Abstract
Introduction
Despite the rising trend in breast cancer incidence and mortality across Sub-Saharan Africa, there remains a critical knowledge gap about the burden and patterns of breast disease and breast cancer screening practices at the population level. This study aimed to identify socioeconomic factors associated with knowledge and practice of breast self-examination (BSE) as well as assess the prevalence of breast disease symptoms among a mixed urban-rural population of women in the Southwest region of Cameroon.
Methods
We conducted a household-level community-based study in Southwest Cameroon between January and March 2017, using a three-stage cluster sampling framework. We surveyed 1287 households and collected self-reported data on 4208 female subjects, 790 of whom were household representatives. Each household representative provided information on behalf of all female household members about any ongoing breast disease symptoms. Moreover, female household representatives were questioned about their own knowledge and practice of BSE.
Results
Women demonstrated low frequency of knowledge of BSE, as 25% (n=201) of household representatives reported any knowledge of BSE; and among these only 15% (n=30) practiced BSE on a monthly basis. Age (aOR: 1.04), usage of Liquid Petroleum Gas fuel, a marker of higher socioeconomic status (aOR: 1.86), and speaking English as a primary language in the household (aOR: 1.59) were significant predictors of knowledge of BSE. Eleven women reported ongoing breast disease symptoms resulting in an overall prevalence of 2.3 cases of breast disease symptoms per 1000 women.
Conclusions
Socioeconomic disparities in access to health education may be a determinant of knowledge of BSE. Community-based strategies are needed to improve dissemination of breast cancer screening methods, particularly for women who face barriers to accessing care.
1. Introduction
Benign and malignant conditions of the breast are of primary concern to women's health worldwide. Breast cancer, in particular, is the most common cancer and one of the highest contributors to disability-adjusted life years (DALYs) in women. In 2013, breast cancer accounted for 464,000 deaths and 13.1 million DALYs globally, with 63% of DALYs occurring in developing countries [1]. Across Sub-Saharan Africa, the incidence and mortality of breast cancer have been continuously rising, primarily due to aging and population growth, increased urbanization, and a higher prevalence of risk factors associated with economic development (e.g., smoking, obesity, physical inactivity, and changing reproductive behaviors) [2]. This growing trend has also been observed in the central African country of Cameroon, where breast cancer is now the leading cancer among women in Yaoundé, comprising 18.5% of all cancers and 32.5% of female cancers [3]. According to the International Agency for Research on Cancer, there were 2,625 new cases of breast cancer per 100,000 women in Cameroon during 2012 [4]. This estimate, however, was based on data extrapolated from a regional hospital-based cancer registry covering part of the country [5]. Thus, currently available data may not accurately reflect the true burden and patterns of breast disease across all regions of Cameroon, especially since registries do not account for individuals who fail to present to formal care.
Despite this limitation, data from hospital-based studies indicate that a majority of breast cancers in Cameroon are diagnosed at advanced stages and result in poor outcomes [6–8]. A recent review of breast cancer data suggests that the lack of early detection programs and limited access to surgical care, the primary treatment modality for breast cancer in Sub-Saharan Africa, are likely contributors to poor overall survival among breast cancer patients in the region [9]. A retrospective cohort study conducted in Yaoundé, Cameroon, found an overall 5-year survival rate of 30% and a 10-year survival rate of 13.2% among breast cancer patients treated between 1995 and 2007 [8]. In contrast, breast cancer survival rates in high-income countries are reported to be over 80% [10–12]. This disparity highlights the critical role early detection and access to surgical treatment represent in improving breast cancer outcomes and survival, as they remain central components of breast cancer control strategies [13].
Early diagnosis of breast cancer is critical to reducing cancer-related mortality, particularly where radiation, hormonal, and chemotherapy are not widely available. Early detection relies on breast awareness and utilization of screening methods. While mammography is the only screening modality proven to reduce breast cancer mortality, mammography is neither affordable nor feasible in many low- and middle-income countries (LMICs) [14, 15]. Consequently, alternative screening methods, such as breast self-examination (BSE), have been recommended in these settings to promote breast health awareness and allow for the early detection of breast abnormalities [15]. BSE is a simple screening method that can be performed at no cost. Positive associations have been found between the practice of BSE and the detection of breast cancer, and previous studies have indicated that a majority of early-stage breast tumors are self-detected [16, 17]. Yet, the main barrier to practicing BSE has been demonstrated to be a lack of knowledge or awareness of BSE [18].
