Abstract
The global meat industry is characterised by a growing interest in natural preservative additives. This study determined the effect of sweet basil (Ocimum basilicum L.) essential oil (SBEO) on colour and lipid oxidation in minced beef. The phytoconstituents of SBEO were analyzed by gas chromatography mass spectrometry. Thereafter, minced beef samples from Nguni and Boran cattle were treated with either no additives (control, C) or SBEO added at 2% (SB2), 4% (SB4), or 6% (SB6). The meat samples were aerobically packaged and stored (4 ± 1°C) for seven days for measurement of lightness (L⁎), redness (a⁎), yellowness (b⁎), hue, chroma, and lipid oxidation (acid-reactive substances, TBARS) on days 0, 4, and 7. Thirty-two bioactive compounds with reported antioxidant and antimicrobial and activities were identified in SBEO, including Estragole (41.40%), 1, 6-Octadien-3-ol, 3,7-dimethyl (29.49%), and trans-.alpha.-Bergamotene (5.32%). On days 0, 4, and 7, SB2, SB4, and SB6 had higher (P < 0.05) L⁎, a⁎, b⁎, hue, and chroma values; and on days 0 and 4 TBARS were lower (P < 0.05) in SB2 and SB4 than C and SB6. The addition of 2% and 4% SBEO improved colour and lipid oxidative stability, demonstrating potential for its use as a natural antioxidant additive in meat.
1. Introduction
Globally, the use of antioxidants as preservatives has been instrumental in improving the quality and extending the shelf life of muscle foods, especially during processing and storage [1]. This is because meat and meat products are easily susceptible to oxidation and microbial contamination due to their chemical composition and rapid depletion of endogenous antioxidants postmortem [2]. Reports have shown that live muscle contains relative amount of endogenous antioxidants, including alpha-tocopherol, histidine-containing dipeptides, ubiquinone, glutathione, carnosine, and anserine, which are capable of scavenging free radicals and disrupting oxidative process in vivo [2–4]. However, after slaughtering, this muscle tissue begins to lose its antioxidative potential due to various postslaughter conditions such as anaerobic environment, presence of free radicals (reactive oxygen and nitrogen species), and lack of enzymatic mechanisms [5, 6].
As postmortem time increases, the activities of these endogenous antioxidants continue to diminish [2, 6], thereby exposing the lipid and protein component of muscle to rapid deterioration. The rate at which the endogenous antioxidants decrease during postmortem may depend on animal species, breed (including heme pigments), nutrition, muscle part, antemortem stress, and physiological functions [7, 8]. In an attempt to boost meat antioxidant content, different antioxidants (natural or synthetic) are used in the meat industry. Recently, the application of synthetic antioxidant in food/meat products has been implicated in causing negative health effect on consumers [6, 9, 10], thereby promoting interest in the utilization of natural antioxidants. Antioxidants are abundantly found in a wide range of natural sources including fruits, herbs grains, spices, nuts, seeds, leaves, and roots [6].
Sweet basil (Ocimum basilicum L.) is one of the frequently used culinary herbs (family of Lamiaceae), known to possess strong antioxidant and antimicrobial activities due to its phenolic acids and aromatic compounds [9, 11]. The plant grows annually or perennially in Asia, India, Africa, and other temperate climate regions throughout the world [9]. Traditionally, every part of the plant is used as medicine to treat headaches, coughs, diarrhea, constipation, warts, worms, kidney malfunction, and digestive problems [12]. According to Marwat et al. [9], basil plant has moderate macro and micro nutritional values including protein (3.15 g/100 g), fat (0.64 g/100 g), energy (23 Kcal), Vitamin C (18 mg/100 g), Vitamin E (0.80 mg/100 g), Vitamin A (5275 IU), Vitamin K (414.8 mcg), Calcium (177 mg/100 g), Iron (3.17 mg/ 100g), Potassium (295 mg/100 g), Magnesium (64 mg/100g), and Sodium (4 mg/100 g).
The in vitro activity of its essential oils has been reported to exhibit antimicrobial, antifungal, anticancer, anticonvulsant, hypnotic, and antioxidant activities [13, 14]. The inclusion of basil leaf extract in minced pork at 0.3 g/kg has been reported to lower the microbial population, oxidative deterioration, and improve the sensory quality compared to the control after 5 days of cold storage at 1°C [15]. However, information on the antioxidant effect of sweet basil essential oil in meat products is rarely available. The consumption of meat products containing antioxidant-rich herbs and spices has been reported to reduce in vivo formation of malondialdehyde and lower the risk of cancer and cardiovascular disease [16]. Therefore, this study was designed to investigate the preservative effect of sweet basil essential oil on physicochemical characteristics and lipid oxidation of minced beef during cold storage.
