Abstract
Context:
Increased circulating insulin levels contribute to hyperandrogenism in polycystic ovarian syndrome (PCOS) which causes a derangement in folliculogenesis, thus contributing to polycystic morphogenesis of the ovaries and a higher than normal anti-Mullerian hormone (AMH). A high AMH is an indicator of either stubborn anovulation or a predictor of ovarian hyperstimulation syndrome. Hence, it is postulated that the use of insulin sensitizers will reduce insulin resistance, hyperandrogenism, and subsequently serum AMH levels and will convert anovulatory cycles to ovulatory.
Aim:
To study the effect of insulin sensitizers on raised serum AMH levels in infertile women with PCOS.
Settings and Design:
This was a prospective interventional randomized single tertiary center study.
Methodology:
The study was conducted from August 2015 to April 2016. Infertile patients with PCOS as defined by the Rotterdam criteria with raised AMH (>5 ng/ml) levels were enrolled in the study under strict inclusion and exclusion criteria. The sample size was 105 patients. Cycle regularity, day 2–antral follicle count (AFC), luteinizing hormone, AMH levels, modified Ferriman–Gallwey score (mFGS), and acne score were recorded before starting the intervention. Patients were randomized into three equal groups of 35 each. Group A received metformin alone, Group B metformin plus myoinositol, and Group C only myoinositol. After completion of 3 months of pretreatment, the same parameters were rechecked.
Statistical Analysis Used:
Univariate analysis and Chi-square test were used for statistical analysis.
Results:
Of 105 patients, 95 completed treatment and the rest 10 dropped out. There was a reduction in AMH in all groups of insulin sensitizers with significant fall in the metformin only group. Cycle regularity, reduction in AFC, mFGS, and grade of acne were also obtained.
Conclusions:
Therapy with insulin sensitizers in PCOS women with raised AMH reduces the AMH levels, converts irregular menstrual cycles to regular, and reduces clinical hyperandrogenism.
KEYWORDS: Anti-Mullerian hormone, insulin sensitizers, polycystic ovarian syndrome
INTRODUCTION
Increased circulating insulin levels contribute to hyperandrogenism in women with polycystic ovarian syndrome (PCOS). Hyperandrogenism causes a derangement in folliculogenesis, thus contributing to the polycystic morphogenesis of the ovaries and a higher than normal anti-Mullerian hormone (AMH). A high AMH is said to be an indicator of either stubborn anovulation or a predictor of ovarian hyperstimulation syndrome. Hence, it is postulated that the use of insulin sensitizers would reduce the insulin resistance, hyperandrogenemia, and subsequently serum AMH levels and would convert anovulatory cycles to ovulatory. The hormonal and metabolic milieu thus corrected would improve response to stimulation in the subsequent in vitro fertilization (IVF) cycles.
Study design
A prospective interventional randomized single-center study conducted from August 2015 to April 2016. The study protocol was approved by the local ethics committee and written informed consent was obtained from all participants.
Inclusion criteria
Infertile patients with PCOS as defined by the Rotterdam criteria, namely at least two out of the following three:
Menstrual disorders defined as oligomenorrhea (cycle length >35 days) or amenorrhea (cycle length >12 weeks)
Clinical hyperandrogenism (modified Ferriman–Gallwey score [mFGS] of ≥6, presence of acne, or seborrhea) and/or biochemical evidence of hyperandrogenemia
Patients with Polycystic ovaries on ultrasound (12 or more measuring 2–9 mm in diameter (mean of both ovaries) and day 2 (D2)/D3 serum AMH levels >5 ng/ml, irrespective of the body mass index (BMI), were included.
Exclusion criteria
Women with other causes of PCOs on ultrasound due to anovulation such as hyperprolactinemia, thyroid disorders, late-onset congenital adrenal hyperplasia, and Cushing's syndrome by the use of appropriate tests
PCOS patients with AMH <5 ng/ml
Patients with contraindications to use of insulin sensitizers such as metformin due to prior adverse effects
Usage of oral contraceptive and/or any other insulin sensitizer in the previous 3 months.
