understand their pathway into food and water sources for consumption. Factors such as soil properties, activities by people, and point sources affect the accumulation of metals in the environment. For example, mining may result in release of substances such as arsenic and mercury [25, 26]. Once in the environment, these substances may contaminate food and water and result in human health hazards, with toxic effects varying depending on the contaminant(s) ingested (Table 1).
Table 1.
Possible human health hazards due to exposure to food contaminants.
| Food contaminants | Possible hazards | References |
|---|---|---|
| Metals/metalloids | ||
|
| ||
| Lead | Complications in the nervous system and red blood cells | [27] |
| Reduction in cognitive development and intellectual performance | [28] | |
| Death among children | [29] | |
|
| ||
| Cadmium | Renal tubular dysfunction, associated with high risk of lung and breast cancer | [30] |
| Osteomalacia and osteoporosis | ||
|
| ||
| Arsenic | Associated with dermal, respiratory, nervous, mutagenic, and carcinogenic effects | [31] |
|
| ||
| Nickel | Associated with dermatotoxicity, lower body weight, and fetotoxicity among pregnant women | [32] |
|
| ||
| Mercury | Linked to cardiovascular, reproductive, and developmental toxicity, neurotoxicity, nephrotoxicity, immunotoxicity, and carcinogenicity | [33] |
|
| ||
| Mycotoxins | ||
|
| ||
| Aflatoxin | Immunodeficiency | [34] |
| Aflatoxicosis | [35] | |
| Primary hepatocellular carcinoma | [36] | |
| Liver cirrhosis | [37] | |
|
| ||
| Ochratoxin | Nephropathy | [38] |
|
| ||
| Deoxynivalenol | Impaired intestinal integrity | [39] |
| Impaired gut-associated immune system | ||
|
| ||
| Zearalenone | Hyperestrogenism and reproductive dysfunction | [40] |
|
| ||
| Fumonisins | Esophageal cancer and birth defects | [41] |
|
| ||
| Antimicrobials | ||
|
| ||
| Tetracyclines | Impaired intestinal flora | [42] |
|
| ||
| Quinolones | Drug-resistant pathogens | [43] |
|
| ||
| Macrolides | Hypersensitivity and anaphylactic shock | [44] |
|
| ||
| Sulfonamides | Kidney damage and nephropathy | [45] |
|
| ||
| Polycyclic aromatic hydrocarbons (PAHs) | ||
|
| ||
| Benzo[a]pyrene | Mutagenicity and carcinogenicity | [46] |
| DNA damage and oxidative stress | [47] | |
| Impaired male fertility | [48] | |
| Respiratory diseases | [49] | |
| Cognitive dysfunction among children | [50] | |
|
| ||
| Pesticides | ||
|
| ||
| Chlorpyrifos | Neurological symptoms | [51] |
|
| ||
| DDTs | Neurological symptoms | [52] |
| Endocrine disruption | [53] | |
|
| ||
| DDT and other OCPs | Infertility and fetal malformation | [54] |
|
| ||
| Dioxins and polychlorinated biphenyls | ||
|
| ||
| Dioxins and PCBs | Language delay | [55] |
| Disturbances in mental and motor development | [56] | |
|
| ||
| PCBs | Neurological disorders | [57] |
2.1. Metals and Metalloids
Metals and metalloids in the environment have various sources. One source of mercury and lead is artisanal gold mining. For example, in the gold mining area of Tongguan, Shaanxi, China, concentrations of these metals in locally produced grains and vegetables exceeded governmental tolerance limits and posed a potential health risk to people from consumption [58]. Lead and cadmium from an iron mine in Morocco resulted in concentrations of cadmium in livestock organs higher than acceptable limits [59]. Likewise, in Spain, sheep near a mine were found to have lead contamination, with levels in 87.5% liver samples above European Union Maximum Residue Levels (MRL) [60].
Industrial regions often have extensive environmental contamination by metals. In Romania, lead, cadmium, copper, and zinc contaminated crops, exceeding maximum acceptable levels in some samples [61]. In China, cadmium from a zinc smelter contaminated leaf and root vegetables particularly [62]. Arsenic, selenium, lead, and other metal and metalloid contaminants were found near a coking plant in China, contaminating soil and food, and detected in blood samples from children [63]. In that case, ingestion of food was determined to be the major exposure pathway for local children. In Belgium, cadmium was detected in locally produced food items grown near nonferrous metal plants [64]. Thallium from a steel plant in south China was found to contaminate soil and thence vegetables, exceeding German standards for the maximum permissible level and showing hyperaccumulation in plants such as leaf lettuce, chard, and pak choi [65].
Many fruits and vegetables have been shown to be contaminated by metals. For example, cadmium in soil was detected in navel oranges in China and lead and cadmium in soybeans in Argentina [66, 67]. Also in China, various metals were detected in edible seeds, with levels of copper sufficiently high to show an increased health risk to people consuming them [68]. On the contrary, contamination levels of mercury in rice samples from a city in eastern China were below levels likely to affect human health [69]. In the global arena, methylmercury has been detected in fish and other seafood around the world [70, 71]. Fish tissues from Turkey were shown to be contaminated with copper, iron, zinc, and manganese [72]. Various metals have also been detected in fish from Sicily, with some concentrations exceeding European regulation limits [73]. In Asia, food species of turtles have been shown to contain mercury [74].
With regard to water, endemic arsenism from contaminated drinking water has been reported in China [11]. Monitoring has detected nickel in drinking water in New South Wales, Australia, but levels do not appear to pose any health risk for the local population [75].
Further evidence of potential health risks to people from metals are surveys of human samples. Mercury and monomethylmercury were detected in human hair samples from French Guiana, associated with a diet rich in fish, with 57% of people tested having mercury levels higher than the WHO safety limit [70]. In Spain, mercury, lead, and cadmium have also been detected in human milk samples, with increased levels of lead associated with higher consumption of potatoes [76].
2.2. Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) primarily occur after organic matter undergoes incomplete combustion or pyrolysis, or from industrial processes [77]. Food contamination comes from the environment, industry, or home cooking (such as when using biomass fuels). These compounds appear to be genotoxic and carcinogenic. Oil spills from transporter ships in the ocean are all too common and will result in contamination of seafood. Besides the petroleum-related polycyclic aromatic hydrocarbon (PAH) compounds, chemical dispersants are often used to mitigate effects of oil in the ocean. After the BP Deepwater Horizon oil spill in Louisiana, USA, in 2010, the Federal government responded to seafood safety concerns by instigating protocols for sampling and analysis of food to determine its safety [78]. Lessons learned after this scenario included recognition of the need to improve risk assessments to adequately protect vulnerable populations, including pregnant women [79].
2.3. Industrial Chemicals
Persistent organic pollutants (POPs) are synthetic organic chemicals; some are used in industry, some as pesticides, and some are by-products from industry or combustion. They include pesticides like aldrin, chlordane and DDT, industrial chemicals like PCBs and HCBs, and unintended by-products like dibenzodioxins and dibenzofurans. They persist in the environment, are distributed globally in air and ocean currents, and accumulate in animals in the food chain (including in humans). Their side effects depend on the chemical and the contaminated species; for example, they may have effects on reproductive or immune systems, or increase cancer risks [80].
Chlorpyrifos is an organophosphate pesticide that affects vision and causes other neurological toxic effects in humans [81]. It has been detected in dietary samples, and foods have been shown to be responsible for approximately 13% of daily exposure to this chemical [51]. Organochlorine pesticides such as DDT have been used in agriculture and vector-transmitted disease control for decades, though their use now is restricted due to known persistence in the environment and toxic effects such as neurological dysfunction and endocrine disruption [82]. Pyrethroids such as permethrin and deltamethrin are widely used for control of vector insects and aircraft disinfection, as they are relatively safe for people [83]. However, their use near foods can result in contamination and studies are ongoing to reduce potential toxic effects [84, 85]. Although neonicotinoids are widespread in the environment and contaminate consumable items, their toxic effects are still not yet well understood [86, 87].
Polychlorinated biphenyls (PCBs) have a variety of uses in industry, including in transformers, as heat exchange fluids or paint additives, or in plastics. Ingestion of PCB residue-contaminated food—especially meat, fish, and poultry—is the main source for people, with ready absorption from the gastrointestinal tract [88, 89]. Contaminated breast milk is a potential source for nursing infants. Chloracne is reported after extensive exposure to PCBs, but immune and carcinogenic effects may also result.
Polybrominated and polychlorinated compounds may originate from anthropogenic and natural sources. They have many uses such as flame retardants and dielectric/coolant fluids in electrical apparatus. Toxic effects include endocrine disruption, neurotoxicity, and cancer. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) have been detected in human milk in China [90]. This is a particular concern due to the high susceptibility of nursing infants to toxic effects. According to a study in Germany, dietary exposure is the most significant pathway for PBDEs in people [91]. In particular, seafood has been cited as a major contributor [92].
