Abstract
Multidrug-resistant pathogens are a significant clinical problem. Efflux pump inhibitors (EPIs) can restore the activities of existing antibiotics by interfering with drug efflux pumps located in bacterial cell membranes. Seaweeds are important sources of biologically active metabolites of natural origin; however, their potential as EPIs remains uninvestigated. Here, functional extracts from the brown seaweeds Laminaria japonica and Sargassum horneri and the red seaweeds Gracilaria sp. and Porphyra dentata were evaluated as potential EPIs against drug-resistant Escherichia coli. All these extracts were found to potentiate the activities of drugs in modulation tests, although not to the same extent. Synergistic effects of the extracts and the drug clarithromycin were observed from the onset of Time-kill assays, with no evidence of bacterial regrowth. Ethidium bromide accumulation studies revealed that the efflux decreased in the presence of each extract, as indicated by the presence of EPIs. Most identified EPIs that have been discovered to date have aromatic structures, and the seaweed extracts were found to contain various terpenes, terpenoids, phenolic compounds, indoles, pyrrole derivatives, alkaloids, and halogenated aromatic compounds. Our study highlights the potential of these compounds of the seaweeds as drug EPIs.
1. Introduction
Multidrug-resistant (MDR) pathogens are a significant clinical problem. O'Neill [1], commissioned by the British government, estimated that, by 2050, global deaths due to drug-resistant infections will increase from 700,000 to 10 million annually and that the global economic loss may reach US$100 trillion. Attempts by the pharmaceutical industry to reverse this trend have achieved limited success, and the development and release of novel antimicrobial agents have faltered [2, 3]. Efflux pumps in bacteria are major contributors to drug resistance; they extrude a broad spectrum of antibiotics to the exterior of the organism. Hence, infections caused by these pathogens can be difficult to treat [4]. For example, the inner membrane transporter AcrB (resistance-nodulation-division family) is the major MDR efflux pump in Escherichia coli and can assemble with a periplasmic adaptor protein AcrA and an outer membrane factor TolC. This assembly confers drug resistance by translocating various types of antibiotics, such as macrolides [5], tetracyclines [6, 7], fluoroquinolones [8], and β-lactams [9], across the inner and outer membranes [10–12]. The use of EPIs has been rapidly gaining attention as a novel approach to treat infections caused by pathogens expressing MDR efflux pumps [13, 14]. Their use in adjunctive therapy may restore the activities of existing antibiotics by interfering with efflux pumps, thereby allowing therapeutically ineffective antibiotics to be reintroduced into clinical practice. EPIs may affect the function of efflux pumps by (i) regulating the expression of the pump, (ii) inhibiting the functional assembly of the membrane transporter complex involved in drug efflux, (iii) interfering with the energy required for active drug transport, and (iv) inhibiting drug transport via competitive/noncompetitive binding or by physically blocking the efflux channel [15]. Several classes of EPIs, including antibiotic (tetracyclines, aminoglycosides, and fluoroquinolones) analogs [16–18], amide derivatives (aromatic nitrogen-containing compounds) [19, 20], indoles [21], alkaloids [22–24]), flavonoids [25, 26], aromatic ketones [27], terpenes [28], and oligosaccharides [29], have been reported to date. Several well-known EPIs, such as phenylalanine-arginine β-naphthylamide (PAβN) [30], carbonyl cyanide m-chlorophenylhydrazone (CCCP) [31], verapamil [32], and reserpine [33], have seen limited clinical use and development owing to their toxicity [14]. Plants are a promising source of novel EPIs owing to their low toxicity and compound diversity. In addition, plants are natural, sustainable, and largely unexplored [34]. Capsaicin (8-methyl-N-vanillyl-6-nonenamide), extracted from hot chilies (genus Capsicum), has been shown to significantly reduce the minimum inhibitory concentration of ciprofloxacin by targeting the transporter NorA of Staphylococcus aureus strains [35]. Piperine, a plant alkaloid found in black pepper (Piper nigrum) and long pepper (Piper longum) and belonging to the family Piperaceae, potentiates the activity of ciprofloxacin against S. aureus strains expressing drug efflux pumps [22, 36]. Lanatoside C and daidzein were chosen from a phytochemical database via in silico screening. Both compounds were shown to potentiate the activities of levofloxacin and carbenicillin and increase the accumulation of ethidium bromide (EB) in E. coli [37]. Seaweeds have been used as food and medicine since centuries. They have a short generation cycle and can be easily cultivated in various aquatic environments [38, 39]. Seaweeds are an important source of biologically active metabolites owing to their availability, diversity, and productivity [40]. They are rich in phenolics [41], terpenoids [42], alkaloids [43], flavonoids [44], and polysaccharides [45], some of which possess specific characteristics that are rare or absent in terrestrial plants. Seaweeds are thought to possess antioxidant [46], antimicrobial [47], anti-inflammation [48, 49], antidiabetic [50, 51], and anticancer activities [52]. Despite these factors, their potential as EPIs has not yet been evaluated. Gram-negative pathogen E. coli is one of the most troublesome clinical bacterial species. Moreover, to date, very few EPIs for Gram-negative bacteria have been reported [53]. To our knowledge, this study was the first to evaluate the potential of ethanolic extracts of the brown seaweeds Laminaria japonica and Sargassum horneri and red seaweeds Gracilaria sp. and Porphyra dentata as EPIs against drug-resistant E. coli.
2. Materials and Methods
2.1. Preparation of Ethanolic Extracts from Seaweeds
L. japonica, Gracilaria sp., and P. dentata were purchased from a local market in Chao-Ching Park. S. horneri was harvested in May 2017 along the north east coast of Taiwan. The seaweeds were washed, air-dried (50°C), ground, and sieved through 0.25 mm pores and stored in a freezer until use. The ethanolic seaweed extracts were obtained by maceration. In brief, the seaweed powders were soaked in 95% ethanol (solid/solvent ratio = 1/10) with slow stirring at room temperature for 24 h for extraction. The mixture was filtered through, and the filtered algal residue was extracted once again using the previous procedure. The ethanolic extracts were obtained by rotary evaporation and lyophilization and stored in dark at -20°C until use.
2.2. Bacterial Strains, Media, and Chemicals
E. coli Kam3 (DE3) which is the acrB deletion strain was used in drug susceptibility, modulation and drug accumulation assays [54]. The bacteria were grown in Luria-Bertani broth (LB) and Mueller-Hinton broth (MH broth) for cultivation and broth microdilution experiments.
2.3. Cloning of E. coli Efflux Pump AcrB
acrB gene was cloned from the E. coli K12 chromosome by using PCR method. The acrB gene amplified by using primers 5′-AAAACCCATATG1CCTAATTTCTTTATCGATCGCC-3′ and 5′-AAAACCGTCGAC2TCAATGATGATCGACAGTAT-3′ which was digested with NdeI and XhoI restriction enzymes and inserted onto pSYC vector (pQE100 derivative, T5 promoter) at the NdeI-XhoI site. The pSYC plasmid encoding acrB was transform into E. coli Kam3 (DE3) in drug susceptibility, modulation, and drug accumulation assays. The ampicillin (100 μg/mL) was used in the experiments.