Though prior studies in the Southwest region of Cameroon have assessed knowledge and practice of BSE among small urban samples [19, 20], it is not clear whether their findings are broadly applicable to the mixed urban-rural Southwest region as a whole. Women residing in rural or difficult to access settings might be at greater risk for poor health awareness. Moreover, the burden and patterns of breast disease in Southwest Cameroon are unknown due to a lack of population level data [5]. To rectify this knowledge gap and better inform policy, a large-scale community based study is needed. The purpose of this study was to assess the knowledge and practice of BSE among women in the Southwest region of Cameroon and identify its associated factors. Additionally, this study sought to estimate the prevalence and describe patterns of breast disease symptoms among symptomatic women in the region.
2. Materials and Methods
2.1. Study Design and Setting
This cross-sectional study was conducted as a subanalysis of a larger community-based survey investigating the prevalence of injury and unmet surgical need in the Southwest region of Cameroon. This region is one of two predominantly Anglophone regions in the country, with an estimated population of 1,575,224 [21].
2.2. Study Population
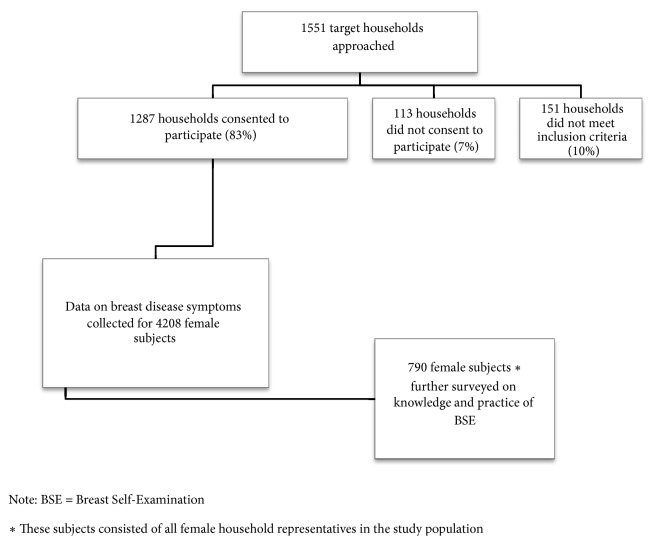
The study population consisted of all female household members residing in Southwest Cameroon. Data on all female household members was collected to estimate the prevalence of breast disease symptoms in the region. Additionally, female subjects designated as household representatives were further surveyed on their knowledge and practice of BSE. Figure 1 outlines the selection process for all study subjects included in the analysis of the prevalence of breast disease symptoms and assessment of knowledge and practice of BSE in Southwest Cameroon.
Figure 1.
Study population selection flow chart.
All households from whom consent was obtained were included in the study. Households without a suitable representative (a household member aged 18 years or older) present after two attempts or any individual not permanently residing in the Southwest region were excluded from the study.
2.3. Sampling Method and Sample Size Calculation
Enumeration areas were selected using a 3-stage clustered sampling framework. Due to safety concerns, two districts within the Southwest region (Akwaya and Bakassi) were excluded from the sampling framework. Probability proportionate to size sampling was used to select nine health districts and four health areas per district. Using geographic coordinates, a starting point was randomly selected within each health area, and contiguous households were approached from that point on until the target household number (n= 200) at each site was reached.
This study was nested in a larger community based survey whose sample size was calculated to provide 78% power to detect a 6% prevalence of injury. This prevalence estimate is based on findings from prior population-based surveys carried out in Sub-Saharan Africa [22–24]. The target sample size calculation was adjusted by a design effect of 2 to account for a loss in effectiveness and a larger variance when using a multi-clustering sampling framework, as well as a predicted 20% nonresponse rate. The minimum calculated target sample size was then deliberately exceeded by 50% to allow for multiple subanalyses of rare events. Finally, to verify that this final sample was large enough to conduct a subanalysis on breast disease, a separate sample size calculation was conducted to provide 78% power to detect a 2.9% prevalence estimate of breast disease, which was derived from a prior population-based study conducted in Sierra Leone [25].
2.4. Data Collection
Data collection was carried out between January 3 and March 3, 2017. Each household was asked to identify a household representative over the age of 18 years, who was then approached using a standard oral consent script. If consent was obtained, the household representative was given a pretested, context-adapted instrument based on the Surgeons OverSeas Assessment of Surgical Need (SOSAS) survey. The SOSAS survey is a tool designed to measure the prevalence of surgically treatable conditions. The validation process of the SOSAS tool, which was carried out in several developing countries, has been previously described [26, 27]. Household representatives were asked to provide information on sociodemographics, breast disease symptoms, defined as any alterations to the breast such as lumps, cancer, or skin or nipple changes or discharge, and care-seeking behaviors for female household members. Female household representatives were further asked about their own knowledge and practice of breast self-examination (BSE).
Socioeconomic status (SES) variables assessed in this study were selected based on an economic clusters model developed using nationally representative household assets data from Cameroon's Demographic and Health Survey [28]. Cooking fuel usage, more specifically the use of Liquid Petroleum Gas (LPG), has been demonstrated to correlate with higher SES in Sub-Saharan Africa, as it is a clean cooking fuel option that is prohibitively expensive for many households in the region [29, 30].
