Key Clinical Message
A surgical approach is the choice in young infants with MTH, who are furthest from the time of physiological involution of the thymus, and when the thymus achieves the largest relative size, a surgical approach is the choice. Steroid therapy has been shown to be ineffective (4, 9, 16, 18‐20). No surgical complications have been reported, and the outcome is excellent. Recurrence has been seen in only one case.
Keywords: computerized tomography scan, pediatric, thymectomy, true thymic hyperplasia
1. CASE REPORT
A 15‐month‐old boy was admitted to the Emergency Room with a history of 3 days of fever, cough, and suspected pneumonia. In his past history, only a slight decline in appetite was reported. Physical examination revealed diminished air entry and crackles at the right hemithorax. A chest X‐ray showed a large intrathoracic radiopaque thickening occupying the right hemithorax (Figure 1). Laboratory tests revealed: leukocyte count 16.36 × 109/L (of which 74% lymphocytes), normal C‐reactive protein, and normal biochemical profile. He was admitted to our pediatric clinic and treated with ceftriaxone (80 mg/kg/d) and clarithromycin (15 mg/kg/d). A chest X‐ray after 5 days of treatment revealed an improvement in the thickening of the right lung, but persistence of mediastinal enlargement. A chest computerized tomography scan (CT scan) was done and showed enlargement of the anterior mediastinum, occupied by solid inhomogeneous predominantly hypodense hypovascularized tissue, with a total dimension of 9.6 × 6 × 10 cm, with left paramedian development with minimal imprint on the jugulo‐subclavian confluence and on the homolateral anonymous vein. This was associated with pleural effusion of a maximum thickness of about 7 mm. The thymus was not well recognized (Figure 2).
Figure 1.
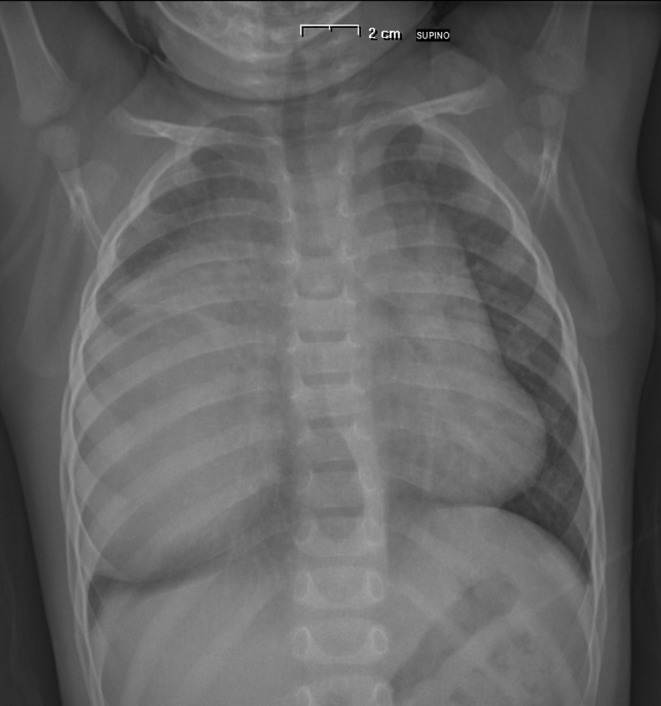
Chest X‐ray of the infant at presentation, showing a large intrathoracic radiopaque mass occupying the right hemithorax
Figure 2.
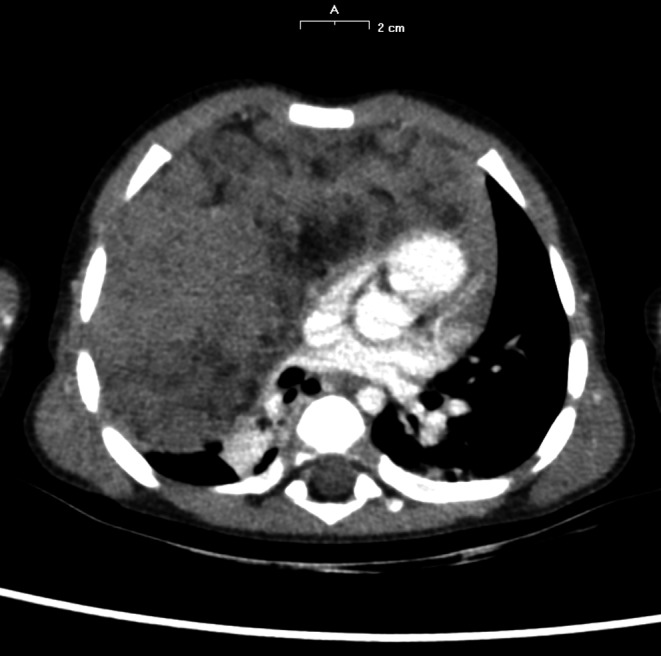
Chest CT scan showing the large mediastinal mass
Abdominal and neck ultrasounds were normal. Echocardiography showed minimum pericardial effusion but no heart chamber compression by the mediastinal mass. Oncologic markers (α‐fetoprotein, vanillylmandelic acid, human chorionic gonadotropin, urinary vanillylmandelic acid, homovanillic acid and 5‐hydroxic‐indoleacetic acid) were negative. Subsequent complete blood count revealed an increase in lymphocytosis (81.9% of 21.78 × 109/L leukocytes).