2.4. IC50 and Modulation Tests
The IC50 experiments and modulation tests were carried out as previously described with some modifications [55]. The IC50 of the antibiotic erythromycin, clarithromycin, and tetracycline, and the ethanolic seaweed extracts against drug-sensitive and drug-resistant E. coli strains were determined by using microdilution methods (MH broth), individually, with an inoculum of logarithmic-phase cells (cell density of an OD600 of 0.05 to 0.1). For the modulation tests, the IC50 of the antibiotic erythromycin, clarithromycin, and tetracycline were determined in the presence of 1/2 or 1/4 IC50 of the seaweed extracts, individually. 500 μg/mL was chosen as the IC50 concentration of the seaweed extracts in the modulation assays when the IC50 > 500 μg/mL.
2.5. Time-Kill Assays
The Time-kill experiments were carried out according to a previous study [27], with some modifications. Time-kill study of clarithromycin (1/2 IC50) alone or in the presence of seaweed extract (IC50, 1/2 IC50 or 1/4 IC50) was performed in 50 ml volume conical flasks containing 20 ml E. coli cells (7 Log CFU/mL). 500 μg/mL were chosen as the IC50 concentration for the seaweed extracts in the Time-kill assays when the IC50 > 500 μg/mL. Each analysis was done in triplicate with a control without seaweed extract.
2.6. EB Accumulation Assay
The EB accumulation assay was performed according to previous studies [56, 57], with the following modifications. The E. coli cells were grown to mid-log phase in MH broth and collected by centrifugation (5000 × g, 5 min and 4°C). The cells were resuspended twice in phosphate buffered saline (PBS) (10 mM Na2HPO4, 1.8 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, 1 mM CaCl2, and 0.5 mM MgCl2 at pH 7.4) and diluted in PBS in a final OD600 of 0.5. The cell suspension was incubated in 96 well plate with the filter-sterilized glucose to a final concentration of 25 mM at room temperature for 3 min. The EB was added to a final concentration of 25 μM and the fluorescence was measured over 38 min using at excitation and emission wavelengths of 520 nm and 600 nm. The effects of CCCP (final concentration of 20 μg/mL) and algal ethanolic extracts (1/2 IC50), individually, were added to bacterial suspension before the fluorescence was measured.
2.7. Gas-Chromatography-Mass Spectrometry
Gas Chromatography tandem Mass Spectrometry (GC-MS/MS) was performed by a PolarisQ Ion Trap GC-MS/MS system with a split/splitless injector and Equity®-5 capillary GC column (L × I.D. 30 m × 0.25 mm, df 0.25 μm). The carrier gas used was Helium (He) at a flow rate of 1 ml/min, and the injection port was maintained at 280°C. The temperature programming at 50°C for 2 min and increased at a rate of 15°C/min until 250°C and held at 250°C for 2 min. Electron impact ionization (EI) of the GC column eluents screening ranges from 100 to 1000 m/z, and the mass spectrometry data was analyzed by database provided by National Institute of Standards and Technology.
2.8. Statistical Analysis
Data are analyzed statistically by using SPSS version 12 (Chicago, IL, USA) and presented as means ± standard deviation. One-way analysis of variance (ANOVA) was used to determine statistical differences between samples means, with the level of significance set at p < 0.05, and multiple comparisons of means were accomplished by Tukey test.
3. Results and Discussion
3.1. Ethanol Extraction of the Four Seaweeds and Their IC50 against Drug-Susceptible and Drug-Resistant E. coli
The chemical structures of several EPIs derived from natural sources have been identified. These include the alkaloids reserpine [58] and piperine [22], the flavonolignans 5′-methoxyhydnocarpin [59] and silybin [60], the flavones baicalein [61] and chrysosplenol-D [62], and the diterpene carnosol [63]. These compounds were initially extracted using organic solvents owing to their lipophilic nature. We used ethanol to extract potential EPIs from L. japonica, S. horneri, Gracilaria sp., and P. dentata and obtained yields of 5.2%, 4.3%, 6.3%, and 3.9%, respectively (Table 1). This is in agreement with the findings of Dickson et al. [64], who used ethanol to extract drug-potentiating substances from the terrestrial plants Microglossa pyrifolia, Mezoneuron benthamianum, and Securinega virosa; they obtained yields of 5.9%, 4.3%, and 3.6%, respectively.
Table 1.
Ethanol extraction yield of the seaweed extracts and their IC50 against drug-susceptible and drug-resistant E. coli.
| Macroalgae | Extraction yield (%) | IC50 (μg/mL) | |
|---|---|---|---|
| Kam3 | Kam3-AcrB | ||
| Brown | |||
| Laminaria japonica | 5.2 | 125 | >500 |
| Sargassum horneri | 4.3 | 250 | >500 |
| Red | |||
| Gracilaria sp. | 6.3 | 62.5 | >500 |
| Porphyra dentata | 3.9 | 250 | >500 |
The ethanolic extracts were tested using the microdilution method against drug-susceptible (Kam3) and drug-resistant (Kam-AcrB) E. coli strains based on their IC50 measurements. As shown in Table 1, the IC50 values of the L. japonica, S. horneri, Gracilaria sp., and P. dentata extracts against Kam3 were 125, 250, 62.5, and 250 μg/mL, respectively, thereby indicating that the extracts might contain some antibacterial compounds. The naturally occurring aromatic organic compound p-cymene found in some algal extracts [65] has been shown to exhibit antibacterial activity against E. coli O157: H7 [66].
Interestingly, the ethanolic extracts at the tested concentrations showed no inhibitory effects against the drug-resistant E. coli strain. The IC50 values of all the four extracts against Kam3-AcrB were >500 μg/mL, indicating that the antibacterial substances in the extracts are expelled by the multidrug transporter AcrB. Lomovskaya et al. [30] reported that Mex pumps in Pseudomonas aeruginosa confer resistance to EPI PAβN, thereby indicating that PAβN is efficiently extruded.
3.2. Seaweed Extracts Potentiate the Activities of Macrolides against Drug-Susceptible and Drug-Resistant E. coli
AcrAB-TolC in E. coli is the most studied tripartite pump to date; it is linked to a wide range of drugs, including macrolides [5], tetracyclines [6, 7], fluoroquinolones [8], and β-lactams [9], and dyes, such as EB [67]. The four ethanolic extracts were added at subinhibitory concentrations of 1/2 and 1/4 IC50 in the modulation assays with the macrolides erythromycin and tetracycline, both of which are known to be substrates of the RND drug transporter AcrB.
As shown in Table 2, the extracts from brown seaweeds L. japonica and S. horneri and red seaweeds Gracilaria sp. at a concentration of 1/2 IC50 were able to potentiate the activity of erythromycin against the Kam3 strain, with a modulation factor of 4, and the S. horneri and Gracilaria sp. extracts were even found to have potentiating activities at 1/4 IC50. In addition, the S. horneri and Gracilaria sp. extracts were able to potentiate the activity of erythromycin against the Kam3-AcrB strain, with a modulation factor of 8 and 2 at 1/2 IC50, respectively, and 2 at 1/4 IC50. Intriguingly, the potentiating activities of the extracts were not observed in the modulation assays using tetracycline (data not shown); this antibiotic shares its mechanism of action (i.e., protein synthesis inhibition) with erythromycin. Lomovskaya et al. [30] demonstrated that PAβN did not potentiate the activities of all antibiotic substrates of the MexAB-OprM efflux pump to the same extent. They speculated that different antibiotics may have different binding sites on the pump and that PAβN-induced inhibition is binding site specific.