2. Materials and Methods
2.1. Collection of Essential Oil and GC-MS Analysis
Organic essential oil from sweet basil leaf, extracted by hydrodistillation, was obtained (Faithful to Nature, South Africa). The phytoconstituents of the oil were analyzed by gas chromatography mass spectrometry (GC-MS). The GC-MS analysis of essential oils was quantitatively performed using an Agilent 7890B GC system coupled with an Agilent 5977A, Chemetrix (pty) Ltd.; Agilent Technologies, DE (Germany) with a Zebron-5MS column (ZB-5MS 30 m × 0.25 mm × 0.25 μm) (5%-phenylmethylpolysiloxane). GC-grade helium was used as carrier gas at a constant flow rate of 2mL/min and splitless was also used at 1ml injection. The injector, source, and oven temperature were set at 280°C, 280°C and 70°C, respectively. The ramp setting was initially programmed at 15°C/min to 120°C, then 10°C/min to 180°C, and then 20°C/min to 270°C and held for 3 min. The identification of the chemical constituents of the essential oil was determined by their GC retention time, percentage composition (area %), and retention indices. The Kovat indices were calculated according to a set of standard hydrocarbons (C9-C20) [17]. The interpretation and identification of their mass spectra were confirmed by mass spectral incorporated library. Likewise, the retention indices were calculated in line with a homologous series of n-alkanes (C8–C32) under similar operating conditions by using a standard equation. Further identification of the components was made based on computer matching of the mass spectra with the National Institute of Standards and Technology (NIST) database (NIST/EPA/NIH) mass spectral library 2014) with those of published data [18]. Empirical searches were conducted using the PubChem Project (https://pubchem.ncbi.nlm.nih.gov/) and DrugBank (www.drugbank.ca/) to identify the known pharmacological properties associated with these compounds components.
2.2. Collection and Preparation of Meat Samples
Fresh beef samples (longissimus thoracis et lumborum muscle) were obtained from Boran and Nguni cattle reared on a natural grazing pasture and slaughtered at the age of 18 months with an average live weight of 380 and 260 kg, respectively, in high throughput commercial abattoir (East London, Eastern Cape Province, South Africa). The meat samples from each breed were cut into small cubes after removal of visible fat and connective tissues and minced in a sterile meat grinder. A portion (500 g) from the ground meat was randomly assigned to one of the following treatments: (1) C (control, meat without additives); (2) SB2 (meat with 2% sweet basil essential oil); (3) SB4 (meat with 4% sweet basil essential oil); and (4) SB6 (meat with 6% sweet basil essential oil); with four replicates per treatment. Immediately after adding the essential oil, the ground meat samples were aerobically packed in polyethylene bags (O2 permeability = 6000–8000 cm3/(24 h × m2 × atm), water vapour transmission = 83 g/ (24 h × m2) and 50% relative humidity), stored at 4 ± 1°C, and analyzed on 0, 2, 4, and 7 days of storage for colour and thiobarbituric acid-reactive substances (TBARS).
2.3. Physicochemical Characteristics
2.3.1. Instrumental Colour Determination
Colour changes in fresh meat during storage were performed using Hunter Lab Minolta colorimeter (BYK-Gardener GmbH, USA) with 20 mm aperture set for illumination D65 at 100 standard observer angles. The CIE colour coordinates L∗ (lightness), a∗ (redness), and b∗ (yellowness) were measured perpendicular to the meat surface at three different points after calibration using the standard green, black, and white colour samples. All the colour parameters (L∗, a∗, and b∗) were obtained from the mean of readings taken from four replicates per treatment. Hue (an indicator of the angle at which a vector radiates into the red-yellow quadrant) and chroma (a measure of colour saturation) were calculated as follows [19]:
| (1) |
2.3.2. Determination of Lipid Oxidation (TBARS)
The lipid oxidation of the ground beef was measured using TBARS, by a modified acid precipitation method. Two grams of each sample was weighed in triplicate into 50 mL tubes, 6.25 mL trichloroacetic acid (TCA, 0.001M) and 6.25 mL distilled water (dH2O) were added, and samples were homogenised (Ultraturax) for 20 sec. Slurry was left to filter through a Wattman no1 filter paper. From a stock solution of 1,1,3,3-Tetramethoxypropan (TMP, 0.001M), a standard curve was prepared in duplicate by adding 0, 5, 10, and 20 μL TMP in 1 mL of dH2O. Three tubes were allocated to each sample and 1 mL of filtered slurry was added to each tube. One millilitre of TBA was added to each standard and to 2 tubes for each sample, while 1 mL of dH2O was added to the third sample tube to act as a turbidity blank. All tubes were capped, vortexed, and incubated in a water bath at 70°C for 1 h. Thereafter, samples were allowed to cool, 200 μL, and the absorbance was read at 530 nm. TBARS, expressed as mg of malondialdehyde (MDA)/kg meat, were calculated as
| (2) |
All TBARS analysis was carried out on four replicates per each treatment and storage day.
