Abstract
Objective:
The objective of this study is to measure levels of Vitamin D3 and leptin and assess their relation of each to the pathogenesis of polycystic ovary syndrome (PCOS).
Design:
This was a cohort observational study.
Settings:
This study was conducted at the Department of Obstetrics and Gynecology, Tanta University.
Materials and Methods:
Ninety lean women were enrolled in this study and were allocated into two groups with 45 patients in each group: the first group (study group) who are lean women with PCOS and the second group (control group) who are the lean infertile patients without PCOS. Blood samples were collected and tested for study parameters.
Results:
There were no significant differences regarding demographic characteristics between both groups. The differences were in ovarian volume and hormonal profiles. Serum leptin was found to be significantly increased in lean PCOS than in control groups. Vitamin D3 levels were found to be lower in the lean PCOS group than in control group.
Conclusion:
Lean PCOS women are a unique group with specific hormonal profiles different from the typical PCOS profiles. Leptin and Vitamin D3 may have a role in the pathogenesis of lean PCOS, but large studies are still required regarding this unique group.
KEYWORDS: Lean, leptin, pathogenesis, polycystic ovary syndrome, Vitamin D3
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a state of polyendocrinopathy affecting 5%–10% of patients in childbearing period and occurs in about 45% of infertility cases. Polycystic ovaries are associated with hyperandrogenemia, especially testosterone, caused by either high luteinizing hormone (LH) produced by the anterior pituitary gland or due to hyperinsulinemia.[1]
The great majority of PCOS cases are obese (60%–65%), and the severity of PCOS symptoms is correlated to obesity. Aromatase enzyme present in adipose tissue converts androstenedione to estrone and testosterone to estradiol, so excess fatty tissue in obese women leads to excess androgens (which are responsible for hirsutism and virilization) and hyperestrogenemia (which inhibits follicle-stimulating hormone [FSH] through negative feedback).[2]
Recent studies focused on the role of hypovitaminosis D in the pathogenesis of metabolic disorders such as insulin resistance (IR) and diabetes mellitus. Hypovitaminosis D was investigated in PCOS patients, and the results suggest that Vitamin D deficiency had a role of in the pathogenesis of PCOS.[3] Obese PCOS patients have been tested for Vitamin D level and shown to have lower serum levels of 25-hydroxyvitamin D than nonobese women with PCOS. The mechanism by which Vitamin D deficiency causes PCOS was suggested to be through the development of IR and impaired glucose tolerance in PCOS patients. However, results of studies in this point are conflicting about Vitamin D deficiency as a causative factor for IR or as a consequence of obesity in PCOS patients.[4,5] Hypovitaminosis D has been reported in lean PCOS women, who are reported to be less insulin resistant than PCOS obese patients.[6]
Leptin is a hormone derived from an adipocytes encoded by “ob” gene that signals the energy magnitude to the brain and recently has an important effects on the reproductive functions of rodents. Circulating leptin levels are directly correlated to obesity, which is frequently associated with PCOS. Hyperinsulinemia and IR are often linked to leptin and its receptors. However, the relationship between leptin, gonadotropins, and insulin in PCOS is still not understood.[7]
In the current study, Vitamin D3 and leptin levels in lean infertile patients with PCOS were measured and compared to normal controls.
MATERIALS AND METHODS
Study design and settings
This study is a cohort observational study that was conducted at the Department of Obstetrics and Gynecology, Tanta University in the period from March 1, 2016, to February 28, 2017.
Eligibility
The study was carried on 90 women in the reproductive age recruited from fertility unit and outpatient clinic of Department of Obstetrics and Gynecology, Tanta University The inclusion criteria were as follows: (i) age from 18 to 35 years; (ii) PCOS diagnosed according to Rotterdam criteria[8] indicating PCOS to be present if any 2 out of 3 criteria are met from the following: (1) oligoovulation and/or anovulation, (2) excess androgen activity (clinical or biochemical), (3) polycystic ovaries (by ultrasound); (iii) body mass index (BMI) <25; and (iv) infertile patients either primary or secondary infertility. The exclusion criteria were as follows: (i) obese women with BMI ≥25, (ii) other endocrinological disorders as thyroid or adrenal dysfunctions, (iii) pregnant women, (iv) patients with chronic diseases such as hepatic or renal diseases and cancers, and (v) patients with history of ovarian surgery.
Allocations
Patients were allocated into two groups: study group (n = 45) – lean PCOS patients and control group (n = 45) – healthy infertile patients with no PCOS.