Perfluorinated compounds (PFCs) are synthetic chemicals with friction-resistant properties that make them useful in many materials and industries. Toxic effects include endocrine and immune system disruption and developmental problems. Some precursors or metabolic intermediates for perfluoroalkyl and polyfluoroalkyl substances (PFASs) are toxic, for example, estrogen-like activities [93]. The PFAS group includes perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA); these have been detected in many food sources including seafood in China and Germany [94, 95]. Drinking water and food are the main sources of exposure to PFOS and PFOA, although levels are usually low [96].
Acrylamide occurs in many foods—generally associated with high heat cooking processes (e.g., in breads and baked or fried potatoes)—and is also manufactured for commercial and industrial uses (such as in paper and dye production, in wastewater treatment, and as a chemical grouting agent) [97]. The IARC has classified acrylamide as a probable human carcinogen, placed in group 2A since 1994 [98].
2.4. Pharmaceuticals and Personal Care Products
The term pharmaceuticals and personal care products (PPCPs) includes a wide range of substances that may enter the environment and thence food or water sources. Antimicrobials and other drugs may originate from use in both humans and animals. For example, swine waste containing antimicrobials may contaminate both water and food [99]. Aside from the very real threat of increased antimicrobial resistance through exposure to extraneous sources of these chemicals, it has also been shown that many drugs have other side effects including endocrine disruption [100]. In some circumstances, the medicinal products themselves may be contaminated, for example, in many herbal products [101].
2.5. Radioactive Elements
Most radioactive elements did not exist naturally, and soil contamination with such material has only become a problem since nuclear weapons and reactors have been developed [102].
After tsunami damage affected the Fukushima nuclear plant in Japan in 2011, monitoring of food and water samples detected contamination above provisional regulation values and restrictions were put in place [103]. Radionucleotides have also been detected in seafood in India, various foods in the Balkans, and food and drinking water in Switzerland [104–106]. Risk assessments are conducted to ensure that levels remain within acceptable limits. Furthermore, experimental models are undertaken to assess safety in ingestion pathways, considering several different food intakes [107]. In the US, there is an FDA rule pertaining to uranium, radium, alpha particle, beta particle, and photon radioactivity in bottled water [108].
2.6. Electronic Waste
Modern society has become encumbered with many electrical devices, and electronic waste (or e-waste) has become a major problem. Inappropriate processing, for example, incomplete combustion, of such products releases a variety of pollutants covered above, including PBDEs, dioxins/furans (PCDD/Fs), PAHs, PCBs, and metals/metalloids [109]. In addition, contamination from such devices can enter drinking water and food [110].
2.7. Plastics
In recent times, we rely more and more on packaging materials—in particular plastics—to transport and help preserve food. These materials are not inert and may themselves contaminate food and drinks as multiple chemicals are released into foods and beverages from food contact materials. These are termed “migrants” and include such chemicals as phthalate plasticizers which have been detected in bottled water [111]. Factors such as higher storage temperatures and prolonged contact time with the packaging were linked to higher levels of contamination, but a health risk assessment showed that the risk for consumers was low [111].
2.8. Nanoparticles
Another recent development is that of nanoparticles. These have one dimension less than 1 x 10−7 m, and engineered nanoparticles have been used in a wide range of products, such as paints, cosmetics, and pesticides [102]. Pathways and effects of these in biota are as yet unclear, but they have been shown to travel in the food chain [112]. Nanosized materials have been detected in foods such as wheat-based products [113].
3. Risk Assessment and Monitoring
As shown in Table 1, each of the possible contaminants in food can be linked to a variety of toxic effects. Any adverse effects seen depend on multiple factors, including whether exposure is acute or chronic, the dose received, the route of exposure, and details of the individual person such as age and health. As an example, lead toxicity affects almost all organs, but the most severely affected is the nervous system [114]. In adults, long-term exposure results in reduced cognitive performance. More severe signs such as learning difficulties and behavioural problems are seen in infants and young children as they are more sensitive during this phase of neurodevelopment [115]. High levels of contamination with lead may also cause kidney damage; chronic exposure may cause anaemia and hypertension, and reduced fertility in males [116]. In pregnant women, high blood lead levels are associated with premature birth or babies with a low birth weight, and this risk is increased in emaciated women [117]. On an individual level, blood sampling is a quick and easy method of assessing circulating levels of lead and can be used to indicate recent or current exposure. However, this does not account for lead stored elsewhere in the body, particularly in bones. X-ray fluorescence can measure whole-body lead in bones, and x-rays may show lead-containing foreign materials [118–120]. Treatment of clinical cases is by using chelating agents, which will reduce blood lead levels, yet neurological effects may remain [121].
On the other hand, at a community level, it may be more important to identify contaminated sites and assess health risks to the general population and thereafter aim to reduce or remove exposure to contaminants such as lead. Thus, monitoring plays a vital role in food safety. Such monitoring has identified contamination of many foods (examples are shown in Table 2). In order to monitor effectively, samples should be analysed from a variety of sources: human samples to detect levels after exposure, diverse foods from the total diet and drinking water sources, and also the environment itself (to identify the source of food contamination). Samples from people frequently include blood, urine, feces, breast milk, hair, and/or semen [122]. Human biomonitoring is notably useful to facilitate risk assessment. A combination of environmental monitoring and biomonitoring may identify risk factors, such as detection of higher levels of cadmium in umbilical cord blood from mothers consuming more than two portions of fish each week [123]. In the case of metals, environmental sampling has shown hotspots of contamination around mining (such as gold, lead, and zinc), electronic waste sites, and industrial areas [110, 124, 125]. Contamination in soils at these sites has been linked to bioaccumulation in agricultural crops and associated increase in human health risk.
Table 2.
Examples of food contamination with different chemicals around the world.
| Food contaminants | Foods | Country | References |
|---|---|---|---|
| Metals/metalloids | |||
|
| |||
| Pb, Hg | Grains and vegetables | China | [58, 62, 67] |
|
| |||
| Pb, Cd | Livestock organs | Morocco | [59] |
|
| |||
| Pb | Sheep livers | Spain | [60] |
|
| |||
| Pb, Cd, Cu, Zn | Agricultural crops | Romania | [61] |
|
| |||
| Cd | Locally produced foods | Belgium | [64] |
|
| |||
| Tl | Lettuce and chard | Germany | [65] |
|
| |||
| Pb, Cd | Soybeans | Argentina | [66] |
|
| |||
| Cu, Zn, Mn, Fe | Fish | Turkey | [72] |
|
| |||
| Mycotoxins | |||
|
| |||
| Deoxynivalenol, zearalenone, T2 toxin and HT-2 toxin | Wheat, barley, Japanese retail foods | Japan | [126] |
|
| |||
| Aflatoxin, ochratoxin | Wheat flour | China | [127] |
|
| |||
| Fumonisins | Maize | South Africa | [128] |
|
| |||
| Nivalenol | Cereals and cereal products | Tunisia | [129] |
|
| |||
| Aflatoxins | Ground nut oil | Sudan | [130] |
|
| |||
| Antimicrobials | |||
|
| |||
| Antimicrobials | Pork meat | Madagascar | [131] |
| Table eggs | Sudan | [132] | |
| Milk | Peru | [133] | |
| Beef | Nigeria | [134] | |
| Meat | Brazil | [135] | |
|
| |||
| Polycyclic aromatic hydrocarbons (PAHs) | |||
|
| |||
| Benzo[a]pyrene | Barbecued foods | Sweden | [136] |
| Chrysene | |||
|
| |||
| Anthracene | Yogurt | Italy | [137] |
| Fluoranthene | |||
|
| |||
| 19 PAHs | Grains, flour, and bran | Poland | [138] |
|
| |||
| Total PAHs | Oyster | Japan | [139] |
|
| |||
| Pesticides and polychlorinated biphenyls (PCBs) | |||
|
| |||
| Chlorpyrifos | Catfish | Australia | [140] |
| Vegetables | China | [141] | |
| Food plant | Algeria | [142] | |
|
| |||
| DDTs and other OCPs | Edible offal | Egypt | [143, 144] |
| Milk | |||
| Chicken products | South Africa | [145] | |
| Milk | Ethiopia | [146] | |
| Fish | Mozambique | [6] | |
|
| |||
| PCBs and OCPs | Baby foods | Korea | [147] |
|
| |||
| OCPs and pyrethroids | Honey | Egypt | [148] |
|
| |||
| PCBs and OCPs | Cereals | Poland | [149] |
|
| |||
| PCBs and OCPs | Milk, yak muscle and liver | Tibet Plateau | [150] |
|
| |||
| Radioactive substances | |||
|
| |||
| Radioactive substances | Water and food | Japan | [103] |
|
| |||
| Radionuclides | Seafood | India | [104] |
|
| |||
| Uranium isotopes | Food | Balkans | [105] |
|
| |||
| Radioactive substances | Water and food | Switzerland | [106] |
Examples of indirect monitoring methods for contaminants in the environment and food include measurement of biomarkers such as proteomics in oysters contaminated with mercury, transcriptome effects in the hepatopancreas of clams, or mutagenicity of seawater in seafood farms associated with PAHs and PCBs [151–153]. High throughput and ultrasensitive screening using nanoparticles has also been utilized for detection of environmental pollutants [154]. Moving forward, testing of chemicals to evaluate potential toxicities before registration and authorised use in the environment may employ tools and concepts such as biomonitoring equivalents and threshold of toxicologic concern, alongside generic and physiologically-based toxicokinetic models [155]. Since 2006, the European Commission has implemented new legislation, called REACH (EC 1907/2006), to identify properties—including toxicities—of chemicals and thus better protect human health and the environment [156]. Other similar legislation exists elsewhere in the world; for example, the Environmental Protection Agency runs a registration process for pesticides to comply with federal laws in the US [157].