3. Statistical Analysis
Data obtained on antioxidant contents of the sweet basil essential oil were analyzed using PROC ANOVA procedures of the Statistical Analysis System (SAS, version 9.1.3 of 2007). The colour and TBARS values were analyzed using PROC GLM procedures of SAS (version 9.1.3 of 2007). Significant differences between the least square means for meat samples were performed using the Fishers' least significance difference (LSD) method of SAS, with significance level of p < 0.05.
4. Results and Discussion
4.1. Chemical Constituent of the Essential Oil of Ocimum basilicum L.
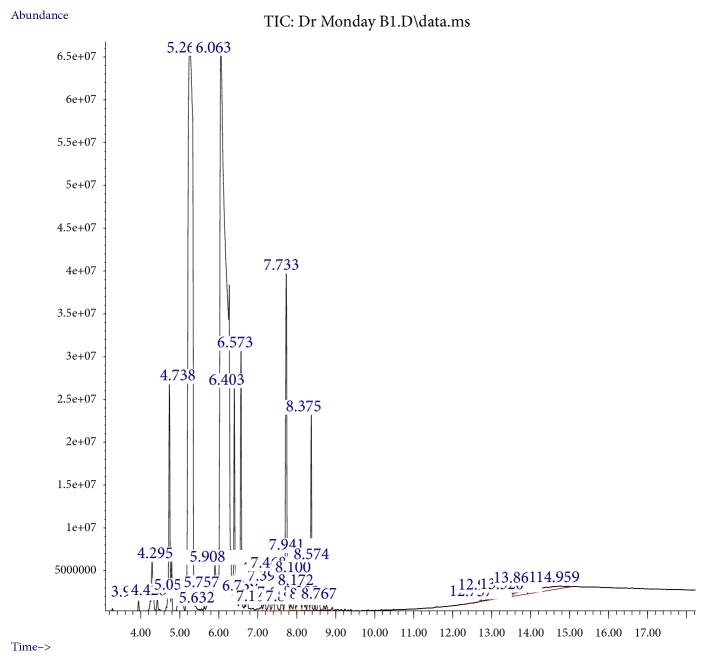
The chemical composition of the sweet basil essential oils is presented in Table 1. The GC-MS analysis of the oil indicated a total of thirty-two (32) individual compounds (Figure 1), along with their retention indices, retention time, and percentage of composition. Our result demonstrated that the main constituents in the essential oil were Estragole (41.40%), 1,6-Octadien-3-ol, 3,7-dimethyl (29.49%), trans-.alpha.-Bergamotene (5.32%), Eucalyptol (3.51), Citral (3.31%), N-Cyano-3-methylbut-2-enamine (3.08%), cis-.alpha.-Bisabolene (1.92%), Levomenthol (1.81%), and beta.-Myrcene (1.11%). Other important constituents identified in the oil with evidence of biological activities as antioxidant and antimicrobial were alpha.-Pinene (0.13%), cis-Linaloloxide (0.75%), Eugenol (0.40%), Copaene (0.38%), Humulene (0.59%), and Nerolidol (0.08%) (Table 2). The amount of compounds detected from this study equalled those reported by Złotek et al. [20], Al Abbasy et al. [21], and Okoye et al. [22] but were slightly lower than those reported by Widyawati et al. [23] and Tsasi et al. [12]. This variation could be attributed to physiological status, climate change, geographic location, harvesting time, mode, and method of extraction [24, 25]. The presence of essential oils and their composition determines the specific aroma and colour of plant and also an array of flavors when consumed [26].
Table 1.
Chemical composition of sweet basil leaf essential oil.