Table 2.
Erythromycin-modulation activity of the seaweed extracts for Kam3 and Kam3-AcrB.
| E. coli | Macroalgal extracts |
Extracts conc. |
IC50 of Erythromycin (μg/mL) | Modulation factor |
|
|---|---|---|---|---|---|
| No extract | With extract | ||||
| Kam3 | Laminaria japonica | IC50/2 | 15.63 | 3.90 | 4 |
| IC50/4 | 15.63 | 15.63 | 1 | ||
| Sargassum horneri | IC50/2 | 15.63 | 3.90 | 4 | |
| IC50/4 | 15.63 | 7.81 | 2 | ||
| Gracilaria sp. | IC50/2 | 15.63 | 3.90 | 4 | |
| IC50/4 | 15.63 | 3.90 | 4 | ||
| Porphyra dentata | IC50/2 | 15.63 | 15.63 | 1 | |
| IC50/4 | 15.63 | NA | NA | ||
|
| |||||
| Kam3-AcrB | Laminaria japonica | IC50/2 | 62.5 | 62.5 | 1 |
| IC50/4 | 62.5 | 62.5 | 1 | ||
| Sargassum horneri | IC50/2 | 62.5 | 7.81 | 8 | |
| IC50/4 | 62.5 | 31.25 | 2 | ||
| Gracilaria sp. | IC50/2 | 62.5 | 31.25 | 2 | |
| IC50/4 | 62.5 | 31.25 | 2 | ||
| Porphyra dentata | IC50/2 | 62.5 | 125 | 0.5 | |
| IC50/4 | 62.5 | 62.5 | 1 | ||
NA, not applicable.
We further investigated the potentiating activities of the extracts with another macrolide clarithromycin in the modulation assay (Table 3). The extracts from L. japonica, S. horneri, and Gracilaria sp. at 1/2 IC50 exhibited potentiating activities with clarithromycin against Kam3 (modulation factor, 8). In addition, all the extracts were found to potentiate the activity of clarithromycin against Kam3-AcrB at 1/2 and 1/4 IC50. The extract from S. horneri appeared to possess the greatest potentiating activity (modulation factor, 16 at 1/2 IC50).
Table 3.
Clarithromycin-modulation activity of the seaweed extracts for Kam3 and Kam3-AcrB.
| E. coli | Macroalgal extracts |
Extracts conc. |
IC50 of Clarithromycin | Modulation factor |
|
|---|---|---|---|---|---|
| No extract | With extract | ||||
| Kam3 | Laminaria japonica | IC50/2 | 21.88 | 2.73 | 8 |
| IC50/4 | 21.88 | 21.88 | 1 | ||
| Sargassum horneri | IC50/2 | 21.88 | 2.73 | 8 | |
| IC50/4 | 21.88 | 21.88 | 1 | ||
| Gracilaria sp. | IC50/2 | 21.88 | 2.73 | 8 | |
| IC50/4 | 21.88 | 5.47 | 4 | ||
| Porphyra dentata | IC50/2 | 21.88 | 21.88 | 1 | |
| IC50/4 | 21.88 | NA | NA | ||
|
| |||||
| Kam3-AcrB | Laminaria japonica | IC50/2 | 175 | 43.75 | 4 |
| IC50/4 | 175 | 87.5 | 2 | ||
| Sargassum horneri | IC50/2 | 175 | 10.94 | 16 | |
| IC50/4 | 175 | 43.75 | 4 | ||
| Gracilaria sp. | IC50/2 | 175 | 87.5 | 2 | |
| IC50/4 | 175 | 87.5 | 2 | ||
| Porphyra dentata | IC50/2 | 175 | 87.5 | 2 | |
| IC50/4 | 175 | 87.5 | 2 | ||
NA, not applicable.
3.3. Effect of Seaweed Extracts on Time-Kill Curves
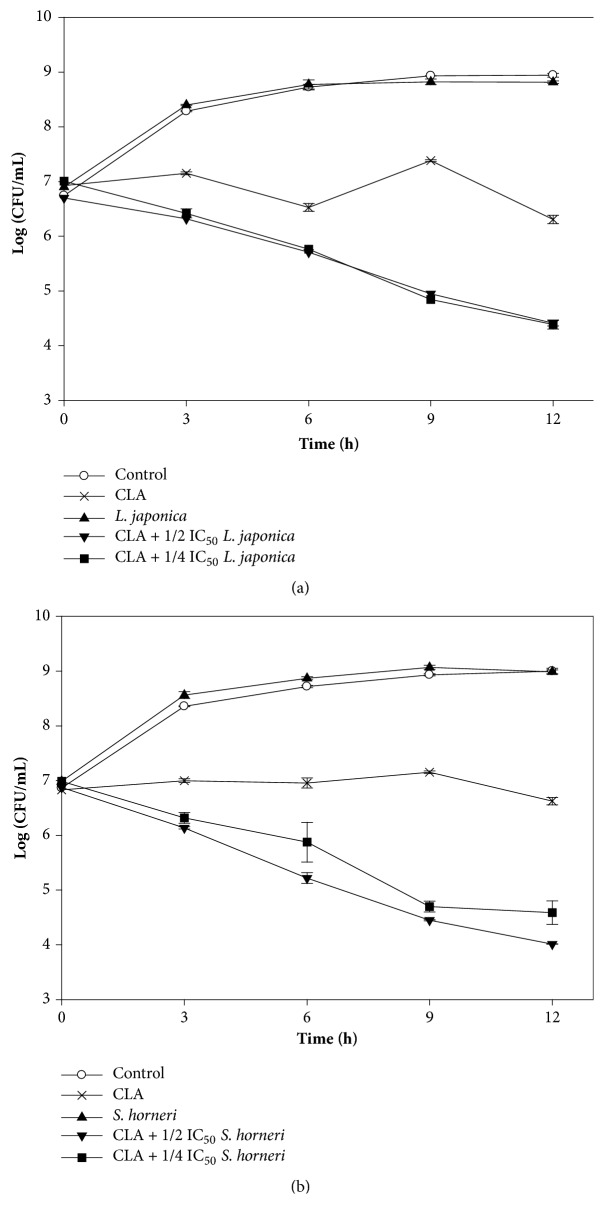
To determine the synergistic effect of the seaweed extracts with macrolide over time, the growth of Kam3-AcrB was monitored in the presence of clarithromycin and clarithromycin + a seaweed extract. Figure 1(a) indicates that Kam-AcrB was allowed to grow to log phase and then exposed to clarithromycin, the L. japonica extract, or the clarithromycin + L. japonica extract. The bacteria exposed to the extract alone showed a growth pattern similar to the control group (no addition), whereas the cells exposed to clarithromycin gradually decreased in number.
Figure 1.
Time-kill curves of clarithromycin alone and combined with brown seaweed (a) L. japonica extract and (b) S. horneri extract against drug-resistant E. coli. The Kam3-AcrB E. coli cells at a cell density of 7 Log CFU/mL were added with clarithromycin alone or combined with seaweed extracts, and the cell numbers were monitored every 3 h for 12 h.
