Abstract
Background
Pathogenic infection in broilers has become an important issue in the development of poultry industry. Xylooligosaccharides released from xylan via xylanase and fermented polysaccharide of Hericium caputmedusae (FPHC) have antimicrobial potential against many pathogens.
Objective
We aimed to explore the effects of xylanase and FPHC on pathogenic infection in the broilers (Gallus gallus domesticus).
Methods
Three hundred and thirty 21-day male broilers were assigned into four groups: control group (CG, basic diet), xylanase group (XG, basic diet + xylanase), FPHC group (HG, basic diet + FPHC), and XHG group (basic diet + xylanase + FPHC). Average daily feed intake (ADFI) and daily gain (ADG) were measured. Microflora from broiler feces was analyzed using 16S rRNA sequencing. Serum tumor necrosis factor- (TNF-) α, interleukin-1β (IL-1β), IL-1 receptor antagonist (IL-1ra), IL-10, total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) contents were detected using kits. The variables were compared using the Student t-test between two groups.
Results
Microbiological investigations showed that 75% of broilers were affected by bacterial pathogens in the CG group, most notably by coagulase-negative staphylococci. Comparatively, 15%, 26%, and 5% of broilers were affected by bacterial pathogens in the XG, HG, and XHG groups, respectively. Xylanase and FPHC treatment increased the ratio of ADG to ADFI and antioxidant capacity by increasing the levels of T-AOC, SOD, and GSH-Px and reducing the levels of MDA (P < 0.05). Xylanase and FPHC treatment improved anti-inflammatory capacity by increasing serum levels of IL-1ra and IL-10 and reducing the levels of IL-1β and TNF-α. On the other hand, the treatment increased probiotic concentration of Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum (P < 0.05), which were also proved in cell culture.
Conclusions
Xylanase and FPHC ameliorate pathogen infection by increasing antioxidant and anti-inflammatory activities of broilers via the increase of probiotics.
1. Introduction
Avian pathogens have become an important issue in the development of poultry industry. Antibiotic is often considered and immeasurable in poultry industry. However, antibiotic-resistant pathogens have become a public health issue and affect the composition of microbiota in poultry [1] and poultry production [2, 3]. It is highly demanded to explore antibiotic alternatives in poultry industry [4].
As emerging prebiotics, the production of xylooligosaccharide from biomass by xylanases was also widely reported [5–7]. The xylooligosaccharides, released from xylan via xylanase, have been proved to exhibit beneficial commensals by decreasing pathogenic bacteria and increasing bifidobacteria [5]. Xylooligosaccharides had antibacterial potential against many pathogens, including Klebsiella pneumoniae, Enterococcus faecalis [8], and Helicobacter pylori [9]. On the other hand, xylooligosaccharide has been reported to maintain gut flora balance by promoting the growth of probiotics, such as Lactobacillus spp. and Bifidobacterium spp., and eliminating enteric pathogens, such as Clostridium perfringens [10]. Polysaccharide from Hericium caputmedusae was also reported to improve gut microflora [11].
On the other hand, the composition of intestinal probiotics will affect broiler immune [12], intestinal microarchitecture [13], and microbial profiles [14]. Furthermore, probiotics have antioxidant [15] and anti-inflammatory [16] properties. Oligosaccharide can improve probiotic effect of intestinal flora [17], and probiotics can use xylooligosaccharides produced from xylan by xylanase [18].
Furthermore, xylooligosaccharide in diets can enhance the growth rate, modulate endocrine metabolism, and improve immune function in poultry [19]. Nonstarch polysaccharide was also found to improve the growth performance of poultry [20]. More work also showed that supplementing pelleted diets with thermoresistant multienzyme improved broiler performance [21]. However, little data are available for the effects of polysaccharide from H. caputmedusae and xylanase on pathogenic infection in broilers. Poultry infection has been found to be associated with antioxidant and anti-inflammatory activities. Therefore, the effects of xylanase and polysaccharides from Hericium caputmedusae on pathogenic infection in broiler were explored by investigating antioxidant and anti-inflammatory activities and changes of intestinal microbiota.
2. Materials and Methods
2.1. Broilers and Diets
Before the experiment, all procedures were approved by the Animal Research Committee of Jilin Agricultural University (approval no. 20150123JAUA1, Changchun, China). H. caputmedusae was purchased from Jilin University Pharmaceutical Factory (Changchun, China). Xylanase was purchased from Hunan New Century Biochemical Co. Ltd. (Yueyang, China). Three hundred and thirty 21-day male broilers (Gallus gallus domesticus) (0.9–1.0 kg) were purchased from Changchun Yongxu Animal Husbandry and Veterinary Company (Changchun, China), and randomly and evenly assigned into four groups according to different treatments (Table 1): control group (CG, the broilers received basic diet), xylanase group (XG, the broilers received basic diet and xylanase with activity of 1200 IU/kg), polysaccharide group (HG, the broilers received basic diet and 0.1% fermented polysaccharides H. caputmedusae (FPHC, w/w)), and xylanase combined with FPHC group (XHG, the broilers received basic diet, 1200 IU/kg of xylanase, and 0.1% FPHC (w/w)). Each of the groups was assigned into three subgroups with 30 broilers in a room (250 cm × 230 cm × 250 cm, length × width × height) and given ad libitum access to feed and water. Each room was controlled at 20°C. The basal diets were fed and prepared in a feed mill. A basal diet was formulated to meet the requirements by National Research Council (NRC) (Table 1, in the CG group) [22]. All broilers were reared in natural light and dark. Average daily gain, mortality, average daily feed intake (ADF), and feed-to-gain ratio were measured.
Table 1.
