Abstract
OBJECTIVE:
The anti-inflammatory limb of immune response is crucial for dampening inflammation. Spontaneous parturition at term and preterm labor are mediated by inflammation in the cervix, membranes and myometrium. This study focuses on the changes in the amniotic fluid concentrations of the anti-inflammatory cytokine IL-10. The objectives of this study were to determine whether there is a relationship between amniotic fluid concentrations of IL-10 and gestational age, parturition (at term and preterm) and intra-amniotic infection/inflammation (IAI).
STUDY DESIGN:
A cross-sectional study was conducted including 301 pregnant women in the following groups: 1) mid-trimester of pregnancy who delivered at term (n=112); 2) mid-trimester who delivered preterm neonates (n=30); 2) term not in labor, without intra-amniotic infection/inflammation (IAI) (n=40); 3) term in labor without IAI (n=24); 4) term in labor with IAI (n=20); 4) preterm labor (PTL) who delivered at term (n=31); 5) PTL without IAI, who delivered preterm (n=30); 6) PTL, with IAI, who delivered preterm (n=14). IL-10 concentrations in amniotic fluid were determined by a specific and sensitive immunoassay. Non-parametric statistics were used for analysis.
RESULTS:
1) IL-10 was detectable in amniotic fluid and its median concentration did not change with gestational age from mid-trimester to term; 2) Patients in labor at term, had a significantly higher median amniotic fluid IL-10 concentration than that of patients at term not in labor (p=0.04); 3) women at term in labor with IAI had a significantly higher median amniotic fluid IL-10 concentration than that of patients at term in labor without IAI (p=0.02); 4) women with PTL and IAI who delivered preterm had a significantly higher median amniotic fluid concentration of IL-10 than those without IAI who delivered preterm and than those who delivered at term (p=0.009 and p<0.001, respectively); 5) Among patients with preterm labor without IAI, those who delivered preterm had a significantly higher median amniotic fluid IL-10 concentration than those who delivered at term (p=0.03).
CONCLUSIONS:
The anti-inflammatory cytokine IL-10 is detectable in the amniotic fluid of normal pregnant women. Spontaneous parturition-at term and in preterm gestation is associated with increased amniotic fluid concentrations of IL-10. Intra-amniotic infection/inflammation (preterm and at term) is also associated with increased amniotic fluid concentrations of IL-10. We propose that IL-10 has a role in the regulation of the immune response in vivo by initiating actions which dampen inflammation.
Keywords: Pregnancy, inflammation, anti-inflammation, IL-10, cytokine, amniotic fluid, mid-trimester
INTRODUCTION
Pro-inflammatory cytokines, such as interleukin (IL)-1,[1–8] IL-6,[6–12] tumor necrosis factor (TNF),[5,6,13–18] IL-18,[19] IL-16,[20] and chemokines such as IL-8,[7,8,21–23] monocyte chemotactic protein-1 (MCP-1),[24,25] macrophage inflammatory protein-1 alfa (MIP-1α),[26] RANTES,[27] and epithelial cell-derived neutrophil-activating peptide-78,[28] are involved in the mechanisms responsible for preterm labor. Indeed, strong evidence suggests that these cytokines are produced by gestational tissues in response to microbial products,[1,29–33] are present in high concentrations in the amniotic fluid of patients with intra-amniotic infection/inflammation (IAI),[1,3,6,9–11,13,13,14,16,19–22,24,26,27,34–38] stimulate uterine contractility by inducing the production of prostaglandins[39–50] and can induce preterm labor and delivery.[2,5,51–53]
IL-10 is an anti-inflammatory cytokine which participates in a negative feedback loop to dampen inflammation.[54] Lymphocytes,[55,56] macrophages[57,58] and dendritic cells[58] are the major sources of this cytokine, however, cyto-, syncitiotrophoblast[59,60] and decidual mononuclear cells[61,62] are additional sources during pregnancy.
A role for IL-10 in pregnancy is supported by the observations that IL-10 null mice (−/−) are more susceptible to LPS induced fetal loss[63] and that the administration of recombinant IL-10 to pregnant mice, rats and rhesus monkeys challenged with lipopolysaccharide (LPS), E. Coli or IL-1β, reduces the intra-amniotic concentrations of pro-inflammatory cytokines and prostaglandins, as well as the proportion of preterm fetal losses, preterm deliveries, and the severity of fetal white matter lesions when compared to non IL-10 treated controls.[63–66] In addition, some polymorphisms in the IL-10 gene appeared to confer susceptibility to preterm birth[67–69] and to neonatal inflammation-associated adverse outcome.[70,71]
IL-10 administration has been proposed as an anti-inflammatory agent in the treatment of bacterial sepsis,[72] PTL[63,65] and in the prevention of the neonatal brain injury in the context of IAI.[73] The objectives of this study were to determine whether there is a relationship between amniotic fluid concentrations of IL-10 and gestational age, parturition (at term and preterm) and IAI.