2.5. Statistical Analyses
All population estimates were adjusted for clustering methodology using a methodology derived from the World Health Organization's guidelines for conducting community based surveys on injuries and violence [29]. Descriptive statistics were carried out using frequencies, proportions, medians, and interquartile ranges. Comparisons between population estimates were conducted using the Adjusted Wald test and Pearson Chi Square test as appropriate. Univariate and multivariate logistic regression analyses were used to identify significant factors associated with the knowledge of BSE. A multivariate logistic regression model was built using sociodemographic and socioeconomic indicators, as well as variables relating to spoken language in the household and at health facilities. These variables were selected based on a review of prior studies investigating knowledge of BSE in LMICs [31, 32]. Covariates included in the final logistic regression model were selected using a backward stepwise regression procedure. All data were stored in REDcap, a secure online database, and were analyzed using STATA version14 [33, 34].
2.6. Ethical Considerations
Ethical approval for this study was obtained from the Committee for Human Research at the University of California, San Francisco, as well as the Institutional Review Board of the University of Douala.
3. Results
Household representatives from 1287 surveyed households provided information on 4208 female subjects. The median age of female subjects was 21 years, with a range of 0 to 115 years. Most subjects belonged to households where at least one family member had completed a secondary (36.9%) or tertiary level of education (40.6%). Over two-thirds of subjects reported residing in a rural setting (69.9%). A majority of subjects belonged to households that owned a cellular telephone (95.4%), owned agricultural land (64.2%), and used wood as a source of cooking fuel (92.4%). As compared to the overall female population of Southwest Cameroon (based on data from the 2011 Cameroon Demographic Health Survey [21]), subjects in this study belonged to households reporting a higher ownership of cellphones (95.4% vs. 70.2%) and more members with a secondary level of education or higher (77.5% vs. 38%) (Table 1).
Table 1.
Sociodemographic characteristics of 4208 female subjects and their households, in Southwest Cameroon.
| Characteristics | Frequency |
|---|---|
| (%) or | |
| Median [IQR] | |
| Age | 21 [10, 34] |
|
| |
| Household members | 7 [5, 10] |
|
| |
| Household Setting | |
| Urban | 1244 (30.1%) |
| Rural | 2886 (69.9%) |
|
| |
| Household possesses a cellphone | 3901 (95.4%) |
|
| |
| Household owns agricultural land | 2650 (64.2%) |
|
| |
| Use of cooking fuel in household | |
| Wood | 3849 (92.4%) |
| Liquid Petroleum Gas (LPG) fuel | 1823 (43.7%) |
| Charcoal | 701 (16.8%) |
| Kerosene | 697 (16.7%) |
| Other | 9 (0.2%) |
|
| |
| Highest educational level achieved by a household member | |
| No formal education | 84 (2.0%) |
| Primary | 834 (20.3%) |
| Secondary | 1517 (36.9%) |
| Tertiary | 1668 (40.6%) |
|
| |
| Household members believe some cancers can be surgically treated | 2624 (63.0%) |
|
| |
| Household members believe deformities can be surgically treated | 2968 (71.2%) |
Note: IQR = interquartile range.
3.1. Patterns of Breast Disease Symptoms and Care Seeking Behaviors
Among the 4208 study subjects, household representatives identified 11 subjects with ongoing symptoms of breast disease at the time of the survey, resulting in an overall prevalence of 2.3 cases of self-reported breast disease symptoms per 1000 women (95% CI 1.13-4.84) in the Southwest region of Cameroon. The estimated prevalence of breast disease symptoms increased to 4.02 cases per 1000 women (95% CI 0.93-17.10) when the sample population was restricted to female household representatives (N=790).
Study subjects reporting breast disease symptoms had a median age of 27 years (IQR 21, 44). Most (72.7%, n=8) reported that their symptoms had developed slowly over time for a mean duration of 7.8 years (SD±10.1). Over half of subjects with ongoing breast disease symptoms described the problem as a breast mass and over a quarter reported having non-lactation-related nipple discharge. Breast disease symptoms were predominantly unilateral and developed outside of pregnancy or lactation (Table 2).
Table 2.