| No | Compounds | Retention indices | Formula | Retention Time (min) | % Peak Area |
|---|---|---|---|---|---|
| 1 | alpha.-Pinene | 935 | C10H16 | 3.946 | 0.13 |
| 2 | beta.-Myrcene | 991 | C10H16 | 4.295 | 1.11 |
| 3 | 4-Hexen-1-ol, acetate | 857 | C8H14O2 | 4.428 | 0.22 |
| 4 | Eucalyptol | 1033 | C10H18O | 4.738 | 3.51 |
| 5 | cis-Linaloloxide | 1090 | C10H18O2 | 5.055 | 0.75 |
| 6 | 1,6-Octadien-3-ol, 3,7-dimethyl | 1099 | C13H22O2 | 5.261 | 29.49 |
| 7 | Methyl ethyl cyclopentene | 1113 | C8H14 | 5.632 | 0.08 |
| 8 | l-Menthone | 1128 | C10H18O | 5.757 | 0.48 |
| 9 | Levomenthol | 1172 | C10H20O | 5.908 | 1.81 |
| 10 | Estragole | 1206 | C10H12O | 6.063 | 41.40 |
| 11 | N-Cyano-3-methylbut-2-enamine | 1238 | C6H10N2 | 6.403 | 3.08 |
| 12 | Citral | 1270 | C10H16O | 6.573 | 3.13 |
| 13 | Cyclohexene, 4-methyl-1-(1-methyle thyl) | 977.5 | C10H18 | 6.739 | 0.42 |
| 14 | Phenol, 2,3,5-trimethyl | 1492 | C9H12O | 7.101 | 0.18 |
| 15 | Eugenol | 1358 | C10H12O2 | 7.185 | 0.40 |
| 16 | Formic acid, cyclohexyl ester | 1304 | C7H12O2 | 7.300 | 0.40 |
| 17 | Copaene | 1495 | C15H24 | 7.395 | 0.38 |
| 18 | cis-7,10,13,16-Docosatetraenoic acid, methyl ester | 1393 | C23H38O2 | 7.468 | 0.55 |
| 19 | Neoisolongifolene | 1411 | C15H24 | 7.635 | 0.20 |
| 20 | trans-.alpha.-Bergamotene | 1433 | C15H24 | 7.733 | 5.32 |
| 21 | Alloaromadendrene | 1452 | C15H24 | 7.852 | 0.12 |
| 22 | Humulene | 1432 | C15H24 | 7.941 | 0.59 |
| 23 | beta.-copaene | 1477 | C15H24 | 8.100 | 0.71 |
| 24 | beta.-Bisabolene | 1509 | C15H24 | 8.172 | 0.38 |
| 25 | cis-muurola-3,5-diene | 1502 | C15H24 | 8.309 | 0.18 |
| 26 | cis-.alpha.-Bisabolene | 1504 | C15H24 | 8.375 | 1.92 |
| 27 | Nerolidol | 1535 | C15H26O | 8.471 | 0.08 |
| 28 | trans-4-Methoxycinnamaldehyde | 1569.8 | C10H10O2 | 8.574 | 0.71 |
| 29 | Benzeneacetic acid,.alpha.-hydrox | 1517 | C8H8O3 | 8.767 | 0.06 |
| 30 | Phenylethanolamine | 1298 | C8H11NO | 8.767 | 0.09 |
| 31 | 3-Methyl-2-phenylindole | 1710 | C15H13N | 12.737 | 0.01 |
| 32 | N-Benzyl-N-ethyl-p-isopropylbenzamide | 1973 | C19H23NO | 13.520 | 0.38 |
Figure 1.
GC-MS chromatogram of sweet basil essential oil.
Table 2.
Bioactivity of phytocomponents identified in the essential oil of Sweet basil by GC-MS.
| No | Compound | Compound Structure | Molecular Weight (g/mol) | Biological activities |
|---|---|---|---|---|
| 1 | alpha.-Pinene | C10H16 | 136.24 | antimicrobial |
| 2 | beta.-Myrcene | C10H16 | 136,23 | Antioxidant, antimicrobial |
| 3 | Eucalyptol | C10H18O | 154.25 | Antimicrobial |
| 4 | cis-Linaloloxide | C10H18O2 | 170.25 | Nematicidal |
| 5 | Levomenthol | C10H20O | 156.27 | antimicrobial |
| 6 | Citral | C10H16O | 152.24 | Antioxidant, antimicrobial |
| 7 | Eugenol | C10H12O2 | 164.20 | Antioxidants, antimicrobial |
| 8 | Copaene | C15H24 | 204,36 | Antioxidant |
| 9 | Humulene | C15H24 | 204.36 | Anti-inflammatory |
| 10 | Nerolidol | C15H26O | 222.37 | Antioxidant, anticancer, antimicrobial |
| 11 | Estragole | C10H12O | 148.2 | Antimicrobial, anti-inflammatory, |
4.2. Physicochemical Characteristics of Meat
Meat colour is one of the most important parameters that influence consumer's decision in buying, selecting, and acceptability of meat and meat products during display [27]. The addition of sweet basil essential oil improved (P < 0.05) the colour of the meat samples compared to control. The results revealed a significant breed effect in meat colour parameters (lightness (L∗), yellowness (b∗), redness (a∗), and chroma and hue values in the meat samples across the storage days (Table 3)).
Table 3.
