Abstract
OBJECTIVES:
This study aimed to evaluate the role of nonsurgical periodontal therapy in improving glycemic control among type 2 diabetes mellitus (T2DM) patients.
MATERIALS AND METHODS:
Adult T2DM patients with mild-to-moderate periodontal disease, reporting to a tertiary care diabetes center in South India, from January to June 2014, were enrolled in the study. Medical management of T2DM along with diet and physical exercise was an inclusion criterion. Patients with factors affecting periodontal health and an inability to follow-up were excluded from the study. All patients underwent nonsurgical periodontal therapy (scaling, root planing, and irrigation of chlorhexidine [0.12%]). Periodontal status and glycated hemoglobin A1c (HbA1c) were assessed preoperatively and 6 months posttreatment. Dental status, diabetic history, and demographic characteristics were recorded to evaluate confounding roles.
RESULTS:
A total of 266 T2DM patients (91 females/175 males; mean age 47.65 ± 5.93 years/range 25–55 years), fulfilling the inclusion criteria, were enrolled. The mean pre- and post-treatment HbA1c levels were respectively, 8.44 ± 1.87 and 7.98 ± 1.81, with a mean reduction of 0.46 ± 0.26 (P < 0.001). Significant HbA1c reduction (P < 0.001) was observed in patients with good pretreatment glycemic control (0.54 ± 0.26; 7.9%), regular follow-up (0.51 ± 0.28; 6.2%), and good oral hygiene (0.60 ± 0.49; 8.0%).
CONCLUSION:
Nonsurgical periodontal therapy is associated with significant HbA1c reduction among T2DM patients with mild-to-moderate periodontitis after a 6-month follow-up period.
Keywords: Diabetes mellitus, glycemic control, hemoglobin A1c, periodontal therapy, periodontitis
Introduction
Diabetes mellitus (DM) and its biological association with periodontal diseases (PDs) has been a point of scientific introspection in the last two decades. PD and especially periodontitis, characterized by inflammation of the tooth-supporting tissues, are regarded as the sixth major diabetic complication worldwide. Contemporary diabetic management with oral hypoglycemic agents (OHAs) and/or insulin has demonstrated reduction in the incidence of systemic complications provided meticulous glycemic control through optimum hemoglobin A1c levels (HbA1c <7%) are achieved.[1,2,3] However, conventional therapies are challenged by the risks of lack of pharmacological responsiveness, developing insulin resistance, and rising production costs. Hence, adjunctive modalities are speculated to minimize diabetic complications.
Reduction in systemic inflammation is one of the proven methods to achieve glycemic control. While DM and poor glycemic control lead to an increased incidence of PD, chronic periodontitis results in a systemic inflammatory state due to persistent bacteremia and increased levels of pro-inflammatory cytokines.[4] Engebretson and Kocher,[5] based on a meta-analysis, reported a 0.36% reduction in HbA1c levels following periodontal therapy in diabetic patients. India ranks second in the global diabetes map, with 65.1 million DM patients and majority among them are adult type 2 diabetes mellitus (T2DM) patients.[6] Early diagnosis and treatment of PD among diabetic patients as an adjunctive modality could potentially help patients in achieving glycemic control, thereby reducing the economic burden of diabetes treatment in a developing country like India.[7] Therefore, the aim of the present study was to evaluate whether nonsurgical periodontal therapy plays a role in reducing HbA1c levels among diabetic patients with mild-to-moderate periodontitis.
Materials and Methods
The study was designed as an interventional study involving a cross-section of adult T2DM patients at a tertiary care diabetes center in Chennai, India. Following institutional ethical approval and informed consenting, T2DM patients with clinical evidence of mild-to-moderate periodontitis reporting for dental evaluation between January 2014 and June 2014 were enrolled in the study, using a convenience random sampling methodology. T2DM patients diagnosed before 1 year and on continuous medical management with OHAs and self-reported compliance to medication, diet, and physical exercise along with the presence of at least 12 or more teeth in the oral cavity were included in the study. The exclusion criteria were presence of local or systemic factors affecting periodontal health such as orthodontic braces, multiple subgingival dental restorations and pharmacological agents (phenytoin sodium and nifedipine), and an inability to report for follow-up after 6 months. Patients with systemic complications due to DM and those whose diabetic medications were altered by the diabetologist during the follow-up period were also excluded, in addition to patients who reportedly followed alternative medicine therapies for DM (e.g., herbal remedies).
