Abstract
Background
The prognostic value of CD133 and SOX2 expression in advanced cancer remains unclear. This study was first conducted to investigate the association between CD133 or SOX2 positivity and clinical outcomes for advanced cancer patients.
Methods
Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated to evaluate the correlation between CD133 or SOX2 positivity and overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), cancer-specific survival (CSS), or recurrence-free survival (RFS) from multivariable analysis. Trial sequential analysis (TSA) was also performed.
Results
13 studies with 1358 cases (CD133) and five studies with 433 cases (SOX2) were identified. CD133 positivity was correlated with worse CSS and OS, but there was no correlation between CD133 positivity and DFS. SOX2 positivity was associated with poor DFS and RFS but was not linked to PFS. Stratified analysis by study source showed that only CD133 positivity can decrease OS for Chinese patients. Stratified analysis by treatment regimens indicated that CD133 positivity was linked to poor OS in patients treated with adjuvant therapy. TSA showed that additional studies were necessary.
Conclusions
CD133 and SOX2 might be associated with worse prognosis in advanced cancer. More prospective studies are strongly needed.
Impact
CD133 and SOX2 may be promising targeted molecular therapy for advanced cancer patients.
1. Introduction
Cancer is still one of the most threatening diseases worldwide [1]. Although surgery, chemotherapy, and/or radiotherapy have greatly improved the clinical survival for early cancer patients, therapies for patients with advanced or metastatic cancer still have a major challenge [2]. Improvements in the treatment of advanced or metastatic cancer patients (surgical technique, chemotherapy, radiotherapy, targeted molecular therapy, and immunotherapy regimens) have extended patients' median survival, but such as 5-year overall survival is still poor [3–5]. Thus the development of new and novel therapeutic regimens for advanced or metastatic cancer patients is important.
Increasing evidence has been suggested regarding cancer stem cells (CSCs) in various cancers. The major characteristics of CSCs are the capability of self-renewal, unlimited proliferation and differentiation, and resistance to conventional treatments like chemotherapy or radiation [6, 7]. Recently, some stem cell markers have been described, such as CD44, CD166, EpCAM, CD133, and SOX2 [8–10]. CD133, also named as prominin-1, is a member of pentaspan transmembrane cell surface glycoproteins [11, 12]. Sex-determining region Y-box protein 2 (SOX2), a High Mobility Group (HMG) domain transcription factor, is involved in the regulation of stem cells self-renewal and pluripotency [13]. CD133 expression has been reported and contributes to malignant transformation and chemo- and radioresistance [14]. SOX2 has been studied in some types of human cancers and facilitates tumor initiation and progression [15–17]. Some meta-analyses investigated the prognostic value of CD133 and SOX2 expression in some human cancers [18–21], but the prognostic significance of CD133 and SOX2 expression in advanced cancer patients remains unclear and unknown.
To our knowledge, the expression of CD133 and SOX2 is hitherto undescribed in advanced cancer by a meta-analysis. To clarify the correlation between the expression of stem cell markers (CD133 and SOX2) and the prognosis in advanced or metastatic cancer patients, we investigated the relationship between the expression of these two markers and survival of the samples.
2. Materials and Methods
2.1. Literature Selection
The present meta-analysis was reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline [22]. The potential studies were identified through searching online databases including PubMed, EMBASE, EBSCO, Web of Science, and Cochrane Library before April 2018 without language restrictions. The main key words and search items were “CD133 OR PROM1 OR prominin-1 OR AC133 antigen OR SOX2 OR Sex determine region Y-box 2 OR SRY box-2 OR SRY-Related HMG-Box Gene 2”, “metastatic OR advanced OR metastasized OR recurrent”, “cancer OR tumor OR carcinoma OR neoplasm”, and “survival OR outcome OR prognosis”. Additional potential articles were also manually searched by the reference lists of the eligible studies.
2.2. Eligibility Criteria
Papers identified for the inclusion criteria in this study for the current analysis were as follows: (1) studies reported the patients with advanced, metastatic, or recurrent cancer; (2) studies investigated the prognostic value of expression of CD133 or SOX2; (3) studies presented sufficient data on hazard ratio (HR) with 95% confidence interval (CI) from multivariable analysis for overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), cancer-specific survival (CSS), relapse/recurrence-free survival (RFS), or metastasis-free survival (MFS); (4) unclear data (HR with 95% CI) such as only P value with HR or 95% CI, survival data calculated based on the described method [23, 24], or contacting the corresponding author via email to request the available information. If two or more papers used the overlapping or same cancer samples, only the study with the largest patient numbers or the most recent article was selected. Case report, reviews, animal studies, unrelated articles, or survival data using univariable analysis were excluded.
2.3. Data Extraction and Study Assessment
The methodology of each eligible study was conducted following REMARK guidelines (Reporting Recommendations for Tumor Marker Prognostic Studies) [25]. 20 criteria were listed in REMARK; each item had scores 0, 1, and 2, with a maximal score of 40 (Table S1). The value was 2 scores when each item was clearly described in the article, 1 score when each item was incompletely defined, and 0 score when each item was not defined or not applicable. We did not define a threshold for the REMARK score of study quality because multivariable survival measures are more valuable than studies using univariable analysis, done in the present meta-analysis. REMARK scores can be used and evaluated for sensitivity analyses. The following information was extracted from eligible studies: first author's name, publication year, study population, study source, mean or median age, type of cancer, detection method, therapy regime, study design, sample type, cut-off value, median or mean follow-up period, survival rate, adjusted variables, and clinical outcomes, etc. All authors resolved the discrepancy when information was controversial.
2.4. Data Analysis
To estimate the effect of CD133 or SOX2 expression status on advanced cancer survival (OS, DFS, PFS, CSS, RFS, or MFS of multivariable analysis), the result with an HR >1 demonstrated an unfavorable prognosis, whereas an HR <1 stood for a good prognosis. The Cochran's Q statistic was used to evaluate heterogeneity among the included studies [26]. The random-effects model (DerSimonian-Laird) was used in the meta-analysis (heterogeneity: P < 0.1) [27, 28]. For the results (> seven studies) with substantial heterogeneity, subgroup analyses based on tumor type, study source, survival rate, sample type, age (years), testing method, and study center design were performed to explain the potential heterogeneity and different strength of the association between subgroups. If all relevant P values of heterogeneity were greater than 0.1 among different subgroups, it indicates the source of heterogeneity from a subgroup variable. The Egger's and Begg's funnel plots were used to evaluate publication bias [29, 30]. Pooled data were analyzed using Stata software, version 12.0 (Stata Corp., College Station, TX, USA).