Patterns of breast disease symptoms among symptomatic study subjects in Southwest Cameroon (n=11).
| Patterns | Frequency (%) |
|---|---|
| Symptom | |
| Lump | 6 (52.6%) |
| Abnormal breast discharge | 3 (27.3%) |
| New asymmetry | 1 (9.1%) |
| Other | 1 (9.1%) |
|
| |
| Location of Symptom | |
| Unilateral | 6 (54.6%) |
| Bilateral | 5 (45.5%) |
|
| |
| Symptom started while pregnant or breast feeding | |
| Yes, while pregnant | 2 (18.2%) |
| Yes, while breast feeding | 3 (27.3%) |
| No | 6 (54.6%) |
|
| |
| Progression of Symptom | |
| Slowly | 8 (72.7%) |
| Suddenly | 2 (18.2%) |
| Unknown | 1 (9.1%) |
|
| |
| Symptom disabling | |
| Yes | 2 (18.2%) |
| No | 9 (81.8%) |
|
| |
| Feelings of depression or shame | |
| Yes | 1 (9.1%) |
| No | 10 (90.9%) |
|
| |
| Breast symptom having household impact ∗ | |
| Yes | 8 (72.7%) |
| No | 3 (27.7%) |
|
| |
| Family has spent assets, savings, or borrowed money to treat current symptom | |
| Yes | 8 (72.7%) |
| No | 3 (27.7%) |
Although 63.6% (n=7) of subjects with breast disease symptoms had sought formal care for their problem, four participants with ongoing breast symptoms had not yet presented to formal care at the time of the survey (36.4%). The reasons provided for not seeking formal care were the perception that the symptom was not serious (n=2), a lack of awareness that the symptom could be treated (n=1), and the belief that formal care was too expensive (n=1). Among the seven subjects with breast symptoms who had previously presented to formal care, four had not yet received any surgical intervention and three reported recurrent disease following an operative intervention. The most common reason provided for not obtaining surgery as a treatment option was the patient's perception that surgery was not needed (75%, n=3).
3.2. Knowledge and Practice of Breast Self-Examination
Information regarding the knowledge and practice of BSE was collected from all female household representatives (n=790) in the study population. A majority of these household representatives (74.6%) indicated having no knowledge of BSE. Of these, 46.3% (n=357) had never heard of BSE, while 26.7% (n=211) had previously heard of BSE but lacked the knowledge to perform it. Among women reporting knowledge of BSE (n=201, 25.4%), 16% practiced it once per year (n= 32), 56% practiced it several times per year (n=113), and 15% practiced BSE on a monthly basis (n= 30). The majority of women who practiced BSE (93.8%) did not wait to perform it at a specific time of the month, whereas a small minority performed BSE the week (3.1%) or the second week (0.3%) following their menses.
Women with knowledge of BSE were significantly older (median: 34 years vs. 31 years) and reported a higher rate of LPG usage as a source of cooking fuel as compared to women without knowledge of BSE. LPG is an indicator of higher SES. Significant differences in household education patterns were also observed between women with and without knowledge of BSE (p=0.03). A higher proportion of women with knowledge of BSE reported having a family member who achieved a tertiary level of education as compared to women without knowledge of BSE (44.4% vs. 33.1%) (Table 3).
Table 3.
Comparison of female household representatives by knowledge of breast self-examination (n= 790).
| Knowledge of BSE a (n=201) | No Knowledge of BSE (n= 589) | P value b | |
|---|---|---|---|
| Age (median [IQR]) | 34 [27, 45] | 31 [24, 40] | p=0.01∗ |
| Use of Cooking Fuel in Household | |||
| Wood | 181 (90.1%) | 529 (90.7%) | p=0.99 |
| Charcoal | 45 (22.3%) | 92 (15.8%) | p=0.22 |
| Liquid Petroleum Gas (LPG) fuel | 113 (56.2%) | 247 (42.4%) | p=0.02∗ |
| Kerosene | 36 (17.9%) | 102 (17.5%) | p=0.89 |
| Other | 2 (1.0%) | 2 (0.3%) | p=0.38 |
| Highest Education level achieved by any household member | p=0.03∗ | ||
| No formal education | 3 (1.5%) | 22 (3.8%) | |
| Primary | 31 (15.7%) | 134 (23.2%) | |
| Secondary | 76 (38.4%) | 227 (39.3%) | |
| Tertiary | 88 (44.4%) | 191 (33.1%) | |
| Primary barrier to seeking Formal care | |||
| None | 69 (34.3%) | 220 (37.7%) | p=0.25 |
| Too Expensive | 42 (20.9%) | 104 (17.8%) | p=0.15 |
| Inaccessible (Too Far) | 5 (2.5%) | 38 (6.5%) | p=0.05∗ |
| Belief that treatment is ineffective | 1 (0.5%) | 13 (2.2%) | p=0.11 |
| Rude or Inattentive staff | 56 (27.9%) | 126 (21.6%) | p=0.24 |
| Preference for traditional medicine | 1 (0.5%) | 5 (0.9%) | p=0.14 |
| Preference for faith healing | 0 (0%) | 4 (0.7%) | p=0.12 |
| Other | 45 (22.4%) | 107 (18.4%) | p=0.33 |
| Transport used by household to seek formal care after Injury | p=0.02∗ | ||
| Walking | 85 (42.5%) | 288 (50.7%) | |
| Private car | 1 (0.5%) | 3 (0.5%) | |
| Private Motorcycle | 0 (0%) | 4 (0.7%) | |
| Taxi | 27 (13.5%) | 64 (11.3%) | |
| Mototaxi | 84 (42.0%) | 209 (36.8%) | |
| Bus | 3 (1.5%) | 0 (0%) | |