Clinical evidence of periodontitis was assessed using the technique of basic periodontal examination (BPE) adapted from the Community Periodontal Index for Treatment Needs (CPITN).[8] Based on BPE screening method using a CPITN probe, patients were identified as having mild-to-moderate periodontitis when there were calculus deposits on the teeth either with or without periodontal pockets with probing depths up to 5.5 mm. The pretreatment HbA1c levels were recorded prior to periodontal therapy for all the patients fulfilling the inclusion criteria, which was followed by nonsurgical periodontal therapy consisting of scaling, root planing, and local irrigation of chlorhexidine 0.12%. Upon completion of the periodontal therapy, patients were educated to follow ideal oral hygiene practices and advised to use chlorhexidine 0.12% mouthwash 4 times daily for 2 weeks and twice daily for another 2 weeks. Patients were recalled after 6 months to assess their periodontal status and during that time posttreatment HbA1c levels were recorded.
In addition to periodontal status and HbA1c levels, measured as an index of glycemic control, data pertaining to dental care and hygiene, history of DM, and demographic characteristics of the patients were also recorded to evaluate their confounding roles if any. The collected data were tabulated for descriptive analysis, and bivariate analysis was done to identify statistical associations between the different independent variables and the pretreatment HbA1c level. Furthermore, paired sample t-test was done to evaluate the role of periodontal therapy in altering the HbA1c levels (pretreatment vs. posttreatment). Statistical analysis was performed using SPSS (Version 21, IBM, Armonk, NY, USA) with a 95% significance level (P < 0.05).
Results
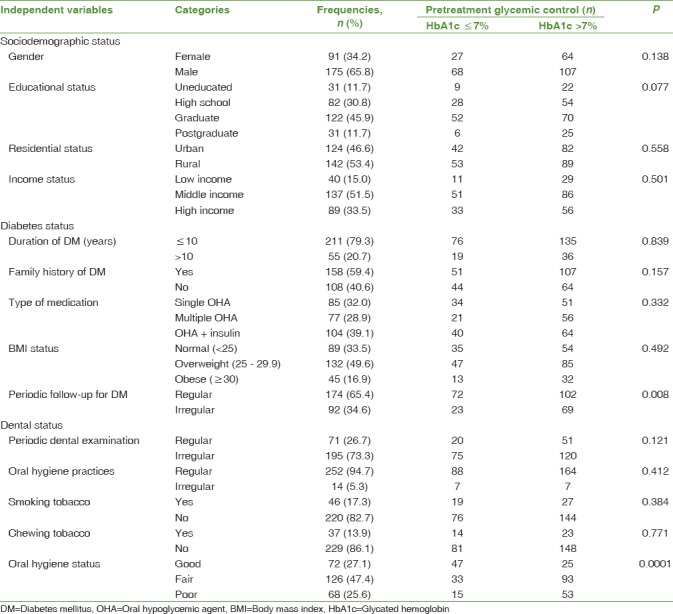
A total of 266 patients (91 females –34.2%; 175 males –65.8%) fulfilling the inclusion criteria were enrolled in the study. The mean age of the study sample was 48 years (standard deviation [SD] 5.9 years; range 25–55 years). While the mean pretreatment HbA1c level was 8.4% (SD 1.9; range 5.1–15.7), the mean posttreatment HbA1c level was 8.0% (SD 1.81; range 4.9–15.0). Based on pretreatment HbA1c levels, it was found that 64.3% (n = 171) of the study sample had poor glycemic control (HbA1c >7.0%). Analyzing the sociodemographic characteristics of the study sample, it was found that majority of the patients were educated (n = 235; 88.3%) and belonged to the middle income group (n = 137; 51.5%). The urban (n = 124; 46.6%) versus rural (n = 142; 53.4%) division among the study sample was minimal.
Data pertaining to diabetic status of the study sample revealed 79.3% (n = 211) of the patients to have been diagnosed with DM within the preceding 10 years and the majority of them with a positive family history for DM (n = 158; 59.4%). Nearly two-thirds of the patients were under treatment with OHAs either single (n = 85; 32%) or multiple (n = 77; 28.9%). Although patients with self-reported compliance to diet and physical exercise were only included to the study, majority of the study patients were either overweight (n = 132; 49.6%) or obese (n = 45; 16.9%). Nevertheless, majority of the patients were regular in getting their diabetic status checked by a diabetologist at least once in 6 months (n = 174; 65.4%).