Once sources of contaminants have been identified, it is vital to minimize contamination of food. For this purpose, regulations are in place at both national and international levels to restrict contaminated food entering the human food chain. In some cases, legislation exists to assess levels of food contamination. For example, the Marine Strategy Framework Directive in Spain monitors for contaminants in edible tissues of seafood destined for human consumption, assessing levels against established EU standards for food safety [158]. The German Federal Environment Agency monitors both the environment—using the German Environmental Survey (GerES)—and human biomonitoring—using the German Environmental Specimen Bank (ESB) [159]. Amongst others, these have, respectively, been used to detect lead in drinking water and exposure to phthalates and bisphenol A. National monitoring systems may cooperate at an international level. To maintain and improve food safety globally, the Codex Alimentarius contains a set of international food standards, guidelines, and codes of practice [20]. These are based on science from risk assessment bodies or organized by consultations with FAO and WHO. These are voluntary but often form the basis of national legislation.
Food standards and legislation focus on individual food products. To understand the combined risk that someone has from one or many chemicals, a complete dietary risk assessment can be conducted to assess the total potential risk of a typical diet. For example, Zhou et al. assessed the levels of organochlorine pesticides (OCPs) in a total diet from China [160]. The study found that aquatic foods, meats, and cereals were the major foods contributing to contamination of the diet with these chemicals. Multilevel risk assessment can also be used to identify critical points in contamination sources. For example, a study of metals in soil and food in Taiwan identified more than 600 metal-contaminated sites over a period of two decades which could then be targeted for remediation efforts [161].
4. Remediation
Once sources of contamination have been identified, it is possible to consider how best to improve food safety through various methods. Methods of remediation vary depending on the type of contaminants present and in which environment. These can be expensive on a large scale. Remediation may focus on reducing contaminants in the environment overall or reducing concentrations in foods specifically.
A common method used to reduce environmental exposure to contaminants is soil remediation. One simple method is to remove contaminated topsoil, which typically contains higher levels of contaminants than subsoil, from agricultural areas [161]. Alternatively, soil turnover and mixing in situ may be sufficient to dilute contaminant, such as metals, concentrations to an acceptable level. Thermal treatment or landfill can also be used to remediate a site. Different soil properties can affect contaminant levels. For example, metal (cadmium, mercury, and chromium) accumulation in flowering Chinese cabbage was shown to be controlled by total metal concentrations in soil and available calcium [162]. It is well known that soil science can be used to improve food quality and quantity [163]. It can similarly be used to reduce contamination of crops. The predominant congener of technical DDT, p,p'-DDT, is susceptible to microbial metabolism and rarely accumulates in aerobic soils [164]. Long-term gardening has been shown to result in lower levels of PAHs, possibly due to PAH degradation by enhanced microbial activity, and/or dilution [9]. Microbial bioremediation may also be used to reduce levels of metal contamination of soils in an environmentally-friendly manner [165].
Different forms of phytoremediation may be used to either remove contamination from soils or to reduce contamination of plants. If the plants are crops for consumption, reduced uptake is beneficial. One example of phytoremediation is selection of plants to specifically remove contaminants from agricultural land, such as using black nightshade (Solanum nigrum L.) for removal of thallium from soil [166]. A study by Yu et al. on cadmium-contaminated agricultural land showed differential accumulation of cadmium in two oilseed rape cultivars [167]. Interestingly, the study also showed increased uptake of cadmium in rice crops planted after the oilseed rape harvest, with contamination of rice higher compared to a crop after a fallow period. Another mechanism of plants which can be used advantageously is that of reduced accumulation of unwanted chemicals in certain cultivars or altered plant hybrids—for example in Chinese kale—and these can be selected to produce safer food [168].
Crop management techniques can affect contamination of plants. Use of slow-release nitrogen fertilizers can reduce cadmium levels in plants such as pak choi, as the plants appear to have stronger tolerance to the metal and a lower efficiency of translocation to edible plant parts compared to those grown using typical fertilizers [169]. Contaminated water used to flood paddy fields is a huge problem in countries that rely on rice crops. Water management—such as drying the paddy field for a period of days between late tillering and young ear differentiation stages—has been shown to reduce cadmium and arsenic levels in rice crops of different rice species [170]. Human health risks from medicinal products contaminating food and water for consumption may be modelled, for example, using pond aquaculture, to identify potential health risks [171].
Exposure to contaminants on foods prepared for consumption can also be reduced by using safer storage alternatives, such as edible films and coatings [172]. Contamination of foods with PAHs from cooking can be greatly reduced by avoiding smoking or open fires but rather replacing them with gas stoves for cooking [77]. Several nongovernmental organizations and charities offer gas stoves to families to help alleviate this source of food contamination, which is a risk particularly for women and children who spend more time at home [173].
5. Summary and Conclusions
Attitudes in society towards food safety and contamination are often rooted in tradition and habit. Although consumers select their diet based on social and financial factors, the remit for food safety remains firmly with regulatory bodies. These bodies can monitor for contaminants and enforce legislation. Aspects of food contamination also have political implications. As mentioned above, food safety laws are necessary, with monitoring of food and water contamination, as well as enacting measures to reduce and eliminate exposure to environmental pollutants. Publicity after environmental pollution-related incidents behoves a government to have public health, legal, and ethical frameworks in place in a timely manner. Education of society regarding safer crop cultivation and livestock rearing, selection of a balanced diet, and safer cooking methods should also be encouraged. On a nationwide scale, governments also should endeavour to reduce urban disparities in environmental exposures. Although some contaminants have focal effects, many are transported globally. For this reason, an international stance on food safety is necessary by reducing environmental and food contamination and ensuring trade of safer food products on a global scale.