A percutaneous biopsy was carried out to exclude malignancy. Fragment analysis was compatible with thymic tissue, making the diagnosis of true thymic hyperplasia (TTH).
Because of clinical improvement and radiological stability of the size of the mass, the patient was discharged. A steroidal therapy (prednisone 2.5 mg/kg/d) was prescribed for 40 days. Radiological investigations showed initially minimal change in tumor size, but subsequently a rapid increase in dimension that reached 10.8 × 11 × 9 cm, with compression of the heart chamber and right main bronchus with atelectasis. The child underwent surgical excision of the mass via right thoracotomy. The mass weighed 492 g and measured 15 × 11.5 × 5 cm. Microscopic finding showed preservation of the normal thymic architecture. Immunohistochemical analysis revealed TTH associated with extramedullary myelopoiesis. Postoperative recovery was uneventful, and the patient was discharged from hospital five days after surgery. Laboratory results (complete blood count and inflammatory indices) at 1 month were normal. A chest X‐ray 3 months after surgery was normal. At 10 months of follow‐up, the child was asymptomatic and showed regular growth; no recurrence was detected.
2. DISCUSSION AND LITERATURE REVIEW
The thymus is a gland situated in the anterior mediastinum, embryologically derived from the third and fourth pairs of pharyngeal pouches.1, 2, 3 Its size varies with age. From a birth mean weight of 15 g, the thymus grows in size until puberty to a mean weight of 30‐40 g,4, 5 and then, it undergoes progressive atrophy, to no more than 5‐15 g in the elderly.3 In early infancy, the thymus reaches its largest relative size, because its rate of increase is less than the rest of the body in a growing child.1, 6 After the age of 2 years, the thymic shadow is less frequently visible.6
True thymic hyperplasia is a rare but significant pathology in pediatric age because of its potentially serious consequences. It is characterized by an increase in size and weight of the thymus gland with preservation of normal thymic architecture and immunohistochemical appearance, but without an apparent cause.1, 3, 4, 5 Its etiology remains unclear.7 It must be differentiated from other anterior mediastinal masses (including thymic lymphoma, thymoma, and germ cell tumors) and from other causes of thymus enlargement (thymus lymphofollicular hyperplasia typical of myasthenia gravis3, 8, 9 or thymus enlargement after treatment for malignant tumors, stress, and steroid therapy).1, 5, 6, 7, 10 Clinical and instrumental diagnosis is difficult. Chemical shift magnetic resonance imaging is reported to be helpful in differentiating thymic lymphoid hyperplasia from thymic neoplasm, but it is not always enough.11 Therefore, separating these entities requires fine‐needle aspiration or biopsy.1, 2, 3, 4, 5, 10
Massive true thymic hyperplasia (MTH) is a variant of TTH. As there are no generally accepted criteria for defining “massive,” in literature, the following guidelines are suggested: (a) the thymus should be greater than the heart shadow on posterior‐anterior chest radiograph, (b) it should weigh several times the expected weight for the age of the patient, and (c) it should represent more than 2% of the body mass.1, 2, 5 About 50 cases of MTH have been recorded.5, 10, 12 The majority of cases occur between 1 and 15 years, rarely later.5
Clinically, MTH could present with effects of mediastinal compression, for instance, respiratory distress, dysphagia or airway obstruction, acute or recurrent pulmonary infections, or less commonly as an incidental finding.4
Being a rare condition, there are no guidelines on its management.A review of the literature revealed that most patients (80%) were managed surgically with complete excision of the mass, with no postoperative complications.4 Some authors reported that a course of steroid therapy could decrease the size of an enlarged thymus gland, because of its immunosuppressive effect on T and B lymphocytes that mature in the thymus gland particularly in infancy.4, 13, 16 But in MTH there is no evidence of efficacy; some of these masses do not respond or continue to growth after steroid therapy ends, sometimes to sizes greater than pretreatment.2, 4, 13, 14, 15, 17, 18, 19, 20, 21 Both normal thymic tissue and lymphomas may show rebound growth after the suspension of steroids.4, 13, 19, 20, 21 Furthermore, there are no clear indications regarding duration and dosage of the corticosteroid. A small number of patients were reported to be treated conservatively, mostly for incidental findings, or in children with few symptoms and with no acute complications.4, 10 In fact, the rate of atrophy of an MTH is often very slow and it could potentially lead to complications such as acute airway obstruction.4 This is true especially in infants, who are furthest from the time of the physiological involution of the thymus and when the thymus has the largest relative size.1
In our case, the decision as to the best management was not easy to take, because of the few cases described and the absence of clear guidelines on therapy. Following confirmation of TTH by biopsy, a period with steroid therapy was tried. It proved ineffective, with subsequent enlargement of the mass. Subsequently, we performed total surgical excision of the mass easily and with no complications.