Ingredients and chemical composition of diets among different groups.
| Ingredients (g/kg) | CG | XG | HG | XHG |
|---|---|---|---|---|
| Corn | 595.0 | 595.0 | 595.0 | 595.0 |
| Soybean meal (47% CP) | 330.0 | 330.0 | 330.0 | 330.0 |
| Corn oil | 35.0 | 35.0 | 35.0 | 35.0 |
| CaHPO4·2H2O | 13.0 | 13.0 | 13.0 | 13.0 |
| Limestone | 13.0 | 13.0 | 13.0 | 13.0 |
| Xylanase (IU/kg)∗ | 0 | 1200 | 0 | 1200 |
| FPHC∗ | 0 | 0 | 1 | 1 |
| Salt | 3.0 | 3.0 | 3.0 | 3.0 |
| DL-Met | 1.0 | 1.0 | 1.0 | 1.0 |
| Premixa | 10.0 | 10.0 | 10.0 | 10.0 |
| Chemical analysis (g/kg) | ||||
| CP | 209.9 | 209.8 | 209.8 | 209.9 |
| Crude fiber | 23.2 | 23.3 | 23.2 | 23.3 |
| Ether extract | 55.2 | 55.3 | 55.2 | 55.2 |
| Crude ash | 62.3 | 62.5 | 62.4 | 62.4 |
| Ca | 8.8 | 8.9 | 8.9 | 8.8 |
| P | 3.5 | 3.6 | 3.6 | 3.6 |
| Calculated analysis | ||||
| ME (MJ/kg) | 13.3 | 13.3 | 13.3 | 13.3 |
| Lys (%) | 1.10 | 1.10 | 1.10 | 1.10 |
| Thr (%) | 0.78 | 0.78 | 0.78 | 0.78 |
| TSAA (%) | 0.83 | 0.83 | 0.83 | 0.83 |
Note: xylanase was added at 1200 IU/kg. FPHC: fermented polysaccharides of Hericium caputmedusae. aPremix provided the following per kilogram of diet: vitamin A (retinyl palmitate), 9000 IU; vitamin D3, 2000 IU; vitamin E (DL-α-tocopheryl acetate), 10.0 mg; vitamin K, 0.5 mg; vitamin B1, 1.8 mg; vitamin B6, 3.5 mg; vitamin B12, 0.01 mg; riboflavin, 3.6 mg; niacin, 35.0 mg; pantothenic acid, 10.0 mg; folic acid, 0.55 mg; biotin, 0.15 mg; choline chloride, 250 mg; Mn, 60.0 mg; Zn, 40.0 mg; Fe, 80.0 mg; Cu, 8.0 mg; I, 0.35 mg; and Se, 0.15 mg. CG: the broiler received basic diet; XG: the broiler received basic diet and 1200 IU/kg xylanase (Hunan New Century Biochemical Co. Ltd., Yueyang, China); HG: the broiler received basic diet and 0.1% polysaccharides from the fermentation extract of Hericium caputmedusae (FPHC, w/w); XHG: the broiler received basic diet, 1200 IU/kg of xylanase, and 0.1% FPHC (w/w). ∗P < 0.05 among four groups.
2.2. H. caputmedusae Culture
H. caputmedusae was transferred from a slant medium to a PDA medium and cultured in a 24°C incubator. After one-week culture, one cm2 of medium with mycelia was transferred to 400 mL of a liquid medium and cultured in a shaker for 10 d at 140 rpm and 25°C. After 30-day fermentation, the mycelium was separated from the fermentation broth and dried. The mycelium was broken using an ultrasonic device, and polysaccharides were isolated by adding distilled water and placed at room temperature for 2 h. The mixture was centrifuged at 12,000g for 10 min, and the supernatants were collected. The steps were repeated for 3 times.
2.3. Preparation of Polysaccharides from H. caputmedusae
Three-fold volume of 95% ethanol was added to the fermentation broth and placed at room temperature for 36 h. Precipitates were collected via centrifugation at 12,000g for 30 min. Protein was removed by adding the mixed solution (n-butanol : chloroform volume, 1 : 4) at 1 : 10 w/v. Finally, purified polysaccharide was obtained.
2.4. Sample Collection
On the days 21 and 42, eight broilers in each group were slaughtered by severing the jugular veins. Small intestinal contents were harvested immediately and transported to the laboratory for counting microbial colonies. Intestinal mucosa was nipped by forceps and rinsed in 0.85% saline solution. Mucosa was scraped by blunt side of surgical knife blades, collected in microtubes immediately, frozen in liquid nitrogen, and stored at −80°C until the next steps.
2.5. Biochemical Analysis
Approximately two-milliliter blood was taken from per broiler, and serum was prepared via centrifugation at 1500 rpm for 10 min. The levels of superoxide dismutase (T-SOD) [23], total antioxidant capacity (T-AOC) [24], glutathione peroxidase (GSH-Px) [25], and malondialdehyde (MDA) [26] have been reported as the biomarkers of oxidative stress. Therefore, the levels of all these molecules were measured in serum samples using the kits from Beyotime Institute of Biotechnology (Jiangsu, China). Inflammation is closely associated with the changes of the distribution of intestinal flora. Therefore, inflammatory situation was detected by measuring serum (tumor necrosis factor) TNF-α, (interleukin) IL-1β, IL-1 receptor antagonist (IL-1ra), and IL-10 via chicken ELISA Kit from Cusabio (College Park, MD, USA).
2.6. Amplification of 16S rRNA
Ten-milligram feces were collected from intestine of each broiler and diluted in water by 100-fold. Genomic DNA was extracted from the sample using a DNA Isolation Kit (Promega, Madison, WI, USA). DNA samples were analyzed using an ND-2000 spectrophotometer (NanoDrop Inc., Wilmington, DE, USA).
Isolated DNA was used as a template to amplify 16S rRNA gene regions using universal primers: forward primer, 5′-AGRGTTYGATYMTGGCTCAG-3′, and reverse primer, 5′-TTACCGCGGCTGCTGGCAC-3′. PCR mixture consisted of 39 μL ddH2O, 1 μL DNA genome, 5 μL 10x buffer, 0.5 μL Taq DNA polymerase (Takara, Dalian, China), 0.5 μL forward and reverse primers (20 μM), and 4 μL dNTP, in total of 50 μL. PCR was performed with the following condition: 95°C for 5 min, 30 cycles of 95°C sec for 20 sec, 55°C for 30 sec, 72°C for 1 min 30 sec, and 72°C for 5 min for final extension. PCR products were determined on 1% agarose gel and gel-purified.