Acknowledgments
This work was supported by the Faculty of Veterinary Medicine, Hokkaido University.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Stasinos S., Nasopoulou C., Tsikrika C., Zabetakis I. The bioaccumulation and physiological effects of heavy metals in carrots, onions, and potatoes and dietary implications for Cr and Ni: a review. Journal of Food Science. 2014;79(5):R765–R780. doi: 10.1111/1750-3841.12433. [DOI] [PubMed] [Google Scholar]
- 2.Price P., Zaleski R., Hollnagel H., Ketelslegers H., Han X. Assessing the safety of co-exposure to food packaging migrants in food and water using the maximum cumulative ratio and an established decision tree. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 2014;31(3):414–421. doi: 10.1080/19440049.2013.865145. [DOI] [PubMed] [Google Scholar]
- 3.Leibson T., Lala P., Ito S. Drug and Chemical Contaminants in Breast Milk: Effects on Neurodevelopment of the Nursing Infant. In: Slikker W. J., Paule M. G., and., Wang C., editors. Handbook of Developmental Neurotoxicology. Elsevier; 2018. pp. 275–284. [Google Scholar]
- 4.Vafeiadi M., Georgiou V., Chalkiadaki G., et al. Association of prenatal exposure to persistent organic pollutants with obesity and cardiometabolic traits in early childhood: the Rhea mother-child cohort (Crete, Greece) Environmental Health Perspectives. 2015;123(10) doi: 10.1289/ehp.1409062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmood A., Malik R. N. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arabian Journal of Chemistry. 2014;7:91–99. doi: 10.1016/j.arabjc.2013.07.002. [DOI] [Google Scholar]
- 6.Thompson L. A., Ikenaka Y., Yohannes Y. B., et al. Human Health Risk from Consumption of Marine Fish Contaminated with DDT and Its Metabolites in Maputo Bay, Mozambique. Bulletin of Environmental Contamination and Toxicology. 2018:1–5. doi: 10.1007/s00128-018-2323-7. [DOI] [PubMed] [Google Scholar]
- 7.da Araújo C. F., Lopes M. V., Vasquez M. R., et al. Cadmium and lead in seafood from the Aratu Bay, Brazil and the human health risk assessment. Environmental Modeling & Assessment. 2016;188(4) doi: 10.1007/s10661-016-5262-y. [DOI] [PubMed] [Google Scholar]
- 8.Kohrman H., Chamberlain C. P. Heavy metals in produce from urban farms in the San Francisco Bay Area. Food Additives & Contaminants: Part B. Surveillance. 2014;7(2):127–134. doi: 10.1080/19393210.2013.859740. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser M. L., Williams M. L., Basta N., Hand M., Huber S. When vacant lots become urban gardens: Characterizing the perceived and actual food safety concerns of urban agriculture in Ohio. Journal of Food Protection. 2015;78(11):2070–2080. doi: 10.4315/0362-028X.JFP-15-181. [DOI] [PubMed] [Google Scholar]
- 10.Enault J., Robert S., Schlosser O., de Thé C., Loret J.-F. Drinking water, diet, indoor air: Comparison of the contribution to environmental micropollutants exposure. International Journal of Hygiene and Environmental Health. 2015;218(8):723–730. doi: 10.1016/j.ijheh.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Jin Y., Liang C., He G., Cao J. Study on distribution of endemic arsenism in China. Wei sheng yan jiu = Journal of hygiene research. 2003;32(6):519–540. [PubMed] [Google Scholar]
- 12.Peters R. J. B., Beeltje H., Van Delft R. J. Xeno-estrogenic compounds in precipitation. Journal of Environmental Monitoring. 2008;10(6):760–769. doi: 10.1039/b805983g. [DOI] [PubMed] [Google Scholar]
- 13.Nelms S. E., Galloway T. S., Godley B. J., Jarvis D. S., Lindeque P. K. Investigating microplastic trophic transfer in marine top predators. Environmental Pollution. 2018 doi: 10.1016/j.envpol.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Enander R. T., Cohen H. J., Gute D. M., Brown L. C., Desmaris A. M. C., Missaghian R. Lead and methylene chloride exposures among automotive repair technicians. Journal of Occupational and Environmental Hygiene. 2004;1(2):119–125. doi: 10.1080/15459620490275911. [DOI] [PubMed] [Google Scholar]
- 15.Järup L. Hazards of heavy metal contamination. British Medical Bulletin. 2003;68(1):167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y., Song S., Wang R., et al. Impacts of soil and water pollution on food safety and health risks in China. Environment International. 2015;77:5–15. doi: 10.1016/j.envint.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Q., Wang Y., Cao Y., et al. Potential health risks of heavy metals in cultivated topsoil and grain, including correlations with human primary liver, lung and gastric cancer, in Anhui province, Eastern China. Science of the Total Environment. 2014;470-471:340–347. doi: 10.1016/j.scitotenv.2013.09.086. [DOI] [PubMed] [Google Scholar]
- 18.Jonker D., Freidig A., Groten J., et al. Safety Evaluation of Chemical Mixtures and Combinations of Chemical and Non-Chemical Stressors. Reviews on Environmental Health. 2004;19(2):83–140. doi: 10.1515/REVEH.2004.19.2.83. [DOI] [PubMed] [Google Scholar]
- 19.The Stockholm Convention. Status of Ratification. http://chm.pops.int/Countries/StatusofRatifications/PartiesandSignatoires/tabid/4500/Default.aspx.
- 20.FAO/WHO Codex Alimentarius Commission. Food and Cosmetics Toxicology. 1966;4:p. 601. doi: 10.1016/S0015-6264(66)80695-8. [DOI] [Google Scholar]
- 21.JECFA. 80th Joint FAO/WHO Expert Committee on Food Additives (JECFA) meeting - Food additives and contaminants. Summary and Conclusions. 2015;71:1–16. [Google Scholar]
- 22.JECFA. Safety evaluation of certain food additives and contaminants. 2011.
- 23.Codex Alimentarius Commssion. Pesticide Residues 50th session in Haikou China.
- 24.JMPR. AGP - JMPR Reports and evaluations. [DOI]
- 25.Smedley P. L., Kinniburgh D. G. A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry. 2002;17(5):517–568. doi: 10.1016/S0883-2927(02)00018-5. [DOI] [Google Scholar]
- 26.Bortey-Sam N., Nakayama S. M. M., Ikenaka Y., et al. Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs) Ecotoxicology and Environmental Safety. 2015;111:160–167. doi: 10.1016/j.ecoenv.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Darwish W. S., Atia A. S., Khedr M. H., Eldin W. F. Metal contamination in quail meat: residues, sources, molecular biomarkers, and human health risk assessment. Environmental Science and Pollution Research. 2018;25(20):20106–20115. doi: 10.1007/s11356-018-2182-0. [DOI] [PubMed] [Google Scholar]
- 28.Szkup-Jabłońska M., Karakiewicz B., Grochans E., et al. Effects of blood lead and cadmium levels on the functioning of children with behaviour disorders in the family environment. Annals of Agricultural and Environmental Medicine. 2012;19(2):241–246. [PubMed] [Google Scholar]
- 29.Yabe J., Nakayama S. M. M., Ikenaka Y., et al. Lead poisoning in children from townships in the vicinity of a lead-zinc mine in Kabwe, Zambia. Chemosphere. 2015;119:941–947. doi: 10.1016/j.chemosphere.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Nordberg G. F., Nogawa K., Nordberg M., Friberg L. T. Cadmium. Handbook on the Toxicology of Metals. 2007:445–486. [Google Scholar]
- 31.Lin H.-J., Sung T.-I., Chen C.-Y., Guo H.-R. Arsenic levels in drinking water and mortality of liver cancer in Taiwan. Journal of Hazardous Materials. 2013;262:1132–1138. doi: 10.1016/j.jhazmat.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 32.United States Environmental Protection Agency (US EPA) Quantification of toxicologic effects for nickel. Prepared by the office of health and environmental assessment, environmental criteria and assessment office, Cincinnati, OH for the office of water, office of science and technology. Washington, DC, USA: 1991. [Google Scholar]
- 33.Genchi G., Sinicropi M. S., Carocci A., Lauria G., Catalano A. Mercury exposure and heart diseases. International Journal of Environmental Research and Public Health. 2017;14(1) doi: 10.3390/ijerph14010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darwish W. S., Ikenaka Y., Nakayama S. M. M., Ishizuka M. An overview on mycotoxin contamination of foods in Africa. Journal of Veterinary Medical Science. 2014;76(6):789–797. doi: 10.1292/jvms.13-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azziz-Baumgartner E., Lindblade K., Gieseker K., et al. Case–Control Study of an Acute Aflatoxicosis Outbreak, Kenya, 2004. Environmental Health Perspectives. 2005;113(12):1779–1783. doi: 10.1289/ehp.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner P. C., Loffredo C., Kafrawy S. E., et al. Pilot survey of aflatoxin–albumin adducts in sera from Egypt. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 2008;25(5):583–587. doi: 10.1080/02652030701713939. [DOI] [PubMed] [Google Scholar]
- 37.Kuniholm M. H., Lesi O. A., Mendy M., et al. Aflatoxin exposure and viral hepatitis in the etiology of liver cirrhosis in The Gambia, West Africa. Environmental Health Perspectives. 2008;116(11):1553–1557. doi: 10.1289/ehp.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hmaissia Khlifa K., Ghali R., Mazigh C., Aouni Z., Machgoul S., Hedhili A. Ochratoxin A levels in human serum and foods from nephropathy patients in Tunisia: Where are you now? Experimental and Toxicologic Pathology. 2012;64(5):509–512. doi: 10.1016/j.etp.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Robert H., Payros D., Pinton P., Théodorou V., Mercier-Bonin M., Oswald I. P. Impact of mycotoxins on the intestine: are mucus and microbiota new targets? Journal of Toxicology and Environmental Health - Part B: Critical Reviews. 2017;20(5):249–275. doi: 10.1080/10937404.2017.1326071. [DOI] [PubMed] [Google Scholar]
- 40.Chang H., Kim W., Park J., et al. The Occurrence of Zearalenone in South Korean Feedstuffs between 2009 and 2016. Toxins. 2017;9(7):p. 223. doi: 10.3390/toxins9070223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J., Chang H., Hong S., Kim D., Chung S., Lee C. A decrease of incidence cases of fumonisins in south Korean feedstuff between 2011 and 2016. Toxins. 2017;9(9) doi: 10.3390/toxins9090286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darwish W. S., Eldaly E., El-Abbasy M., Ikenaka Y., Ishizuka M. Antibiotic residues in food: African scenario. Japanese Journal of Veterinary Research2. 2013;61:S13–S22. [PubMed] [Google Scholar]
- 43.Kools S. A. E., Moltmann J. F., Knacker T. Estimating the use of veterinary medicines in the European Union. Regulatory Toxicology and Pharmacology. 2008;50(1):59–65. doi: 10.1016/j.yrtph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 44.El Bayomi R. M., Darwish W. S., El-Moaty A. M. A., Gad T. M. Prevalence, antibiogram, molecular characterization and reduction trial of Salmonella typhimurium isolated from different fish species. Japanese Journal of Veterinary Research. 2016;64:S181–S186. [Google Scholar]