With the aim of analyzing clinical presentation and management of giant thymic hyperplasia in young infants, we reviewed the cases of MTH reported in the literature between 1976 and March 2018 in children aged <2 years of life, the age when the thymus is relatively largest.1, 6
A total of 14 cases met the inclusion criteria, and they are all reported in Table 1.1, 2, 3, 4, 5, 6, 7, 12, 14, 17, 18, 22, 23
Table 1.
A total of 14 cases of MTH in children <2 y old reported in literature, including our index case
| No. | Ref. | Sex | Age | Weight (kg) | Presenting symptoms | Treatment | Thymus size | Surgical complications | Outcome (follow‐up reported) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | O Shea et al (1978)17 | M | 1 y | ‐ | Respiratory distress | 1. Steroid (regrowth after therapy suspension) 2. Surgical thymectomy | 420 g | No | Asymptomatic (5 mo) |
| 2 | Lamesch (1982)13 | F | 7 mo | 5.7 | Respiratory distress |
1. Steroid and ventilation (ineffective) 2. Surgical thimectomy |
230 g 18 × 11 × 8.5 cm |
No | Asymptomatic (7 y) |
| 3 | Parker et al (1984)6 | M | 15 mo | ‐ | Respiratory tract infection | Surgical thimectomy | 200 g | No | Asymptomatic (not listed the time of follow‐up) |
| 4 | Linegar et al (1993)4 | F | 2 mo | 3.6 | Chest infection, respiratory distress, acute airway obstruction, splenomegaly | Surgical thimectomy | 220 g | No | Asymptomatic (3 mo) |
| 5 | Lee et al (1996)1 | M | 11 mo | 8 | Fever and upper respiratory symptoms | Surgical thimectomy | 500 g | No | Asymptomatic (not listed the time of follow‐up) |
| 6 | Szarf et al (2010)23 | M | 2 y | ‐ | Respiratory infections and persistent tachyonoea | Surgical thimectomy | 830 g | No | Asymptomatic (not listed the time of follow‐up) |
| 7 | Tan et al (2010)2 | F | 9 mo | 8 | Fever and upper respiratory symptoms |
1. Steroid (2 mg/kg/d for 2 wk, unsuccessful) 2. Surgical thymectomy |
200 g 17.5 × 11 × 5 cm |
No | Asymptomatic at the discharge from hospital (fu not listed) |
| 8 | Katz et al, (1977)12 | M | 7 mo | ‐ | Hepatomegaly | surgical thimectomy |
224 g 9 × 8 × 6 cm |
No | Asymptomatic (4 y) |
| 9 | Lee et al (1979)18 | F | 22 mo | 11.8 | Asymptomatic (incidental finding) |
1. Steroid (prednisone 1.5 mg/kg/d for 5 d, unsuccessful) 2. Surgical thymectomy |
550 g 19 × 12 × 4.5 cm |
No | Asymptomatic (6 mo) |
| 10 | Weis et al (2017)13 | M | 1 mo | ‐ | Respiratory insufficiency, cardiocirculatory instability |
1. Steroid (prednisolone at high doses for 12 d, ineffective) 2. Surgical thymectomy |
200 g 8.5 × 3.8 × 7.5 cm |
No | Recurrence[Link] → 2nd resection → asymptomatic (1 y) |
| 11 | Regal et al (2007)5 | M | 5 mo | ‐ | Respiratory distress | Surgical thymectomy | 380 g | No | Asymptomatic (2 y) |
| 12 | Woywodt et al (1999)7 | M | 11 mo | ‐ | Pneumonia | Surgical thymectomy |
550 g 17 × 5 × 3 cm |
No | Asymptomatic (6 y) |