While 94.7% of the patients (n = 252) reportedly practiced routine oral hygiene techniques, only a fourth of the study patients (n = 71; 26.7%) underwent routine dental examination in the preceding year. Tobacco-related habits, such as smoking (n = 220; 82.7%) and chewing (n = 229; 86.1%), were not reported among a majority of the patients. Dental examination during BPE revealed good oral hygiene in only 27.1% (n = 72) of the patients. While majority of the patients had a fair oral hygiene (n = 126; 47.4%), the oral hygiene was poor in 25.6% (n = 68) of the patients.
Bivariate analysis revealed no significant associations between the pretreatment Hba1c level and the variables related to sociodemographic status. However, within the variables related to diabetic status, regular follow-up for DM was significantly associated with lower pretreatment HbA1c levels (P < 0.01). Similar statistically significant association (P < 0.001) was found between the pretreatment oral hygiene status and HbA1c levels, wherein patients with good oral hygiene had lower mean pretreatment HbA1c levels (7.49 ± 1.82) compared to those with fair oral hygiene (8.52 ± 1.58) and poor oral hygiene (9.28 ± 1.98). Results of the descriptive and bivariate analyses are shown in Table 1.
Table 1.
Bivariate analysis for statistical association between the independent variables and pretreatment glycemic control (n=266)
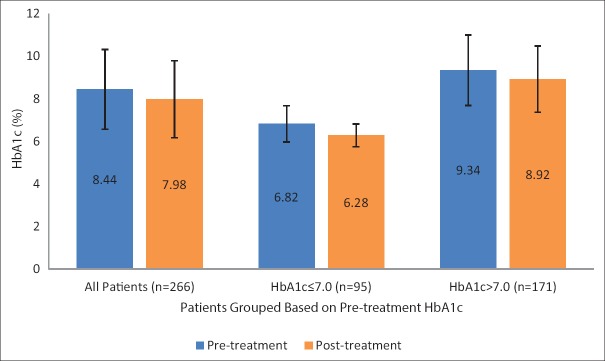
Paired samples t-test between the pre- and post-treatment HbA1c levels revealed statistically significant reduction in the HbA1c levels of the entire study sample following periodontal therapy (mean pretreatment HbA1c – 8.44 ± 1.87; mean posttreatment HbA1c – 7.98 ± 1.81; P < 0.001; mean reduction – 0.46 ± 1.05). Interestingly, patients with good glycemic control (HbA1c ≤7.0) prior to periodontal therapy showed a greater reduction in HbA1c levels compared to those with poor pretreatment glycemic control [Table 2 and Figure 1]. Stratifying the patients according to the confounding variables based on bivariate analysis, namely regularity of diabetic follow-up and pretreatment oral hygiene status, greater reduction in HbA1c was observed among patients who were reportedly regular for diabetic follow-up and those who had good oral hygiene status. Interestingly, among patients who had poor pretreatment oral hygiene status, the reduction in HbA1c was not only the least, but was also statistically insignificant (P = 0.208) [Tables 3 and 4].
Table 2.
Paired samples (t-test) comparison between pre- and post-treatment glycemic control (n=266)

Figure 1.
Bar graph with standard error bars showing the posttreatment reduction in hemoglobin A1C levels
Table 3.
Paired samples (t-test) comparison between pre- and post-treatment glycemic control stratified by regularity of diabetic follow-up (n=266)

Table 4.