- 45.Darwish W. S., Atia A. S., Reda L. M., Elhelaly A. E., Thompson L. A., Saad Eldin W. F. Chicken giblets and wastewater samples as possible sources of methicillin-resistant Staphylococcus aureus: Prevalence, enterotoxin production, and antibiotic susceptibility. Journal of Food Safety. 2018;38(4) doi: 10.1111/jfs.12478. [DOI] [Google Scholar]
- 46.Darwish W. S., Ikenaka Y., Nakayama S., Mizukawa H., Ishizuka M. Mutagenicity of modelled-heat-treated meat extracts: Mutagenicity assay, analysis and mechanism of mutagenesis. Japanese Journal of Veterinary Research. 2015;63(4):173–182. doi: 10.14943/jjvr.63.4.173. [DOI] [PubMed] [Google Scholar]
- 47.Darwish W. S., Ikenaka Y., Nakayama S., Mizukawa H., Thompson L. A., Ishizuka M. β-carotene and retinol reduce benzo[a]pyrene-induced mutagenicity and oxidative stress via transcriptional modulation of xenobiotic metabolizing enzymes in human HepG2 cell line. Environmental Science and Pollution Research. 2018;25(7):6320–6328. doi: 10.1007/s11356-017-0977-z. [DOI] [PubMed] [Google Scholar]
- 48.Dai J. B., Wang Z. X., Qiao Z. D. The hazardous effects of tobacco smoking on male fertility. Asian Journal of Andrology. 2015 doi: 10.4103/1008-682X.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jumpponen M., Rönkkömäki H., Pasanen P., Laitinen J. Occupational exposure to gases, polycyclic aromatic hydrocarbons and volatile organic compounds in biomass-fired power plants. Chemosphere. 2013;90(3):1289–1293. doi: 10.1016/j.chemosphere.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Jedrychowski W. A., Perera F. P., Camann D., et al. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environmental Science and Pollution Research. 2015;22(5):3631–3639. doi: 10.1007/s11356-014-3627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang Y., MacIntosh D. L., Camann D. E., Ryan P. B. Analysis of aggregate exposure to chlorpyrifos in the NHEXAS-Maryland investigation. Environmental Health Perspectives. 2002;110(3):235–240. doi: 10.1289/ehp.02110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson L. A., Darwish W. S., Ikenaka Y., Nakayama S. M. M., Mizukawa H., Ishizuka M. Organochlorine pesticide contamination of foods in Africa: Incidence and public health significance. Journal of Veterinary Medical Science. 2017;79(4):751–764. doi: 10.1292/jvms.16-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Windham G. C., Lee D., Mitchell P., Anderson M., Petreas M., Lasley B. Exposure to organochlorine compounds and effects on ovarian function. Epidemiology. 2005;16(2):182–190. doi: 10.1097/01.ede.0000152527.24339.17. [DOI] [PubMed] [Google Scholar]
- 54.Bretveld R. W., Hooiveld M., Zielhuis G. A., Pellegrino A., van Rooij I. A. L. M., Roeleveld N. Reproductive disorders among male and female greenhouse workers. Reproductive Toxicology. 2008;25(1):107–114. doi: 10.1016/j.reprotox.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Caspersen I. H., Haugen M., Schjølberg S., et al. Maternal dietary exposure to dioxins and polychlorinated biphenyls (PCBs) is associated with language delay in 3 year old Norwegian children. Environment International. 2016;91:180–187. doi: 10.1016/j.envint.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 56.Nakajima S., Saijo Y., Kato S., et al. Effects of prenatal exposure to polychlorinated biphenyls and dioxins on mental and motor development in Japanese children at 6 months of age. Environmental Health Perspectives. 2006;114(5):773–778. doi: 10.1289/ehp.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winneke G. Developmental aspects of environmental neurotoxicology: lessons from lead and polychlorinated biphenyls. Journal of the Neurological Sciences. 2011;308(1-2):9–15. doi: 10.1016/j.jns.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 58.Xiao R., Wang S., Li R., Wang J. J., Zhang Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicology and Environmental Safety. 2017;141:17–24. doi: 10.1016/j.ecoenv.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Nouri M., Haddioui A. Human and animal health risk assessment of metal contamination in soil and plants from Ait Ammar abandoned iron mine, Morocco. Environmental Modeling & Assessment. 2016;188(1):1–12. doi: 10.1007/s10661-015-4999-z. [DOI] [PubMed] [Google Scholar]
- 60.Pareja-Carrera J., Mateo R., Rodríguez-Estival J. Lead (Pb) in sheep exposed to mining pollution: Implications for animal and human health. Ecotoxicology and Environmental Safety. 2014;108:210–216. doi: 10.1016/j.ecoenv.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 61.Nedelescu M., Baconi D., Neagoe A., et al. Environmental metal contamination and health impact assessment in two industrial regions of Romania. Science of the Total Environment. 2017;580:984–995. doi: 10.1016/j.scitotenv.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 62.Li B., Wang Y., Jiang Y., et al. The accumulation and health risk of heavy metals in vegetables around a zinc smelter in northeastern China. Environmental Science and Pollution Research. 2016;23(24):25114–25126. doi: 10.1007/s11356-016-7342-5. [DOI] [PubMed] [Google Scholar]
- 63.Cao S., Duan X., Zhao X., et al. Health risks from the exposure of children to As, Se, Pb and other heavy metals near the largest coking plant in China. Science of the Total Environment. 2014;472:1001–1009. doi: 10.1016/j.scitotenv.2013.11.124. [DOI] [PubMed] [Google Scholar]
- 64.Vromman V., Saegerman C., Pussemier L., et al. Cadmium in the food chain near non-ferrous metal production sites. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 2008;25(3):293–301. doi: 10.1080/02652030701509980. [DOI] [PubMed] [Google Scholar]
- 65.Liu J., Luo X., Wang J., et al. Thallium contamination in arable soils and vegetables around a steel plant—A newly-found significant source of Tl pollution in South China. Environmental Pollution. 2017;224:445–453. doi: 10.1016/j.envpol.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 66.Salazar M. J., Rodriguez J. H., Nieto G. L., Pignata M. L. Effects of heavy metal concentrations (Cd, Zn and Pb) in agricultural soils near different emission sources on quality, accumulation and food safety in soybean [Glycine max (L.) Merrill] Journal of Hazardous Materials. 2012;233-234:244–253. doi: 10.1016/j.jhazmat.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 67.Cheng J., Ding C., Li X., Zhang T., Wang X. Heavy metals in navel orange orchards of Xinfeng County and their transfer from soils to navel oranges. Ecotoxicology and Environmental Safety. 2015;122:153–158. doi: 10.1016/j.ecoenv.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 68.Chen Z.-F., Zhao Y., Zhu Y., et al. Health risks of heavy metals in sewage-irrigated soils and edible seeds in Langfang of Hebei province, China. Journal of the Science of Food and Agriculture. 2010;90(2):314–320. doi: 10.1002/jsfa.3817. [DOI] [PubMed] [Google Scholar]
- 69.Wang G., Gong Y., Zhu Y.-X., Miao A.-J., Yang L.-Y., Zhong H. Assessing the risk of Hg exposure associated with rice consumption in a typical city (Suzhou) in eastern China. International Journal of Environmental Research and Public Health. 2017;14(5) doi: 10.3390/ijerph14050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fréry N., Maury-Brachet R., Maillot E., Deheeger M., De Mérona B., Boudou A. Gold-mining activities and mercury contamination of native Amerindian communities in French Guiana: Key role of fish in dietary uptake. Environmental Health Perspectives. 2001;109(5):449–456. doi: 10.1289/ehp.01109449. doi: 10.1289/ehp.01109449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peng Q., Greenfield B. K., Dang F., Zhong H. Human exposure to methylmercury from crayfish (Procambarus clarkii) in China. Environmental Geochemistry and Health. 2016;38(1):169–181. doi: 10.1007/s10653-015-9701-4. [DOI] [PubMed] [Google Scholar]
- 72.Tekin-Özan S. Determination of heavy metal levels in water, sediment and tissues of tench (Tinca tinca L., 1758) from Beyşehir Lake (Turkey) Environmental Modeling & Assessment. 2008;145(1–3):295–302. doi: 10.1007/s10661-007-0038-z. [DOI] [PubMed] [Google Scholar]
- 73.Copat C., Bella F., Castaing M., Fallico R., Sciacca S., Ferrante M. Heavy metals concentrations in fish from Sicily (Mediterranean Sea) and evaluation of possible health risks to consumers. Bulletin of Environmental Contamination and Toxicology. 2012;88(1):78–83. doi: 10.1007/s00128-011-0433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green A. D., Buhlmann K. A., Hagen C., Romanek C., Whitfield Gibbons J. Mercury contamination in turtles and implications for human health. Journal of Environmental Health. 2010;72(10):14–22. [PubMed] [Google Scholar]
- 75.Alam N., Corbett S. J., Ptolemy H. C. Environmental health risk assessment of nickel contamination of drinking water in a country town in NSW. Public Health Research & Practice . 2008;19(9-10):170–173. doi: 10.1071/NB97043. [DOI] [PubMed] [Google Scholar]
- 76.García-Esquinas E., Pérez-Gómez B., Fernández M. A., et al. Mercury, lead and cadmium in human milk in relation to diet, lifestyle habits and sociodemographic variables in Madrid (Spain) Chemosphere. 2011;85(2):268–276. doi: 10.1016/j.chemosphere.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 77.Alexander J., Benford D., Cockburn A., et al. Polycyclic Aromatic Hydrocarbons in Food Scientific Opinion of the Panel on Contaminants in the Food Chain (Question N° EFSA-Q-2007-136) The European Food Safety Authority Journal. 2008;724:1–114. [Google Scholar]
- 78.Ylitalo G. M., Krahn M. M., Dickhoff W. W., et al. Federal seafood safety response to the Deepwater Horizon oil spill. Proceedings of the National Acadamy of Sciences of the United States of America. 2012;109(50):20274–20279. doi: 10.1073/pnas.1108886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rotkin-Ellman M., Wong K. K., Solomon G. M. Seafood contamination after the BP gulf oil spill and risks to vulnerable populations: A critique of the FDA risk assessment. Environmental Health Perspectives. 2012;120(2):157–161. doi: 10.1289/ehp.1103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Q. Q., Loganath A., Chong Y. S., Tan J., Obbard J. P. Persistent organic pollutants and adverse health effects in humans. Journal of Toxicology and Environmental Health, Part A. Current Issues. 2006;69(21):1987–2005. doi: 10.1080/15287390600751447. [DOI] [PubMed] [Google Scholar]
- 81.National Pesticide Information Center. Chlorpyrifos Technical Fact Sheet. http://npic.orst.edu/factsheets/archive/chlorptech.html.