2.7. Microbiota Analysis
Sample for bead-based sequencing was set up according to an earlier report [27] and sequenced using Roche 454 GS FLX platform on GS FLX instruments from Roche (Roche, Nutley, NJ, USA) [28]. The heat map of 16S rRNA gene sequences on the 21st day and 42nd day was created using Genomics Viewer at http://www.broadinstitute.org/igv. The R packages stats were used to perform statistical analysis. Simpson index and Shannon index were used to analyze the community diversity among different groups [29].
2.8. Microorganism
Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum were purchased from Institute of Microbiology, Chinese Academy of Sciences (Beijing, China). The seed was transferred to 100 mL of a basal medium with glucose 10.0 g/L, peptone 10.0 g/L, K2HPO4 1.0 g/L, MgSO4 0.2 g/L, and Na2CO3 5.0 g/L (pH 7.0) in a 250 mL Erlenmeyer flask. 1200 IU/kg of xylanase or 0.1% FPHC was added to the medium. The strains were cultured in a thermostatic orbital shaker for 48 h, at 37°C and 200 rpm. Samples were withdrawn at regular intervals, and probiotics were counted by serial dilution of the material in sterile distilled water and plating on a LB agar plate. The bacterial numbers were counted by observing the colony on the plate after one-day culture at 37°C.
2.9. Statistical Analysis
All data were presented as mean value ± S.D. The Student t-test was used to compare the variables between two groups using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). There is a significant difference if P < 0.05.
3. Results
3.1. Growth Performance
The statistical difference of the ratio of feed to gain was insignificant among four groups before the 21st day (Table 2, P > 0.05). Compared with CG, Table 2 showed that the broilers had a higher ratio of feed to gain when they received xylanase or FPHC (P < 0.05). The ratio was highest when the broilers received both xylanase and FPHC (P < 0.05). No mortality was observed during the whole experimental period.
Table 2.
Effects of xylanase and FPHC on growth performance of broilers (n = 8).
| Groups | ADG (g) | ADFI (g) | Feed/gain | |
|---|---|---|---|---|
| CG | 21 d | 35.8 ± 3.6 | 72 ± 8 | 1.85 ± 0.16 |
| 42 d | 71.0 ± 6.5 | 151 ± 13 | 2.13 ± 0.18b,c,d | |
|
| ||||
| XG | 21 d | 35.1 ± 3.3 | 70 ± 9 | 1.79 ± 0.15 |
| 42 d | 76.8 ± 6.1 | 148 ± 15 | 1.93 ± 0.17a,d | |
|
| ||||
| HG | 21 d | 36.1 ± 3.4 | 73 ± 7 | 1.90 ± 0.18 |
| 42 d | 77.5 ± 7.3 | 146 ± 12 | 1.88 ± 0.15a,d | |
|
| ||||
| XHG | 21 d | 35.5 ± 3.5 | 70 ± 6 | 1.87 ± 0.14 |
| 42 d | 81.9 ± 7.8 | 142 ± 12 | 1.73 ± 0.16a,b,c | |
Note: ADFI: average daily feed intake; ADG: average daily gain. Xylanase and FPHC were added from days 22 to 42. aP < 0.05 vs. a CG group; bP < 0.05 vs. a XG group; cP < 0.05 vs. a HG group; dP < 0.05 vs. a XHG group.
3.2. Xylanase and FPHC Treatment Increases the Numbers of Intestinal Bacterial Species of Broilers
The numbers of bacterial species of broilers were similar among four groups before the 21st day (Figure 1(a), P > 0.05). Xylanase and FPHC treatment increased the numbers of bacterial species of broilers while the number was reduced significantly in the control group on the 42nd day when compared with other groups (Figure 1(b), P < 0.05).
Figure 1.
The effects of xylanase and FPHC treatment on the numbers of intestinal bacterial species of broilers. (a) The numbers of bacterial species of broilers among four groups before the 21st day. (b) The numbers of bacterial species of broilers among four groups on the 42nd day.
3.3. Xylanase and FPHC Treatment Increases the Concentration of Probiotics
Heat map analysis showed that xylanase and FPHC treatment increased the concentration of probiotics, including Lactobacillus and Bacillus species. For other species, Anaerotruncus, Candidatus Arthromitus, Pseudomonas, Lachnospiraceae, Enterococcus, Stenotrophomonas, and Acinetobacter were increased in the control group while Lactococcus, Blautia, Subdoligranulum, Flavonifractor, and Lachnoclostridium were increased in xylanase and FPHC groups.
The concentration of L. plantarum [30], B. licheniformis [31], and B. subtilis [32] was measured in the intestine of broilers and compared on the 21st day and 42nd day using feces among four groups (Table 3). The statistical difference for the concentration was insignificant among four groups before the 21st day (Table 3, P > 0.05). Compared with the broilers in the control group, the concentration of these probiotics was higher in the xylanase or FPHC group on the 42nd day (P < 0.05). These results suggest that xylanase and FPHC treatment increases the concentration of probiotics.
Table 3.
Effect of xylanase and FPHC on the intestinal microbiota of broilers (n = 8).
| Item | Lactobacillus plantarum | Bacillus licheniformis | Bacillus subtilis | |
|---|---|---|---|---|
| CG | 21 d | 5.34 ± 0.39 | 2.68 ± 0.32 | 2.35 ± 1.29 |
| 42 d | 6.26 ± 0.83b,c,d | 3.51 ± 0.57b,c,d | 5.39 ± 0.64b,c,d | |
|
| ||||
| XG | 21 d | 5.05 ± 0.26 | 2.51 ± 0.30 | 2.89 ± 1.36 |
| 42 d | 7.04 ± 0.76a,c,d | 3.00 ± 0.69a,c,d | 5.58 ± 0.52a,c,d | |
|
| ||||
| HG | 21 d | 5.58 ± 0.31 | 2.55 ± 0.28 | 2.47 ± 1.15 |
| 42 d | 7.96 ± 0.75a,b,d | 5.44 ± 0.61a,b,d | 5.62 ± 0.49a,b,d | |
|
| ||||
| XHG | 21 d | 5.16 ± 0.35 | 2.62 ± 0.34 | 2.46 ± 1.21 |
| 42 d | 6.58 ± 0.87a,b,c | 4.73 ± 0.75a,b,c | 5.01 ± 0.32a,b,c | |
Note: From days 0 to 21, all groups received basal diets. Xylanase and FPHC were added from days 22 to 42. Bacterial numbers were represented as log10 cfu per gram of tissues. aP < 0.05 vs. a CG group; bP < 0.05 vs. a XG group; cP < 0.05 vs. a HG group; dP < 0.05 vs. a XHG group.