2.5. Trial Sequential Analysis
In the meta-analysis involving a small number of participants, random errors can lead to spurious results [31, 32]. Trial sequential analysis (TSA) was conducted to control random errors and to estimate the required study population [33]. The optimal a priori anticipated information size (APIS) method was set in our study. We calculated diversity-adjusted TSA based on the relative risk reduction (RRR) of 20%, the prespecified type I error of 5%, and the type II error (20% or 10%). We also calculated diversity-adjusted TSA based on a RRR of 15%, the prespecified type I error (α) of 5%, and a type II error (β) of 20%. Monitoring boundaries are applied to decide whether a clinical trial could be terminated early. When the cumulative Z curve was more than the trial sequential monitoring boundary or required information size (RIS) boundary, it suggested the firm evidence. Otherwise, more clinical studies are needed. Meta-analysis of HR estimates was performed using Stata software, version 12.0 (Stata Corp., College Station, TX, USA) and R software, version 3.4.2 (The R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Study Characteristics
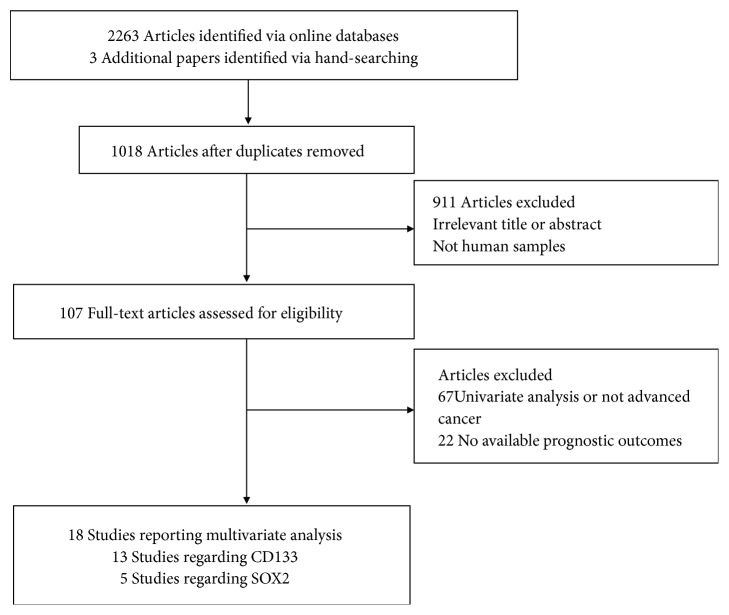
Flowchart describing the study selection process is shown in Figure 1. After the described inclusion criteria, 18 eligible studies involving 1791 advanced cancer patients were selected for the current meta-analysis [34–51]. Of these studies, 13 studies published from 2006 to 2017 (one prospective study and 12 retrospective studies) evaluated the prognostic role of CD133 positivity [35, 37, 38, 40, 42, 44–51], including 1358 cases. Five studies (one prospective study and four retrospective studies) assessed the prognostic role of SOX2 positivity [34, 36, 39, 41, 43], including 433 cases. The mean REMARK scores were 21, with a range from 12 to 28. Most studies (78%) reported patients treated with adjuvant therapy. All articles published were from 2006 to 2017, and six studies were conducted in China, six studies in Japan, one study in Korea, and the remaining five studies in Europe. The characteristics of the eligible studies using multivariable analysis are listed in Table 1 and Table S2.
Figure 1.
Flow chart for identification of eligible studies.
Table 1.
Main characteristics of studies included in the meta-analysis.
| First author | Study source | Age | Testing method | Cancer type | Study design | Specimen type | Cases | Survival rate | Outcomes | Therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| Mehra 2006 | The Netherlands | NA | NASBA | Cancer with bone metastases | Retrospective, multicentre | Blood | 50 | < 3 years | OS | Part (adjuvant therapy) |
|
| ||||||||||
| Li 2009 | China | NA | IHC | Advanced colon carcinoma | Retrospective, single-center | Tissue | 104 | 5 years | OS | Adjuvant chemotherapy |
|
| ||||||||||
| Fusi 2011 | Germany | 54 | Flow cytometry analysis | Metastatic melanoma | Retrospective, single-center | Blood | 32 | NA | OS | Neoadjuvant chemotherapy |
|
| ||||||||||
| Pilati 2012 | Italy | 63 | qRT-PCR | Colorectal liver metastasis | Retrospective, single-center | Blood | 50 | 3 years | CSS | Surgery and chemotherapy |
|
| ||||||||||
| Sakai 2012 | Japan | NA | IHC | Colorectal cancer with liver metastasis | Retrospective, single-center | Tissue | 92 | 3 years | OS, DFS | Surgery |
|
| ||||||||||
| Qin 2012 | China | NA | IHC | Advanced serous ovarian cancer | Retrospective, multicentre | Tissue | 123 | NA | OS | Adjuvant chemotherapy |
|
| ||||||||||
| Lee 2012 | Korea | 61.5 | IHC | Advanced gastric cancer | Retrospective, single-center | Tissue | 100 | 5 years | OS, DFS | Surgery and adjuvant chemotherapy |
|
| ||||||||||
| Sprenger 2013 | Germany | 63 | IHC, blind | Advanced rectal adenocarcinoma | Prospective, multicentre | Tissue | 126 | NA | CSS, DFS | Surgery and radiochemotherapy |
|
| ||||||||||
| Yamamoto 2014 | Japan | NA | IHC, blind | Colorectal cancer liver metastasis | Retrospective, single-center | Tissue | 103 | 5 years | OS | Surgery and chemotherapy |
|
| ||||||||||
| Liu 2014 | China | 57 | IHC, blind | Epithelial ovarian cancer with central nervous system metastasis | Retrospective, single-center | Tissue | 29 | < 3 years | OS | Surgery and adjuvant therapy |
|
| ||||||||||
| Kazama 2015 | Japan | 67.1 | IHC | Colorectal cancer with lymph node metastasis | Retrospective, single-center | Tissue | 138 | > 5 years | OS | Surgery and adjuvant chemotherapy |
|
| ||||||||||
| Kishikawa 2016 | Japan | 59.4 | IHC | Colorectal cancer with synchronous liver metastases | Retrospective, single-center | Tissue | 88 | NA | OS, DFS | Surgery and adjuvant chemotherapy |
|
| ||||||||||
| Pei 2016 | China | NA | IHC, blind | Advanced colorectal cancer | Retrospective, single-center | Tissue | 323 | NA | OS, DFS | Surgery and adjuvant chemotherapy |
|
| ||||||||||
| Huang 2014 | China | NA | IHC, blind | Breast cancer with axillary lymph nodes | Retrospective, multicentre | Tissue | 107 | NA | DFS | NA |
|
| ||||||||||
| Shen 2014 | China | 51 | IHC, blind | Advanced cervical squamous cell carcinoma | Retrospective, multicentre | Tissue | 132 | 5 years | PFS | Radiotherapy |
|
| ||||||||||
| Udagawa 2015 | Japan | 66 | IHC | Lung squamous cell carcinoma with lymph node metastasis | Retrospective, single-center | Tissue | 113 | NA | RFS | Surgery |
|
| ||||||||||
| Sodja 2016 | Slovenia | 65 | qRT-PCR | Advanced small-cell lung cancer | Prospective, single-center | Blood | 50 | NA | DFS, PFS | Chemotherapy |
|
| ||||||||||
| Yamawaki 2017 | Japan | NA | IHC | Advanced endometrial cancer | Retrospective, single-center | Tissue | 31 | NA | PFS | NA |
NA: not applicable; NASBA: nuclear acid sequence-based amplification; IHC: immunohistochemistry; qRT-PCR: Real-Time Quantitative PCR; OS: overall survival; DFS: disease-free survival; PFS: progression-free survival; CSS: cancer-specific survival; RFS: recurrence-free survival (RFS).