- 82.UNEP. The Stockholm Convention on Persistent Organic Pollutants, Geneva, Switzerland. 2001, http://chm.pops.int/Portals/0/Repository/convention_text/UNEP-POPS-COP-CONVTEXT-FULL.English.PDF.
- 83.WHOPES. Safety of Pyrethroids for Public Health Use. 2005.
- 84.Akoto O., Gavor S., Appah M. K., Apau J. Estimation of human health risk associated with the consumption of pesticide-contaminated vegetables from Kumasi, Ghana. Environmental Modeling & Assessment. 2015;187(5) doi: 10.1007/s10661-015-4471-0. [DOI] [PubMed] [Google Scholar]
- 85.Abdel-Daim M. M., Abuzead S. M. M., Halawa S. M. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0072991.e72991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cimino A. M., Boyles A. L., Thayer K. A., Perry M. J. Effects of neonicotinoid pesticide exposure on human health: A systematic review. Environmental Health Perspectives. 2017;125(2):155–162. doi: 10.1289/EHP515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ikenaka Y., Fujioka K., Kawakami T., et al. Contamination by neonicotinoid insecticides and their metabolites in Sri Lankan black tea leaves and Japanese green tea leaves. Toxicology Reports. 2018;5:744–749. doi: 10.1016/j.toxrep.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.World Health Organization. Polychlorinated biphenyls: human health aspects. http://www.who.int/pcs/pubs/pub_cicad.htm.
- 89.Agency for Toxic Substances & Disease Registry. Toxicological Profile for Polychlorinated Biphenyls (PCBs) 2000. [PubMed]
- 90.Zhang L., Li J., Zhao Y., et al. A national survey of polybrominated diphenyl ethers (PBDEs) and indicator polychlorinated biphenyls (PCBs) in Chinese mothers' milk. Chemosphere. 2011;84(5):625–633. doi: 10.1016/j.chemosphere.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 91.Fromme H., Körner W., Shahin N., et al. Human exposure to polybrominated diphenyl ethers (PBDE), as evidenced by data from a duplicate diet study, indoor air, house dust, and biomonitoring in Germany. Environment International. 2009;35(8):1125–1135. doi: 10.1016/j.envint.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Cruz R., Cunha S. C., Casal S. Brominated flame retardants and seafood safety: A review. Environment International. 2015;77:116–131. doi: 10.1016/j.envint.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 93.Buck R. C., Franklin J., Berger U., et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated Environmental Assessment and Management. 2011;7(4):513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Y., Wang Y., Li J., et al. Perfluorinated compounds in seafood from coastal areas in China. Environment International. 2012;42(1):67–71. doi: 10.1016/j.envint.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Schuetze A., Heberer T., Effkemann S., Juergensen S. Occurrence and assessment of perfluorinated chemicals in wild fish from Northern Germany. Chemosphere. 2010;78(6):647–652. doi: 10.1016/j.chemosphere.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 96.Van Asselt E. D., Kowalczyk J., Van Eijkeren J. C. H., et al. Transfer of perfluorooctane sulfonic acid (PFOS) from contaminated feed to dairy milk. Food Chemistry. 2013;141(2):1489–1495. doi: 10.1016/j.foodchem.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 97.Kumar J., Das S., Teoh S. L. Dietary Acrylamide and the Risks of Developing Cancer: Facts to Ponder. Frontiers in Nutrition. 2018;5 doi: 10.3389/fnut.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.IARC (International Agency for Research on Cancer) Agents Classified by the IARC Monographs, Volumes 1-123. https://monographs.iarc.fr/wp-content/uploads/2018/09/List_of_Classifications.pdf.
- 99.He L.-Y., Ying G.-G., Liu Y.-S., et al. Discharge of swine wastes risks water quality and food safety: Antibiotics and antibiotic resistance genes from swine sources to the receiving environments. Environment International. 2016;92-93:210–219. doi: 10.1016/j.envint.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 100.Kabir E. R., Rahman M. S., Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environmental Toxicology and Pharmacology. 2015;40(1):241–258. doi: 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 101.Posadzki P., Watson L., Ernst E. Contamination and adulteration of herbal medicinal products (HMPs): an overview of systematic reviews. European Journal of Clinical Pharmacology. 2013;69(3):295–307. doi: 10.1007/s00228-012-1353-z. [DOI] [PubMed] [Google Scholar]
- 102.Walker J. S., Don G. W. Mathematics and music. CRC Press, Boca Raton, FL; 2013. p. xiv+318. [Google Scholar]
- 103.Hamada N., Ogino H., Fujimichi Y. Safety regulations of food and water implemented in the first year following the Fukushima nuclear accident. Journal of Radiation Research. 2012;53(5):641–671. doi: 10.1093/jrr/rrs032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khan M. F., Godwin Wesley S. Assessment of health safety from ingestion of natural radionuclides in seafoods from a tropical coast, India. Marine Pollution Bulletin. 2011;62(2):399–404. doi: 10.1016/j.marpolbul.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 105.Carvalho F. P., Oliveira J. M. Uranium isotopes in the Balkan's environment and foods following the use of depleted uranium in the war. Environment International. 2010;36(4):352–360. doi: 10.1016/j.envint.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 106.Brennwald M. S., van Dorp F. Radiological risk assessment and biosphere modelling for radioactive waste disposal in Switzerland. Journal of Environmental Radioactivity. 2009;100(12):1058–1061. doi: 10.1016/j.jenvrad.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 107.Pröhl G., Olyslaegers G., Kanyar B., et al. Development and comparison of five site-specific biosphere models for safety assessment of radioactive waste disposal. Journal of Radiological Protection. 2005;25(4):343–373. doi: 10.1088/0952-4746/25/4/001. [DOI] [PubMed] [Google Scholar]
- 108.Food and Drug Administration HHS. Beverages: bottled water. Direct final rule. Federal register. 2003;68(41):9873–9882. [PubMed] [Google Scholar]
- 109.Wong M. H., Wu S. C., Deng W. J., et al. Export of toxic chemicals - A review of the case of uncontrolled electronic-waste recycling. Environmental Pollution. 2007;149(2):131–140. doi: 10.1016/j.envpol.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 110.Liu Q., Cao J., Li K. Q., et al. Chromosomal aberrations and DNA damage in human populations exposed to the processing of electronics waste. Environmental Science and Pollution Research. 2009;16(3):329–338. doi: 10.1007/s11356-008-0087-z. [DOI] [PubMed] [Google Scholar]
- 111.Zare Jeddi M., Rastkari N., Ahmadkhaniha R., Yunesian M., Nabizadeh R., Daryabeygi R. A margin of exposure approach to assessment of non-cancerous risk of diethyl phthalate based on human exposure from bottled water consumption. Environmental Science and Pollution Research. 2015;22(24):19518–19528. doi: 10.1007/s11356-015-5076-4. [DOI] [PubMed] [Google Scholar]
- 112.Kalman J., Paul K. B., Khan F. R., Stone V., Fernandes T. F. Characterisation of bioaccumulation dynamics of three differently coated silver nanoparticles and aqueous silver in a simple freshwater food chain. Environmental Chemistry. 2015;12(6):662–672. doi: 10.1071/EN15035. [DOI] [Google Scholar]
- 113.Gatti A. M., Tossini D., Gambarelli A., Montanari S., Capitani F. Investigation of the presence of inorganic micro- and nanosized contaminants in bread and biscuits by environmental scanning electron microscopy. Critical Reviews in Food Science and Nutrition. 2009;49(3):275–282. doi: 10.1080/10408390802064347. [DOI] [PubMed] [Google Scholar]
- 114.Wani A. L., Ara A., Usmani J. A. Lead toxicity: A review. Interdisciplinary Toxicology. 2015;8(2):55–64. doi: 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rubin R., Strayer D. S. Environmental and Nutritional pathology. in. Rubins pathology. Clinicopathologic Foundations of Medicine; 2008. [Google Scholar]