Preservative effect of sweet basil essential oil on colour stability of minced beef from Boran and Nguni cattle during cold storage at 4°C.
| Parameters | Days | Treatment (T) | Breed (B) | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SB2 | SB4 | SB6 | Boran | Nguni | T | B | B x T | |||
| Lightness (L) | 0 | 34.93d | 34.39c | 35.51b | 36.64a | 34.92b | 35.82a | 0.56 | 0.01 | 0.03 | 0.04 |
| 4 | 37.88a | 38.13a | 38.49a | 38.18a | 37.58a | 38.72a | 0.89 | 0.89 | 0.07 | 0.78 | |
| 7 | 35.18c | 37.38b | 37.82b | 39.38a | 36.78b | 38.09a | 0.40 | <0.01 | <0.01 | <0.01 | |
|
| |||||||||||
| Yellowness (b) | 0 | 16.43b | 16.32b | 16.91ab | 17.42a | 16.52b | 17.02a | 0.31 | 0.03 | 0.003 | 0.33 |
| 4 | 12.03c | 13.16b | 13.96a | 14.25a | 12.91b | 13.79a | 0.20 | <0.01 | <0.01 | 0.01 | |
| 7 | 9.97c | 12.04b | 12.33b | 13.87a | 11.79b | 12.32a | 0.30 | 0.01 | <0.02 | 0.04 | |
|
| |||||||||||
| Redness (a) | 0 | 17.89c | 18.45b | 18.71b | 19.18a | 18.80a | 18.32a | 0.34 | 0.01 | 0.54 | 0.01 |
| 4 | 11.44d | 11.53c | 12.15b | 12.77a | 11.72b | 12.22a | 0.40 | 0.01 | <0.01 | 0.001 | |
| 7 | 10.83b | 11.30a | 10.39b | 10.82b | 11.08a | 10.58b | 0.37 | 0.05 | 0.03 | 0.15 | |
|
| |||||||||||
| Chroma | 0 | 24.30c | 24.65bc | 35.24a | 25.91b | 25.05a | 25.02a | 0.43 | 0.01 | 0.91 | 0.03 |
| 4 | 16.73d | 17.50c | 18.52b | 19.14a | 17.45b | 18.43a | 0.31 | <0.01 | <0.01 | 0.05 | |
| 7 | 14.73c | 16.55b | 16.23b | 17.62a | 16.32a | 16.25a | 0.32 | <0.01 | 0.16 | 0.12 | |
|
| |||||||||||
| Hue | 0 | 0.74a | 0.73a | 0.74a | 0.74a | 0.72b | 0.75a | 0.00 | 0.14 | 0.01 | 0.01 |
| 4 | 0.83a | 0.85a | 0.85a | 0.84a | 0.84a | 0.85a | 0.01 | 0.32 | 0.72 | 0.01 | |
| 7 | 0.73c | 0.81b | 0.88a | 0.90a | 0.80b | 0.85a | 0.02 | <0.01 | <0.01 | <0.01 | |
C: no additives; SB2: 2% sweet basil essential oil; SB4: 4% sweet basil essential oil; SB6: 6% sweet basil essential oil.
Means not sharing a common superscript (with a-b) in a row for each treatment are significantly different at p < 0.05.
In Table 3, L∗- and b∗- values varied significantly among treatments and storage period. From days 0 to 7, all the meat samples treated with basil EO reflected the higher L∗ and b∗ values than control group. This result is in agreement with the findings of Ünal et al. [28] and Karabagias et al. [29] who reported that meat treated with oregano, sage, rosemary, and thyme essential oil essential oils had higher L∗ and b∗ values than the control group throughout the storage period. This is probably due to the protective effect of essential oil treatments on colour lightness.
The result of the a-value of the beef samples in this study are shown in Table 3, with the value progressively decreasing across the days of storage within the treatments. This is in agreement with other studies who have reported remarkable decrease in redness intensity of meat samples (beef, mutton, and chicken) treated with essential oil during cold storage period [28, 30, 31]. At day 0, meat sample treated with 6% sweet basil EO had the highest redness values (19.18), followed by sample containing 4% (18.71) and 2 (18.45) sweet basil EO and least in control (17.89). Similar trend was also observed in day 4, with meat sample treated with sweet basil EO showing intense red colour (12.77), higher than the control group (11.44) and other treatments. Ünal et al. [28] in their study also found higher a-value in fresh minced beef treated with essential oil compared to control. However, at the 7, meat sample treated with 2% basil EO had the highest redness (11.30), followed by the control group and least in sample treated with 4 and 6% basil EO. This reduction in redness colour intensity of beef samples treated with 4 and 6% sweet basil EO at day 7 compared to control could be due to self-oxidation of basil EO to now act as prooxidants to increase pigment oxidation. Reports have revealed that, at high concentration, essential oil can behave as prooxidant, damaging cellular biomolecules [32, 33].
Increase in oxidation is strongly related to decrease in a-values of meat products or denaturation the myoglobin molecules thereby negatively reducing the colour of the meat product during cold storage and display [28, 34, 35]. In overall, fresh ground meat from Boran cattle breed exhibited higher colour stability than Nguni cattle breed. This could be due to differences in their genotypic characteristic. Lynch et al. [36] had earlier reported a significant difference in colour of beef from three breeds during storage period.