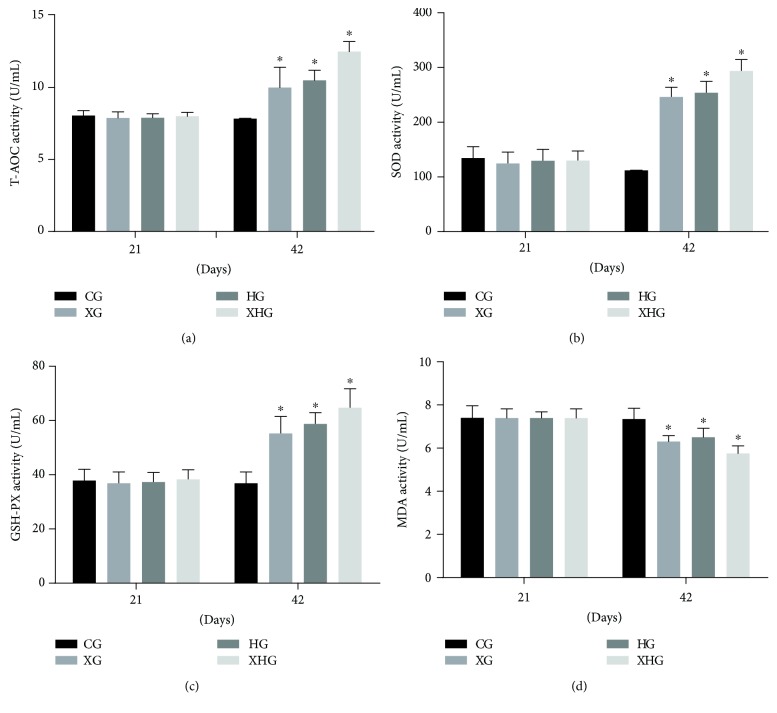
3.4. Xylanase and FPHC Treatment Increases Antioxidant Activities of Broilers
The antioxidant properties were measured by investigating the activities of T-AOC, SOD, GSH-px, and MDA in the intestine of broilers among four groups. Before xylanase and FPHC treatment, there was no significant difference for antioxidant activities among four groups (Figure 2, P > 0.05). Compared with the broilers in the CG group, the activities of T-AOC (Figure 2(a)), SOD (Figure 2(b)), and GSH-PX (Figure 2(c)) were increased in XG, HG, and XHG groups while the activity of MDA (Figure 2(d)) was reduced in XG, HG, and XHG groups after xylanase and FPHC treatment (P < 0.05). These results suggest that xylanase and FPHC treatment increases the antioxidant activities of broilers.
Figure 2.
The effects of xylanase and FPHC on antioxidant activities of broilers. (a) The effects of xylanase and FPHC on T-AOC activities of broilers. (b) The effects of xylanase and FPHC on SOD activities of broilers. (c) The effects of xylanase and FPHC on GSH-PX activities of broilers. (d) The effects of xylanase and FPHC on MDA activities of broilers.
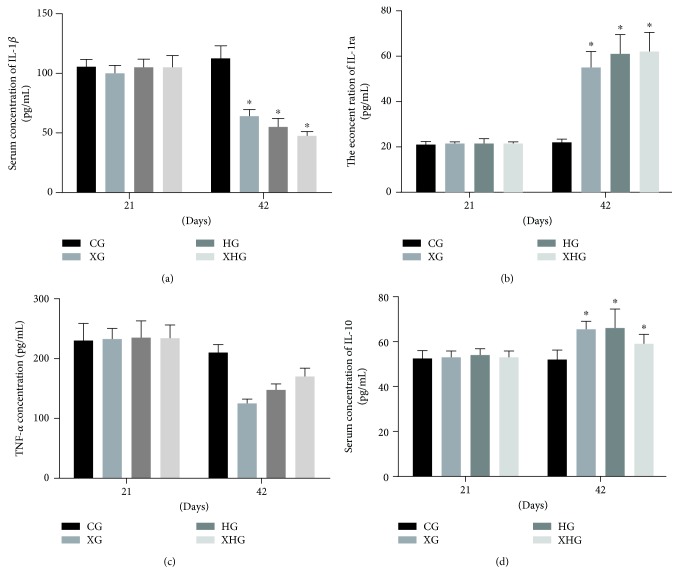
3.5. Xylanase and FPHC Treatment Increases Anti-Inflammatory Activities of Broilers
The anti-inflammatory properties were measured by investigating the serum concentrations of IL-1β, IL-1ra, TNF-α, and IL-10 in the intestine of broilers among four groups. Before xylanase and FPHC treatment, there was no significant difference for the concentrations of the cytokines among four groups (Figure 3, P > 0.05). Compared with the broilers in the control group, the concentrations of IL-1ra (Figure 3(b)) and IL-10 (Figure 3(d)) were increased in XG, HG, and XHG groups while the concentrations of IL-1β (Figure 3(a)) and TNF-α (Figure 3(c)) were reduced in the three groups after xylanase and FPHC treatment (P < 0.05). These results suggest that xylanase and FPHC treatment increases the anti-inflammatory activities of broilers.
Figure 3.
The effects of xylanase and FPHC on anti-inflammatory activities of broilers. (a) The effects of xylanase and FPHC on the concentration of IL-1β of broilers. (b) The effects of xylanase and FPHC on the concentration of IL-1ra of broilers. (c) The effects of xylanase and FPHC on the concentration of TNF-α of broilers. (d) The effects of xylanase and FPHC on the concentration of IL-10 of broilers.