- 116.Sokol R. Z., Berman N. The effect of age of exposure on lead-induced testicular toxicity. Toxicology. 1991;69(3):269–278. doi: 10.1016/0300-483X(91)90186-5. [DOI] [PubMed] [Google Scholar]
- 117.Cleveland L. M., Minter M. L., Cobb K. A., Scott A. A., German V. F. Pregnant Women. American Journal of Nursing. 2008;108(10):40–49. doi: 10.1097/01.NAJ.0000337736.76730.66. [DOI] [PubMed] [Google Scholar]
- 118.Grant L. D. Environmental toxicants: human exposures and their health effects. In: Lippman M., editor. Wiley-Interscience. 2009. pp. 757–809. [Google Scholar]
- 119.Kosnett M. J. In: Poisoning and Drug Overdose. Olson K. R., editor. McGraw Hill Medical; 2012. [Google Scholar]
- 120.Kosnett M. J. Heavy metal intoxication and chelators. In: Katzung B. G., editor. Basic and Clinical Pharmacology. McGraw Hill Professional; 2007. [Google Scholar]
- 121.Rogan W. J., Dietrich K. N., Ware J. H., et al. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. The New England Journal of Medicine. 2001;344(19):1421–1426. doi: 10.1056/NEJM200105103441902. [DOI] [PubMed] [Google Scholar]
- 122.Yusa V., Millet M., Coscolla C., Roca M. Analytical methods for human biomonitoring of pesticides. A review. Analytica Chimica Acta. 2015;891:15–31. doi: 10.1016/j.aca.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 123.Dursun A., Yurdakok K., Yalcin S. S., et al. Maternal risk factors associated with lead, mercury and cadmium levels in umbilical cord blood, breast milk and newborn hair. The Journal of Maternal-Fetal and Neonatal Medicine. 2016;29(6):954–961. doi: 10.3109/14767058.2015.1026255. [DOI] [PubMed] [Google Scholar]
- 124.Bortey-Sam N., Nakayama S. M. M., Akoto O., et al. Accumulation of heavy metals and metalloid in foodstuffs from agricultural soils around Tarkwa area in Ghana, and associated human health risks. International Journal of Environmental Research and Public Health. 2015;12(8):8811–8827. doi: 10.3390/ijerph120808811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bose-O'Reilly S., Yabe J., Makumba J., Schutzmeier P., Ericson B., Caravanos J. Lead intoxicated children in Kabwe, Zambia. Environmental Research. 2018;165:420–424. doi: 10.1016/j.envres.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 126.Yoshinari T., Takeuchi H., Aoyama K., et al. Occurrence of four Fusarium mycotoxins, deoxynivalenol, zearalenone, T-2 toxin, and HT-2 toxin, in wheat, barley, and Japanese retail food. Journal of Food Protection. 2014;77(11):1940–1946. doi: 10.4315/0362-028X.JFP-14-185. [DOI] [PubMed] [Google Scholar]
- 127.Liu Y., Lu Y., Wang L., Chang F., Yang L. Survey of 11 mycotoxins in wheat flour in Hebei province, China. Food Additives & Contaminants: Part B. Surveillance. 2015;8(4):250–254. doi: 10.1080/19393210.2015.1074291. [DOI] [PubMed] [Google Scholar]
- 128.Burger H.-M., Lombard M. J., Shephard G. S., Rheeder J. R., van der Westhuizen L., Gelderblom W. C. A. Dietary fumonisin exposure in a rural population of South Africa. Food and Chemical Toxicology. 2010;48(8-9):2103–2108. doi: 10.1016/j.fct.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 129.Serrano A. B., Font G., Ruiz M. J., Ferrer E. Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chemistry. 2012;135(2):423–429. doi: 10.1016/j.foodchem.2012.03.064. [DOI] [PubMed] [Google Scholar]
- 130.Elshafie S. Z. B., ElMubarak A., El-Nagerabi S. A. F., Elshafie A. E. Aflatoxin B1 Contamination of Traditionally Processed Peanuts Butter for Human Consumption in Sudan. Mycopathologia. 2011;171(6):435–439. doi: 10.1007/s11046-010-9378-2. [DOI] [PubMed] [Google Scholar]
- 131.Rakotoharinome M., Pognon D., Randriamparany T., et al. Prevalence of antimicrobial residues in pork meat in Madagascar. Tropical Animal Health and Production. 2014;46(1):49–55. doi: 10.1007/s11250-013-0445-9. [DOI] [PubMed] [Google Scholar]
- 132.Sirdar M. M., Picard J., Bisschop S., Jambalang A. R., Gummow B. A survey of antimicrobial residues in table eggs in Khartoum State, Sudan, 2007-2008. Onderstepoort Journal of Veterinary Research. 2012;79(1):1–9. doi: 10.4102/ojvr.v79i1.360. [DOI] [PubMed] [Google Scholar]
- 133.Redding L., Cubas-Delgado F., Sammel M., et al. Antibiotic residues in milk from small dairy farms in rural Peru. Food Additives & Contaminants: Part A. 2014;31(6):1001–1008. doi: 10.1080/19440049.2014.905877. [DOI] [PubMed] [Google Scholar]
- 134.Omeiza G. K., Ajayi I. E., Ode O. J. Assessment of antimicrobial drug residues in beef inabuja, the federal capital territory, Nigeria. Veterinaria Italiana. 2012;48(3):283–289. [PubMed] [Google Scholar]
- 135.Nonaka C., Oliveira A., Paiva C. Occurrence of antimicrobial residues in Brazilian food animals in 2008 and 2009. Food Additives & Contaminants: Part A. 2012;29(4):526–534. doi: 10.1080/19440049.2011.625649. [DOI] [PubMed] [Google Scholar]
- 136.Abramsson-Zetterberg L., Darnerud P. O., Wretling S. Low intake of polycyclic aromatic hydrocarbons in Sweden: Results based on market basket data and a barbecue study. Food and Chemical Toxicology. 2014;74:107–111. doi: 10.1016/j.fct.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 137.Battisti C., Girelli A. M., Tarola A. M. Polycyclic aromatic hydrocarbons (PAHs) in yogurt samples. Food Additives & Contaminants: Part B. Surveillance. 2015;8(1):50–55. doi: 10.1080/19393210.2014.968880. [DOI] [PubMed] [Google Scholar]
- 138.Ciecierska M., Obiedziński M. W. Polycyclic aromatic hydrocarbons in the bakery chain. Food Chemistry. 2013;141(1):1–9. doi: 10.1016/j.foodchem.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 139.Nakata H., Uehara K., Goto Y., et al. Polycyclic aromatic hydrocarbons in oysters and sediments from the Yatsushiro Sea, Japan: Comparison of potential risks among PAHs, dioxins and dioxin-like compounds in benthic organisms. Ecotoxicology and Environmental Safety. 2014;99:61–68. doi: 10.1016/j.ecoenv.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 140.Huynh H. P. V., Nugegoda D. Effects of chlorpyrifos exposure on growth and food utilization in Australian catfish, Tandanus tandanus. Bulletin of Environmental Contamination and Toxicology. 2012;88(1):25–29. doi: 10.1007/s00128-011-0431-8. [DOI] [PubMed] [Google Scholar]
- 141.Lu M., Jiang W. W., Wang J., et al. Persistence and Dissipation of Chlorpyrifos in Brassica Chinensis, Lettuce, Celery, Asparagus Lettuce, Eggplant, and Pepper in a Greenhouse. PLoS ONE. 2014;9(6):p. e100556. doi: 10.1371/journal.pone.0100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Benzidane C., Dahamna S. Chlorpyrifos residues in food plant in the region of Setif-Algeria. Communications in Agricultural and Applied Biological Sciences. 2013;78(2):157–160. [PubMed] [Google Scholar]
- 143.Mahmoud A. F. A., Ikenaka Y., Yohannes Y. B., et al. Distribution and health risk assessment of organochlorine pesticides (OCPs) residue in edible cattle tissues from northeastern part of Egypt: High accumulation level of OCPs in tongue. Chemosphere. 2016;144:1365–1371. doi: 10.1016/j.chemosphere.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 144.Raslan A. A., Elbadry S., Darwish W. S. Estimation and Human Health Risk Assessment of Organochlorine Pesticides in Raw Milk Marketed in Zagazig City, Egypt. Journal of Toxicology. 2018;2018:1–8. doi: 10.1155/2018/3821797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thompson L. A., Ikenaka Y., Yohannes Y. B., et al. Concentrations and human health risk assessment of DDT and its metabolites in free-range and commercial chicken products from KwaZulu-Natal, South Africa. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 2017;34(11):1959–1969. doi: 10.1080/19440049.2017.1357209. [DOI] [PubMed] [Google Scholar]