3.2. Association between CD133 Positive Expression and the Prognosis
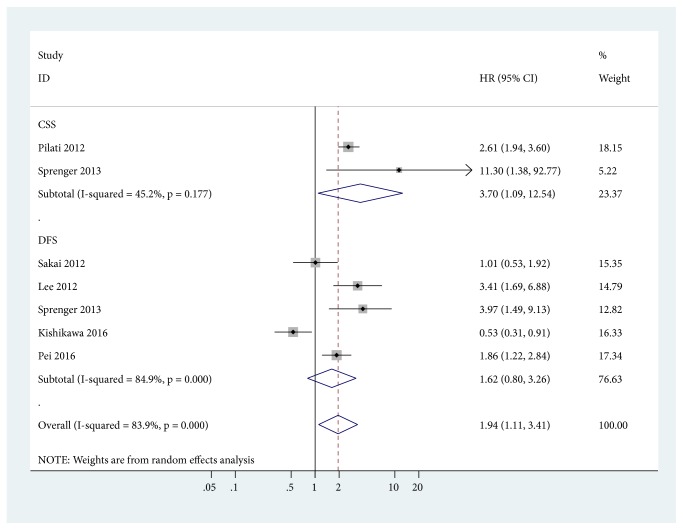
The pooled data from two studies involving 176 advanced cancer patients showed that CD133 positive expression was associated with a worse cancer-specific survival (CSS) (HR = 3.70, 95% CI = 1.09-12.54, P = 0.036) (Figure 2). Data from five studies involving 729 patients with advanced cancer demonstrated no association between CD133 positive expression and DFS (HR = 1.62, 95% CI = 0.80-3.26, P = 0.178) (Figure 2).
Figure 2.
Forest plot for the correlation between CD133 positive expression and cancer-specific survival (CSS) and disease-free survival (DFS).
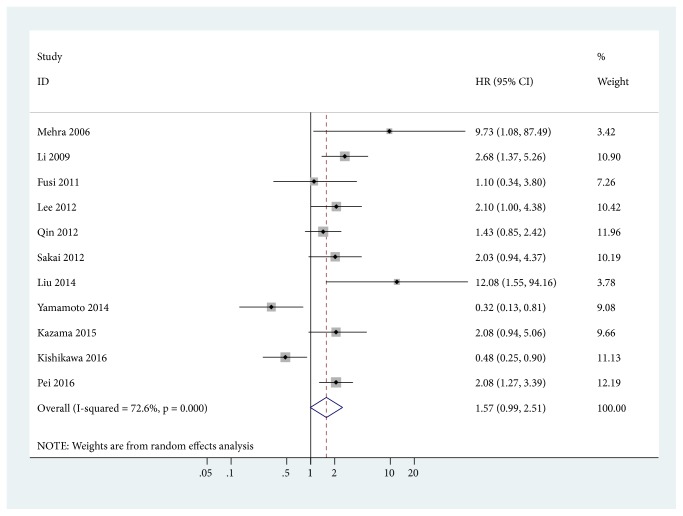
11 studies with 1182 cases were included in the final analysis of CD133 positivity and OS. Data showed that CD133 positivity was slightly correlated with an unfavorable OS (HR = 1.57, 95% CI = 0.99-2.51, P = 0.057) (Figure 3).
Figure 3.
Forest plot for the correlation between CD133 positive expression and overall survival (OS).
3.3. Subgroup and Sensitivity Analyses of CD133 Positive Expression in OS
We summarized the results of the subgroup analyses among several related clinical parameters (tumor type, study source, survival rate, sample type, age (years), testing method, study center design, treatment regimens, and sample size) for OS in Table 2. All P values of heterogeneity were not more than 0.1 between different subgroups; subgroup analyses did not find the potential sources of heterogeneity.
Table 2.
Subgroup analyses of CD133 positivity in overall survival (OS).