Hormonal assays
Morning venous blood samples were obtained between 8 and 10 am after 8–12 h overnight fasting and between the 3rd and 5th day of a spontaneous or progesterone-induced menstrual cycle.
The hormones measured in this study were pituitary hormones: LH, FSH, thyroid-stimulating hormone (TSH), and prolactin; ovarian hormones: estrogen; adrenal hormones: testosterone and dehydroepiandrosterone sulfate (DHEA-S); and the study parameters such as serum leptin and Vitamin D3 levels. Fasting blood sugar sample and other samples were sent immediately to Tanta University central laboratory for measuring hormonal assays.
Data acquisition
Demographic characteristics, type and duration of infertility were obtained. Pelvic ultrasonography was done for all patients. Hormonal levels were acquired and registered for all cases.
Ethical approval
All patients were informed about study aims and the ethics committee of Faculty of Medicine, Tanta University approved this study on April 1, 2016, and given this study the code of 30895/4//16.
Statistical methods
Data were analyzed using SPSS Software version 18 (IBM Inc., Chicago, IL, USA). Results were expressed as mean ± standard deviation. The student t-test was used to compare continuous variables. P < 0.05 was considered statistically significant.
RESULTS
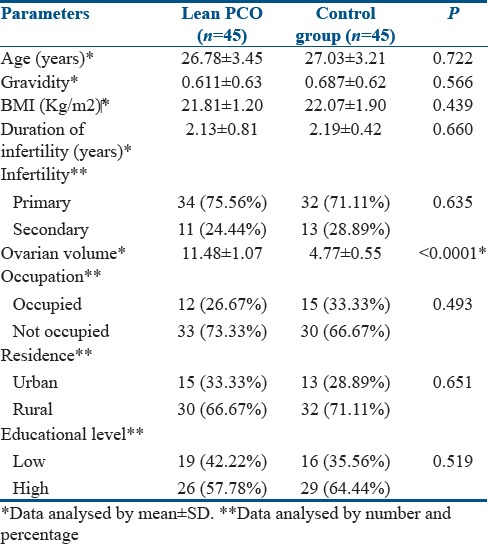
The demographic characteristics of enrolled patients were demonstrated in Table 1. There was no significant difference regarding age, gravidity, duration, or type of infertility in both groups. The majority of cases were nonoccupied of high educational levels and from rural areas. The differences were in ovarian volume with increase in in PCOS group than in control group (P < 0.0001) as shown in Table 1.
Table 1.
Demographic characteristics of enrolled patients
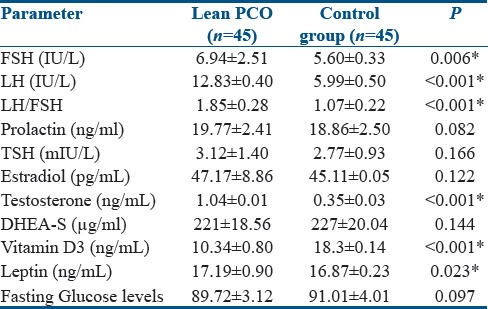
The hormonal profiles of enrolled patients were demonstrated in Table 2. The FSH, LH, and FSH/LH ratio were higher in the PCOS group. Similarly, testosterone and estradiol levels were found to be higher in PCOS group than in control group. DHEA-S, prolactin, and TSH levels were not significantly different in both groups. Leptin levels in study and control groups were 17.19 ± 0.90 and 16.87 ± 0.23 ng/ml, respectively. Leptin levels were significantly increased in PCOS than in control groups (P = 0.0232). The Vitamin D3 levels were 10.34 ± 0.80 and 18.3 ± 0.14 ng/ml in study and control groups, respectively. Vitamin D3 levels were found to be lower in PCOS group rather than in control group (P < 0.0001). Glucose levels were not significantly different in both groups denoting absent IR in lean PCOS patients.
Table 2.
Hormonal profiles of enrolled patients
DISCUSSION
PCOS is recognized as the most common endocrine disorder of reproductive-aged women around the world. PCOS affects approximately 4%–12% of women in their reproductive age. It is a leading cause of infertility and other metabolic disorders. Most patients with PCOS are obese with typical IR and hormonal abnormalities. Lean patients with PCOS are unique group with special characteristics regarding IR, hormonal profile, and management.[9]
In the current study, lean PCOS patients were compared to matched lean infertile patients without PCOS where most demographic characteristics were not significantly different in both groups except for ovarian volume as shown in Table 1. Typical hormonal profiles were observed in the PCOS group regarding FSH, LH, elevated LH/FSH ratio, and testosterone. The hormones that were not significantly increased in lean PCOS group were estradiol, DHEA-S, prolactin, and TSH. Leptin was found to be significantly increased in PCOS group than in non-PCOS, denoting that it may has a role in pathogenesis of the disease in lean patients. On the contrary, Vitamin D3 levels were found to be lower in lean PCOS group. Glucose levels were not significantly increased in lean PCOS group than in control group as shown in Table 2.