3.6. Xylanase and Polysaccharide Reduce Pathogen Infection Rates of Broilers
Microbiological investigations demonstrated that 75% of broilers were affected by bacterial pathogens in the CG group, most notably by coagulase-negative staphylococci (Table 4). Comparatively, 15%, 26%, and 5% of broilers were affected by bacterial pathogens in the XG, HG, and XHG groups, respectively. These findings suggest that coagulase-negative staphylococci are prevalent in the local area. FPHC and xylanase can control and prevent bacterial pathogen prevalence.
Table 4.
Infected bacterial pathogens in broilers (cases).
| Intestinal pathogens | CG | XG | HG | XHG |
|---|---|---|---|---|
| Coagulase-pos. | ||||
| S. aureus | 5 | 3 | 4 | 1 |
| S. intermedius | 2 | 1 | 1 | 0 |
| Coagulase-neg. | 0 | |||
| S. lentus | 19 | 4 | 3 | 2 |
| S. simulans | 16 | 5 | 2 | 1 |
| S. cohnii | 10 | 3 | 2 | 1 |
| S. gallinarum | 5 | 3 | 3 | 2 |
| S. capitis | 4 | 2 | 1 | 0 |
| S. xylosus | 1 | 1 | 0 | 0 |
| S. hominis | 1 | 0 | 1 | 0 |
| S. auricularis | 1 | 1 | 1 | 0 |
| S. carnosus | 2 | 0 | 0 | 0 |
| S. caseolyticus | 2 | 0 | 0 | 0 |
| S. kloosi | 1 | 0 | 0 | 0 |
| S. epidermidis | 1 | 0 | 0 | 1 |
| S. arlettae | 1 | 0 | 1 | 0 |
| S. piscifermentans | 1 | 0 | 0 | 1 |
| Gram-positive | ||||
| Corynebacterium sp. | 3 | 1 | 1 | 1 |
| Stomatococcus sp. | 4 | 1 | 1 | 1 |
| Micrococcus sedentarius | 2 | 0 | 1 | 0 |
| Micrococcus varians | 1 | 0 | 0 | 0 |
| Micrococcus luteus | 2 | 0 | 1 | 0 |
| Streptococcus sp. | 1 | 0 | 0 | 0 |
| Gram-negative | ||||
| Escherichia coli | 6 | 1 | 2 | 1 |
| Moraxella sp. | 4 | 1 | 1 | 0 |
| Proteus mirabilis | 1 | |||
| Acinetobacter sp. | 2 | 2 | 1 | 1 |
| Pseudomonas sp. | 6 | 0 | 2 | 1 |
| Yersinia sp. | 2 | 1 | 1 | 0 |
Note: CG: the broiler received basic diet; XG: the broiler received basic diet and 1200 IU/kg xylanase; HG: the broiler received basic diet and 0.1% polysaccharides from the fermentation extract of Hericium caputmedusae (FPHC, w/w); XHG: the broiler received basic diet, 1200 IU/kg of xylanase, and 0.1% FPHC (w/w). CG: basic diet; XG: basic diet + xylanase; HG: basic diet + FPHC; and XHG: basic diet + xylanase + FPHC.
3.7. Xylanase and FPHC Treatment Promoted the Growth of Probiotics
The growth-promoting properties of xylanase and FPHC were measured using B. licheniformis, B. subtilis, and L. plantarum via cell culture. At 0-hour culture, there was no significant difference for cell concentration among three species (Figure 4, P > 0.05). At 24-hour culture, xylanase and FPHC treatment increased the cell concentrations of B. licheniformis (Figure 4(a)), B. subtilis (Figure 4(b)), and L. plantarum (Figure 4(c)) when compared with the controls (P < 0.05). At 48-hour culture, xylanase and FPHC treatment also further increased more cell concentrations than controls (Figure 4, P < 0.05). These results suggested that xylanase and FPHC treatment promoted the growth of these probiotics.
Figure 4.
The effects of xylanase and FPHC treatment on the growth of probiotics. (a) The effects of xylanase and FPHC treatment on the growth of Bacillus licheniformis. (b) The effects of xylanase and FPHC treatment on the growth of Bacillus subtilis. (c) The effects of xylanase and FPHC treatment on the growth of Lactobacillus plantarum. ∗P < 0.05 vs. a control group.
4. Discussion
Xylanase and FPHC have a beneficial effect on the physiology, health, and productivity of broilers. Early studies demonstrated that xylanase resulted in higher weight gain in broilers when compared to controls without xylanase addition [33]. The diets with xylanase will affect the animal growth rate by improving the utilization of nutrient [34]. Xylanase prolongs the retention time of fiber in the intestinal tract, and more nutrient can be absorbed. Furthermore, longer duration of fiber in the intestinal tract will result in better microbial adaptation [35].
It has been well known that the intestinal microbiota is an important determinant for gastrointestinal health of broilers. Probiotics have the potential to improve the beneficial bacteria and inhibit pathogenic bacteria. Supplementation of prebiotic will eliminate pathogenic bacteria and increase probiotics, which have beneficial effects on broiler growth and immune-related gene expression [12]. Probiotics also provide protection against bacterial infection [36]. B. subtilis supplementation in diet will affect the diversity, composition, and functional diversity of the fecal microbiota in broiler [37]. B. licheniformis improves the growth and antioxidant abilities of broilers. Meanwhile, the probiotics can affect the expression of genes associated with fatty acid synthesis and oxidation [38]. L. plantarum can effectively replace in-feed antibiotic and improve the intestinal health by changing intestinal villus morphology and inhibiting the pathogenic loading [39]. Lactobacillus species effectively absorb and expel heavy metal toxicity from the gastrointestinal tract of broilers [40]. The present study showed that xylanase and FPHC treatment increased the concentrations of L. plantarum, B. licheniformis, and B. subtilis in the small intestine (Table 3). B. licheniformis and B. subtilis are aerobes and use oxygen in the intestine, resulting in an oxygen-free environment for the proliferation of anaerobic probiotics such as Lactobacillus. The probiotics will produce more acidic environments, which control the growth of potential pathogens.