| Factors | Subgroups | Studies | HR with 95% CI | Heterogeneity (P) | P value | Cases | TSA |
|---|---|---|---|---|---|---|---|
| Tumor type | Colorectal cancer | 6 | 1.27 (0.64-2.50) | < 0.001 | 0.493 | 848 | |
| Ovarian cancer | 2 | 3.27 (0.43-25.03) | 0.049 | 0.254 | 152 | ||
| Melanoma | 1 | 1.1 (0.34-3.8) | NA | > 0.05 | 32 | ||
| Cancer with bone metastases | 1 | 9.73 (1.08-87.49) | NA | < 0.05 | 50 | More | |
| Gastric cancer | 1 | 2.097 (1.003-4.383) | NA | < 0.05 | 100 | More | |
| Study source | Japanese | 4 | 0.90 (0.36-2.26) | 0.001 | 0.823 | 421 | |
| Chinese | 4 | 2.12 (1.35-3.33) | 0.154 | 0.001 | 579 | More | |
| Others | 3 | 2.07 (0.90-4.78) | 0.229 | 0.089 | 182 | ||
| Survival rate | 5 years | 3 | 1.26 (0.38-4.17) | 0.001 | 0.703 | 307 | |
| < 3 years | 2 | 10.92 (2.44-48.96) | 0.888 | 0.002 | 79 | More | |
| Others | 6 | 1.38 (0.85-2.26) | 0.01 | 0.197 | 796 | ||
| Sample type | Tissue | 9 | 1.51 (0.92-2.49) | < 0.001 | 0.103 | 1100 | |
| Blood | 2 | 2.68 (0.33-21.83) | 0.088 | 0.358 | 82 | ||
| Age (years) | > 60 | 2 | 2.09 (1.20-3.64) | 0.989 | 0.009 | 238 | More |
| ≤ 60 | 3 | 1.40 (0.31-6.27) | 0.01 | 0.661 | 149 | ||
| NA | 6 | 1.64 (0.92-2.91) | 0.003 | 0.093 | 795 | ||
| Treatment regimens | Adjuvant therapy | 4 | 1.91 (1.08-3.39) | 0.173 | 0.026 | 309 | More |
| Surgery and adjuvant therapy | 6 | 1.34 (0.62-2.93) | < 0.001 | 0.457 | 781 | ||
| Testing method | Blind | 3 | 1.64 (0.32-8.37) | < 0.001 | 0.553 | 455 | |
| NA | 8 | 1.62 (1.00-2.63) | 0.005 | 0.048 | 727 | More | |
| Study center design | Multicentre | 2 | 2.74 (0.46-16.19) | 0.097 | 0.266 | 173 | |
| Single-center | 8 | 1.54 (0.85-2.79) | < 0.001 | 0.154 | 977 | ||
| NA | 1 | 1.1 (0.34-3.8) | NA | > 0.05 | 32 | ||
| Sample size | ≥ 100 | 6 | 1.58 (0.97-2.57) | 0.007 | 0.066 | 891 | |
| < 100 | 5 | 1.93 (0.66-5.65) | 0.001 | 0.232 | 291 |
HR: hazard ratio; 95% CI: 95% confidence interval; NA: not applicable; TSA: trial sequential analysis.
Based on tumor type, significant difference was not found in 848 patients with colorectal cancer (six studies: HR = 1.27, 95% CI = 0.64-2.50, P = 0.493), 152 patients with ovarian cancer (two studies: HR = 3.27, 95% CI = 0.43-25.03, P = 0.254), and 32 patients with melanoma (one study: HR = 1.1, 95% CI = 0.34-3.8). There was statistical significance in patients with 50 cancer patients with bone metastases (one study: HR = 9.73, 95% CI = 1.08-87.49) and 100 patients with gastric cancer (one study: HR = 2.097, 95% CI = 1.003-4.383).
Subgroup analysis by treatment regimens indicated that CD133 positivity was slightly linked to poor OS in patients treated with adjuvant therapy (4 studies with 309 cases: HR = 1.91, 95% CI = 1.08-3.39, P = 0.026). Subgroup analysis of study source showed that only Chinese with CD133 positivity was significantly correlated with a worse OS (four studies with 579 cases: HR = 2.12, 95% CI = 1.35-3.33, P = 0.001). Subgroup analysis of survival rate indicated that CD133 positivity was significantly related to a less than 3-year OS (two studies with 79 cases: HR = 10.92, 95% CI = 2.44-48.96, P = 0.002). Stratified analysis by age demonstrated that CD133 positivity was significantly associated with shorter OS in patients aged more than 60 years (two studies with 238 cases: HR = 2.09, 95% CI = 1.20-3.64, P = 0.009). Significant difference was not noted between other subgroup analyses (sample type, study center design, and sample size) and CD133 positivity (Table 2).
Sensitivity analysis was performed by omitting an individual study by turn to detect the robustness of the result. The result showed that two studies conducted by Yamamoto 2014 et al. [42] and Kishikawa 2016 et al. [37] in Japan significantly affected the pooled HR value, with the significant HR (2.02, 95% CI = 1.56-2.60, P < 0.001) and no evidence of heterogeneity (P = 0.413).
3.4. Association between SOX2 Positive Expression and the Prognosis
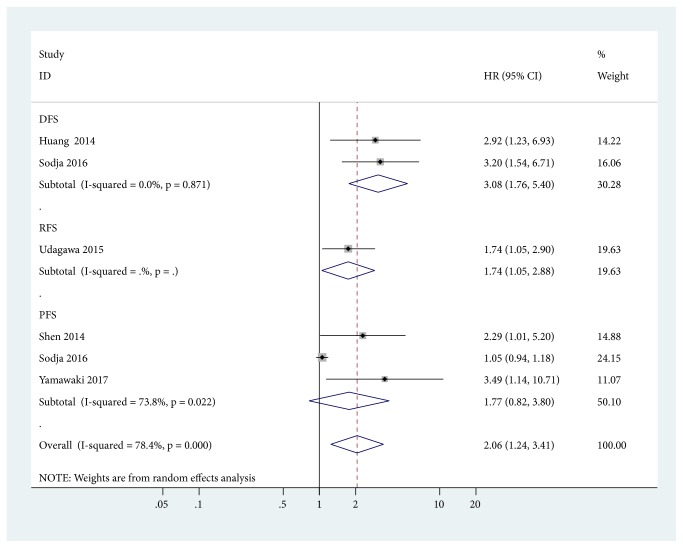
SOX2 positivity was associated with worse DFS (two studies with 157 cases: HR = 3.08, 95% CI = 1.76-5.40, P < 0.001) and RFS (one study with 113 cases: HR = 1.736, 95% CI = 1.055-2.901, P = 0.033), but no relationship was found between SOX2 positivity and PFS (three studies with 213 cases: HR = 1.77, 95% CI = 0.82-3.80, P = 0.145) (Figure 4).
Figure 4.
Forest plot for the association between SOX2 positivity and disease-free survival (DFS), relapse/recurrence-free survival (RFS), and progression-free survival (PFS).
3.5. Publication Bias
Publication bias was detected for OS and DFS of CD133 positive expression. No evidence of publication bias was noted using Egger's test (P = 0.564 > 0.05) and Begg's test (P = 0.876 > 0.05) in OS (Figure S1). Moreover, we did not find publication bias for DFS of CD133 positive expression (P > 0.1) (Figure S1).