METHODOLOGY
Infertile PCOS patients visiting the outpatient department were evaluated in the early follicular phase following spontaneous or progesterone-induced menstruation (cycle day 2–3). Their age, type of infertility (primary or secondary), cycle regularity, height, weight, BMI, clinical evidence of hyperandrogenism (based on mFGS), presence and grade of acne (based on the score by the Indian Acne Society), and prior response to ovarian stimulation (if any) were noted. Peripheral venipuncture to assess serum hormonal concentrations of AMH was done. Transvaginal scan was done to assess the base line antral follicle count (AFC). The sample size was 105 patients. Once the patients met the inclusion criteria and did not fall into any exclusion criteria, as described above, drug randomization was done using the computer-generated tables. They received any one of the following insulin sensitizers:
Group A: Tablet metformin 850 mg twice a day alone (n = 35)
Group B: Tablet metformin 850 mg twice a day plus tablet myoinositol 2 g twice a day (n = 35)
Group C: Tablet myoinositol 2 g twice a day alone (n = 35).
The therapy was continued for 3 months.
The test subjects were their own controls.
At the end of this period, the following parameters were rechecked.
Cycle regularity
BMI
Clinical evidence of hyperandrogenism (based on mFGS)
Presence and grade of acne (based on the score by the Indian Acne Society)
Serum AMH
AFC.
The primary outcome was:
Change in serum AMH levels.
The secondary outcomes were:
Cycle regularity
Change in AFC
Change in mFGS
Change in acne score.
RESULTS
There were 10 dropouts of the study with 95 subjects remaining. Statistical analysis was done using univariate analysis and Chi-square tests in SPSS Version 20 (IBM, Armonk, NY, USA).
Table 1 summarizes the baseline parameters at the start of the study. As can be seen, the age is comparable in all the three groups with no statistically significant difference. Serum AMH levels were comparable in all the three groups with no statistically significant difference. The number of patients in the primary and secondary infertility group was also comparable in all the three groups. The AFC, however, had a statistically significant difference in all the three groups at the start with the highest AFC in myoinositol only group.
Table 1.
Baseline characteristics of the study groups

Table 2 shows the results at the end of 3 months of treatment. There was a significant fall in serum AMH levels in all the three groups. The maximum fall was in the group that received only metformin.
Table 2.
Comparison of anti-Mullerian hormone levels and antral follicle count before and after treatment with insulin sensitizers
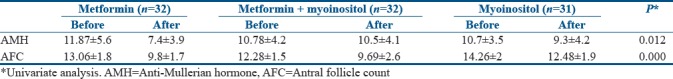
Table 3 compares the cycle regularity before and after treatment with insulin sensitizers. Cycles became regular in all the three groups with the maximum numbers seen in the group which received both the insulin sensitizers.
Table 3.
Comparison of cycle regularity before and after treatment with insulin sensitizers

Table 4 shows the change in the grade of hirsutism as shown by the mFGS. There was a significant reduction in the hirsutism score in all the groups. In Group A, the percentage of patients with grade 2 hirsutism reduced and the percentage in grade 1 increased as compared to before treatment. Similarly, in Group B, the percentage of patients with grade 2 hirsutism reduced and the percentage in grade 1 increased. In Group C, the percentage of patients with grade 1 hirsutism reduced and the percentage of grade 0 increased. Overall, there was reduction in hirsutism with the use of insulin sensitizers.
Table 4.
Comparison of modified Ferriman-Gallwey score before and after treatment with insulin sensitizers

Table 5 shows the change in the grade of acne as shown by the acne score. There was a significant reduction in the acne score in all the groups. In Group A, the percentage of patients with grade 2 acne reduced and the percentage in grade 1 increased as compared to before treatment. Similarly, in Group B, the percentage of patients with grade 2 acne reduced and the percentage in grade 1 increased. In Group C, the percentage of patients with grade 2 acne reduced and the percentage of grade 1 increased. Overall, there was reduction in acne score with the use of insulin sensitizers.
Table 5.