| Primary spoken language in Household | p=0.05∗ | ||
| Pidgin English | 57 (28.5%) | 200 (34.7%) | |
| English | 76 (38.0%) | 153 (26.5%) | |
| French | 6 (3.0%) | 17 (3.0%) | |
| Local dialect | 54 (27%) | 180 (31.2%) | |
| Other | 7 (3.5%) | 27 (4.7%) | |
| Primary spoken language used to communicate with health providers: | |||
| Pidgin English | 80 (39.8%) | 313 (53.7%) | p=0.03∗ |
| English | 143 (71.1%) | 363 (62.3%) | p<0.01∗ |
| French | 10 (5.0%) | 38 (6.5%) | p=0.37 |
| Local dialect | 4 (2.0%) | 20 (3.4%) | p=0.70 |
| Other | 2 (0.3%) | 1(0.5%) | p=0.55 |
Note: BSE = breast self-examination. An asterisk (∗) represents a p value of ≤ 0.05. Frequencies and percentages may not add up as a result of missing data.
aKnowledge of BSE indicates that the study subject reported knowing how to perform BSE on herself.
bAll p value estimates were calculated using the adjusted Wald test and Pearson Chi Square test. The Wald test was adjusted for all variables in the data.
Women without knowledge of BSE were significantly more likely to report a lack of proximity to formal medical care as a primary barrier to care (p=0.05) and these participants were also more likely to cite walking as their means of transport to formal care (50.7% vs. 42.5%). In contrast, women with knowledge of BSE were more likely to report the use of a motorized vehicle as a means of transport to access formal care (p=0.02) (Table 3).
Women with knowledge of BSE were more likely to report English as the primary spoken language in their households in addition to the language used to communicate with health providers. Conversely, women without knowledge of BSE were more likely to report the use of Pidgin English to communicate with health providers (Table 3). These participants were also less likely to believe that injuries (78.6% vs. 89.1%, p=0.03) and deformities (64.7% vs. 79.6%, p<0.01) could be treated with surgery. No significant differences were found between the two groups regarding the treatability of some cancers by surgery (63.1% vs. 63.0%, p=0.20).
A multivariate logistic regression analysis indicated that age (aOR=1.04, p<0.01), English as the primary household language (aOR=1.59, p=0.045), and LPG use (aOR=1.86, p= 0.034) were all significant independent predictors of knowledge of BSE (Table 4).
Table 4.
Predictors of knowledge of breast self-examination among female household representatives in Southwest Cameroon.
| Predictor a | Adjusted OR | 95% CI | P value |
|---|---|---|---|
| Age b | 1.04 | (1.01-1.06) | p <0.01∗ |
|
| |||
| Use of Liquid Petroleum Gas (LPG)c | 1.86 | (1.06 -3.25) | p=0.03∗ |
| as cooking fuel in the household | |||
|
| |||
| Primary barrier to seeking Formal Care: Inaccessibility (Too far) d | 0.33 | (0.10 -1.04) | p=0.06 |
|
| |||
| English is primary spoken language in household e | 1.59 | (1.01 -2.48) | p=0.05∗ |
Note: OR = odds ratio; CI = confidence interval. An asterisk represents a p value of ≤ 0.05
aThe final multivariable logistic regression model was built using a backward stepwise regression procedure.
bThe reference group consisted of subjects of a younger age.
cThe reference group consisted of subjects belonging to households where LPG was not used as a source of cooking fuel.
dThe reference group consisted of subjects who did not report inaccessibility as a primary barrier to seeking formal care.
eThe reference group consisted of subjects who did not primarily speak English in the household.
4. Discussion
In this study, we demonstrate that knowledge and practice of breast self-examination (BSE) is extremely poor among women in the Southwest region of Cameroon. Our findings indicate that women in this region are significantly more likely to know how to perform BSE if they use LPG as a source of cooking fuel, belong to a household where English is the primary spoken language, or are of an older age. These results are consistent with an array of previously published literature from developed countries demonstrating that higher SES, education, and age are all predictive factors of BSE knowledge and compliance [31, 32]. Although it is not possible to definitively establish why these factors associate with improved BSE knowledge in a cross-sectional survey, we can speculate that women who utilize LPG and are of a higher SES have better access to health services and more opportunities to engage with health professionals, resulting in a greater health literacy about breast screening methods. Indeed, it is common for women to receive health education lectures from health providers, particularly during antenatal care visits [35]. Conversely, participants who lacked knowledge about how to perform BSE reported increased barriers to accessing healthcare, including greater distance to healthcare facilities and use of walking as the most likely transport modality to seek care following serious injury.