3.6. TSA
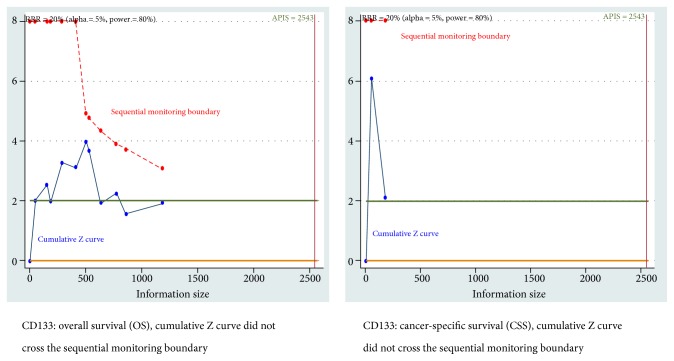
When the prespecified type I error α (5%), a RRR of 20%, and a type II error β of 20% (80% power) were set, the TSA showed that cumulative Z curve did not cross the sequential monitoring boundary for CSS and OS of CD133 positive expression (Figure 5). For DFS of SOX2 positivity, cumulative Z curve was not more than the sequential monitoring boundary (Figure S2A). For positive results of OS of CD133 positivity among subgroups, the TSA also demonstrated that cumulative Z curve did not cross the trial sequential monitoring boundary (Table 2).
Figure 5.
Trial sequential analysis (TSA) for cancer-specific survival (CSS) and overall survival (OS) of CD133 positive expression (α = 5%, β = 20%, and the relative risk reduction (RRR) = 20%).
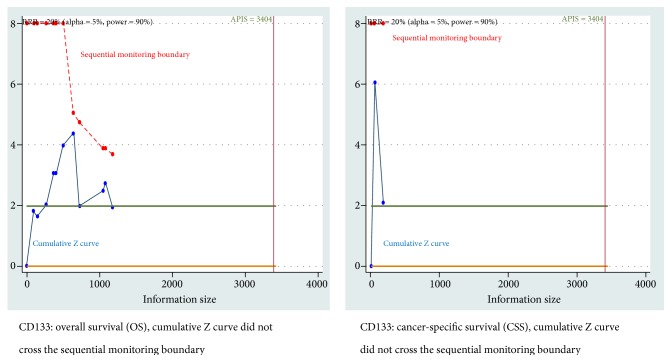
When the type I error of 5%, a RRR of 20%, and a type II error of 10% (90% power) were used, TSA also demonstrated that the cumulative Z curve did not reach the sequential monitoring boundary between CD133 positivity and CSS and OS (Figure 6). The TSA showed that the cumulative Z curve did not cross the trial sequential monitoring boundary between SOX2 positivity and DFS (Figure S2B).
Figure 6.
Trial sequential analysis (TSA) for cancer-specific survival (CSS) and overall survival (OS) of CD133 positive expression (α = 5%, β = 10%, and the relative risk reduction (RRR) = 20%).
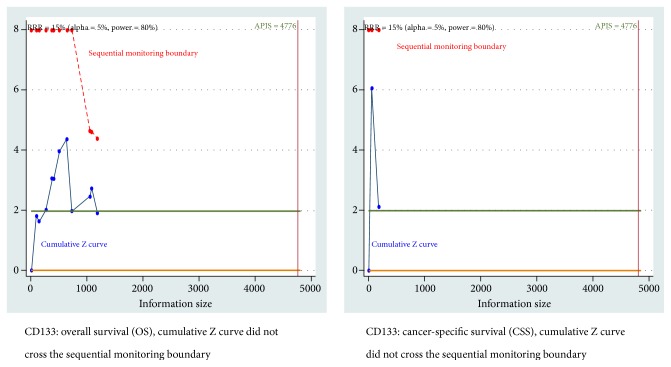
When the type I error of 5%, type II error of 20%, and a more conservative RRR of 15% were set, the results remained consistent, and the TSA also showed that cumulative Z curve did not reach the trial sequential monitoring boundary (Figure 7 and Figure S2C).
Figure 7.
Trial sequential analysis (TSA) for cancer-specific survival (CSS) and overall survival (OS) of CD133 positive expression (α = 5%, β = 20%, and the relative risk reduction (RRR) = 15%).
4. Discussion
CSCs, a small subpopulation of tumor cells, drive the growth and progression of cancers [52]. More importantly, CSCs are considered to be involved in chemotherapy/radiotherapy resistance, metastasis, and postoperative recurrence [53, 54]. Some meta-analyses showed that CD133 was a biomarker of putative CSCs in many solid tumors and its positivity may be associated with poor overall survival in nonsmall-cell lung cancer [55], worse prognosis in patients with glioblastoma [20], and reduced overall survival in colorectal cancer [56]. SOX2 expression may be correlated with better overall survival in nonsmall cell lung cancer [21], but worse overall survival in head and neck cancer [57]. However, some results were contradictory, for example, patients with CD133-positive is correlated with a better prognosis in colorectal liver metastasis [42]. Patients with CD133-positive are associated with an unfavorable prognosis in advanced colorectal cancer [35]. The conventional prognostic factors such as tumor stage or grade could not well predict clinical outcome based on an individual basis [58]. To date, there are still no effective markers available for the prognosis of patients with advanced cancer. Therefore, it remains important to better understand the characteristics of CSCs, CD133, and SOX2 for valuable therapeutic and prognostic targets in clinical practice to predict disease outcomes in advanced or metastatic cancer patients. In our meta-analysis, we have attempted to estimate the prognostic effect of CSCs, CD133, and SOX2 using multivariable analysis in patients with advanced or metastatic cancer.