MATERIAL AND METHODS
Study design:
This is a cross-sectional study designed to examine the relationship among amniotic fluid concentrations of IL-10 and gestational age, spontaneous labor at term, preterm parturition and intra-amniotic infection/inflammation.
Amniotic fluid was collected by trans-abdominal amniocentesis from 301 women with singleton pregnancies in the following groups: 1) Patients undergoing mid-trimester amniocentesis for clinical indications who delivered at term (n=112); 2) women who had a mid-trimester amniocentesis and subsequently delivered a preterm neonate (n=30); 3) women at term not in labor without IAI (n=40); 4) patients at term in labor without evidence of intra-amniotic infection/inflammation (n=24); 5) women at term in labor with IAI (n=20); 6) women with preterm labor and intact membranes without IAI at the time of amniocentesis, who delivered at term (n=31); 7) women in PTL without IAI, who delivered preterm neonates (n=30); 6) women in PTL with IAI, who delivered preterm neonates (n=14).
Clinical definitions:
Patients were considered to have a normal pregnancy if they did not have obstetrical, medical or surgical complications of pregnancy, and delivered a term (≥ 37 weeks) neonate with a birth weight above the 10th percentile for gestational age. PTL was defined in the presence of regular uterine contractions occurring at a frequency of at least 2 every 10 minutes combined with documented cervical change, prior 37 weeks of gestation. Intra-amniotic infection was defined by a positive amniotic fluid culture for microorganisms. Intra-amniotic inflammation was defined in the presence of white blood cell (WBC) count > 100 cells/mm3.
Amniotic fluid sample collection and analysis:
Amniotic fluid was collected by trans-abdominal amniocentesis and fluid not required for clinical purposes was centrifuged to remove cellular and particulate matter and the supernatant stored at −70° C until analysis. Amniotic fluid WBC count, Gram stain, and glucose concentrations were used in the management of patients with preterm labor. Amniotic fluid samples from the term and preterm groups were cultured for aerobic, anaerobic species as well as genital mycoplasmas (Ureaplasma urealyticum and Mycoplasma hominis). Pregnant patients were enrolled at Hutzel Hospital, Detroit, MI, USA, Pennsylvania Hospital, Philadelphia, Pennsylvania, USA, and Sotero del Rio Hospital, Puente Alto, Cile. The collection of samples for research was approved by the Institutional Review Boards of Wayne State University, Pennsylvania Hospital, and Sotero del Rio Hospital, as well as the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NIH/DHHS). All participants provided written informed consent for collection of clinical data and biological materials. Many of these samples have been previously employed to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations in non-pregnant women, normal pregnant women and those with complications.
Human interleukin-10 (IL-10) Immunoassay:
Amniotic fluid concentrations of IL-10 were determined by specific and sensitive enzyme-linked immunoassays (Endogen Inc., Pierce Biotechnology, Rockford, IL). Validation included spike and recovery experiments, which produced parallel curves indicating that amniotic fluid matrix constituents did not interfere with antigen-antibody binding in this assay system. The calculated inter- and intra-assay coefficients of variation for the IL-10 immunoassay in our laboratory were 2.8% and 4.4%, respectively. The lower limit of detection (sensitivity) was 2.6 pg/ml.
Statistical analysis:
Data were tested for normality using the Kolmogorov-Smirnov test. Kruskal-Wallis test was used for analysis of variance. Comparisons between two groups were performed using Mann-Whitney rank sum tests. A p value <0.05 was considered statistically significant. SPSS v.14.0 (SPSS Inc., Chicago, IL, USA) was used for analysis.
RESULTS
Three hundred and one patients were included in this study. The demographic and clinical characteristics of patients in different groups of this study are displayed in Table I.