Most diseases of broilers are associated with oxidative damage [41]. The studies focused on antioxidant molecules in broilers. Dietary antioxidants can minimize the negative effect of oxidized oil on meat qualities of broilers [42]. Our results showed that xylanase and FPHC treatment increased the level of T-AOC, SOD, and GSH-PX and reduced the level of MDA (Figure 2). These changes had beneficial effects on broilers by improving their antioxidant activities.
Reducing enteric inflammation and maintaining intestinal homeostasis are very important to improve the growth of broilers. Probiotics can improve immunomodulatory activity and are effective in controlling Salmonella colonization, invasion, and the induced inflammation [43]. The levels of lymphocyte phenotypes (including B and T lymphocytes) and plasma immunoglobulin in broilers are also associated with their infected diseases [44]. Present findings demonstrated that xylanase and FPHC treatment increased the anti-inflammatory activities of broilers by increasing the levels of IL-1ra and IL-10 and reducing the levels of IL-1β and TNF-α (Figure 3). Furthermore, cell culture showed that xylanase and FPHC treatment also promoted the growth of probiotics (Figure 4).
T-AOC is the antioxidant capacity of the body's defense system and can fully reflect the antioxidant capacity of both enzyme and nonenzymatic systems. Although it does not clearly represent the activity of an antioxidant or antioxidant enzyme, it reflects antioxidant capacity better than a single indicator. SOD, one of the members of the enzymatic system, specifically and efficiently scavenges superoxide radicals. It is the only enzyme known to directly eliminate O2− and protect cells. This study showed that xylanase + FPHC treatment increased serum T-AOC levels, SOD, and GSH-PX activity and reduced the serum MDA level. MDA is one of the most toxic lipid peroxides, and it can not only destroy the membrane structure and membrane protein function and affect the function and metabolism of nucleic acids but also cause autoimmune disorders. Therefore, the determination of MDA can reflect the degree of lipid peroxidation and help to understand damage degree of tissue and cells. Xylanase and FPHC not only have a wide range of activity and diversity but also have abundant sources, low costs, and good safety. They have a good application prospect in animal husbandry production.
There are some limitations of the present work: (1) the changes for tissue morphology and intestinal barriers were not investigated here. The work only reflected the changes for inflammatory and infection situations in broilers; (2) the functions of most species of the microbiota in broilers were not analyzed in the present study; (3) the effects of xylanase and HPFC on antioxidant and anti-inflammatory signaling pathway were not explored either. Thus, further work is still needed to perfect present results in the future.
5. Conclusions
Xylanase and FPHC can effectively increase the serum T-AOC, SOD, and GSH-PX activity and reduce the MDA content to improve the broiler's antioxidant activities. Xylanase and FPHC treatment also maintained intestinal species in a healthy situation. Meanwhile, the addition of xylanase and FPHC in diet increased broiler's anti-inflammatory capacity by increasing the levels of IL-1ra and IL-10 and reducing the levels of IL-1β and TNF-α. Broilers were affected by bacterial pathogens, most notably by coagulase-negative staphylococci. Xylanase and FPCH treatment ameliorated pathogen infection of broilers by increasing the amounts of probiotics B. licheniformis, B. subtilis, and L. plantarum. For poultry, because of its special digestive tract structure characteristics, the role of the antioxidant effect of xylanase and FPHC may be different and remains to be studied.
Acknowledgments
We are grateful to Prof. Bo Liu and his group for the assistance with the use of instruments. The project was supported by the program of Agricultural Science and Technology Achievements Transformation of Ministry of Science and Technology (no. 2013G B2B100110) and Jilin Province Comprehensive Utilization of Straw Technology Innovation Platform under special foundation (2014C-1).
Data Availability
The data for the current study are available from the corresponding author upon reasonable request.
Ethical Approval
Before the experiment, all procedures were approved by the Animal Research Committee of Jilin Agricultural University (Changchun, China, approval no. 20150123JAUA1).
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
SZ and CW designed and performed the experiment. YS and GW drafted the manuscript. HC contributed to manuscript revision. DL carried out the data analysis. XY and GC revised the manuscript.
References
- 1.Seitz M., Valentin-Weigand P., Willenborg J. Use of antibiotics and antimicrobial resistance in veterinary medicine as exemplified by the swine pathogen Streptococcus suis. Current Topics in Microbiology and Immunology. 2016;398:103–121. doi: 10.1007/82_2016_506. [DOI] [PubMed] [Google Scholar]
- 2.Park J. W., Jeong J. S., Lee S. I., Kim I. H. Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens. Poultry Science. 2016;95(12):2829–2835. doi: 10.3382/ps/pew241. [DOI] [PubMed] [Google Scholar]
- 3.Shen X., Yi D., Ni X., et al. Effects of Lactobacillus plantarum on production performance, immune characteristics, antioxidant status, and intestinal microflora of bursin-immunized broilers. Canadian Journal of Microbiology. 2014;60(4):193–202. doi: 10.1139/cjm-2013-0680. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y., Iji P. A., Choct M. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World's Poultry Science Journal. 2009;65(1):97–114. doi: 10.1017/S0043933909000087. [DOI] [Google Scholar]