- 146.Deti H., Hymete A., Bekhit A. A., Mohamed A. M. I., Bekhit A. E.-D. A. Persistent organochlorine pesticides residues in cow and goat milks collected from different regions of Ethiopia. Chemosphere. 2014;106:70–74. doi: 10.1016/j.chemosphere.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 147.Jeong Y., Lee S., Kim S., et al. Occurrence and exposure assessment of polychlorinated biphenyls and organochlorine pesticides from homemade baby food in Korea. Science of the Total Environment. 2014;470-471:1370–1375. doi: 10.1016/j.scitotenv.2013.07.071. [DOI] [PubMed] [Google Scholar]
- 148.Malhat F. M., Haggag M. N., Loutfy N. M., Osman M. A. M., Ahmed M. T. Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemosphere. 2015;120:457–461. doi: 10.1016/j.chemosphere.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 149.Roszko M., Jedrzejczak R., Szymczyk K. Polychlorinated biphenyls (PCBs), polychlorinated diphenyl ethers (PBDEs) and organochlorine pesticides in selected cereals available on the Polish retail market. Science of the Total Environment. 2014;466-467:136–151. doi: 10.1016/j.scitotenv.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 150.Pan J., Gai N., Tang H., et al. Organochlorine pesticides and polychlorinated biphenyls in grass, yak muscle, liver, and milk in Ruoergai high altitude prairie, the eastern edge of Qinghai-Tibet Plateau. Science of the Total Environment. 2014;491-492:131–137. doi: 10.1016/j.scitotenv.2014.03.074. [DOI] [PubMed] [Google Scholar]
- 151.Ruiz Y., Suárez P., Alonso A., Longo E., San Juan F. Mutagenicity test using Vibrio harveyi in the assesment of water quality from mussel farms. Water Research. 2013;47(8):2742–2756. doi: 10.1016/j.watres.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Q.-H., Huang L., Zhang Y., Ke C.-H., Huang H.-Q. Proteomic approach for identifying gonad differential proteins in the oyster (Crassostrea angulata) following food-chain contamination with HgCl2. Journal of Proteomics. 2013;94:37–53. doi: 10.1016/j.jprot.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 153.Milan M., Ferraresso S., Ciofi C., et al. Exploring the effects of seasonality and chemical pollution on the hepatopancreas transcriptome of the Manila clam. Molecular Ecology. 2013;22(8):2157–2172. doi: 10.1111/mec.12257. [DOI] [PubMed] [Google Scholar]
- 154.Koedrith P., Thasiphu T., Weon J.-I., Boonprasert R., Tuitemwong K., Tuitemwong P. Recent trends in rapid environmental monitoring of pathogens and toxicants: Potential of nanoparticle-based biosensor and applications. The Scientific World Journal. 2015;2015 doi: 10.1155/2015/510982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Caldwell J. C., Evans M. V., Krishnan K. Cutting edge PBPK models and analyses: Providing the basis for future modeling efforts and bridges to emerging toxicology paradigms. Journal of Toxicology. 2012;2012 doi: 10.1155/2012/852384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.The European Commission, “REACH". http://ec.europa.eu/environment/chemicals/reach/reach_en.htm.
- 157.US Environmental Protection Agency. Pesticide Registration Manual Helps Applicants. https://www.epa.gov/pesticide-registration. [Google Scholar]
- 158.Gago J., Viñas L., Besada V., Bellas J. The link between descriptors 8 and 9 of the Marine Strategy Framework Directive: lessons learnt in Spain. Environmental Science and Pollution Research. 2014;21(23):13664–13671. doi: 10.1007/s11356-014-3283-z. [DOI] [PubMed] [Google Scholar]
- 159.Kolossa-Gehring M., Becker K., Conrad A., Schröter-Kermani C., Schulz C., Seiwert M. Environmental surveys, specimen bank and health related environmental monitoring in Germany. International Journal of Hygiene and Environmental Health. 2012;215(2):120–126. doi: 10.1016/j.ijheh.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 160.Zhou P., Zhao Y., Li J., et al. Dietary exposure to persistent organochlorine pesticides in 2007 Chinese total diet study. Environment International. 2012;42(1):152–159. doi: 10.1016/j.envint.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 161.Lai H.-Y., Hseu Z.-Y., Chen T.-C., Chen B.-C., Guo H.-Y., Chen Z.-S. Health risk-based assessment and management of heavy metals-contaminated soil sites in Taiwan. International Journal of Environmental Research and Public Health. 2010;7(10):3595–3614. doi: 10.3390/ijerph7103596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu Y., Kong G. T., Jia Q. Y., et al. Effects of soil properties on heavy metal accumulation in flowering chinese cabbage (brassica campestris L. ssp. chinensis var. utilis tsen et lee) in pearl river delta, China. Journal of Environmental Science and Health, Part B: Pesticides, Food Contaminants, and Agricultural Wastes. 2007;42(2):219–227. doi: 10.1080/03601230601125404. [DOI] [PubMed] [Google Scholar]
- 163.Zhu Y.-G. Soil science in the understanding of the security of food systems for health. Asia Pacific Journal of Clinical Nutrition. 2009;18(4):516–519. [PubMed] [Google Scholar]
- 164.Boul H. L. DDT residues in the environment—A review with a New Zealand perspective. New Zealand Journal of Agricultural Research. 1995;38(2):257–277. doi: 10.1080/00288233.1995.9513126. [DOI] [Google Scholar]
- 165.Mishra G. K. Microbes in heavy metal remediation: A review on current trends and patents. Recent Patents on Biotechnology. 2017;11(3):188–196. doi: 10.2174/1872208311666170120121025. [DOI] [PubMed] [Google Scholar]
- 166.Wu Q., Leung J. Y. S., Huang X., et al. Evaluation of the ability of black nightshade Solanum nigrum L. for phytoremediation of thallium-contaminated soil. Environmental Science and Pollution Research. 2015;22(15):11478–11487. doi: 10.1007/s11356-015-4384-z. [DOI] [PubMed] [Google Scholar]
- 167.Yu L., Zhu J., Huang Q., Su D., Jiang R., Li H. Application of a rotation system to oilseed rape and rice fields in Cd-contaminated agricultural land to ensure food safety. Ecotoxicology and Environmental Safety. 2014;108:287–293. doi: 10.1016/j.ecoenv.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 168.Guo J.-J., Tan X., Fu H.-L., et al. Selection for Cd Pollution-Safe Cultivars of Chinese Kale (Brassica alboglabra L. H. Bailey) and Biochemical Mechanisms of the Cultivar-Dependent Cd Accumulation Involving in Cd Subcellular Distribution. Journal of Agricultural and Food Chemistry. 2018;66(8):1923–1934. doi: 10.1021/acs.jafc.7b05123. [DOI] [PubMed] [Google Scholar]
- 169.Zhang R.-R., Liu Y., Xue W.-L., Chen R.-X., Du S.-T., Jin C.-W. Slow-release nitrogen fertilizers can improve yield and reduce Cd concentration in pakchoi (Brassica chinensis L.) grown in Cd-contaminated soil. Environmental Science and Pollution Research. 2016;23(24):25074–25083. doi: 10.1007/s11356-016-7742-6. [DOI] [PubMed] [Google Scholar]
- 170.Liao G., Wu Q., Feng R., et al. Efficiency evaluation for remediating paddy soil contaminated with cadmium and arsenic using water management, variety screening and foliage dressing technologies. Journal of Environmental Management. 2016;170:116–122. doi: 10.1016/j.jenvman.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 171.Rico A., Geng Y., Focks A., Van den Brink P. J. Modeling environmental and human health risks of veterinary medicinal products applied in pond aquaculture. Environmental Toxicology and Chemistry. 2013;32(5):1196–1207. doi: 10.1002/etc.2153. [DOI] [PubMed] [Google Scholar]
- 172.Maftoonazad N., Badii F. Use of edible films and coatings to extend the shelf life of food products. Recent Patents on Food, Nutrition & Agriculture. 2009;1(2):162–170. doi: 10.2174/2212798410901020162. [DOI] [PubMed] [Google Scholar]
- 173.Roshan D. R., Isaifan R. J. Household Air Pollution from Burning Biomass for Cooking and Heating – A Review of Health Hazards and Intervention Programs. Journal of Environmental Science and Pollution Research. 2018;4(3):289–296. doi: 10.30799/jespr.139.18040302. [DOI] [Google Scholar]