Chemotherapy and radiotherapy are major treatment strategies to eliminate cancer cells, but chemoresistance, radioresistance, and cancer recurrence are major obstacles for the long-term survival of cancer patients [59, 60]. Recent studies show that CSCs are resistant to chemotherapy and radiotherapy and targeting CSCs may become a promising opportunity to cure patients with cancer [54, 61]. The studies of 78% (14 studies) reported patients with adjuvant therapy such as chemotherapy and radiotherapy in this meta-analysis. According to a comprehensive analysis of published studies (CD133: 13 studies with 1358 patients and SOX2: five studies with 433 patients). We found that patients with CD133-positive advanced cancer was correlated with poorer CSS (HR = 3.70, P = 0.036) and showed a trend towards poor OS (HR = 1.57, P = 0.057), but no relationship was reported between CD133 positivity and DFS (HR = 1.62, P = 0.178). For the analyses of CD133 in OS, we performed sensitivity and subgroup analyses. The removal of the study by Yamamoto 2014 [42] used blinding of the detection and the removal of the study by Kishikawa 2016 [37] did not report blinding of the detection (Table 1). We did not find that the possible factors and reasons can influence the pooled HR of OS in CD133. Because these two retrospective studies [37, 42] reported that CD133 positivity was linked to favorable OS. SOX2 positivity was related to shorter DFS (HR = 3.08, P < 0.001) and RFS (HR = 1.736, P = 0.033), but SOX2 positivity was not correlated with PFS (HR = 1.77, P = 0.145). In addition, no publication bias was observed in OS and DFS of CD133. These positive results were further proven by TSA, and the data suggested that additional clinical trials were needed to confirm these conclusions.
We further performed subgroup analyses of CD133 expression stratified by cancer type, study source, survival rate, sample type, age (years), testing method, study center design, and sample size in OS. Subgroup analysis by cancer type showed that CD133 expression was associated with shorter OS in cancer with bone metastases and gastric cancer but no relationship in colorectal cancer, ovarian cancer, and melanoma. Stratified analysis by study source indicated that only CD133 positivity could significantly reduce OS in Chinese patients (HR = 2.12, P = 0.001), suggesting that CD133 may play a more important role in the prognosis of advanced cancer for Chinese. Stratified analysis by survival rate showed that only CD133 positivity might significantly decrease OS in patients with < 3-year survival rate (HR = 10.92, P = 0.002), which suggested that the expression of CD133 may be correlated with shorter OS within 3 years. Subgroup analysis by age indicated that only CD133 positivity can significantly shorten OS in patients aged more than 60 years (HR = 2.09, P = 0.009), suggesting that CD133 may play a more key role in the prognosis for elderly patients. However, no significant difference was found between CD133 positivity and other subgroups such as sample type, study center design, and sample size. We further used TSA to achieve more meaningful results among different subgroups. TSA suggested that the available sample data were insufficient to draw firm conclusions regarding the expression of CD133 to OS.
Our meta-analysis had some limitations. First, the number of the included studies was not very large and some of these eligible studies had small sample sizes. TSA confirmed that cumulative Z curve did not cross the sequential monitoring boundary. Thus, more trials are needed for more reliable results. Second, studies were mainly conducted in China, Japan, and Europe; thus, other study sources (USA) are lacking. Third, most studies were of retrospective design; only two studies were of prospective design. Additional prospective clinical studies (such as blinded detection of CD133 and SOX2 expression) are essential to obtain more firm results in different cancer types, such as colorectal, lung, breast, and head-neck cancer. Finally, there was considerable heterogeneity in this meta-analysis. Although we analyzed several factors that may influence heterogeneity, these variables could not clearly explain the sources of heterogeneity. Thus, clinical practice should interpret our results with caution.
To conclude, our meta-analysis showed that CD133-positive expression may be associated with worse CSS and OS. Subgroup analysis by tumor type showed that CD133 positivity was linked to worse OS in cancer with bone metastases and gastric cancer. Subgroup analysis by study source demonstrated that only CD133 positivity was related to poor OS for Chinese. Subgroup analysis by survival rate showed that CD133 positivity was correlated with a less than 3-year OS. Subgroup analysis by age demonstrated that the expression of CD133 was associated with shorter OS in patients > 60 years. SOX2 positivity may be related to poor DFS and RFS. Further TSA suggested the need for additional clinical studies. Herein, more high-quality prospective studies are essential to obtain more reliable evidence and help stratify advanced cancer patients who can benefit from different therapies.
Acknowledgments
This research was supported by grants from the Natural Science Foundation of China (81473624) and the Shanghai Science and Technology Innovation Action Plan Project (No. 16401970500-3).
Contributor Information
Susu Han, Email: anyasue@163.com.
Tao Huang, Email: huangtao334@163.com.
Fenggang Hou, Email: fghou555@126.com.
Data Availability
The data used to support the findings of this study are included within the article.
Disclosure
Susu Han and Tao Huang are co-first authors of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Susu Han, Tao Huang, and Fenggang Hou contributed to the conception and design of this research. Susu Han, Xing Wu, Xiyu Wang, Shanshan Liu, Wei Yang, and Qi Shi contributed to the drafting of the article and final approval of the submitted version. Susu Han, Tao Huang, Xing Wu, Xiyu Wang, Shanshan Liu, Wei Yang, Qi Shi, Hongjia Li, and Fenggang Hou contributed to data analyses and the interpretation and completion of the figures and tables. All authors read and approved the final manuscript.
Supplementary Materials
Table S1: REMARK guidelines.
Table S2: Detailed characteristics of studies included in the meta-analysis.
Figure S1: Publication bias using Egger's and Begg's tests for overall survival (OS) and disease-free survival (DFS) of CD133 positive expression.
Figure S2: Trial sequential analysis (TSA) for disease-free survival (DFS) of SOX2 positivity.