A study by Carmina et al. (2009) found that patients with PCOS have an increase in lean mass that is highly associated with hyperinsulinemia and altered fat parameters and less so with androgen. IR and hyperinsulinemia are present in up to 65% of obese women with PCOS and in up to 20% of lean women with PCOS. In the current study glucose levels were not increased in the lean PCOS denoting absence of IR.[10]
Rizk et al. (2015) conducted a study to assess the concentrations of the leptin and its receptors in PCOS and its relation to adiposity, IR, and androgens. They found that PCOS is associated with increased leptin levels denoting the role of leptin in the pathogenesis of PCOS. They found also that leptin is not significantly increased in lean PCOS patients if compared to lean non-PCOS patients.[11] Similarly, Olszanecka-Glinianowicz et al. (2013) demonstrated higher leptin levels in PCOS patients compared with age- and BMI-matched control group. Furthermore, they observed more elevated leptin in obese patients than in lean PCOS patients.[12] On the contrary, other studies showed no significant difference of leptin between patients with and without PCOS.[13,14]
Dagogo-Jack et al. (1996) in their study found that circulating leptin levels are not affected by glucose levels or hyperinsulinemia. They concluded that insulin is nonstimulating leptin secretion in humans and that increased leptin levels in obese individuals are not due to hyperinsulinemia.[15]
On the other hand, Saleh et al. (2004) found that leptin levels differ in obese than in nonobese patients. They concluded that leptin plays an important role in in the pathogenesis of obesity related IR and perhaps in the ovarian dysfunction found in PCOS.[16]
The other parameter in this study is the Vitamin D3 levels, where levels of Vitamin D3 were found to be lower in lean PCOS than in control group (P < 0.0001). Similarly, Sahin et al. (2014) conducted a study to investigate the correlation between IR and serum Vitamin D3 concentrations and hormonal parameters in lean women with PCOS. They found that Vitamin D deficiency is not implicated in pathogenesis of PCOS in lean women. They also correlated IR to intrinsic mechanisms and genetic predisposition in lean PCOS patients.[17]
On the other hand, Hahn et al. (2006) found that levels of Vitamin D3 are significantly higher in lean women than in obese ones. They concluded that low levels of Vitamin D3 in PCOS women are associated with obesity and IR but not with PCOS per s e. Similar results were obtained by Wehr et al. (2009).[18,19]
Controversy is still present on the value of Vitamin D3 supplementation and its effect in improving ovulation in women with PCOS.[20] A study by Rashidi et al. (2009) found that administration of Vitamin D and calcium in addition to metformin in women with PCOS may have beneficial effects on menstrual regularity and ovulation.[21] On the other hand, Garg et al. (2015) found that Vitamin D3 supplementation had no significant beneficial effect on insulin kinetics and cardiovascular risk factors among women with PCOS treated with metformin.[22]
The debate is still present whether levels of Vitamin D3 is increased or decreased in lean and obese women with PCOS. This was stated by Thomson et al. (2012) in their review study where they stated that the available data are insufficient to identify the basis of the difference in Vitamin D status between lean and obese women with PCOS.[23]
CONCLUSION
Lean PCOS patients are a unique group with specific hormonal profile than typical profiles of PCOS. In lean PCOS, leptin levels were increased and Vitamin D3 levels were decreased if compared to healthy controls. The role of leptin and Vitamin D3 in the pathogenesis of PCOS needs to be reinvestigated by larger studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Barber TM, Dimitriadis GK, Andreou A, Franks S. Polycystic ovary syndrome: Insight into pathogenesis and a common association with insulin resistance. Clin Med (Lond) 2015;15(Suppl 6):s72–6. doi: 10.7861/clinmedicine.15-6-s72. [DOI] [PubMed] [Google Scholar]
- 2.Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction. 2015;149:R219–27. doi: 10.1530/REP-14-0435. [DOI] [PubMed] [Google Scholar]
- 3.Lathief S, Pal L. Polycystic Ovary Syndrome. New York, NY: Springer; 2014. Emerging concepts: Role of Vitamin D deficiency in the pathogenesis of PCOS; pp. 317–31. [Google Scholar]