Although this study did not specifically investigate sources of BSE knowledge, our conclusion that knowledge of BSE may be associated with access to healthcare may suggest that health professionals perform an important role in teaching BSE. If this is indeed the case, emphasizing provider training in clinical encounters could be exploited as a tool to increase breast-screening rates and quality in the Southwest region of Cameroon. Furthermore, the association of household English utilization with increased awareness of BSE may indicate a disproportionate dissemination of health information in English as opposed to Pidgin or other local dialects. Expanding language competency of healthcare providers in Pidgin also may hold promise as a means of increasing dissemination of BSE knowledge. Future population-based studies should investigate sources of health care knowledge regarding breast screening.
The prevalence of breast disease symptoms in this study population was lower than anticipated based on results from numerous studies suggesting women in Sub-Saharan Africa present at late stages [9]. Currently available data, though restricted in their generalizability, also point to a rising trend in breast cancer incidence in Cameroon [5]. Consequently, a community-based study was expected to identify a significant amount of undiagnosed breast disease cases across the Southwest region, given the absence of a population-based mammography-screening program and evidence demonstrating poor practice of alternative early detection methods [19, 20]. Yet, as compared to recent population-based surveys in Rwanda and Sierra Leone which detected a 4.4% and 2.9% prevalence of self-reported breast masses in women, respectively, the prevalence of self-reported breast disease symptoms found in this study was relatively low [25]. Our findings therefore call into question whether sociocultural factors, such as stigma, may have contributed to reduced reporting of breast disease symptoms among study subjects. Internalized stigma associated with breast cancer has been identified in parts of Sub-Saharan Africa [36]; however more rigorous studies are needed to better ascertain how stigma contributes to delaying cancer care engagement [37].
Surgical intervention is currently the primary modality of breast cancer treatment in Africa; and in most cases, surgical biopsy is also the means of establishing diagnosis for breast disease symptoms [9]. Yet, over a third of study participants reported that they did not believe that cancers could be surgically treated. Poor understanding of the therapeutic role of surgery in cancer care may contribute to delayed presentation or treatment among persons with breast symptoms. Although only a small number of subjects in this study were identified as having ongoing breast symptoms, those who failed to seek care or obtain surgery primarily cited a belief that their symptoms were not a serious health risk or that surgery was unnecessary. Given the high documented prevalence of late-stage presentation and poor outcomes of breast cancer in Sub-Saharan Africa [9], these findings warrant further investigation into the role of treatment perceptions in determining presentation and timing of formal-care seeking.
4.1. Limitations
Reliance on self-reported data, particularly from a single member of the household on behalf of other family members, is a limitation of our study. Designated household representatives may not have been aware of some breast disease symptoms in female members of their households, and representative knowledge likely varied based on family dynamics and the relationship of the representative to female family members. This factor could have led to an underestimation of the true prevalence of breast disease in the study population. To gauge the magnitude of this effect, we compared the prevalence of breast symptoms above with the prevalence of self-reported breast disease among female household representatives. Notably, restricting the sample population to female household representatives resulted in a near doubling of the estimated prevalence of breast disease. On the basis of this discrepancy in finding, we would recommend that future studies determining the prevalence of breast disease symptoms should collect data only directly from individual subjects, given the sensitive nature of the disease topic.
Importantly, it should be noted that this limitation did not affect the primary outcome of this study, as breast self-examination was only assessed among female household representatives; however, as with all survey-based data, knowledge of BSE was self-reported. Household representatives were not objectively assessed regarding their ability to correctly perform BSE, which could have potentially resulted in overestimation of BSE knowledge.
Finally, although summary data is reported for subjects identified as having ongoing breast symptoms, the size of this cohort is too small to support in-group comparisons or for extensive generalization to the larger study population.
5. Conclusions
Socioeconomic factors predict knowledge of BSE, raising concerns about disparities in access to health education, particularly as it pertains to women's health issues. Findings from this study emphasize the need to develop community-based strategies for improved and equitable dissemination of health information to ensure that breast cancer prevention and screening tools reach populations living in remote areas, who face greater barriers to accessing formal care. Furthermore, it is critical that this health information be communicated in a language that a majority of people in the region can easily comprehend, such as Pidgin English or local dialects in this case. Adopting alternative models to create awareness, such as community health worker interventions, could be potential solutions to spreading health information on breast cancer prevention in underserved communities.