Paired samples (t-test) comparison between pre- and post-treatment glycemic control stratified by oral hygiene status (n=266)

Discussion
The metamorphosis of DM into an epidemic of global scale requires a multifaceted approach toward comprehensive management.[6] While strict glycemic control with the help of insulin and OHAs is the indicator of successful management, public health systems shall be benefited by studying the role of adjunctive modalities in helping achieve glycemic control.[9] The objective of the present study was to evaluate the role of periodontal therapy as an adjunctive modality for achieving glycemic control in T2DM patients. Although studies have reported an increased risk of PD and especially periodontitis among T2DM patients with chronically elevated HbA1c levels,[10,11] the two-way association between DM and periodontitis has not been evaluated completely.[12,13]
Periodontitis is a progressive inflammatory disease of the periodontal tissues, namely gingiva (gums), periodontal ligament, and alveolar bone (tooth-supporting bone). It begins with gingivitis in response to the microorganisms in dental plaque and progresses to periodontitis characterized by soft-tissue inflammation, loss of attachment between the periodontal tissues and teeth, and alveolar bone recession.[14] The loss of tissue attachment and bone resorption in the periodontal tissues is orchestrated through collagen breakdown by the reactive oxygen molecules, proteolytic enzymes, and matrix metalloproteinases synthesized by the neutrophils in response to inflammation.[14] Inability to achieve glycemic control in T2DM leads to impaired function of neutrophils, accumulation of advanced glycation end products, and oxidative stress pathways, thereby contributing to an increased risk for developing periodontitis.[15]
The peculiar aspect of periodontitis in diabetic patients lies in the fact that the body's immune system, instead of completely eliminating the source of inflammation (microorganisms), keeps the inflammatory process continuously activated, leading to a chronic inflammatory reaction.[14] This chronic inflammatory response leads to systemic upregulation of pro-inflammatory cytokines such as interleukins (IL-1β, 4, 6, 8, and 10) and tumor necrosis factor-alpha.[4,13,16,17] These pro-inflammatory cytokines have been reported to play a role in inducing insulin resistance, initiating pancreatic beta-cell destruction, and altering lipid metabolism, leading to hyperlipidemia with low-density lipoproteins and triglycerides.[16,18] Based on a long-term prospective study among nondiabetic patients, Saito et al.[19] reported that patients with periodontitis had significantly higher frequency of impaired glucose tolerance in comparison to patients without periodontitis. All of these findings make it alluring to hypothesize a causative role for periodontal inflammation, resulting in poor glycemic control.
Removal of the causative factors for periodontal inflammation, namely plaque and calculus, and preventing their accumulation form the cornerstone of periodontal therapy.[20] Treatment primarily involves mechanical procedures, which include professional cleaning (scaling) and mechanical debridement of plaque and calculus both in the supragingival and subgingival regions. In addition, mechanical planing of the affected root surfaces (root planing) is carried out to remove the existing plaque and calculus deposits and to prevent future accumulation.[14,20] Furthermore, surgical treatment of periodontal pockets (deepened gingival crevices) and administration of systemic and local antibiotics and antiseptic mouthrinses are advocated in patients with severe periodontitis.[14] Based on a retrospective controlled trial, Stewart et al.[21] reported a 17.1% improvement in glycemic control, after a 10-month follow-up period, among T2DM patients who underwent treatment for adult periodontitis in comparison to only a 6.7% improvement among matched controls. Similarly, Kiran et al.[22] reported a 10.94% reduction in HbA1c levels after mechanical treatment for PD and a 3-month follow-up period among T2DM patients. The above results are in coherence with the results of the present study, wherein a 5.5% reduction in HbA1c levels was observed following nonsurgical periodontal therapy over a 6-month follow-up period. Moreover, reevaluation after a 6-month follow-up period was chosen in the present study due to the periodicity of half-yearly follow-up visits in the center where the study was conducted.
The periodontal therapy procedures followed in this study were similar to those reported in previous studies.[21,22,23,24] However, instead of comparing the outcomes of periodontal therapy against that of a control group, the present study involved periodontal therapy for all the study patients and stratification of their outcomes based on pretreatment glycemic control. In addition, the confounding effect of several variables was also examined. It was observed that patients with good pretreatment glycemic control (HbA1c <7.0%) had a 7.9% reduction in their HbA1c levels following periodontal therapy. This was significantly higher compared to the 4.6% HbA1c reduction observed among patients with poor pretreatment glycemic control (HbA1c >7.0%) [Table 2]. Interestingly, stratification of the outcomes based on variables with a confounding effect on pretreatment glycemic control, namely diabetic follow-up and oral hygiene status, showed that patients with a history of regular diabetic follow-up and good oral hygiene status had significant reduction in their HbA1c levels compared to that of the others [Tables 3 and 4].
Although previously reported studies had similar findings, the results of the present study provide substantive evidence to the adjunctive role of periodontal therapy in achieving better glycemic control owing to its large sample size and optimum follow-up period.[5,7,21,22,25] Interestingly, studies have also reported that periodontal therapy plays no significant role in improving glycemic control among diabetic patients than it does among healthy controls.[23,24] Nevertheless, the same studies reported a reduction in circulating pro-inflammatory cytokine levels among T2DM patients who underwent periodontal therapy[23] and better periodontal health following periodontal therapy among well-controlled patients with diabetes comparable to that of healthy controls.[24] While the protocol of the present study was based primarily on clinical periodontal assessments, the significantly higher reduction in HbA1c among well-controlled patients with diabetes is suggestive of a decrease in PD-induced systemic inflammation similar to what would be observed among healthy patients.