Comparison of grade of acne before and after treatment with insulin sensitizers

DISCUSSION
Insulin resistance is a common feature in obese and to a lesser extent in lean PCOS. Insulin sensitivity is decreased up to 35%–40% in women with PCOS in comparison to normal women.[1] Increased circulating insulin levels cause hyperandrogenism in these women by stimulating increased ovarian androgen production and by inhibiting hepatic sex hormone-binding globulin production.[1] Insulin and luteinizing hormone (LH) act synergistically to cause hyperandrogenism.[1]
High local androgen concentrations contribute to the polycystic morphogenesis of the ovaries. They do so by conversion to more potent 5a-reduced androgens. These cannot be aromatized to estrogen. They also inhibit aromatase activity and follicle-stimulating hormone (FSH) induction of LH receptors on Granulosa cells. This impedes progressive follicular development. Thus, new follicular growth does occur but arrests before full maturation. This leads to multiple small cysts measuring 2–10 mm in diameter surrounded by hyperplastic theca cells. These atretic follicles contribute to an expanding ovarian stroma that increases in volume over time and starts a self-propagating cycle of hyperandrogenism and chronic anovulation.[1]
AMH, also known as Mullerian-inhibiting substance, belongs to the transforming growth factor-beta superfamily of proteins that includes activins, inhibins, bone morphogenetic proteins, and growth determining factors. The gene for AMH is found on chromosome 19. Initially, it was known to have a role in the sexual differentiation in male fetus in utero but has recently been found to have a role in female reproductive physiology. Produced in the highest concentration by the preantral and small antral follicles from 36 weeks of intrauterine life till menopause, the role of AMH has been extensively studied. AMH is a known test of the ovarian reserve and reflects the leftover follicular pool. It serves as a predictor of the IVF success by indirectly correlating with the oocyte yield and quantity. AMH prevents the recruitment of primordial follicles. Low AMH levels thus allow more primordial follicular recruitment and faster exhaustion of the ovarian reserve. AMH prevents the FSH-mediated estrogen production from the preantral and small antral follicles by inhibiting the aromatase enzyme and reducing the levels of LH receptors, thereby preventing the follicular growth. Levels start reducing once the follicle reaches 10 mm size and further reduces as it attains dominance. High AMH prevents dominance.
AMH levels in women with PCOS are 2–3 times higher than normal women. AMH production is 75 times higher from granulosa cells from anovulatory PCOS than normal granulosa cells. The levels in anovulatory PCOS are 5 times higher than ovulatory PCOS. The reason for the increased AMH in PCOS could either be due to the increase in the number of the preantral and small antral follicles in women with PCOS as compared to non-PCOS or due to the associated hyperandrogenism and insulin resistance as explained above. It has been demonstrated that granulosa cells of women with PCOS intrinsically produce more AMH than non PCOS women.
PCOS women can be ovulatory or anovulatory. The levels of AMH in ovulatory PCO are higher than normal but lower than anovulatory PCOS in whom it is 18 times higher. The reason could be higher androgens in the anovulatory subgroup than the ovulatory ones.
Thus, it was postulated that the use of insulin sensitizer drugs such as metformin and myoinositol would reduce the insulin resistance and consequently hyperandrogenism, serum AMH levels, and D2–AFC and would convert anovulatory PCO patients to ovulatory. The hormonal and metabolic milieu thus corrected was postulated to improve response to stimulation in the subsequent IVF cycles.
On reviewing the literature, we found no study comparing all the three groups. Hence, each of the insulin sensitizers were considered individually and studies comparing the parameters under study were searched.
When the literature was reviewed for metformin alone, four studies similar to our study were studied. The serum AMH levels fell in the study by Saleh et al.,[2] Neagu and Cristescu,[3] and Tomova et al.[4] similar to what we had observed in our study. However, in the study by Nascimento et al.,[5] there was no significant change in the serum AMH levels.
When the AFC was compared between the studies, Saleh et al.[2] and Neagu and Cristescu[3] saw a drop in the same. This parameter was not studied by Tomova et al.[4] and Nascimento et al.[5] We saw a fall in the AFC, but whether it was statistically significant in one group as compared to the other cannot be commented upon as there was a significant intergroup variation at the start.
In all the studies discussed above, cycle regularity was attained after treatment. This is indirect evidence to the conversion of anovulatory to ovulatory status.