4.3. Lipid Oxidative Characteristics of Meat
The results of the oxidative changes (TBARS) of samples treated with basil essential oil aregiven in Table 4. The findings show that adding sweet basil essential oil can protect ground beef against lipid oxidation. In comparison to control group, meat samples containing sweet basil EO at 2 and 4% had lower the TBARS values from day 0 to 4. The inhibitory effects of the sweet basil essential oil against the TBAS formation could be attributed to the inherent phenolic content, phytoconstituents and antioxidant activity. Many studies have reported a positive correlation between phytochemical content or antioxidant activity of plant essential oil and reduction in lipid oxidation in meat products [15, 28]. The antioxidant activity of phytochemical compounds in essential oil has been associated with the hydroxyl group linked to the aromatic ring, which is capable of donating hydrogen atoms with electrons and neutralizing free radicals [26].
Table 4.
Preservative effect of sweet basil essential oil on oxidative stability of minced beef from Boran and Nguni cattle during cold storage at 4°C.
| Days | Treatment (T) | Breed (B) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | SB2 | SB4 | SB6 | Boran | Nguni | T | B | B x T | |
| 0 | 0.60±0.31 | 0.19±0.28 | 0.22±0.28 | 0.65±0.24 | 0.31±0.20 | 0.53±0.19 | 0.66 | 0.46 | 0.04 |
| 4 | 1.03±0.09 | 0.90±0.11 | 1.00±0.09 | 1.13±0.10 | 0.90±0.07 | 1.14±0.06 | 0.77 | 0.42 | 0.78 |
| 7 | 1.00±0.09 | 0.96±0.08 | 1.11±0.11 | 1.24±0.08 | 0.96±0.06 | 1.19±0.07 | 0.17 | 0.15 | <0.01 |
At day 7 of storage meat sample treated with higher concentration (4 and 6% sweet basil EO) reflected higher TBARs values than the control group. This result is similar to the finding of Kuzelov et al. [15] who reported that meat sample treated higher concentration of holy basil extract above 3 mg/kg exhibited higher TBARS values than the control after 5th of cold storage at 1°C. In general, the addition of sweet basil essential oil exhibited higher antioxidant activity at 2% than the control and those treated with 4 and 6% essential oil at day 7 of storage.
In overall, the TBARS values of the Boran beef were slightly lower than Nguni beef samples across the treatment and storage period. Since both animals were raised on natural pasture, this difference in their TBARS values could be attributed to factors such as inherent endogenous antioxidants, and composition and distribution of unsaturated fatty acids in triacylglycerol molecule, which has been reported to influence the rate of lipid oxidation in muscle food [37]. This result is in agreement with the report of Xie et al. [38] who found that Limousin beef sample has significant lower TBARS values than Qinchuan cattle breed.
5. Conclusion
A total of 32 bioactive compounds were identified in SBEO, with reported preservative functions including antioxidant and antimicrobial activities, as well as anti-inflammatory, nematicidal, and anticancer compounds. The addition of 2% and 4% SBEO improved colour stability of minced beef during 7 days of refrigerated storage compared to the control. The TBARS were significantly lower in SB2 and SB4 on days 0 and 4, while SB6 was similar to or higher than C. The results indicate the potential of SBEO at 2 and 4% as a natural antioxidant additive to improve colour and lipid oxidative stability during refrigerated storage of aerobically packaged minced beef, and further studies investigating its antimicrobial activity are recommended.
Acknowledgments
The authors are grateful to the Govan Mbeki Research Development Centre (GMRDC), University of Fort Hare, and the DST-NRF Centre of Excellence in Food Security (Project ID: 170275) for financial assistance.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
There are no conflicts of interest declared by the authors.
Authors' Contributions
Andrew Bamidele Falowo, Felicitas Esnart Mukumbo, Emrobowansan Monday Idamokoro, Anthony Jide Afolayan, and Voster Muchenje conceptualized and designed the work; Andrew Bamidele Falowo, Felicitas Esnart Mukumbo, and Emrobowansan Monday Idamokoro collected and analyzed the data, visualized the results, and proofread the paper; Andrew Bamidele Falowo and Felicitas Esnart Mukumbo wrote the paper; Anthony Jide Afolayan and Voster Muchenje provided laboratory facilities and financial support for the experiment.
References
- 1.Metsovas S. Synthetic Antioxidants: Longer Shelf-Life May Lead to Shorter Lifespan. 2013, http://pontoniere.blogautore.espresso.repubblica.it/2013/05/20/synthetic-antioxidants-longer-shelf-life-may-lead-to-shorter-lifespan.