Evidences from the literature indicate a definitively increased risk of PDs among diabetic patients.[4,10,12,14,15,19] However, public health systems shall be benefited by studying the influence of periodontitis on poor glycemic control among diabetic patients.[5,7,15] With increasing evidence toward the detrimental effects of poor periodontal health, especially among diabetic patients,[13,17,18,26] it is imperative that elimination of periodontal inflammation is considered an essential element of contemporary diabetes management. This is easily achievable through a minimally invasive procedure such as nonsurgical periodontal therapy as evidenced in this study. The levels of HbA1c reduction through periodontal therapy observed in the present study are comparable to the addition of an OHA for achieving glycemic control.[5] Therefore, in spite of the absence of a matched control group, the present results are highly suggestive of the efficacy of periodontal therapy in helping achieve favorable glycemic control among T2DM patients. Moreover, the inclusion of a simple treatment regimen such as periodontal therapy in the diabetic treatment protocol would benefit a diabetic-dense developing country like India, wherein equitable care for all diabetic patients is still a challenge.[27,28]
The potential implications of the present study were aimed toward periodontal health promotion as an adjunct to achieve better glycemic control among T2DM patients. Unlike prevention of tooth decay, dental public health activities do not offer primary focus toward prevention of PDs, recognizing populations at risk and mitigating its prevalence.[29] The present study results imply the importance of routine screening for PD among diabetic patients as they not only belong to a specific population at risk for developing periodontitis, but also alleviation of periodontal inflammation might help them achieve good glycemic control. Although it may be alluring to hypothesize and recommend that treating periodontitis among T2DM patients can help achieve glycemic control (HbA1c <7%), further multicenter, controlled clinical trials in diverse populations are required to definitively establish this causal relationship.
Conclusion
The results of the present study indicate a significant role for nonsurgical periodontal therapy in helping achieve glycemic control among T2DM patients with mild-to-moderate periodontitis after a 6-month follow-up period. Moreover, they could serve as an evidence base for reformation and implementation of public health strategies involving prevention, early diagnosis, treatment, and palliation of periodontitis to achieve good metabolic control in T2DM patients. Adjunctive modalities of diabetes management such as periodontal therapy, as reported in this study, can help achieve optimal glycemic control, prevent complications, and reduce economic burden in developing countries such as India, as they are supposedly an innovative, cost-effective, and noninvasive strategy, which could be reached easily to the population at risk through public health-care facilities.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The findings reported in this article are a part of a research project approved by the Ethical committee at Madras Diabetes Research Foundation and Dr. Mohan's Diabetes Specialities Centre, “WHO collaborating centre for non-communicable diseases prevention and control, and ICMR centre for advanced research on diabetes”, Chennai, India. The ethical and clinical practice code for this study is MDRF/IRB/DENT/CS05052014. The authors would like to acknowledge all the patients who participated in this study and the Department of Research Operations, Madras Diabetes Research Foundation, Chennai, India.