- 4.Grundmann M, von Versen-Höynck F. Vitamin D – Roles in women's reproductive health? Reprod Biol Endocrinol. 2011;9:146. doi: 10.1186/1477-7827-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butts S, Seifer D, Senapati S, Koelper NC, Legro RS, Diamond MP. Vitamin D deficiency is associated with poor reproductive outcomes in PCOS but not unexplained infertility. Fertil Steril. 2017;108:e69–70. [Google Scholar]
- 6.Stovall DW, Bailey AP, Pastore LM. Assessment of insulin resistance and impaired glucose tolerance in lean women with polycystic ovary syndrome. J Womens Health (Larchmt) 2011;20:37–43. doi: 10.1089/jwh.2010.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarti J. Serum leptin level in women with polycystic ovary syndrome: Correlation with adiposity, insulin, and circulating testosterone. Ann Med Health Sci Res. 2013;3:191–6. doi: 10.4103/2141-9248.113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Dawood AS. Debates regarding lean patients with polycystic ovary syndrome: A narrative review. J Hum Reprod Sci. 2017;10:154–61. doi: 10.4103/jhrs.JHRS_77_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmina E, Guastella E, Longo RA, Rini GB, Lobo RA. Correlates of increased lean muscle mass in women with polycystic ovary syndrome. Eur J Endocrinol. 2009;161:583–9. doi: 10.1530/EJE-09-0398. [DOI] [PubMed] [Google Scholar]
- 11.Rizk NM, Sharif E. Leptin as well as free leptin receptor is associated with polycystic ovary syndrome in young women. Int J Endocrinol. 2015;2015:927805. doi: 10.1155/2015/927805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olszanecka-Glinianowicz M, Madej P, Nylec M, Owczarek A, Szanecki W, Skałba P, et al. Circulating apelin level in relation to nutritional status in polycystic ovary syndrome and its association with metabolic and hormonal disturbances. Clin Endocrinol (Oxf) 2013;79:238–42. doi: 10.1111/cen.12120. [DOI] [PubMed] [Google Scholar]
- 13.Mantzoros CS, Dunaif A, Flier JS. Leptin concentrations in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:1687–91. doi: 10.1210/jcem.82.6.4017. [DOI] [PubMed] [Google Scholar]
- 14.Jahanfar S, Maleki H, Mosavi AR, Jahanfar M. Leptin and its association with polycystic ovary syndrome: A twin study. Gynecol Endocrinol. 2004;18:327–34. doi: 10.1080/09513590410001667256. [DOI] [PubMed] [Google Scholar]
- 15.Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M. Plasma leptin and insulin relationships in obese and nonobese humans. Diabetes. 1996;45:695–8. doi: 10.2337/diab.45.5.695. [DOI] [PubMed] [Google Scholar]
- 16.Saleh HA, El-Nwaem MA, El-Bordiny MM, Maqlad HM, El-Mohandes AA, Eldaqaq EM, et al. Serum leptin elevation in obese women with PCOs: A continuing controversy. J Assist Reprod Genet. 2004;21:361–6. doi: 10.1023/B:JARG.0000046204.81682.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahin S, Eroglu M, Selcuk S, Turkgeldi L, Kozali S, Davutoglu S, et al. Intrinsic factors rather than Vitamin D deficiency are related to insulin resistance in lean women with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2014;18:2851–6. [PubMed] [Google Scholar]
- 18.Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, et al. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114:577–83. doi: 10.1055/s-2006-948308. [DOI] [PubMed] [Google Scholar]
- 19.Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, et al. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol. 2009;161:575–82. doi: 10.1530/EJE-09-0432. [DOI] [PubMed] [Google Scholar]
- 20.Irani M, Merhi Z. Role of Vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil Steril. 2014;102:460–8.e3. doi: 10.1016/j.fertnstert.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Rashidi B, Haghollahi F, Shariat M, Zayerii F. The effects of calcium-Vitamin D and metformin on polycystic ovary syndrome: A pilot study. Taiwan J Obstet Gynecol. 2009;48:142–7. doi: 10.1016/S1028-4559(09)60275-8. [DOI] [PubMed] [Google Scholar]
- 22.Garg G, Kachhawa G, Ramot R, Khadgawat R, Tandon N, Sreenivas V, et al. Effect of Vitamin D supplementation on insulin kinetics and cardiovascular risk factors in polycystic ovarian syndrome: A pilot study. Endocr Connect. 2015;4:108–16. doi: 10.1530/EC-15-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomson RL, Spedding S, Buckley JD. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2012;77:343–50. doi: 10.1111/j.1365-2265.2012.04434.x. [DOI] [PubMed] [Google Scholar]