Table I:
Clinical and demographic characteristics and amniotic fluid concentrations of IL-10 in the study groups
| Diagnostic group | Number of cases | GA amniocentesis (weeks) | GA delivery (weeks) | Maternal age (years) | Birthweight (grams) | AF IL-10 concentrations (pg/mL) |
|---|---|---|---|---|---|---|
| Mid-trimester amniocentesis, term delivery | 112 | 16 (16–17) |
39 (38–40) |
36 (35–38) |
3362 (3096–3632) |
11.7 (10.7–13.4) |
| Mid-trimester amniocentesis, preterm delivery | 30 | 16 (15.9–17) |
33.5 (26.8–35.3) |
36.5 (35–39) |
2166 (1281–2846) |
11.5 (10.2–12.9) |
| Term not in labor, no IAI | 40 | 39 (38–40) |
39 (38.4–40) |
24 (21–30) |
3260 (3023–3500) |
10.4 (3.5–19.5) |
| Term in labor, no IAI | 24 | 40 (39–40) |
40 (39–40) |
22 (18.3–27) |
3560 (3173–3760) |
19.3 (5.5–59.7) |
| Term in labor, IAI | 20 | 39 (38–40) |
39 (38.1–40) |
25 (21.8–29) |
3245 (3100–3600) |
58.1 (25.3–151.0) |
| PTL, no IAI, delivered at term | 31 | 31.9 (30.3–33) |
39 (38–40) |
21 (19–26) |
3060 (2807–3350) |
40.0 (32.9–50.9) |
| PTL, no IAI, delivered preterm | 30 | 28.4 (25.8–31.6) |
31 (29–33.2) |
20.5 (18–23.5) |
1618 (1200–2077) |
59.9 (30.8–160.4) |
| PTL, IAI, delivered preterm | 14 | 29.5 (25.8–32.6) |
29 (25.8–33.2) |
25 (20–29.3) |
1250 (843–2049) |
301.3 (68.6–504.8) |
Values are expressed in median and interquartile ranges.
AF, amniotic fluid; GA, gestational age; IAI, intra-amniotic infection/inflammation; IL-10, interleukin-10; PTL, preterm labor.
IL-10 is a physiologic constituent of amniotic fluid:
IL-10 was detectable in 95% (286/301) of cases. Non-detectable IL-10 was found if 15 patients. One had preterm labor without IAI, 6 patients were at term and were not in labor (all without IAI) and 8 patients were at term in labor (5 of which did not have IAI).
Amniotic fluid IL-10 concentration as a function of gestational age:
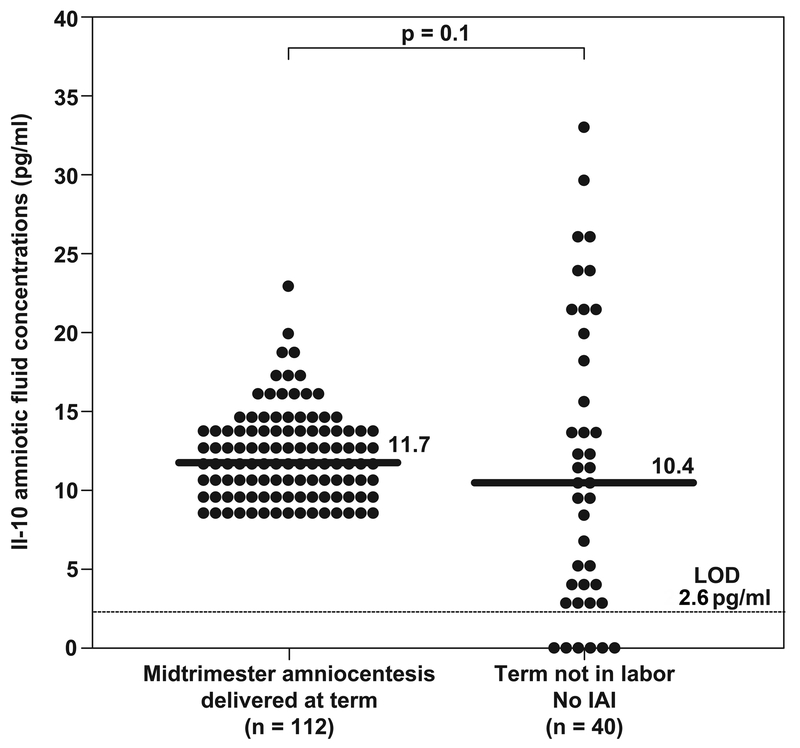
There was no significant difference in the median amniotic fluid concentration of IL-10 between patients in the mid-trimester of pregnancy who delivered at term and patients at term not in labor at the time of amniocentesis. [mid-trimester: median: 11.7 pg/ml, range (8.6–22.9) vs. term not in labor: median: 10.4 pg/ml; range (0–33.1); p=0.1]. (Table I and Figure 1).
Figure 1: Amniotic fluid concentrations of IL-10 in normal pregnancies.
No significant differences were found in amniotic fluid median IL-10 concentrations between patients in the mid-trimester of pregnancy who delivered at term and patients at term not in labor at the time of amniocentesis (mid-trimester who delivered at term: median: 11.7 pg/mL; range 8.6–22.9 versus term no labor, no IAI: median: 10.4 pg/mL; range 0–33.1; p = 0.1.