- 5.Nieto-Domínguez M., de Eugenio L. I., York-Durán M. J., et al. Prebiotic effect of xylooligosaccharides produced from birchwood xylan by a novel fungal GH11 xylanase. Food Chemistry. 2017;232:105–113. doi: 10.1016/j.foodchem.2017.03.149. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopalan G., Shanmugavelu K., Yang K. L. Production of prebiotic-xylooligosaccharides from alkali pretreated mahogany and mango wood sawdust by using purified xylanase of Clostridium strain BOH3. Carbohydrate Polymers. 2017;167:158–166. doi: 10.1016/j.carbpol.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Chang S., Guo Y., Wu B., He B. Extracellular expression of alkali tolerant xylanase from Bacillus subtilis Lucky9 in E. coli and application for xylooligosaccharides production from agro-industrial waste. International Journal of Biological Macromolecules. 2017;96:249–256. doi: 10.1016/j.ijbiomac.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Kallel F., Driss D., Chaabouni S. E., Ghorbel R. Biological activities of xylooligosaccharides generated from garlic straw xylan by purified xylanase from Bacillus mojavensis UEB-FK. Applied Biochemistry and Biotechnology. 2015;175(2):950–964. doi: 10.1007/s12010-014-1308-1. [DOI] [PubMed] [Google Scholar]
- 9.Christakopoulos P., Katapodis P., Kalogeris E., et al. Antimicrobial activity of acidic xylo-oligosaccharides produced by family 10 and 11 endoxylanases. International Journal of Biological Macromolecules. 2003;31(4-5):171–175. doi: 10.1016/S0141-8130(02)00079-X. [DOI] [PubMed] [Google Scholar]
- 10.Lin S. H., Chou L. M., Chien Y. W., Chang J. S., Lin C. I. Prebiotic effects of xylooligosaccharides on the improvement of microbiota balance in human subjects. Gastroenterology Research and Practice. 2016;2016:6. doi: 10.1155/2016/5789232.5789232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang H. M., Song H., Wang L. N., et al. Effects of dietary polysaccharides from the submerged fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. on performance, gut microflora, and cholesterol metabolism in broiler chickens. Livestock Science. 2014;167:276–285. doi: 10.1016/j.livsci.2014.07.004. [DOI] [Google Scholar]
- 12.Pender C. M., Kim S., Potter T. D., Ritzi M. M., Young M., Dalloul R. A. In ovo supplementation of probiotics and its effects on performance and immune-related gene expression in broiler chicks. Poultry Science. 2017;96(5):1052–1062. doi: 10.3382/ps/pew381. [DOI] [PubMed] [Google Scholar]
- 13.Ashraf S., Zaneb H., Yousaf M. S., et al. Effect of dietary supplementation of prebiotics and probiotics on intestinal microarchitecture in broilers reared under cyclic heat stress. Journal of Animal Physiology and Animal Nutrition. 2013;97:68–73. doi: 10.1111/jpn.12041. [DOI] [PubMed] [Google Scholar]
- 14.Zhang T., Xie J., Zhang M., Fu N., Zhang Y. Effect of a potential probiotics Lactococcus garvieae B301 on the growth performance, immune parameters and caecum microflora of broiler chickens. Journal of Animal Physiology and Animal Nutrition. 2016;100(3):413–421. doi: 10.1111/jpn.12388. [DOI] [PubMed] [Google Scholar]
- 15.Spyropoulos B. G., Misiakos E. P., Fotiadis C., Stoidis C. N. Antioxidant properties of probiotics and their protective effects in the pathogenesis of radiation-induced enteritis and colitis. Digestive Diseases and Sciences. 2011;56(2):285–294. doi: 10.1007/s10620-010-1307-1. [DOI] [PubMed] [Google Scholar]
- 16.Rachmilewitz D., Katakura K., Karmeli F., et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126(2):520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Bomba A., Nemcova R., Gancarcikova S., Herich R., Guba P., Mudronova D. Improvement of the probiotic effect of micro-organisms by their combination with maltodextrins, fructo-oligosaccharides and polyunsaturated fatty acids. British Journal of Nutrition. 2002;88(S1):S95–S99. doi: 10.1079/BJN2002634. [DOI] [PubMed] [Google Scholar]
- 18.Chapla D., Pandit P., Shah A. Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresource Technology. 2012;115:215–221. doi: 10.1016/j.biortech.2011.10.083. [DOI] [PubMed] [Google Scholar]
- 19.Zhenping S., Wenting L., Ruikui Y., et al. Effect of a straw-derived xylooligosaccharide on broiler growth performance, endocrine metabolism, and immune response. Canadian Journal of Veterinary Research. 2013;77(2):105–109. [PMC free article] [PubMed] [Google Scholar]
- 20.Montanhini Neto R., N'Guetta E., Gady C., Francesch M., Preynat A. Combined effect of using near-infrared spectroscopy for nutritional evaluation of feed ingredients and non-starch polysaccharide carbohydrase complex on performance of broiler chickens. Animal Science Journal. 2017;88(12):1979–1986. doi: 10.1111/asj.12822. [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi Gheisar M., Hosseindoust A., Kim I. H. Effects of thermo-resistant non-starch polysaccharide degrading multi-enzyme on growth performance, meat quality, relative weights of body organs and blood profile in broiler chickens. Journal of Animal Physiology and Animal Nutrition. 2016;100(3):499–505. doi: 10.1111/jpn.12387. [DOI] [PubMed] [Google Scholar]
- 22.NRC. Nutrient Requirements of Poultry. Washington, DC, USA: National Academy Press; 1994. [Google Scholar]