Furthermore, the number of viable cells exposed to clarithromycin + L. japonica extract (1/2 and 1/4 IC50) sharply decreased from 7 to 4.4 log CFU/mL after 12 h of incubation. Similar results were observed in the assays with S. horneri (Figure 1(b)). Inhibitory effects were not observed when testing the bacteria with S. horneri alone. Moreover, the addition of the clarithromycin + S. horneri extract (1/2 and 1/4 IC50) resulted in a greater inhibitory effect than that of clarithromycin alone. The approximate reduction in cell number was 7 to 4 log CFU/mL after 12 h (1/2 IC50).
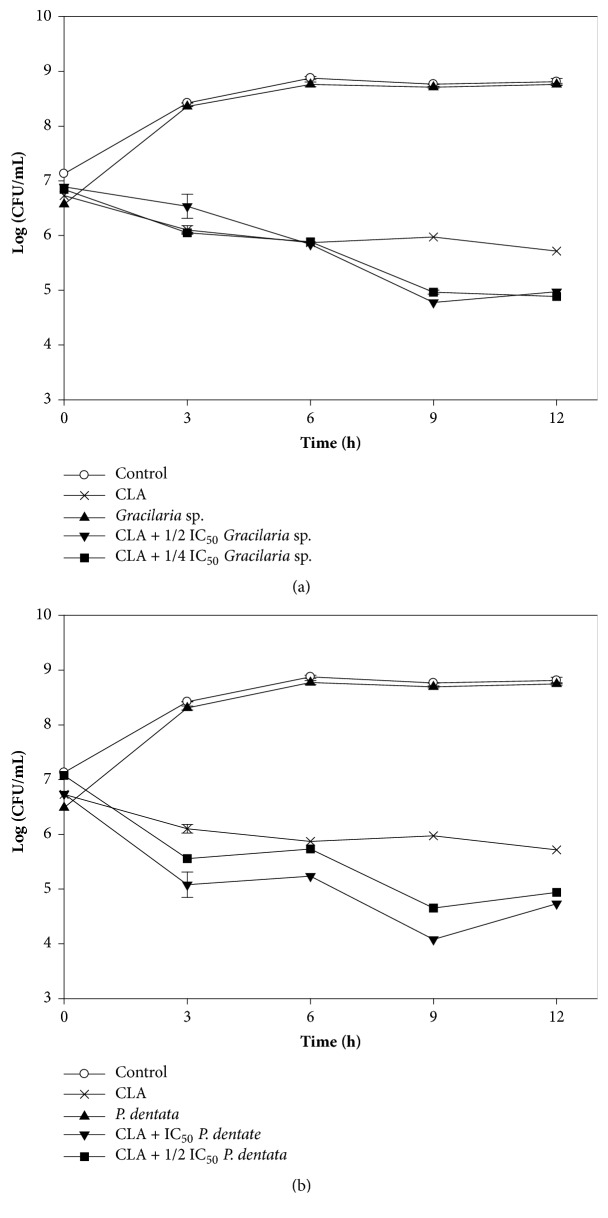
We also investigated the synergistic inhibitory effects of clarithromycin and red seaweed extracts against Kam3-AcrB over time. Figure 2 shows that the addition of Gracilaria sp. or P. dentata extracts to the cells in the presence of clarithromycin resulted in greater inhibition than when clarithromycin was used alone. Our data suggest that the extracts had synergistic effects on clarithromycin from the onset of incubation, with no bacterial regrowth occurring. Zhou et al. [68] reported similar results of Time-kill assays, thus demonstrating the synergistic antibacterial effects of the alkaloid EPI berberine and the antibiotic ciprofloxacin in a MDR clinical isolate of Klebsiella pneumoniae.
Figure 2.
Time-kill curves of clarithromycin alone and combined with red seaweed (a) Gracilaria sp. extract and (b) P. dentata extract against drug-resistant E. coli. The Kam3-AcrB E. coli cells at a cell density of 7 Log CFU/mL were added with clarithromycin alone or combined with seaweed extracts, and the cell numbers were monitored every 3 h for 12 h.
3.4. Efflux-Mediated Properties of the Seaweed Extracts
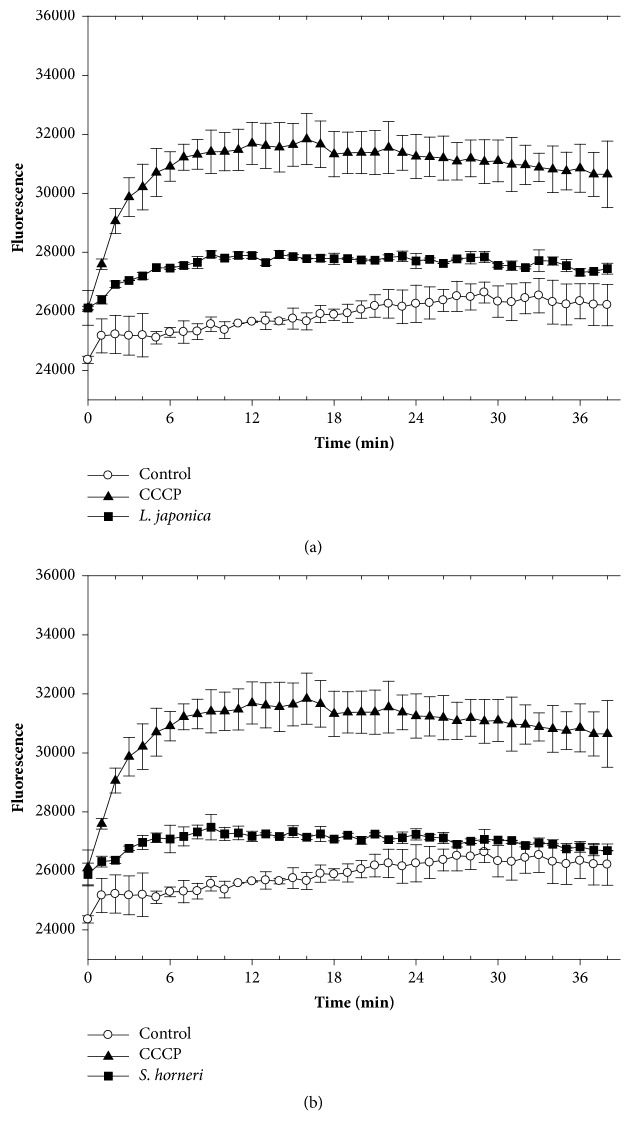
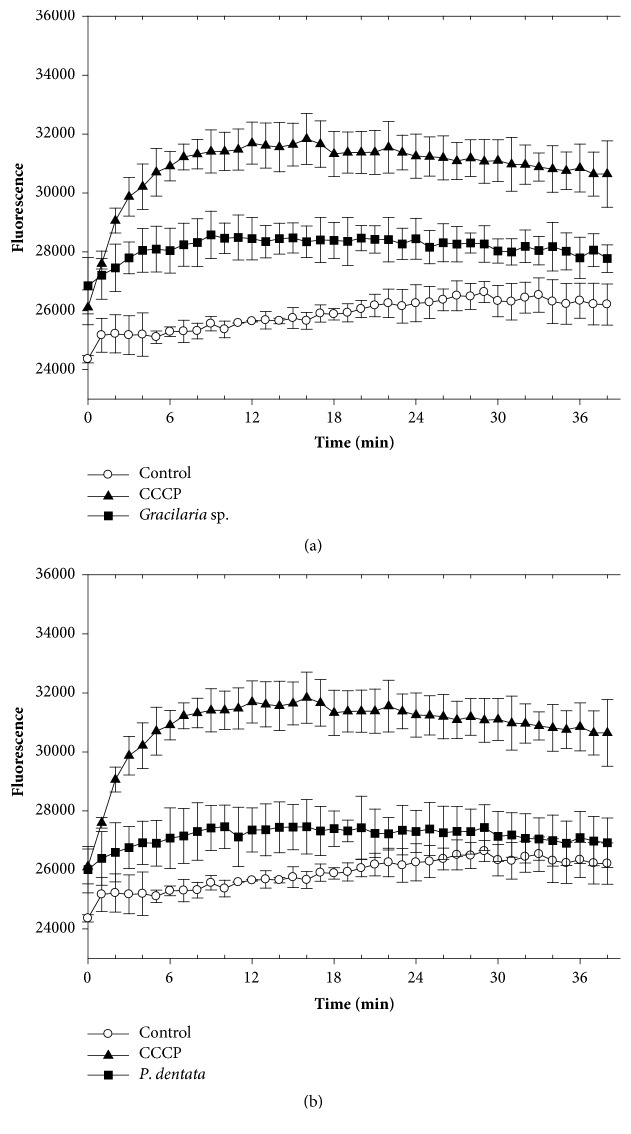
To analyze the potential efflux-mediated properties of the seaweed extracts, we monitored the accumulation of EB into Kam3-AcrB in their presence. EB is a substrate of the multidrug transporter AcrB in E. coli [67] and intensely fluoresces when bound to DNA. Figure 3(a) shows that EB accumulation was greater in cells exposed to the extract and positive control CCCP (20 μg/mL; a proton uncoupler) rather than the control (no addition). This indicates that the L. japonica extract possesses an efflux pump inhibitor that interferes with EB efflux from Kam3-AcrB. Similar results were observed in cells exposed to S. horneri (Figure 3(b)), Gracilaria sp. (Figure 4(a)), and P. dentata extracts (Figure 4(b)). The EB accumulation assays were also performed in Kam3 in the presences of the seaweed extracts and observed no obvious increase of EB accumulation (data not shown). This might suggest that the seaweed extracts increase the EB accumulation possibly by mediating efflux pump rather than by increasing membrane permeability. Our data indicate that all the extracts contain functional compounds that increase EB accumulation in Kam-AcrB. This highlights their potential as EPIs of AcrB, which is known to be overexpressed with a strong promoter in Kam3-AcrB strains. However, we were unable to rule out the possibility that the extracts interfere with other efflux pumps that translocate EB.
Figure 3.
Effects of brown seaweeds (a) L. japonica extract and (b) S. horneri extract on EB accumulation in drug-resistant E. coli. The E. coli Kam3-AcrB cells were added with glucose (25 mM) and EB (25 μM) in presence or absence of CCCP (20 μg/mL) or seaweed extracts (1/2 IC50). The fluorescence was monitored at Ex 520 nm and Em 600 nm.
Figure 4.
Effects of red seaweeds (a) Gracilaria sp. extract and (b) P. dentata extract on EB accumulation in drug-resistant E. coli. The E. coli Kam3-AcrB cells were added with glucose (25 mM) and EB (25 μM) in presence or absence of CCCP (20 μg/mL) or seaweed extracts (1/2 IC50). The fluorescence was monitored at Ex 520 nm and Em 600 nm.