References
- 1.Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Urruticoechea A., Alemany R., Balart J., Villanueva A., Viñals F., Capellá G. Recent advances in cancer therapy: An overview. Current Pharmaceutical Design. 2010;16(1):3–10. doi: 10.2174/138161210789941847. [DOI] [PubMed] [Google Scholar]
- 3.Kroeze S. G. C., Fritz C., Hoyer M., et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: A systematic review. Cancer Treatment Reviews. 2017;53:25–37. doi: 10.1016/j.ctrv.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R. L., Sahar L., Portier K. M., Ward E. M., Jemal A. Cancer death rates in US congressional districts. CA: A Cancer Journal for Clinicians. 2015;65(5):339–344. doi: 10.3322/caac.21292. [DOI] [PubMed] [Google Scholar]
- 5.Pardoll D. M. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty M. R., Smigiel J. M., Junk D. J., Jackson M. W. Cancer stem cell plasticity drives therapeutic resistance. Cancers. 2016;8(1):1–13. doi: 10.3390/cancers8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derwahl M. Linking stem cells to thyroid cancer. The Journal of Clinical Endocrinology & Metabolism. 2011;96(3):610–613. doi: 10.1210/jc.2010-2826. [DOI] [PubMed] [Google Scholar]
- 8.Han S., Yang W., Zong S., et al. Clinicopathological, prognostic and predictive value of CD166 expression in colorectal cancer: A meta-analysis. Oncotarget . 2017;8(38):64373–64384. doi: 10.18632/oncotarget.17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han S., Zong S., Shi Q., et al. Is Ep-CAM Expression a Diagnostic and Prognostic Biomarker for Colorectal Cancer? A Systematic Meta-Analysis. EBioMedicine. 2017;20:61–69. doi: 10.1016/j.ebiom.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safa A. R. Resistance to cell death and its modulation in cancer stem cells. Critical Reviews in Oncogenesis. 2016;21(3-4):203–219. doi: 10.1615/CritRevOncog.2016016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbeil D., Fargeas C. A., Huttner W. B. Rat prominin, like its mouse and human orthologues, is a pentaspan membrane glycoprotein. Biochemical and Biophysical Research Communications. 2001;285(4):939–944. doi: 10.1006/bbrc.2001.5271. [DOI] [PubMed] [Google Scholar]
- 12.Yin A. H., Miraglia S., Zanjani E. D., et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–5012. [PubMed] [Google Scholar]
- 13.Masui S., Nakatake Y., Toyooka Y., et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nature Cell Biology. 2007;9(6):625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J.-X., Liu B.-L., Zhang X. How powerful is CD133 as a cancer stem cell marker in brain tumors? Cancer Treatment Reviews. 2009;35(5):403–408. doi: 10.1016/j.ctrv.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Murray N. R., Justilien V., Fields A. P. SOX2 Determines Lineage Restriction: Modeling Lung Squamous Cell Carcinoma in the Mouse. Cancer Cell. 2016;30(4):505–507. doi: 10.1016/j.ccell.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Keysar S. B., Le P. N., Miller B., et al. Regulation of head and neck squamous cancer stem cells by PI3K and SOX2. Journal of the National Cancer Institute. 2017;109(1) doi: 10.1093/jnci/djw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boumahdi S., Driessens G., Lapouge G., et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511(7508):246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 18.Fan Z., Li M., Chen X., et al. Prognostic Value of Cancer Stem Cell Markers in Head and Neck Squamous Cell Carcinoma: a Meta-analysis. Scientific Reports. 2017;7(1) doi: 10.1038/srep43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng B., Yang G., Jiang R., et al. Cancer stem cell markers predict a poor prognosis in renal cell carcinoma: A meta-analysis. Oncotarget . 2016;7(40):65862–65875. doi: 10.18632/oncotarget.11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W., Chen H., Lv S., Yang H. High CD133 Expression Is Associated with Worse Prognosis in Patients with Glioblastoma. Molecular Neurobiology. 2016;53(4):2354–2360. doi: 10.1007/s12035-015-9187-1. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Huang Y., Huang Y., et al. The Prognostic Value of SOX2 Expression in Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS ONE. 2013;8(8):p. e71140. doi: 10.1371/journal.pone.0071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000100.e1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altman D. G., Bland J. M. How to obtain the confidence interval from a P value. British Medical Journal. 2011;343 doi: 10.1136/bmj.d2090.d2090 [DOI] [Google Scholar]
- 24.Tierney J. F., Stewart L. A., Ghersi D., Burdett S., Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8, article 16 doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McShane L. M., Altman D. G., Sauerbrei W., Taube S. E., Gion M., Clark G. M. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) JNCI: Journal of the National Cancer Institute. 2005;97(16):1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 26.Zintzaras E., Ioannidis J. P. A. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21(18):3672–3673. doi: 10.1093/bioinformatics/bti536. [DOI] [PubMed] [Google Scholar]
- 27.Evangelou E., Ioannidis J. P. A. Meta-analysis methods for genome-wide association studies and beyond. Nature Reviews Genetics. 2013;14(6):379–389. doi: 10.1038/nrg3472. [DOI] [PubMed] [Google Scholar]
- 28.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary Clinical Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Begg C. B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 31.Miladinovic B., Mhaskar R., Hozo I., Kumar A., Mahony H., Djulbegovic B. Optimal information size in trial sequential analysis of time-to-event outcomes reveals potentially inconclusive results because of the risk of random error. Journal of Clinical Epidemiology. 2013;66(6):654–659. doi: 10.1016/j.jclinepi.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Thorlund K., Devereaux P. J., Wetterslev J., et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? International Journal of Epidemiology. 2009;38(1):276–286. doi: 10.1093/ije/dyn179. [DOI] [PubMed] [Google Scholar]
- 33.Brok J., Thorlund K., Gluud C., Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. Journal of Clinical Epidemiology. 2008;61(8):763–769. doi: 10.1016/j.jclinepi.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Yamawaki K., Ishiguro T., Mori Y., et al. Sox2-dependent inhibition of p21 is associated with poor prognosis of endometrial cancer. Cancer Science. 2017;108(4):632–640. doi: 10.1111/cas.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pei Q., Zhu H., Tan F., et al. Intravascular emboli is an independent risk factor for the prognosis of stage III colorectal cancer patients after radical surgery. Oncotarget . 2016;7(35):57268–57276. doi: 10.18632/oncotarget.11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sodja E., Rijavec M., Koren A., Sadikov A., Korošec P., Cufer T. The prognostic value of whole blood SOX2, NANOG and OCT4 mRNA expression in advanced small-cell lung cancer. Radiology and Oncology. 2016;50(2):188–196. doi: 10.1515/raon-2015-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kishikawa J., Kazama S., Oba K., et al. CD133 Expression at the Metastatic Site Predicts Patients’ Outcome in Colorectal Cancer with Synchronous Liver Metastasis. Annals of Surgical Oncology. 2016;23(6):1916–1923. doi: 10.1245/s10434-016-5099-1. [DOI] [PubMed] [Google Scholar]