IAI, intra-amniotic infection/inflammation; LOD, lower limit of detection.
Spontaneous labor at term is associated with higher concentrations of IL-10:
The median amniotic fluid IL-10 concentration was significantly higher in women in spontaneous labor at term than in those not in labor at term. [term in labor: median 19.3 pg/ml; range (0–115.6) vs. term not in labor: median: 10.4 pg/ml; range (0–33.1); p=0.04]. (Table I and Figure 2). These results suggest that spontaneous labor at term is associated with increased availability of IL-10. It is important to stress that patients in labor did not have evidence of intra-amniotic infection (all had negative amniotic fluid cultures for aerobic and anaerobic bacteria and low white blood cell count).
Figure 2: Amniotic fluid concentrations of IL-10 in pregnant women at term.
The median amniotic fluid IL-10 concentration was significantly higher in women in spontaneous labor at term than in those not in labor at term (term in labor: median 19.3 pg/mL; range 0-115.6 versus term not in labor: median 10.4 pg/mL; range (0–33.1); p = 0.04]. Women with spontaneous labor at term and evidence of intra-amniotic infection and/or intra-amniotic inflammation had a significantly higher median amniotic fluid concentration of IL-10 than women in spontaneous labor at term without intra-amniotic infection or inflammation (term in labor, IAI: median 58.1 pg/mL, range 0–426.7 versus term in labor, no IAI: median 19.3 pg/mL; range 0–115.6; p = 0.02).
IAI, intra-amniotic infection/inflammation; LOD, lower limit of detection.
IL-10 is increased in the amniotic fluid of women with intra-amniotic infection/inflammation:
Women with spontaneous labor at term and evidence of intra-amniotic infection and/or inflammation had a significantly higher median amniotic fluid concentration of IL-10 than women in spontaneous labor at term without intra-amniotic infection or inflammation [term in labor, IAI: median 58.1 pg/ml (0–426.7) vs. term in labor, no IAI: median 19.3 pg/ml; range (0–115.6); p=0.02]. (Table I and Figure 2).
Women with PTL and intra-amniotic infection/inflammation had a significantly higher median amniotic fluid concentration of IL-10 than patients with preterm labor and intact membranes without IAI who delivered a preterm neonate. [PTL with IAI: median: 301.27 pg/ml; range (11.3–672.3) vs. PTL without IAI resulting in a preterm delivery: median: 59.9 pg/ml, range (0–580.9); p=0.009]. (Table I and Figure 3).
Figure 3: Amniotic fluid IL-10 concentrations in patients with preterm labor.
The median amniotic fluid IL-10 concentration was significantly higher in women with spontaneous preterm labor, without IAI, and who subsequently delivered a preterm neonate than in those who delivered at term (PTL without IAI resulting in a preterm delivery: median 59.9 pg/mL, range 0–580.9 versus PTL resulting in term delivery: median 40.0 pg/mL, range 13.2–139.8; p < 0.03]. Women with PTL and IAI had a significantly higher median amniotic fluid concentration of IL-10 than patients with preterm labor and intact membranes without IAI who delivered a preterm neonate (PTL with IAI: median 301.3 pg/mL; range 11.3–672.3 versus PTL without IAI resulting in a preterm delivery: median 59.9 pg/mL, range 0–580.9; p = 0.009).
IAI, intra-amniotic infection/inflammation; LOD, lower limit of detection; PTL, preterm labor.
IL-10 is elevated in women with preterm labor in the absence of intra-amniotic infection/inflammation:
The median amniotic fluid IL-10 concentration was significantly higher in women with spontaneous preterm labor, without intra-amniotic infection/inflammation and who subsequently delivered a preterm neonate than in those who delivered at term [(PTL without IAI resulting in a preterm delivery: median: 59.9 pg/ml, range (0–580.9) vs. PTL resulting in term delivery: median 40.0 pg/ml, range (13.2–139.8); p=0.03]. (Table I and Figure 3).
Amniotic fluid IL-10 in the mid-trimester of pregnancy did not differ between women who had a preterm delivery and those who had a term delivery:
There was not a significant difference between the median amniotic fluid concentration of IL-10 in the mid-trimester of women who subsequently delivered preterm and those who delivered at term [mid-trimester amniocentesis who delivered a preterm neonate: median: 11.5 pg/ml, range (8.8–18.8) vs. mid-trimester amniocentesis who delivered at term: median: 11.7 pg/ml, range (8.6–22.9); p=0.3]. (Table I and Figure 4).