3.5. Chemical Compositions of the Seaweed Extracts
Table 4 shows the chemical composition of the seaweed extracts as determined using gas chromatography-mass spectrometry (GC-MS). The classes with the symbol ⌜++⌟ have more chemicals than the classes with the symbol ⌜+⌟ in each seaweed extract. All the extracts contained terpenes, terpenoids, and phenolic compounds. The S. horneri and P. dentata extracts contained indoles, whereas the Gracilaria sp. extract contained pyrrole derivatives. In addition, halogenated aromatic compounds and alkaloids were identified in the red seaweeds.
Table 4.
Chemical composition of the ethanol extracts from the seaweeds.
| Classifications | Brown seaweeds | Red seaweeds | ||
|---|---|---|---|---|
| L. japnoica | S. horneri | Gracilaria sp. | P. dentata | |
| Terpenes | + | ++ | ++ | + |
| Terpenoids | + | + | + | + |
| Phenolic compounds | ++ | ++ | ++ | ++ |
| Indoles | – | + | – | + |
| Pyrrole derivatives | – | – | + | – |
| Halogenated aromatic compounds | – | – | + | + |
| Alkaloids | – | – | + | + |
++, dominantly present in the extract; +, present in the extract; –, absent in the extract.
The chemical structure of several plant-derived and synthetic EPIs has been characterized. The diterpenes carnosol and carnosic acid obtained from the herb Rosmarinus officinalis are known to potentiate erythromycin and tetracycline against S. aureus strains expressing Tet(K) and Msr (A) pumps [63]. Lorenzi et al. [69] indicated that the monoterpene geraniol obtained from Helichrysum italicum increases the efficacy of quinolones and chloramphenicol in E. coli. Synthetic indole derivatives, such as [4-benzyloxy-2-(5-nitro-1H-2-yl)-phenyl]-methanol, have been reported as EPIs for the efflux pump NorA in S. aureus [70].
Furthermore, synthetic halogenated phenothiazine derivatives, such as chlorpromazine, are thought to inhibit drug efflux pumps via various mechanisms [71]. Dwivedi et al. [23] indicated that alkaloid chanoclavine isolated from Ipomoea muricata potentiates the activity of tetracycline against MDR clinical E. coli isolate, possibly by inhibiting drug efflux and downregulating the expression of drug transporters. In addition, catharanthine isolated from the leaves of flowering plant Catharanthus roseus has been shown to potentiate the activity of tetracycline, possibly due to the inhibition of the efflux pumps in P. aeruginosa [24]. 3,4-Dibromopyrrole-2,5-dione, a bacterial halogenated metabolite, is an effective EPI against bacterial strains that overexpress AcrB-TolC, MexAB-OprM, and MexXY-OprM [72]. The plant-derived alkaloids reserpine and piperine are also effective EPIs for fluoroquinolones in S. aureus [22, 33]. EPIs hinder the functions of efflux pumps via several mechanisms.
Seaweeds are rich in bioactive terpenes and aromatic compounds, some of which possess specific characteristics that are absent or rarely found in terrestrial plants. For instance, approximately 25% of all halogenated natural products are alkaloids, and most of them are found in marine organisms [73]. Halogenated compounds obtained from seaweeds are varied and range from indoles, terpenes, and phenols to halogenated hydrocarbons; investigations into their potential use as EPIs would be beneficial [74]. Our IC50 data in Table 1 indicated that some antibacterial substances in the extracts are expelled by the multidrug transporter AcrB, which might suggest that the inhibition of drug transport was a result of competitive binding to AcrB. Further chromatographic purification of the ethanolic extracts obtained from seaweeds is required to identify and produce pure, active compounds. The findings of this study should encourage further research into seaweeds and their extracts with a view to identifying new EPIs with potential clinical applications.