- 23.Dziegielewska-Gesiak S., Wysocka E., Michalak S., Nowakowska-Zajdel E., Kokot T., Muc-Wierzgon M. Role of lipid peroxidation products, plasma total antioxidant status, and Cu-, Zn-superoxide dismutase activity as biomarkers of oxidative stress in elderly prediabetics. Oxidative Medicine and Cellular Longevity. 2014;2014:8. doi: 10.1155/2014/987303.987303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peluso I., Raguzzini A. Salivary and urinary total antioxidant capacity as biomarkers of oxidative stress in humans. Pathology Research International. 2016;2016:14. doi: 10.1155/2016/5480267.5480267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendall A., Woolcock A., Brooks A., Moore G. E. Glutathione peroxidase activity, plasma total antioxidant capacity, and urinary F2-isoprostanes as markers of oxidative stress in anemic dogs. Journal of Veterinary Internal Medicine. 2017;31(6):1700–1707. doi: 10.1111/jvim.14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mumcu U. Y., Kocer I., Ates O., Alp H. H. Decreased paraoxonase1 activity and increased malondialdehyde and oxidative DNA damage levels in primary open angle glaucoma. International Journal of Ophthalmology. 2016;9(10):1518–1520. doi: 10.18240/ijo.2016.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Shehri S. S., Sweeney E. L., Cowley D. M., et al. Deep sequencing of the 16S ribosomal RNA of the neonatal oral microbiome: a comparison of breast-fed and formula-fed infants. Scientific Reports. 2016;6(1, article 38309) doi: 10.1038/srep38309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G. D., Lewis J. D., Hoffmann C., et al. Sampling and pyrosequencing methods for characterizing bacterial communities in the human gut using 16S sequence tags. BMC Microbiology. 2010;10(1):p. 206. doi: 10.1186/1471-2180-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill T. C. J., Walsh K. A., Harris J. A., Moffett B. F. Using ecological diversity measures with bacterial communities. FEMS Microbiology Ecology. 2003;43(1):1–11. doi: 10.1111/j.1574-6941.2003.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 30.Xie J., Yu Q., Nie S., Fan S., Xiong T., Xie M. Effects of Lactobacillus plantarum NCU116 on intestine mucosal immunity in immunosuppressed mice. Journal of Agricultural and Food Chemistry. 2015;63(51):10914–10920. doi: 10.1021/acs.jafc.5b04757. [DOI] [PubMed] [Google Scholar]
- 31.Girija V., Malaikozhundan B., Vaseeharan B., et al. In vitro antagonistic activity and the protective effect of probiotic Bacillus licheniformis Dahb1 in zebrafish challenged with GFP tagged Vibrio parahaemolyticus Dahv2. Microbial Pathogenesis. 2018;114:274–280. doi: 10.1016/j.micpath.2017.11.058. [DOI] [PubMed] [Google Scholar]
- 32.Li Z., Wang W., Lv Z., Liu D., Guo Y. Bacillus subtilis and yeast cell wall improve the intestinal health of broilers challenged by Clostridium perfringens. British Poultry Science. 2017;58(6):635–643. doi: 10.1080/00071668.2017.1370697. [DOI] [PubMed] [Google Scholar]
- 33.Cozannet P., Kidd M. T., Montanhini Neto R., Geraert P. A. Next-generation non-starch polysaccharide-degrading, multi-carbohydrase complex rich in xylanase and arabinofuranosidase to enhance broiler feed digestibility. Poultry Science. 2017;96(8):2743–2750. doi: 10.3382/ps/pex084. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Ortiz G., Sola-Oriol D., Martinez-Mora M., Perez J. F., Bedford M. R. Response of broiler chickens fed wheat-based diets to xylanase supplementation. Poultry Science. 2017;96(8):2776–2785. doi: 10.3382/ps/pex092. [DOI] [PubMed] [Google Scholar]
- 35.Kiarie E., Walsh M. C., Romero L. F., Arent S., Ravindran V. Nutrient and fiber utilization responses of supplemental xylanase in broiler chickens fed wheat based diets are independent of the adaptation period to test diets. Poultry Science. 2017;96(9):3239–3245. doi: 10.3382/ps/pex100. [DOI] [PubMed] [Google Scholar]
- 36.Pender C. M., Kim S., Potter T. D., Ritzi M. M., Young M., Dalloul R. A. Effects of in ovo supplementation of probiotics on performance and immunocompetence of broiler chicks to an Eimeria challenge. Beneficial Microbes. 2016;7(5):699–705. doi: 10.3920/BM2016.0080. [DOI] [PubMed] [Google Scholar]
- 37.Oh J. K., Pajarillo E. A. B., Chae J. P., Kim I. H., Yang D. S., Kang D. K. Effects of Bacillus subtilis CSL2 on the composition and functional diversity of the faecal microbiota of broiler chickens challenged with Salmonella Gallinarum. Journal of Animal Science and Biotechnology. 2017;8(1):p. 1. doi: 10.1186/s40104-016-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou M., Zeng D., Ni X., et al. Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Lipids in Health and Disease. 2016;15(1):p. 48. doi: 10.1186/s12944-016-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vineetha P. G., Tomar S., Saxena V. K., Kapgate M., Suvarna A., Adil K. Effect of laboratory-isolated Lactobacillus plantarum LGFCP4 from gastrointestinal tract of guinea fowl on growth performance, carcass traits, intestinal histomorphometry and gastrointestinal microflora population in broiler chicken. Journal of Animal Physiology and Animal Nutrition. 2017;101(5):e362–e370. doi: 10.1111/jpn.12613. [DOI] [PubMed] [Google Scholar]
- 40.Jahromi M. F., Liang J. B., Ebrahimi R., et al. Protective potential of Lactobacillus species in lead toxicity model in broiler chickens. Animal. 2017;11(5):755–761. doi: 10.1017/S175173111600224X. [DOI] [PubMed] [Google Scholar]
- 41.Song Z., Lv J., Sheikhahmadi A., Uerlings J., Everaert N. Attenuating effect of zinc and vitamin E on the intestinal oxidative stress induced by silver nanoparticles in broiler chickens. Biological Trace Element Research. 2017;180(2):306–313. doi: 10.1007/s12011-017-1016-0. [DOI] [PubMed] [Google Scholar]
- 42.Delles R. M., True A. D., Ao T., Dawson K. A., Xiong Y. L. Fibre type-dependent response of broiler muscles to dietary antioxidant supplementation for oxidative stability enhancement. British Poultry Science. 2016;57(6):751–762. doi: 10.1080/00071668.2016.1232479. [DOI] [PubMed] [Google Scholar]
- 43.Chen C. Y., Tsen H. Y., Lin C. L., Yu B., Chen C. S. Oral administration of a combination of select lactic acid bacteria strains to reduce the Salmonella invasion and inflammation of broiler chicks. Poultry Science. 2012;91(9):2139–2147. doi: 10.3382/ps.2012-02237. [DOI] [PubMed] [Google Scholar]
- 44.Honda B. T. B., Calefi A. S., Costola-de-Souza C., et al. Effects of heat stress on peripheral T and B lymphocyte profiles and IgG and IgM serum levels in broiler chickens vaccinated for Newcastle disease virus. Poultry Science. 2015;94(10):2375–2381. doi: 10.3382/ps/pev192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for the current study are available from the corresponding author upon reasonable request.