- 38.Kazama S., Kishikawa J., Yasuda K., et al. CD133 Expression in Lymph Node Metastases Is Associated with Tumor Aggressiveness During Lymph Node Metastasis in Colorectal Cancer. Anticancer Reseach. 2015;35(12):6599–6605. [PubMed] [Google Scholar]
- 39.Udagawa H., Ishii G., Morise M., et al. Comparison of the expression levels of molecular markers among the peripheral area and central area of primary tumor and metastatic lymph node tumor in patients with squamous cell carcinoma of the lung. Journal of Cancer Research and Clinical Oncology. 2015;141(8):1417–1425. doi: 10.1007/s00432-015-1912-7. [DOI] [PubMed] [Google Scholar]
- 40.Liu B.-L., Liu S.-J., Baskys A., et al. Platinum sensitivity and CD133 expression as risk and prognostic predictors of central nervous system metastases in patients with epithelial ovarian cancer. BMC Cancer. 2014;14(1) doi: 10.1186/1471-2407-14-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen L., Huang X., Xie X., Su J., Yuan J., Chen X. High Expression of SOX2 and OCT4 Indicates Radiation Resistance and an Independent Negative Prognosis in Cervical Squamous Cell Carcinoma. Journal of Histochemistry & Cytochemistry. 2014;62(7):499–509. doi: 10.1369/0022155414532654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto S., Tanaka K., Takeda K., et al. Patients with CD133-negative colorectal liver metastasis have a poor prognosis after hepatectomy. Annals of Surgical Oncology. 2014;21(6):1853–1861. doi: 10.1245/s10434-014-3549-1. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y.-H., Luo M.-H., Ni Y.-B., et al. Increased SOX2 expression in less differentiated breast carcinomas and their lymph node metastases. Histopathology. 2014;64(4):494–503. doi: 10.1111/his.12257. [DOI] [PubMed] [Google Scholar]
- 44.Sprenger T., Conradi L.-C., Beissbarth T., et al. Enrichment of CD133-expressing cells in rectal cancers treated with preoperative radiochemotherapy is an independent marker for metastasis and survival. Cancer. 2013;119(1):26–35. doi: 10.1002/cncr.27703. [DOI] [PubMed] [Google Scholar]
- 45.Lee H. H., Seo K. J., An C. H., Kim J. S., Jeon H. M. CD133 expression is correlated with chemoresistance and early recurrence of gastric cancer. Journal of Surgical Oncology. 2012;106(8):999–1004. doi: 10.1002/jso.23178. [DOI] [PubMed] [Google Scholar]
- 46.Qin Q., Sun Y., Fei M., et al. Expression of putative stem marker nestin and CD133 in advanced serous ovarian cancer. Neoplasma. 2012;59(3):310–315. doi: 10.4149/neo_2012_040. [DOI] [PubMed] [Google Scholar]
- 47.Sakai N., Yoshidome H., Shida T., et al. CXCR4/CXCL12 expression profile is associated with tumor microenvironment and clinical outcome of liver metastases of colorectal cancer. Clinical & Experimental Metastasis. 2012;29(2):101–110. doi: 10.1007/s10585-011-9433-5. [DOI] [PubMed] [Google Scholar]
- 48.Pilati P., Mocellin S., Bertazza L., et al. Prognostic value of putative circulating cancer stem cells in patients undergoing hepatic resection for colorectal liver metastasis. Annals of Surgical Oncology. 2012;19(2):402–408. doi: 10.1245/s10434-011-2132-2. [DOI] [PubMed] [Google Scholar]
- 49.Fusi A., Reichelt U., Busse A., et al. Expression of the stem cell markers nestin and CD133 on circulating melanoma cells. Journal of Investigative Dermatology. 2011;131(2):487–494. doi: 10.1038/jid.2010.285. [DOI] [PubMed] [Google Scholar]
- 50.Li C., Li B., Liang Y., et al. Higher percentage of CD133+ cells is associated with poor prognosis in colon carcinoma patients with stage IIIB. Journal of Translational Medicine. 2009;7(1):p. 56. doi: 10.1186/1479-5876-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehra N., Penning M., Maas J., et al. Progenitor marker CD133 mRNA is elevated in peripheral blood of cancer patients with bone metastases. Clinical Cancer Research. 2006;12(16):4859–4866. doi: 10.1158/1078-0432.CCR-06-0422. [DOI] [PubMed] [Google Scholar]
- 52.Lobo N. A., Shimono Y., Qian D., Clarke M. F. The biology of cancer stem cells. Annual Review of Cell and Developmental Biology. 2007;23(1):675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 53.Govaert K. M., Emmink B. L., Nijkamp M. W., et al. Hypoxia after liver surgery imposes an aggressive cancer stem cell phenotype on residual tumor cells. Annals of Surgery. 2014;259(4):750–759. doi: 10.1097/SLA.0b013e318295c160. [DOI] [PubMed] [Google Scholar]
- 54.Morrison R., Schleicher S. M., Sun Y., et al. Targeting the Mechanisms of Resistance to Chemotherapy and Radiotherapy with the Cancer Stem Cell Hypothesis. Journal of Oncology. 2011;2011:13. doi: 10.1155/2011/941876.941876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen E., Zeng Z., Bai B., Zhu J., Song Z. The prognostic value of CSCs biomarker CD133 in NSCLC: A meta-analysis. Oncotarget . 2016;7(35):56526–56539. doi: 10.18632/oncotarget.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang K., Xu J., Zhang J., Huang J. Prognostic role of CD133 expression in colorectal cancer: a meta-analysis. BMC Cancer. 2012;12(1) doi: 10.1186/1471-2407-12-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W., Li B., Wang R., Huang D., Jin W., Yang S. SOX2 as prognostic factor in head and neck cancer: A systematic review and meta-analysis. Acta Oto-Laryngologica. 2014;134(11):1101–1108. doi: 10.3109/00016489.2014.913311. [DOI] [PubMed] [Google Scholar]
- 58.Bamias A., Chorti M., Deliveliotis C., et al. Prognostic significance of CA 125, CD44, and epithelial membrane antigen in renal cell carcinoma. Urology. 2003;62(2):368–373. doi: 10.1016/S0090-4295(03)00264-4. [DOI] [PubMed] [Google Scholar]
- 59.Nurgali K., Jagoe R. T., Abalo R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Frontiers in Pharmacology. 2018;9 doi: 10.3389/fphar.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delaney G., Jacob S., Featherstone C., Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104(6):1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 61.Kharkar P. S. Cancer stem cell (CSC) inhibitors: a review of recent patents (2012-2015) Expert Opinion on Therapeutic Patents. 2017;27(7):753–761. doi: 10.1080/13543776.2017.1325465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: REMARK guidelines.
Table S2: Detailed characteristics of studies included in the meta-analysis.
Figure S1: Publication bias using Egger's and Begg's tests for overall survival (OS) and disease-free survival (DFS) of CD133 positive expression.
Figure S2: Trial sequential analysis (TSA) for disease-free survival (DFS) of SOX2 positivity.
Data Availability Statement
The data used to support the findings of this study are included within the article.