4. Conclusions
The ethanolic seaweed extracts were able to potentiate the activities of macrolides against E. coli and inhibit the action of efflux pumps in clinically important pathogens, thereby highlighting their potential as effective EPIs. We believe these extracts should be further investigated to exploit their ability to block drug efflux pumps, thereby facilitating the development of novel antimicrobial agents effective against clinically important MDR pathogens.
Acknowledgments
This work was financially supported by the Center of Excellence for the Oceans, National Taiwan Ocean University from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan, the grants from the Ministry of Science and Technology (Taiwan, R.O.C., MOST 107-2221-E-019-023), and the grant from Hsu-Jih Education Foundation (TIARF107A1-002). We thank Professor Adrian R Walmsley and Dr. Maria Ines Borges-Walmsley, Durham University, UK, for kindly providing E. coli Kam3 strain and expression vectors. We thank Professor Yean-Chang Chen, Department of Aquaculture, National Taiwan Ocean University, for kindly providing brown seaweeds S. horneri.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Hong-Ting Victor Lin and Pang-Hung Hsu conceived and designed the experiments. Wen-Jung Lu, Hsuan-Ju Lin, Pang-Hung Hsu, and Margaret Lai performed the experiments. Wen-Jung Lu, Lai, Pang-Hung Hsu, and Hong-Ting Victor Lin analyzed the data. Hong-Ting Victor Lin wrote the paper. Wen-Jung Lu, Hsuan-Ju Lin, and Pang-Hung Hsu contributed equally to this work.
References
- 1.ONeill J. Review on Antimicrobial Resistance. London, UK: 2014. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. [Google Scholar]
- 2.Jasovský D., Littmann J., Zorzet A., Cars O. Antimicrobial resistance—a threat to the world’s sustainable development. Upsala Journal of Medical Sciences. 2016;121(3):159–164. doi: 10.1080/03009734.2016.1195900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dwivedi G. R., Sanchita, Singh D. P., Sharma A., Darokar M. P., Srivastava S. K. Nano particles: Emerging warheads against bacterial superbugs. Current Topics in Medicinal Chemistry. 2016;16(18):1963–1975. doi: 10.2174/1568026616666160215154556. [DOI] [PubMed] [Google Scholar]
- 4.McKeegan K. S., Borges-Walmsley M. I., Walmsley A. R. The structure and function of drug pumps: An update. Trends in Microbiology. 2003;11(1):21–29. doi: 10.1016/S0966-842X(02)00010-0. [DOI] [PubMed] [Google Scholar]
- 5.Chollet R., Chevalier J., Bryskier A., Pagès J.-M. The AcrAB-TolC pump is involved in macrolide resistance but not in telithromycin efflux in Enterobacter aerogenes and Escherichia coli. Antimicrobial Agents and Chemotherapy. 2004;48(9):3621–3624. doi: 10.1128/AAC.48.9.3621-3624.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okusu H., Ma D., Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. Journal of Bacteriology. 1996;178(1):306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Cristóbal R. E., Vincent P. A., Salomón R. A. Multidrug resistance pump AcrAB-TolC is required for high-level, Tet(A)-mediated tetracycline resistance in Escherichia coli. Journal of Antimicrobial Chemotherapy. 2006;58(1):31–36. doi: 10.1093/jac/dkl172. [DOI] [PubMed] [Google Scholar]
- 8.Swick M. C., Morgan-Linnell S. K., Carlson K. M., Zechiedrich L. Expression of multidrug efflux pump genes acrAB-tolC, mdfA, and norE in Escherichia coli clinical isolates as a function of fluoroquinolone and multidrug resistance. Antimicrobial Agents and Chemotherapy. 2011;55(2):921–924. doi: 10.1128/AAC.00996-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim S. P., Nikaido H. Kinetic parameters of efflux of penicillins by the multidrug efflux transporter AcrAB-TolC of Escherichia coli. Antimicrobial Agents and Chemotherapy. 2010;54(5):1800–1806. doi: 10.1128/AAC.01714-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuda N., Sakagawa E., Ohya S., Gotoh N., Tsujimoto H., Nishino T. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy. 2000;44(12):3322–3327. doi: 10.1128/AAC.44.12.3322-3327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H. T., Bavro V. N., Barrera N. P., et al. MacB ABC transporter is a dimer whose ATPase activity and macrolide-binding capacity are regulated by the membrane fusion protein MacA. The Journal of Biological Chemistry. 2009;284(2):1145–1154. doi: 10.1074/jbc.M806964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du D., van Veen H. W., Luisi B. F. Assembly and operation of bacterial tripartite multidrug efflux pumps. Trends in Microbiology. 2015;23(5):311–319. doi: 10.1016/j.tim.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Pagès J.-M., Alibert-Franco S., Mahamoud A., et al. Efflux pumps of gram-negative bacteria, a new target for new molecules. Current Topics in Medicinal Chemistry. 2010;10(18):1848–1857. doi: 10.2174/156802610793176620. [DOI] [PubMed] [Google Scholar]
- 14.Lomovskaya O., Bostian K. A. Practical applications and feasibility of efflux pump inhibitors in the clinic - A vision for applied use. Biochemical Pharmacology. 2006;71(7):910–918. doi: 10.1016/j.bcp.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Pagès J., Amaral L. Mechanisms of drug efflux and strategies to combat them: challenging the efflux pump of Gram-negative bacteria. Biochimica et Biophysica Acta. 2009;1794(5):826–833. doi: 10.1016/j.bbapap.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Nelson M. L., Park B. H., Andrews J. S., Georgian V. A., Thomas R. C., Levy S. B. Inhibition of the Tetracycline Efflux Antiport Protein by 13-Thio-Substituted 5-Hydroxy-6-deoxytetracyclines. Journal of Medicinal Chemistry. 1993;36(3):370–377. doi: 10.1021/jm00055a008. [DOI] [PubMed] [Google Scholar]
- 17.Van Bambeke F., Pagès J.-M., Lee V. J. Inhibitors of bacterial efflux pumps as adjuvants in antibiotic treatments and diagnostic tools for detection of resistance by efflux. Recent Patents on Anti-Infective Drug Discovery. 2006;1:138–175. doi: 10.2174/157489106777452692. [DOI] [PubMed] [Google Scholar]
- 18.Kerns R. J., Rybak M. J., Kaatz G. W., et al. Piperazinyl-linked fluoroquinolone dimers possessing potent antibacterial activity against drug-resistant strains of Staphylococcus aureus. Bioorganic & Medicinal Chemistry Letters. 2003;13(10):1745–1749. doi: 10.1016/S0960-894X(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 19.Markham P. N., Westhaus E., Klyachko K., Johnson M. E., Neyfakh A. A. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrobial Agents and Chemotherapy. 1999;43(10):2404–2408. doi: 10.1128/AAC.43.10.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piddock L. J. V., Garvey M. I., Rahman M. M., Gibbons S. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in Gram-negative bacteria. Journal of Antimicrobial Chemotherapy. 2010;65(6):1215–1223. doi: 10.1093/jac/dkq079.dkq079 [DOI] [PubMed] [Google Scholar]