Acknowledgments
We wish to acknowledge the following individuals for their dedicated work and invaluable contribution to the implementation of this study: Susan Mbeboh MD, Frida Nganje Embolo MD, William Chendjou MD, Eunice Oben MD, Emerson Wepngong MD, Dr. Alain Etoundi Mballa MD from the Ministry of Public Health in Cameroon, Dr. Victor Mbome MD, Dr. Pius Ekumbu PhD from the University of Buea Faculty of Health Sciences, and finally the directors and staff of Limbe Regional Hospital. This study received funding from the University of California San Francisco Center for Global Surgical Studies.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
None of the authors have any proprietary interests or conflicts of interest related to this submission.
References
- 1.Fitzmaurice C., Dicker D., Pain A., et al. The Global Burden of Cancer. JAMA oncology. 2013;1(4):505–27. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin D. M., Bray F., Ferlay J., Jemal A. Cancer in Africa 2012. Cancer Epidemiology, Biomarkers & Prevention. 2014;23(6):953–966. doi: 10.1158/1055-9965.EPI-14-0281. [DOI] [PubMed] [Google Scholar]
- 3.Enow Orock G. E., Ndom P., Doh A. S. Current Cancer Incidence and Trends in Yaounde, Cameroon. Oncol Gastroenterol Hepatol Reports. 2012;1(1):58–63. [Google Scholar]
- 4.Stimpfel M., Virant-Klun I. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Journal of Cancer Stem Cell Research. 2016;4(3):p. 1. doi: 10.14343/JCSCR.2016.4e1003. [DOI] [Google Scholar]
- 5.Sando Z., Fouelifack Y. F., Fouogue T. J., Fouedjio J. H., Essame-Oyono J. L. Trends in breast and cervical cancer incidence in Cameroon (Central Africa) from 2004 to 2011. Journal Africain du Cancer. 2015;7(3):118–121. doi: 10.1007/s12558-015-0378-5. [DOI] [Google Scholar]
- 6.Nguefack C. T., Biwole M. E., Massom A., et al. Epidemiology and surgical management of breast cancer in gynecological department of Douala general hospital. Pan African Medical Journal. 2012;13(1) [PMC free article] [PubMed] [Google Scholar]
- 7.Kemfang Ngowa J. D., Yomi J., Kasia J. M., Mawamba Y., Ekortarh A. C., Vlastos G. Breast Cancer Profile in a Group of Patients Followed up at the Radiation Therapy Unit of the Yaounde General Hospital, Cameroon. Obstetrics and Gynecology International. 2011;2011:5. doi: 10.1155/2011/143506.143506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngowa J. D., Kasia J. M., Yomi J., et al. Breast cancer survival in cameroon: analysis of a cohort of 404 patients at the yaoundé general hospital. Advances in Breast Cancer Research. 2015;4(02):p. 44. doi: 10.4236/abcr.2015.42005. [DOI] [Google Scholar]
- 9.Sutter S. A., Slinker A., Balumuka D. D., Mitchell K. B. Surgical management of breast cancer in africa: a continent-wide review of intervention practices, barriers to care, and adjuvant therapy. Journal of Global Oncology. 2017;3(2):162–168. doi: 10.1200/JGO.2016.003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bossard N., Velten M., Remontet L., et al. Survival of cancer patients in France: A population-based study from The Association of the French Cancer Registries (FRANCIM) European Journal of Cancer. 2007;43(1):149–160. doi: 10.1016/j.ejca.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Siegel R. L., Miller K. D., Jemal A. Cancer Statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 12.Walters S., Maringe C., Butler J., et al. Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000-2007: A population-based study. British Journal of Cancer. 2013;108(5):1195–1208. doi: 10.1038/bjc.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelband H., Sloan F. A. Cancer Control Opportunities in Low-And Middle-Income Countries. Washington, DC, USA: National Academies Press; 2007. [PubMed] [Google Scholar]
- 14.World Health Organization. Breast Cancer: Prevention And Control. 2017. http://www.who.int/cancer/detection/breastcancer/en/ [Google Scholar]
- 15.Anderson B. O., Yip C.-H., Smith R. A., et al. Guideline Implementation for Breast Healthcare in Low‐Income and Middle‐Income Countries. Cancer. 2008;113(S8):2221–2243. doi: 10.1002/cncr.23844. [DOI] [PubMed] [Google Scholar]
- 16.Roth M. Y., Elmore J. G., Yi-Frazier J. P., Reisch L. M., Oster N. V., Miglioretti D. L. Self-detection remains a key method of breast cancer detection for U.S. women. Journal of Women's Health. 2011;20(8):1135–1139. doi: 10.1089/jwh.2010.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith E. M., Francis A. M., Polissar L. The effect of breast self-exam practices and physician examinations on extent of disease at diagnosis. Preventive Medicine. 1980;9(3):409–417. doi: 10.1016/0091-7435(80)90235-2. [DOI] [PubMed] [Google Scholar]