Figure 4: Amniotic fluid IL-10 concentrations in women in the mid-trimester of pregnancy.
There was no significant difference between the median amniotic fluid concentration of IL-10 in the mid-trimester of women who subsequently delivered preterm and those who delivered at term (mid-trimester amniocentesis who delivered a preterm neonate: median 11.5 pg/mLl, range 8.8–18.8 versus mid-trimester amniocentesis who delivered at term: median 11.7 pg/mL, range 8.6–22.9; p = 0.3].
LOD, lower limit of detection.
DISCUSSION
Principal findings of this study:
1) IL-10 is detectable in amniotic fluid and its median concentration does not change with gestational age from mid-trimester to term; 2) Spontaneous labor at term is associated with increased amniotic fluid concentrations of IL-10; 3) Similarly, spontaneous PTL in the absence of IAI, associated with an increased amniotic fluid concentration of IL-10; 4) IAI (at term or in PTL) is also associated with a significantly higher amniotic fluid IL-10 concentration than spontaneous preterm or term labor without IAI. Collectively, our findings suggest that IL-10 participates in the mechanisms of labor (term and preterm) as well as in the host response to intrauterine infection.
What is IL-10?:
Human IL-10 is a 18 kDa cytokine[74,75] encoded by a gene on chromosome 1,[76] originally described in 1989 as a factor produced by mouse Th2 clones (in response to Concavalin A or antigen stimulation) which is capable of inhibiting the production of pro-inflammatory cytokines by activated Th1 cells.[77] For this reason it was termed “Cytokine synthesis inhibitor” or “Cytokine Inhibitory Factor”.[77]
IL-10 was subsequently isolated, expressed and cloned.[78,79] The mouse and human IL-10 genes are 90% homologous, and both proteins are non-covalent homodimers. Human IL-10 is minimally or not glycosilated, which is contrast to its murine counterpart.[77,80]
The IL-10 Cytokines Family:
It is now recognized that IL-10 belongs to a family of cytokines which includes IL-19, IL-20, IL-22, IL-24, IL-26 and a series of herpesviral and poxviral proteins.[81] The IL-10 related cytokines share limited homology with IL-10 (25% identity and 50% similarity on average),[81] share several receptor subunits and are expected to display similar functions,[82] however further research is required to address their biological activities.[82]
Sources of IL-10:
Activated Th2 cells are the major source of IL-10 production[74,77] and IL-10 has been classically considered a Th2 cytokine marker.[83] This was based upon both the observation that IL-10 was a product of Th2 mouse clones,[77] and the classic Th-1 and Th-2 paradigm[84] with a mutually exclusive activation and cytokine production.[85,86] According to this line of thought, Th1 cells would produce IFN-γ, IL-2 and other cytokines involved in the control of cell-mediated immunity (i.e macrophage activation and delayed-type hypersensitivity responses) and Th2 cells, producing IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13 involved in humoral-antibody mediated responses and in the induction of B cells growth and differentiation into plasma cells.[84,87] Recent evidence, however, suggests that IL-10 is produced by both Th1 and Th2 populations,[55,88,89] stimulated B cells,[56,90–93] LPS activated monocytes,[57] macrophages,[58] dendritic cells,[58,94–96] as well as regulatory T cells (Treg),[97–102] which are involved in controlling both the innate and the adaptive immune responses,[103–105] and in the prevention of immunopathology (self-antigens reactivity and/or exaggerated immune responses to pathogens).[99,103,104,106,107] Of note, Treg cells producing IL-10 can be developed under different regimens of antigen stimulation, both in vitro[97,108] and in vivo,[98,109] particularly upon prolonged stimulation, which induces the appearance of “anergic” T-cells, which fail to proliferate and express significantly higher levels of IL-10.[109] There is evidence suggesting that IL-10 produced by Treg cells is involved in controlling some immune responses in vivo,[98,110] including allergic and autoimmune pathologies,[111–113] and in dampening inflammation in different organs such as the brain,[111,113] airways[112] and gut.[110,114]
Homology between IL-10 with viral prodcuts:
It is of interest that some viruses such as the Epstein-Barr virus (EBV),[78,115] and the equine herpes virus type 2,[116] contain in their genome nucleotide sequences which are highly homologous to the human and mouse IL-10 genes.[115,116] In particular, the recombinant protein of the gene “Bam HI C fragment rightward reading frame” (BRCF1), the open reading frame of the EBV genome, is similar in size to the human IL-10, and mimics its activity on both macrophages and B cells.[115] These proteins (in addition to the human IL-10 expression induced by EBV-transformed B cells),[56,92] could represent a mechanism through which viruses interact with the host’s immune system to suppress the local antiviral immune response,[56,92] and favor viral survival by inhibiting the synthesis of macrophages and T cells cytokines with anti-viral properties.[75]
IL-10 production by gestational tissues:
During pregnancy, sources of IL-10 include cytotrophoblasts,[59,60] syncitiotrophoblasts,[59] chorion,[117,118] as well as decidual mononuclear cells/macrophages,[61,62,119,120] and decidual Natural Killer cells.[120,121] IL-10 secretion from decidual mononuclear cells/macrophages has been reported to begin in early pregnancy,[61,120] and has been detected at term gestation.[62] The higher IL-10 secretion by decidual cells, when compared to the non pregnant endometrium, may contribute to the suppression of the local cell-mediated immunity, to enhance embryonic implantation and favor pregnancy maintenance.[122] Hanna et al.[59] examined the expression profile of IL-10, of its receptor, as well as the expression profile of other cytokines (IL-2, IL-4 and IFNγ) in placental explants and cytotrophoblasts. They reported that IL-10 is the predominant cytokine expressed in first and second trimester placental explants in normal pregnancies, and that its expression is gestational age-dependent, followed by a transcriptionally attenuation as term gestation approaches.[59]
IL-10: a cytokine that dampens inflammation:
The resolution of inflammatory response is crucial for survival. Although it was believed for many years that the inflammatory response would resolve passively, it is now clear that this is an active process requiring: 1) removal of the inflammatory stimulus; 2) decrease in the bioavailability of pro-inflammatory mediators and a switch to an anti-inflammatory cytokine milieu and 3) removal of the inflammatory cells and debris allowing for tissue repair.
IL-10 is a pleiotropic anti-inflammatory cytokine involved in limiting and terminating inflammatory responses,[54] recently defined as a “governor” of inflammation.[54,83] Its production by various T cell subsets after prolonged activation is important to prevent excessive inflammation.[123] Cytokines with pro-inflammatory properties such as IL-12, IL-27 and IL-6, in cooperation with TGFβ, induce IL-10 production in various T helper cell subsets.[124–128] Of note, stimulation of macrophages and dendritic cells with LPS and toll-like receptor (TLR)-2 agonists, activates signaling pathways that converge on the IL-10 gene and induce its expression.[83] The production of IL-10 by stimulated human monocytes is relatively late, as compared to the production of IL-1α, IL-1β, IL-6, IL-8, tumor necrosis factor alpha (TNFα), and granulocyte colony-stimulating factor (G-CSF).[57]
A growing body of evidence supports a role for IL-10 in the down-regulation of the immune response to protect the host from an exaggerated, even if effective, inflammatory response. Such evidence includes the following: 1) IL-10 knock out mice infected with Trypanosoma cruzi and Toxoplasma gondii die rapidly because of a massive and sustained inflammatory response,[129,130] and are predisposed to have an exaggerated Th1 immune response in the context of Plasmodium chabaudi chabaudi malaria,[131] and Listeria Monocytogenes meningoencephalitis;[132] 2) In contrast, elevated concentrations of IL-10, or an imbalance between IL-10 and IL-12 in favor of IL-10, have been associated with persistent infection-induced inflammation, resulting in the development of invasive and chronic disease, such as human visceral leishmaniasis,[133] chronic human Candida albicans infections,[134] as well as Paracoccidioidomycosis;[135–140] 3) Upon LPS challenge, IL-10 deficient mice display an uncontrolled production of TNFα,[141] are extremely vulnerable to the generalized Shwartzman reaction,[141] and are more sensitive to lethal acute endotoxin shock, (which occurs with a 20-fold lower dose of LPS).[141] In contrast, mice receiving IL-10 (recombinant IL-10 injection)[72] and transgenic mice over-expressing IL-10 in their macrophages,[142] are protected from a lethal intraperitoneal injection of endotoxin,[72] and display a decreased production of pro-inflammatory cytokines[72,142] (TNFα[72,142] and IL-12[142]) when compared to controls.