| 13 | Sayed et al22 | M | 3 mo | 3.5 | Respiratory distress, failure to thrive | Surgical thymectomy |
219.7 g 12 × 14 × 5 cm |
No | Diagnosis of BWS, hepatic hemangioma |
| 14 | Our case index | M | 15 mo | 10.5 | Respiratory infection |
1. Steroid (prednisone 2 mg/kg/d for 1 mo, ineffective) 2. Surgical thymectomy |
492 g 15 × 11.5 × 5 cm |
No | Asymptomatic (10 mo) |
The first column of the table refers to the cases of MTH included in our review, with the reference of the literature. The 2nd, 3rd, and 4th columns refer, respectively, to gender, age, and weight (if listed) of patients. The 5th, 6th, 7th, and 8th columns report symptoms at onset, management, mass size, and surgical complications. The last column refers to the outcome.
(M, male; F, female. y, years; mo, months; d, day. BWS, Beckwith‐Wiedemann syndrome)
Recurrence of TTH at short distance from the first surgery. A second surgical resection was performed, and then the child has been asymptomatic in the follow‐up at 1 y.
Ten out of fourteen (71.4%) were male and four out of fourteen (28.6%) female. The median age at onset of symptoms was 10.3 months old. Twelve out of fourteen (85.7%) patients presented with respiratory symptoms. MTH was discovered incidentally in only one patient. Six out of fourteen (42.9%) patients were initially given steroid treatment, with no benefits: in one case, the thymus regrew after therapy was suspended; in the others five cases, it was ineffective also due to significant preoperative shrinkage.
Finally, all patients, including our case, were managed surgically. In children aged <2 years of age with MTH, no conservative treatment has been reported. No peri‐ or post‐operative complications have been observed. In our case, the giant mass was well‐distinguished from the surrounding tissues and was not infiltrating; it was easily removed.
Thirteen of the fourteen (92.9%) of patients were asymptomatic during follow‐up. Only in one case, a recurrence was observed during follow‐up and it was treated successfully with a second surgical resection.
CONFLICT OF INTEREST
Neither this paper nor any part of its essential substance has been or will be published or submitted to another scientific journal or is being considered for publication elsewhere. This submission represents original work. All the authors contributed to the writing and/or revision of the manuscript, and we have all read and approved the submission of this manuscript. We declare no conflict of interest in relation to this paper. Parents give their consent for publication of textual material (case history) and radiographic images.
AUTHOR CONTRIBUTION
ET, MC, and LP: revised the literature and drafted the manuscript. DD: contributed to the enrollment of the patient, diagnosis, and management. DD, GP, and AP: revised the article and approved the manuscript in the final form.