- 2.Xiao S., Zhang W. G., Lee E. J., Ahn D. U. Effects of diet, packaging and irradiation on protein oxidation, lipid oxidation of raw broiler thigh meat. Animal Industry Report. 2013;659:p. 2761. doi: 10.3382/ps.2010-01244. [DOI] [PubMed] [Google Scholar]
- 3.Decker E., Livisay S., Zhou S. Mechanisms of endogenous skeletal muscle antioxidants: chemical and physical aspects. In: Decker E., Faustman C., Lopez-Bote C., editors. Antioxidants in Muscle Foods. New York, NY, USA: Wiley Interscience; 2000. pp. 25–60. [Google Scholar]
- 4.Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(4):113–119. doi: 10.1111/j.1747-0080.2007.00197.x. [DOI] [Google Scholar]
- 5.Carlsen C. U., Møller J. K. S., Skibsted L. H. Heme-iron in lipid oxidation. Coordination Chemistry Reviews. 2005;249(3-4):485–498. doi: 10.1016/j.ccr.2004.08.028. [DOI] [Google Scholar]
- 6.Kumar Y., Yadav D. N., Ahmad T., Narsaiah K. Recent trends in the use of natural antioxidants for meat and meat products. Comprehensive Reviews in Food Science and Food Safety. 2015;14(6):796–812. doi: 10.1111/1541-4337.12156. [DOI] [Google Scholar]
- 7.Patrakova I. S., Gurinovich G. V. The study of factors affecting the activity of meat antioxidant system. Foods and Raw Materials. 2015;3(1):33–40. doi: 10.12737/11235. [DOI] [Google Scholar]
- 8.Muhlisin, Utama D. T., Lee J. H., Choi J. H., Lee S. K. Antioxidant enzyme activity, iron content and lipid oxidation of raw and cooked meat of Korean native chickens and other poultry. Asian-Australasian Journal of Animal Sciences. 2016;29(5):695–701. doi: 10.5713/ajas.15.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marwat S. K., Khan M. S., Ghulam S., Anwar N., Mustafa G., Usman K. Phytochemical constituents and pharmacological activities of sweet Basil-Ocimum basilicum L. (Lamiaceae) Asian Journal of Chemistry. 2011;23(9):3773–3782. [Google Scholar]
- 10.Hazra S., Biswas S., Bhattacharyya D., Das S. K., Khan A. Quality of cooked ground buffalo meat treated with the crude extracts of Moringa oleifera (Lam.) leaves. Journal of Food Science and Technology. 2012;49(2):240–245. doi: 10.1007/s13197-011-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taie H. A. A., Salama Z. A.-E. R., Radwan S. Potential activity of basil plants as a source of antioxidants and anticancer agents as affected by organic and bio-organic fertilization. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38(1):119–127. [Google Scholar]
- 12.Tsasi G., Mailis T., Daskalaki A., et al. The effect of harvesting on the composition of essential oils from five varieties of Ocimum basilicum L. cultivated in the Island of Kefalonia, Greece. Plants. 2017;6(3) doi: 10.3390/plants6030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakowienko P., Wójcik-Stopczyńska B., Jadczak D. Antifungal activity of essential oils from two varieties of sweet basil (Ocimum basilicum L.) Vegetable Crops Research Bulletin. 2011;74(1):97–106. doi: 10.2478/v10032-011-0008-4. [DOI] [Google Scholar]
- 14.Joshi R. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Ancient Science of Life. 2014;33(3):p. 149. doi: 10.4103/0257-7941.144618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuzelov A., Andronikov D., Taskov N., Sofijanova E., Saneva D. Oxidative stability effect of basil, garlic and muscat blossom extracts on lipids and microbiology of minced meat. Comptes rendus de l'Academie bulgare des Sciences. 2017;70(9):1227–1236. [Google Scholar]
- 16.Li Z., Henning S. M., Zhang Y., et al. Antioxidant-rich spice added to hamburger meat during cooking results in reduced meat, plasma, and urine malondialdehyde concentrations. American Journal of Clinical Nutrition. 2010;91(5):1180–1184. doi: 10.3945/ajcn.2009.28526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Shazly A., Dorai G., Wink M. Composition and antimicrobial activity of essential oil and hexane-ether extract of Tanacetum santolinoides (DC.) Feinbr. and Fertig. Zeitschrift für Naturforschung. 2002;57(7-8):620–623. doi: 10.1515/znc-2002-7-812. [DOI] [PubMed] [Google Scholar]
- 18.Shahat A. A., Ibrahim A. Y., Hendawy S. F., et al. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules. 2011;16(2):1366–1377. doi: 10.3390/molecules16021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minolta. Precise color communication. Minolta Camera, Ltd: Ramsey, NJ, USA, 1993.