The IL-10 cell of origin appears to have biological importance. IL-10 produced by T-cells is involved in the regulation of T cell responses, whereas IL-10 production from other cell types (i.e macrophages, dendritic cells) is required to dampen the acute inflammatory response (normal innate responses to LPS or skin irritation).[143]
Of interest, IL-10 may participate in mediating immune-regulatory functions of galectin-1, a beta galactoside binding protein that appears to play a pivotal role in feto-maternal tolerance.[144,145] Blois et al.[144] have recently reported that galectin-1 promotes the generation of tolerogenic uterine dendritic cells,[144] and skews the balance toward a Th2 dominant cytokine profile at the fetomaternal interface.[144] The immunomodulatory effects elicited by galectin-1 are partly mediated by induction of the expression of IL-10. Evidence in support of this includes: 1) recombinant galectin-1 induces the production of IL-10 (mRNA and protein) by CD4+ and CD8+ cells;[146] 2) incubation of peripheral blood mononuclear cells with stable galectin-1 homodimers (dGal) induces high production of IL-10, particularly from monocytes and T cells, but not by B cells;[147] 3) administration of recombinant galectin-1 to null mutant mice prevents fetal loss and restores maternal fetal tolerance through multiple mechanisms including the in vivo expansion of IL-10 secreting regulatory T cells;[144] and 4) in IL-10 null mice, administration of galectin-1 does not significantly lower the number of stress-triggered fetal losses.[144]
Galectin-1 expression has been previously demonstrated in the endometrium, decidua and human placentas (villous stroma, endothelial cells, as well as syncytio and cyto-trophoblast).[148–152] Our group has recently demonstrated, by in situ hybridization and immunostaining, that galectin-1 mRNA and protein are abundantly expressed in all the cell types of fetal membranes (amnion epithelial cells, chorionic trophoblasts, mesenchimal cells) and decidual stromal cells in third trimester.[153] Recently we reported a significantly higher galectin-1 mRNA expression (2 fold, p=0.002) in the chorioamniotic membranes of patients with preterm prelabor rupture of the membranes (PPROM) and histological chorioamnionitis, compared to those without it.[153] Thus, we propose that galectin-1 and IL-10 have a role in the resolution of the inflammatory response in chorioamnionitis.
Biological activities of IL-10:
The anti-inflammatory properties of IL-10 are related to its down-regulation of T cell functions, which occurs mainly through an indirect mechanism involving the antigen presenting cells,[154] as well as through a potent suppression of the effector functions of macrophages and NK cells, as well as T cells.[80]
Some of the cellular targets and biological activities of IL-10 include the following: 1) Inactivation of monocytes/macrophages: IL-10 inhibits the production of pro-inflammatory cytokines by LPS activated macrophages (IL-1α and IL-1β, granulocute macrophage-CSF (GM-CSF), G-CSF, TNFα, IL-6, IL-8, IL-10 and IL-12),[57,155] and reduces their intracellular microorganisms killing potential with several mechanisms including the inhibition of TNF production,[54,129,156] and preventing the release of reactive oxygen intermediates.[77,156] IL-10 down-regulates the expression of major histocompatibility complex (MHC) class II antigens on LPS-activated monocytes,[57] inhibits the macrophage co-stimulatory activity by selective inhibiting the up-regulation of B7 expression,[157] and decreases the expression of cell-adhesion molecules such as intercellular adhesion molecule (ICAM)-1 and B7 on monocytes.[158] 2) Effects on maturation and functions of dendritic cells: IL-10 inhibits the maturation of dendritic cells from monocytes precursors.[159,160] In addition, dendritic cells cultured with IL-10 are less efficient as antigen presenting cells to CD4+ T cell clones,[161] have an increased capacity to capture antigens[161] but a concomitant decreased stimulatory activity.[161] Among the proposed mechanisms, is the observation that IL-12 production by dendritic cells upon interaction with CD4+ T cells is down-regulated by IL-10.[162] 3) Suppression of Th1 cell responses: IL-10 inhibits the synthesis of the pro-inflammatory cytokine IL-12 by macrophages and dendritic cells.[54,162,163] Of note, IL-12 plays a central role in the ontogeny of a Th1 type immune responses, directing IFNγ production (a Th1 associated cytokine) rather than IL-4 production (Th2 associated cytokine) from T cells.[75] In addition, IL-10 is an inhibitor of the Th1 cells cytokine production (IL-2, IL-3, TNF, IFNγ and granulocyte-macrophage CSF).[77] It has been reported that the inhibition of Th1 cytokine synthesis by IL-10 is not complete, since it does not affect the early cytokine synthesis, whereas later synthesis is strongly inhibited.[77] 3) Suppression of the effector function of Natural Killer (NK)- cells: IL-10 inhibits the production of IFN-γ by NK cells in response to IL-2 and in the presence of accessory cells.[164] 4) Regulation of the growth and differentiation of mast-cells: IL-10, in synergy with other cytokines such as IL-3 and/or IL-4, enhances growth and proliferation of mast cells,[165] and activates transcription of genes for two mast-cells proteases, mucosal mast cell protease (MMCP)-1 and MMCP-2;[166,167] 5) Generation of T regulatory cells 1 (Tr1) from CD4+ T cells chronically activated. These regulatory cells are characterized by a low proliferative capacity, high production of IL-10, low levels of IL-2 and no IL-4,[97] and may, in turn, play a role in controlling immune responses and tolerance in vivo.[54] 6) Protection of the functional integrity of inflamed tissues: IL-10 protects the epithelial monolayer integrity otherwise disrupted by IFNγ treatment. This is accomplished by maintaining the size selectivity of the epithelial tight junction.[168]
Amniotic fluid IL-10 in normal pregnancy:
Previous studies of amniotic fluid IL-10 found no changes in the IL-10 concentration with advancing gestational age.[169,170] In contrast, Greig et al.,[171] reported a significantly higher median IL-10 amniotic fluid concentration in women at term than those in the second trimester (p<0.001).[171] We found no significant difference between the median IL-10 amniotic fluid concentration in women in the mid-trimester and that of women at term not in labor.