- 21.Zeng B., Wang H., Zou L., Zhang A., Yang X., Guan Z. Evaluation and target validation of indole derivatives as inhibitors of the AcrAB-TolC efflux pump. Bioscience, Biotechnology, and Biochemistry. 2010;74(11):2237–2241. doi: 10.1271/bbb.100433. [DOI] [PubMed] [Google Scholar]
- 22.Khan I. A., Mirza Z. M., Kumar A., Verma V., Qazi G. N. Piperine, a phytochemical potentiator of ciprofloxacin against Staphylococcus aureus. Antimicrobial Agents and Chemotherapy. 2006;50(2):810–812. doi: 10.1128/AAC.50.2.810-812.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dwivedi G. R., Maurya A., Yadav D. K., et al. Synergy of clavine alkaloid ‘chanoclavine’ with tetracycline against multi-drug-resistant. Journal of Biomolecular Structure and Dynamics. 2018:1–19. doi: 10.1080/07391102.2018.1458654. [DOI] [PubMed] [Google Scholar]
- 24.Dwivedi G. R., Tyagi R., Sanchita, et al. Antibiotics potentiating potential of catharanthine against superbug Pseudomonas aeruginosa. Journal of Biomolecular Structure and Dynamics. 2017:1–15. doi: 10.1080/07391102.2017.1413424. [DOI] [PubMed] [Google Scholar]
- 25.Christena L. R., Subramaniam S., Vidhyalakshmi M., Mahadevan V., Sivasubramanian A., Nagarajan S. Dual role of pinostrobin-a flavonoid nutraceutical as an efflux pump inhibitor and antibiofilm agent to mitigate food borne pathogens. RSC Advances. 2015;5(76):61881–61887. doi: 10.1039/C5RA07165H. [DOI] [Google Scholar]
- 26.Stermitz F. R., Scriven L. N., Tegos G., Lewis K. Two flavonols from Artemisa annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Medica. 2002;68(12):1140–1141. doi: 10.1055/s-2002-36347. [DOI] [PubMed] [Google Scholar]
- 27.Dwivedi G. R., Tiwari N., Singh A., et al. Gallic acid-based indanone derivative interacts synergistically with tetracycline by inhibiting efflux pump in multidrug resistant E. coli. Applied Microbiology and Biotechnology. 2016;100(5):2311–2325. doi: 10.1007/s00253-015-7152-6. [DOI] [PubMed] [Google Scholar]
- 28.Gibbons S., Oluwatuyi M., Veitch N. C., Gray A. I. Bacterial resistance modifying agents from Lycopus europaeus. Phytochemistry. 2003;62(1):83–87. doi: 10.1016/s0031-9422(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 29.Pereda-Miranda R., Kaatz G. W., Gibbons S. Polyacylated oligosaccharides from medicinal Mexican morning glory species as antibacterials and inhibitors of multidrug resistance in Staphylococcus aureus. Journal of Natural Products. 2006;69(3):406–409. doi: 10.1021/np050227d. [DOI] [PubMed] [Google Scholar]
- 30.Lomovskaya O., Warren M. S., Lee A., et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrobial Agents and Chemotherapy. 2001;45(1):105–116. doi: 10.1128/aac.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sjuts H., Vargiu A. V., Kwasny S. M., et al. Molecular basis for inhibition of AcrB multidrug efflux pump by novel and powerful pyranopyridine derivatives. Proceedings of the National Acadamy of Sciences of the United States of America. 2016;113(13):3509–3514. doi: 10.1073/pnas.1602472113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Research. 1981;41(5):1967–1972. [PubMed] [Google Scholar]
- 33.Neyfakh A. A., Borsch C. M., Kaatz G. W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrobial Agents and Chemotherapy. 1993;37(1):128–129. doi: 10.1128/AAC.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savoia D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiology. 2012;7(8):979–990. doi: 10.2217/fmb.12.68. [DOI] [PubMed] [Google Scholar]
- 35.Kalia N. P., Mahajan P., Mehra R., et al. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. Journal of Antimicrobial Chemotherapy. 2012;67(10):2401–2408. doi: 10.1093/jac/dks232.dks232 [DOI] [PubMed] [Google Scholar]
- 36.Mirza Z. M., Kumar A., Kalia N. P., Zargar A., Khan I. A. Piperine as an inhibitor of the MdeA efflux pump of Staphylococcus aureus. Journal of Medical Microbiology. 2011;60(Pt 10):1472–1478. doi: 10.1099/jmm.0.033167-0. [DOI] [PubMed] [Google Scholar]
- 37.Aparna V., Dineshkumar K., Mohanalakshmi N., Velmurugan D., Hopper W. Identification of natural compound inhibitors for multidrug efflux pumps of Escherichia coli and Pseudomonas aeruginosa using In Silico high-throughput virtual screening and In Vitro validation. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.John R. P., Anisha G. S., Nampoothiri K. M., Pandey A. Micro and macroalgal biomass: a renewable source for bioethanol. Bioresource Technology. 2011;102(1):186–193. doi: 10.1016/j.biortech.2010.06.139. [DOI] [PubMed] [Google Scholar]
- 39.Jang J.-S., Cho Y. K., Jeong G.-T., Kim S.-K. Optimization of saccharification and ethanol production by simultaneous saccharification and fermentation (SSF) from seaweed, Saccharina japonica. Bioprocess and Biosystems Engineering. 2012;35(1-2):11–18. doi: 10.1007/s00449-011-0611-2. [DOI] [PubMed] [Google Scholar]
- 40.Kim S., Chojnacka K. Marine Algae Extracts: Processes, Products, And Applications. Wiley-VCH; 2015. [DOI] [Google Scholar]
- 41.Montero L., del Pilar Sánchez-Camargo A., Ibáñez E., Gilbert-López B. Phenolic Compounds from Edible Algae: Bioactivity and Health Benefits. Current Medicinal Chemistry. 2018;25(37):4808–4826. doi: 10.2174/0929867324666170523120101. [DOI] [PubMed] [Google Scholar]
- 42.Pérez M., Falqué E., Domínguez H. Antimicrobial Action of Compounds from Marine Seaweed. Marine Drugs. 2016;14(3):p. 52. doi: 10.3390/md14030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Güven K. C., Percot A., Sezik E. Alkaloids in marine algae. Marine Drugs. 2010;8(2):269–284. doi: 10.3390/md8020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamed I., Özogul F., Özogul Y., Regenstein J. M. Marine bioactive compounds and their health benefits: a review. Comprehensive Reviews in Food Science and Food Safety. 2015;14(4):446–465. doi: 10.1111/1541-4337.12136. [DOI] [Google Scholar]
- 45.Garcia-Vaquero M., Rajauria G., O'Doherty J. V., Sweeney T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Research International. 2017;99:1011–1020. doi: 10.1016/j.foodres.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Chew Y. L., Lim Y. Y., Omar M., Khoo K. S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT - Food Science and Technology. 2008;41(6):1067–1072. doi: 10.1016/j.lwt.2007.06.013. [DOI] [Google Scholar]
- 47.Shanmughapriya S., Manilal A., Sujith S., Selvin J., Kiran G. S., Natarajaseenivasan K. Antimicrobial activity of seaweeds extracts against multiresistant pathogens. Annals of Microbiology. 2008;58(3):535–541. doi: 10.1007/BF03175554. [DOI] [Google Scholar]