- 20.Złotek U., Michalak-Majewska M., Szymanowska U. Effect of jasmonic acid elicitation on the yield, chemical composition, and antioxidant and anti-inflammatory properties of essential oil of lettuce leaf basil (Ocimum basilicum L.) Food Chemistry. 2016;213:1–7. doi: 10.1016/j.foodchem.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 21.Al Abbasy D. W., Pathare N., Al-Sabahi J. N., Khan S. A. Chemical composition and antibacterial activity of essential oil isolated from Omani basil (Ocimum basilicum Linn.) Asian Pacific Journal of Tropical Disease. 2015;5(8):645–649. doi: 10.1016/S2222-1808(15)60905-7. [DOI] [Google Scholar]
- 22.Mith H., Yayi-Ladékan E., Sika Kpoviessi S. D., et al. Chemical Composition and Antimicrobial Activity of Essential Oils of. Journal of Essential Oil Bearing Plants. 2016;19(6):1413–1425. doi: 10.1080/0972060X.2014.890076. [DOI] [Google Scholar]
- 23.Widyawati P. S., Wijaya C. H., Hardjosworo P. S., Sajuthi D. Volatile Compounds of Pluchea indica Less and Ocimum basillicum Linn Essential Oiland Potency as Antioxidant. HAYATI Journal of Biosciences. 2013;20(3):117–126. doi: 10.4308/hjb.20.3.117. [DOI] [Google Scholar]
- 24.Figueiredo A. C., Barroso J. G., Pedro L. G., Scheffer J. J. C. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour and Fragrance Journal. 2008;23(4):213–226. doi: 10.1002/ffj.1875. [DOI] [Google Scholar]
- 25.Kayode R. M. O., Afolayan A. J. Cytotoxicity and effect of extraction methods on the chemical composition of essential oils of Moringa oleifera seeds. Journal of Zhejiang University SCIENCE B. 2015;16(8):680–689. doi: 10.1631/jzus.B1400303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radha Krishnan K., Babuskin S., Azhagu Saravana Babu P., et al. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. International Journal of Food Microbiology. 2014;171:32–40. doi: 10.1016/j.ijfoodmicro.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Grunert K. G., Bredahl L., Brunsø K. Consumer perception of meat quality and implications for product development in the meat sector - A review. Meat Science. 2004;66(2):259–272. doi: 10.1016/S0309-1740(03)00130-X. [DOI] [PubMed] [Google Scholar]
- 28.Ünal K., Babaoglu A. S., Karakaya M. Effect of Oregano, Sage and Rosemary Essential Oils on Lipid Oxidation and Color Properties of Minced Beef During Refrigerated Storage. Journal of Essential Oil Bearing Plants. 2014;17(5):797–805. doi: 10.1080/0972060X.2014.956803. [DOI] [Google Scholar]
- 29.Karabagias I., Badeka A., Kontominas M. G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Science. 2011;88(1):109–116. doi: 10.1016/j.meatsci.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Keokamnerd T., Acton J. C., Han I. Y., Dawson P. L. Effect of commercial rosemary oleoresin preparations on ground chicken thigh meat quality packaged in a high-oxygen atmosphere. Poultry Science. 2008;87(1):170–179. doi: 10.3382/ps.2007-00066. [DOI] [PubMed] [Google Scholar]
- 31.AL-Hijazeen M. Effect of direct adding oregano essential oil (Origanum syriacum L.) on quality and stability of chicken meat patties. Food Science and Technology. 2017;(0) doi: 10.1590/1678-457x.17117. [DOI] [Google Scholar]
- 32.Miguel M. G., Cruz C., Faleiro L., et al. Foeniculum vulgare essential oils: Chemical composition, antioxidant and antimicrobial activities. Natural Product Communications (NPC) 2010;5(2):319–328. [PubMed] [Google Scholar]
- 33.Mimica-Dukić N., Orč Ić D., Lesjak M., Šibul F. Essential oils as powerful antioxidants: Misconception or scientific fact? ACS Symposium Series. 2016;1218:187–208. doi: 10.1021/bk-2016-1218.ch012. [DOI] [Google Scholar]
- 34.Mancini R. A., Hunt M. C. Current research in meat color. Meat Science. 2005;71(1):100–121. doi: 10.1016/j.meatsci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Shah M. A., Bosco S. J. D., Mir S. A. Effect of Moringa oleifera leaf extract on the physicochemical properties of modified atmosphere packaged raw beef. Food Packaging and Shelf Life. 2015;3:31–38. doi: 10.1016/j.fpsl.2014.10.001. [DOI] [Google Scholar]
- 36.Lynch A., Buckley D. J., Galvin K., Mullen A. M., Troy D. J., Kerry J. P. Evaluation of rib steak colour from Friesian, Hereford and Charolais heifers pastured or overwintered prior to slaughter. Meat Science. 2002;61(3):227–232. doi: 10.1016/S0309-1740(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 37.Wsowicz E., Gramza A., Heoe M., et al. Oxidation of lipids in food. Polish Journal of Food And Nutrition Sciences. 2004;13/54(1):87–100. [Google Scholar]
- 38.Xie X., Meng Q., Cui Z., Ren L. Effect of cattle breed on meat quality, muscle fiber characteristics, lipid oxidation and fatty acids in China. Asian-Australasian Journal of Animal Sciences. 2012;25(6):824–831. doi: 10.5713/ajas.2011.11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.