There was an improvement in hirsutism in the study by Saleh et al.[2] and Nascimento et al.[5] as was witnessed by us. The other two study groups referred to earlier did not study the parameter.
When a search was made in PubMed, no study was found that showed any effect of insulin sensitizer inositol on serum AMH levels. As for the other parameters such as cycle regularity, improvement in hirsutism, and acne score, the studies by Genazzani et al.,[6] Costantino et al.,[7] and Angik et al.[8] showed results similar to ours.
AFC was studied by Angik et al.[8] and was found reduced after treatment similar to our study result.
In the literature searched, only one study by Ali et al.[9] compared the effect of both metformin and myoinositol combined on the parameters studied. The AMH levels reduced, cycles became regular with an improvement in the clinical hyperandrogenism and acne score. This group however did not study the effect of the drugs on the AFC.
CONCLUSIONS
The following conclusions can be drawn from the study conducted regarding therapy with insulin sensitizers in PCOS:
Reduces serum AMH levels
Converts irregular cycles to regular
Reduces clinical hyperandrogenism
Minimum duration of therapy-3 months.
Merits
First of its kind
Prospective randomized
Single-center
Single observer
Adequate sample size.
Demerits
Effect of longer duration of the treatment needs to be seen in patients who did not respond
Effect of the treated patients in stimulation cycles needs to be seen
The correlation of BMI with insulin resistance could not be seen.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank our patients who agreed to be a part of our study.
REFERENCES
- 1.Speroff L. Clinical Gynecologic Endocrinology and Infertility. 8th ed. Philadelphia: Wolters Kluwer; 2011. Chronic anovulation and the polycystic ovarian syndrome; pp. 495–532. [Google Scholar]
- 2.Saleh BO, Ibraheem WF, Ameen NS. The role of anti-Mullerian hormone and inhibin B in the assessment of metformin therapy in women with polycystic ovarian syndrome. Saudi Med J. 2015;36:562–7. doi: 10.15537/smj.2015.5.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neagu M, Cristescu C. Anti-Műllerian hormone – A prognostic marker for metformin therapy efficiency in the treatment of women with infertility and polycystic ovary syndrome. J Med Life. 2012;5:462–4. [PMC free article] [PubMed] [Google Scholar]
- 4.Tomova A, Deepinder F, Robeva R, Kirilov G, Mechandjiev Z, Kumanov P, et al. Anti-Müllerian hormone in women with polycystic ovary syndrome before and after therapy with metformin. Horm Metab Res. 2011;43:723–7. doi: 10.1055/s-0031-1286307. [DOI] [PubMed] [Google Scholar]
- 5.Nascimento AD, Silva Lara LA, Japur de Sá Rosa-e-Silva AC, Ferriani RA, Reis RM. Effects of metformin on serum insulin and anti-Mullerian hormone levels and on hyperandrogenism in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2013;29:246–9. doi: 10.3109/09513590.2012.736563. [DOI] [PubMed] [Google Scholar]
- 6.Genazzani AD, Lanzoni C, Ricchieri F, Jasonni VM. Myo-inositol administration positively affects hyperinsulinemia and hormonal parameters in overweight patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24:139–44. doi: 10.1080/09513590801893232. [DOI] [PubMed] [Google Scholar]
- 7.Costantino D, Minozzi G, Minozzi E, Guaraldi C. Metabolic and hormonal effects of myo-inositol in women with polycystic ovary syndrome: A double-blind trial. Eur Rev Med Pharmacol Sci. 2009;13:105–10. [PubMed] [Google Scholar]
- 8.Angik R, Jajoo SS, Hariharan C, Chimote A. A comparative study of metabolic and hormonal effects of myoinositol vs. metformin in women with polycystic ovary syndrome: A randomised controlled trial. Int J Reprod Contracept Obstet Gynecol. 2015;4:189–94. [Google Scholar]
- 9.Ali LQ, Luaibi NM, Majeed BJ. Used inositol decreased anti-Müllerian hormone (AMH) in polycystic ovary syndrome women. Int J Adv Res. 2015;3:857–69. [Google Scholar]