- 48.Lin H.-T. V., Lu W.-J., Tsai G.-J., Chou C.-T., Hsiao H.-I., Hwang P.-A. Enhanced anti-inflammatory activity of brown seaweed Laminaria japonica by fermentation using Bacillus subtilis. Process Biochemistry. 2016;51(12):1945–1953. doi: 10.1016/j.procbio.2016.08.024. [DOI] [Google Scholar]
- 49.Kazłowska K., Hsu T., Hou C.-C., Yang W.-C., Tsai G.-J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. Journal of Ethnopharmacology. 2010;128(1):123–130. doi: 10.1016/j.jep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 50.Lin H.-T. V., Tsou Y.-C., Chen Y.-T., Lu W.-J., Hwang P.-A. Effects of low-molecular-weight fucoidan and high stability fucoxanthin on glucose homeostasis, lipid metabolism, and liver function in a mouse model of type II diabetes. Marine Drugs. 2017;15(4, article no. 113) doi: 10.3390/md15040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharifuddin Y., Chin Y.-X., Lim P.-E., Phang S.-M. Potential bioactive compounds from seaweed for diabetes management. Marine Drugs. 2015;13(8):5447–5491. doi: 10.3390/md13085447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moussavou G., Kwak D. H., Obiang-Obonou B. W., et al. Anticancer effects of different seaweeds on human colon and breast cancers. Marine Drugs. 2014;12(9) doi: 10.3390/md12094898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prasch S., Bucar F. Plant derived inhibitors of bacterial efflux pumps: an update. Phytochemistry Reviews. 2015;14(6):961–974. doi: 10.1007/s11101-015-9436-y. [DOI] [Google Scholar]
- 54.Morita Y., Kodama K., Shiota S., et al. NorM, putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrobial Agents and Chemotherapy. 1998;42(7):1778–1782. doi: 10.1128/AAC.42.7.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soothill J. S., Ward R., Girling A. J. The IC50: An exactly defined measure of antibiotic sensitivity. Journal of Antimicrobial Chemotherapy. 1992;29(2):137–139. doi: 10.1093/jac/29.2.137. [DOI] [PubMed] [Google Scholar]
- 56.Lu W.-J., Lin H.-J., Janganan T. K., et al. ATP-binding cassette transporter VcaM from vibrio cholerae is dependent on the outer membrane factor family for its function. International Journal of Molecular Sciences. 2018;19(4) doi: 10.3390/ijms19041000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown A. R., Ettefagh K. A., Todd D., et al. A mass spectrometry-based assay for improved quantitative measurements of efflux pump inhibition. PLoS ONE. 2015;10(5) doi: 10.1371/journal.pone.0124814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poisson J., Le Hir A., Goutarel R., Janot M. M. Isolation of reserpine from roots of Rauwolfia vomitoria Afz. Comptes Rendus Hebdomadaires Des Séances De L'académie Des Sciences. 1954;238(15):1607–1609. [PubMed] [Google Scholar]
- 59.Stermitz F. R., Lorenz P., Tawara J. N., Zenewicz L. A., Lewis K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5'-methoxyhydnocarpin, a multidrug pump inhibitor. Proceedings of the National Acadamy of Sciences of the United States of America. 2000;97(4):1433–1437. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stermitz F. R., Beeson T. D., Mueller P. J., Hsiang J.-F., Lewis K. Staphylococcus aureus mdr efflux pump inhibitors from a berberis and a mahonia (sensu strictu) species. Biochemical Systematics and Ecology. 2001;29(8):793–798. doi: 10.1016/S0305-1978(01)00025-4. [DOI] [PubMed] [Google Scholar]
- 61.Fujita M., Shiota S., Kuroda T., et al. Remarkable synergies between baicalein and tetracycline, and baicalein and β-lactams against methicillin-resistant Staphylococcus aureus. Microbiology and Immunology. 2005;49(4):391–396. doi: 10.1111/j.1348-0421.2005.tb03732.x. [DOI] [PubMed] [Google Scholar]
- 62.Liu K. C.-S. C., Yang S.-L., Roberts M. F., Elford B. C., Phillipson J. D. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Reports. 1992;11(12):637–640. doi: 10.1007/BF00236389. [DOI] [PubMed] [Google Scholar]
- 63.Oluwatuyi M., Kaatz G. W., Gibbons S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry. 2004;65(24):3249–3254. doi: 10.1016/j.phytochem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Dickson R. A., Houghton P. J., Hylands P. J., Gibbons S. Antimicrobial, resistance-modifying effects, antioxidant and free radical scavenging activities of Mezoneuron benthamianum Baill., Securinega virosa Roxb. & Wlld. and Microglossa pyrifolia Lam. Phytotherapy Research. 2006;20(1):41–45. doi: 10.1002/ptr.1799. [DOI] [PubMed] [Google Scholar]
- 65.Ramawat K. G., Mérillon J.-M. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. NY, USA: Springer; 2013. [Google Scholar]
- 66.Kiskó G., Roller S. Carvacrol and p-cymene inactivate Escherichia coli O157:H7 in apple juice. BMC Microbiology. 2005;5, article 36:1–9. doi: 10.1186/1471-2180-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paixão L., Rodrigues L., Couto I., et al. Fluorometric determination of ethidium bromide efflux kinetics in Escherichia coli. Journal of Biological Engineering. 2009;3 doi: 10.1186/1754-1611-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou X.-Y., Ye X.-G., He L.-T., et al. In vitro characterization and inhibition of the interaction between ciprofloxacin and berberine against multidrug-resistant Klebsiella pneumoniae. The Journal of Antibiotics. 2016;69(10):741–746. doi: 10.1038/ja.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lorenzi V., Muselli A., Bernardini A. F., et al. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrobial Agents and Chemotherapy. 2009;53(5):2209–2211. doi: 10.1128/AAC.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samosorn S., Bremner J. B., Ball A., Lewis K. Synthesis of functionalised 2-aryl-5-nitro-1H-indoles and their activity as bacterial NorA efflux pump inhibitors. Bioorganic & Medicinal Chemistry. 2006;14(3):857–865. doi: 10.1016/j.bmc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 71.Bailey A. M., Paulsen I. T., Piddock L. J. V. RamA confers multidrug resistance in Salmonella enterica via increased expression of acrB, which is inhibited by chlorpromazine. Antimicrobial Agents and Chemotherapy. 2008;52(10):3604–3611. doi: 10.1128/AAC.00661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whalen K. E., Poulson-Ellestad K. L., Deering R. W., Rowley D. C., Mincer T. J. Enhancement of antibiotic activity against multidrug-resistant bacteria by the efflux pump inhibitor 3,4-dibromopyrrole-2,5-dione isolated from a Pseudoalteromonas sp. Journal of Natural Products. 2015;78(3):402–412. doi: 10.1021/np500775e. [DOI] [PubMed] [Google Scholar]
- 73.Fattorusso E., Taglialatela-Scafati O. Modern Alkaloids : Structure, Isolation, Synthesis and Biology. Weinheim, Germany: Wiley-VCH; 2008. [DOI] [Google Scholar]
- 74.Cabrita M. T., Vale C., Rauter A. P. Halogenated compounds from marine algae. Marine Drugs. 2010;8(8):2301–2317. doi: 10.3390/md8082301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.