Tadiotto E, Clemente M, Pecoraro L, Piacentini G, Degani D, Pietrobelli A. Massive thymic hyperplasia in a 15‐month‐old boy: Case report and literature review. Clin Case Rep. 2019;7:27–31. 10.1002/ccr3.1896
REFERENCES
- 1. Lee YM, Koh MT, Omar A, Majid A. Hyperplasia of thymic gland. Singapore Med J. 1996;37:288‐290. [PubMed] [Google Scholar]
- 2. Tan Z, Ying LY, Zhang ZW, Li JH, Gao Z, Qi JC. True thymic hyperplasia in an infant. J Pediatr Surg. 2010;45:1711‐1713. [DOI] [PubMed] [Google Scholar]
- 3. Weis CA, Märkl B, Schuster T, Vollerl K, Ströbel P, Marx A. “Echte Thymushyperplasie” Differenzialdiagnose der hymusvergrößerung bei äuglingen und Kindern. Pathologe. 2017;38:286‐293. [DOI] [PubMed] [Google Scholar]
- 4. Linegar AG, Odell JA, Fennell WM, et al. Massive thymic hyperplasia. Ann Thorac Surg. 1993;55(5):1197‐1201. [DOI] [PubMed] [Google Scholar]
- 5. Regal MA. Gigantic enlargement of the thymus gland. Saudi Med J. 2007;28(10):1587‐1589. [PubMed] [Google Scholar]
- 6. Parker LA, Gaisie G, Scatliff JH. Computerized tomography and ultrasonographic findings in massive thymic hyperplasia. Case discussion and review of current concepts. Clin Pediatr (Phila). 1985;24:90‐94. [DOI] [PubMed] [Google Scholar]
- 7. Woywodt A, Verhaart S, Kiss A. Massive true thymic hyperplasia. Eur J Pediatr Surg. 1999;9(5):331‐333. [DOI] [PubMed] [Google Scholar]
- 8. Bouchikh M, Zouaidia F, Benhaddou E, Mahassini N, Achir A, El Malki HO. Expression of receptor for advanced glycation end‐products (RAGE) in thymus from myasthenia patients. Rev Neurol (Paris). 2017;173(6):388‐395. [DOI] [PubMed] [Google Scholar]
- 9. Toret E, Demirag B, Köker SA, et al. Aplastic anemia as an immune‐mediated complication of thymoma: a case report. J Pediatr Hematol Oncol. 2018;40(7):e464–e466. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen R, Coleman JL, Howard SC, Metzger ML. Watchful waiting for some children with a mediastinal mass: the potential role for 1⁸F‐fluorodeoxyglucose positron emission tomography: a case report and review of the literature. BMC Pediatr. 2013;13:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phung T, Nguyen T, Tran D, Phan N, Nguyen H. Thymic hyperplasia case without suppressing on chemical shift magnetic resonance imaging. Case Rep Radiol. 2018;1‐4. 10.1155/2018/7305619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katz SM, Chatten J, Bishop HC, Rosenblum H. Report of a case of gross thymic hyperplasia in a child. Am J Clin Pathol. 1977;68:786‐790. [DOI] [PubMed] [Google Scholar]
- 13. Lamesch AJ. Massive thymic hyperplasia in infants. Z Kinderchir. 1983;38:16‐18. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi T, Hirabayashi Y, Kobayashi Y. Diagnostic value of plain chest roentgenogram and CT scan findings in four cases of massive thymic hyperplasia. Pediatr Radiol. 1986;16:452‐455. [DOI] [PubMed] [Google Scholar]
- 15. Caffey J, Silbey R. Regrowth and overgrowth of the thymus after atrophy induced by the oral administration of adrenocorticosteroids to human infants. Pediatrics. 1960;26:762‐770. [PubMed] [Google Scholar]
- 16. Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: pearls and pitfalls. Radiographics. 2010;30:413‐428. [DOI] [PubMed] [Google Scholar]
- 17. O'Shea PA, Pansatiankul B, Farnes P. Giant thymic hyperplasia in infancy: immunologic, histologic, and ultrastructural observations. Lab Invest. 1978;38:391. [Google Scholar]
- 18. Lee Y, Moallem S, Clauss RH. Massive hyperplastic thymus in a 22‐month‐old infant. Ann Thorac Surg. 1979;27(4):356‐358. [DOI] [PubMed] [Google Scholar]
- 19. Griffiths SP, Levine OR, Baker DH, et al. Evaluation of an enlarged cardiothymic image in infancy: thymolytic effect of steroid administration. Am J Cardiol. 1961;8:311‐318. [DOI] [PubMed] [Google Scholar]
- 20. Gafey J, DiLiberti C. Acute atrophy of the thymus induced by adrenocorticoids observed roentgenographically in living human infants. AJR. 1959;82(530):40. [PubMed] [Google Scholar]
- 21. Pokorny WI, Sherman JO. Mediastinal tumours In: Holder TH, Ashcroft KW, eds. Pediatric Surgery. Philadelphia: W B Saunders; 1980:241‐252. [Google Scholar]
- 22. Sayed S, Sharma V, McBride CA, Levitt D, Alphonso N. Massive thymic hyperplasia in a neonate with Beckwith‐Wiedemann syndrome. J Paediatr Child Health. 2016;52:90‐92. [DOI] [PubMed] [Google Scholar]
- 23. Szarf G, Mussi de Andrade TC, De Oliveira R, Ota LH, Lederman HM. Massive thymic hyperplasia presenting with respiratory insufficiency in a 2‐year‐old child. Thorax. 2010;65(6):555‐557. [DOI] [PubMed] [Google Scholar]