- 18.Birhane K., Alemayehu M., Anawte B., et al. Practices of breast self-examination and associated factors among female Debre Berhan university students. International Journal of Breast Cancer. 2017;2017:6. doi: 10.1155/2017/8026297.8026297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh M. A. B., Atashili J., Fuh E. A., Eta V. A. Breast Self-Examination and breast cancer awareness in women in developing countries: A survey of women in Buea, Cameroon. BMC Research Notes. 2012;5, article no. 627 doi: 10.1186/1756-0500-5-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nde F. P., Assob J. C., Kwenti T. E., Njunda A. L., Tainenbe T. R. Knowledge, attitude and practice of breast self-examination among female undergraduate students in the University of Buea. BMC Research Notes. 2015;8(1, article 43) doi: 10.1186/s13104-015-1004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institut National de la Statistique (INS) and ICF International. Enquête Démographique et de Santé et à Indicateurs Multiples du Cameroun 2011. Calverton, Md, USA: INS and ICF International; 2012. [Google Scholar]
- 22.Mock C. N., Abantanga F., Cummings P., Koepsell T. D. Incidence and outcome of injury in Ghana: A community-based survey. Bull World Health Organ. 1999;77(12):955–964. [PMC free article] [PubMed] [Google Scholar]
- 23.Moshiro C., Heuch I., Astrom A. N., Setel P., Hemed Y., Kvåle G. Injury morbidity in an urban and a rural area in Tanzania: an epidemiological survey. BMC Public Health. 2005;5(11) doi: 10.1186/1471-2458-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olawale O. A., Owoaje E. T. Incidence and pattern of injuries among residents of a rural area in South-Western Nigeria: A community-based study. BMC Public Health. 2007;7(246) doi: 10.1186/1471-2458-7-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ntirenganya F., Petroze R. T., Kamara T. B., et al. Prevalence of breast masses and barriers to care: Results from a population-based survey in Rwanda and Sierra Leone. Journal of Surgical Oncology. 2014;110(8):903–906. doi: 10.1002/jso.23726. [DOI] [PubMed] [Google Scholar]
- 26.Groen R. S., Samai M., Petroze R. T., et al. Pilot Testing of a Population-based Surgical Survey Tool in Sierra Leone. World Journal of Surgery. 2012;36(4):771–774. doi: 10.1007/s00268-012-1448-9. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S., Ranjit A., Shrestha R., et al. Surgical needs of Nepal: Pilot study of population based survey in pokhara, Nepal. World Journal of Surgery. 2014;38(12):3041–3046. doi: 10.1007/s00268-014-2753-2. [DOI] [PubMed] [Google Scholar]
- 28.Eyler L., Hubbard A., Juillard C. Assessment of economic status in trauma registries: A new algorithm for generating population-specific clustering-based models of economic status for time-constrained low-resource settings. International Journal of Medical Informatics. 2016;94:49–58. doi: 10.1016/j.ijmedinf.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Schlag N., Zuzarte F. Market Barriers to Clean Cooking Fuels in Sub-Saharan Africa: A Review of Literature. Stockholm, Sweden: Stockholm Environment Institute; 2008. [Google Scholar]
- 30.Sethi D., Habibula S., McGee K. S., et al. Guidelines for Conducting Community Surveys on Injuries and Violence. World Health Organization; 2004. [DOI] [PubMed] [Google Scholar]
- 31.Madan A. K., Barden C. B., Beech B., Fay K., Sintich M., Beech D. J. Socioeconomic factors, not ethnicity, predict breast self-examination. The Breast Journal. 2000;6(4):263–266. doi: 10.1046/j.1524-4741.2000.99016.x. [DOI] [PubMed] [Google Scholar]
- 32.Holtzman D., Celentano D. D. The practice and efficacy of breast self-examination: A critical review. American Journal of Public Health. 1983;73(11):1324–1326. doi: 10.2105/AJPH.73.11.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J. G. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.
- 35.Mbuagbaw L., Medley N., Darzi A. J., Richardson M., Habiba Garga K., Ongolo-Zogo P. Health system and community level interventions for improving antenatal care coverage and health outcomes. Cochrane Database of Systematic Reviews. 2014;12(12) doi: 10.1002/14651858.CD010994.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meacham E., Orem J., Nakigudde G., Zujewski J. A., Rao D. Exploring stigma as a barrier to cancer service engagement with breast cancer survivors in Kampala, Uganda. Psycho-Oncology. 2016;25(10):1206–1211. doi: 10.1002/pon.4215. [DOI] [PubMed] [Google Scholar]
- 37.Sharma K., Costas A., Shulman L. N., Meara J. G. A systematic review of barriers to breast cancer care in developing countries resulting in delayed patient presentation. Journal of Oncology. 2012;2012:8. doi: 10.1155/2012/121873.121873 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.