References
- 1.Al Habashneh R, Khader Y, Hammad MM, Almuradi M. Knowledge and awareness about diabetes and periodontal health among Jordanians. J Diabetes Complications. 2010;24:409–14. doi: 10.1016/j.jdiacomp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Alba-Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R, et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res. 2007;40:1037–44. doi: 10.1590/s0100-879x2006005000143. [DOI] [PubMed] [Google Scholar]
- 3.Skyler JS. Effects of glycemic control on diabetes complications and on the prevention of diabetes. Clin Diabetes. 2004;22:162–6. [Google Scholar]
- 4.Correa FO, Gonçalves D, Figueredo CM, Bastos AS, Gustafsson A, Orrico SR, et al. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J Clin Periodontol. 2010;37:53–8. doi: 10.1111/j.1600-051X.2009.01498.x. [DOI] [PubMed] [Google Scholar]
- 5.Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: A systematic review and meta-analysis. J Clin Periodontol. 2013;40(Suppl 14):S153–63. doi: 10.1111/jcpe.12084. [DOI] [PubMed] [Google Scholar]
- 6.Federation ID, editor. Belgium, Brussels: International Diabetes Federation; 2015. IDF Diabetes Atlas. [Google Scholar]
- 7.Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: A randomized clinical trial. JAMA. 2013;310:2523–32. doi: 10.1001/jama.2013.282431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tugnait A, Clerehugh V, Hirschmann PN. Use of the basic periodontal examination and radiographs in the assessment of periodontal diseases in general dental practice. J Dent. 2004;32:17–25. doi: 10.1016/s0300-5712(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 9.Deed G, Barlow J, Kuo I. Early and tight glycaemic control – The key to managing type 2 diabetes. Aust Fam Physician. 2012;41:681–4. [PubMed] [Google Scholar]
- 10.Taylor GW, Borgnakke WS. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 11.Morita I, Inagaki K, Nakamura F, Noguchi T, Matsubara T, Yoshii S, et al. Relationship between periodontal status and levels of glycated hemoglobin. J Dent Res. 2012;91:161–6. doi: 10.1177/0022034511431583. [DOI] [PubMed] [Google Scholar]
- 12.Weinspach K, Staufenbiel I, Memenga-Nicksch S, Ernst S, Geurtsen W, Günay H. Level of information about the relationship between diabetes mellitus and periodontitis – Results from a nationwide diabetes information program. Eur J Med Res. 2013;18:6. doi: 10.1186/2047-783X-18-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mealey BL. Periodontal disease and diabetes. A two-way street. J Am Dent Assoc. 2006;137(Suppl):26S–31S. doi: 10.14219/jada.archive.2006.0404. [DOI] [PubMed] [Google Scholar]
- 14.Herring ME, Shah SK. Periodontal disease and control of diabetes mellitus. J Am Osteopath Assoc. 2006;106:416–21. [PubMed] [Google Scholar]
- 15.Chapple IL. Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: Consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Clin Periodontol. 2013;40(Suppl 14):S106–12. doi: 10.1111/jcpe.12077. [DOI] [PubMed] [Google Scholar]
- 16.Sun WL, Chen LL, Zhang SZ, Ren YZ, Qin GM. Changes of adiponectin and inflammatory cytokines after periodontal intervention in type 2 diabetes patients with periodontitis. Arch Oral Biol. 2010;55:970–4. doi: 10.1016/j.archoralbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: A two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 18.Iacopino AM. Periodontitis and diabetes interrelationships: Role of inflammation. Ann Periodontol. 2001;6:125–37. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 19.Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: The Hisayama study. J Dent Res. 2004;83:485–90. doi: 10.1177/154405910408300610. [DOI] [PubMed] [Google Scholar]
- 20.Cugini MA, Haffajee AD, Smith C, Kent RL, Jr, Socransky SS. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol. 2000;27:30–6. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 21.Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001;28:306–10. doi: 10.1034/j.1600-051x.2001.028004306.x. [DOI] [PubMed] [Google Scholar]
- 22.Kiran M, Arpak N, Unsal E, Erdoğan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–72. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 23.Al-Mubarak S, Ciancio S, Aljada A, Mohanty P, Ross C, Dandona P, et al. Comparative evaluation of adjunctive oral irrigation in diabetics. J Clin Periodontol. 2002;29:295–300. doi: 10.1034/j.1600-051x.2002.290404.x. [DOI] [PubMed] [Google Scholar]
- 24.Christgau M, Palitzsch KD, Schmalz G, Kreiner U, Frenzel S. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: Clinical, microbiological, and immunologic results. J Clin Periodontol. 1998;25:112–24. doi: 10.1111/j.1600-051x.1998.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 25.Darré L, Vergnes JN, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: A meta-analysis of interventional studies. Diabetes Metab. 2008;34:497–506. doi: 10.1016/j.diabet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Grossi SG. Treatment of periodontal disease and control of diabetes: An assessment of the evidence and need for future research. Ann Periodontol. 2001;6:138–45. doi: 10.1902/annals.2001.6.1.138. [DOI] [PubMed] [Google Scholar]
- 27.Ali MK, Narayan KM, Mohan V. Innovative research for equitable diabetes care in India. Diabetes Res Clin Pract. 2009;86:155–67. doi: 10.1016/j.diabres.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Venkataraman K, Kannan AT, Mohan V. Challenges in diabetes management with particular reference to India. Int J Diabetes Dev Ctries. 2009;29:103–9. doi: 10.4103/0973-3930.54286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen PE, Ogawa H. Strengthening the prevention of periodontal disease: The WHO approach. J Periodontol. 2005;76:2187–93. doi: 10.1902/jop.2005.76.12.2187. [DOI] [PubMed] [Google Scholar]