Of note, no gestational age related changes in IL-10 concentrations have been described not in maternal plasma (longitudinal study with sampling at 12, 20 and 35 weeks of gestation)[172] or in maternal serum (first and second trimester uncomplicated pregnancies),[173] despite a significantly higher concentration in pregnant than in non-pregnant controls.[172]
The sources of amniotic fluid IL-10 are still unclear, and may include the mother, the fetus (i.e:. keratynocytes, upon stimulation, can produce IL-10[174,175]), as well as a local production by gestational tissues (cytotrophoblasts,[59,60] syncitiotrophoblasts,[59] chorion,[117,118] decidual stromal cells,[176] mononuclear cells/macrophages,[61,62,119,120] and NK cells[120,121]).
Amniotic fluid IL-10 in the mid-trimester:
Among patients in the mid-trimester, there were no significant differences in the median amniotic fluid concentration of IL-10 between those who subsequently delivered at term and those who delivered preterm. Apuzzio et al.,[177] in contrast to our findings, had previously reported higher concentrations of both IL-10 and IL-6 in second trimester amniotic fluid from patients who subsequently delivered preterm, as well as an inverse correlation between amniotic fluid IL-10 concentration at the time of mid-trimester genetic amniocentesis and gestational age at delivery.[177]
IL-10 in spontaneous labor at term:
Women in spontaneous labor at term have a significantly higher median amniotic fluid concentration of IL-10 than women at term not in labor. This finding is consistent with the view that labor at term is an event characterized by activation of the inflammatory cascade,[178–180] as suggested by the evidence of inflammatory cells and a higher production of pro-inflammatory cytokines in the cervix, myometrium, chorioamniotic membranes and amniotic cavity[179,181] as well as by an up-regulation of inflammatory associated genes.[182]
It is unlikely that chorion and decidua contribute to the additional pool of IL-10 which is detectable in amniotic fluid during labor at term. Indeed, previous studies focusing on gestational tissues reported either no significant differences,[59,62,183] or a significant reduction in IL-10 expression in the presence of labor when compared to controls.[184–186] Of note, Hanna et al.,[59] reported a labor-associated up-regulation of TNFα and IL-1β expression in placental explants and freshly isolated cytotrophoblasts in contrast to an absence of appreciable changes in IL-10 expression.[59] In contrast to our findings, two previous studies have reported no significant differences in amniotic fluid IL-10 concentrations between women at term not in labor and those in labor.[170,171] Perhaps the larger sample size in our study is an explanation for the differences.
IL-10 in preterm labor:
Among women with preterm labor who delivered preterm, those with evidence of IAI had a significantly higher median amniotic fluid concentration of IL-10 than those without IAI. However, PTL leading to preterm delivery, even in the absence of infection and inflammation, was associated with higher bioavailability of IL-10 in amniotic fluid. This suggests that an increase in amniotic fluid IL-10 is part of the common pathway of parturition because such a finding was also observed in spontaneous labor at term. Our interpretation is consistent with the view that labor is an inflammatory process.[182,187–189]
The sources and the mechanisms responsible for the higher IL-10 amniotic fluid pool in the presence of infection/inflammation associated preterm birth are still unclear. There is evidence that IL-10 production is induced in gestational tissues in the presence of inflammation and that IL-10 may participate in dampening inflammation. Such evidence includes the following: 1) LPS injection into the cervix of timed-pregnant rats is followed by an increase in Th1 cytokines, such as TNFα but also by a mild, although significant increase in placental IL-10 concentrations;[190] 2) gestational tissues, stimulated with IL-1β, express higher levels of IL-10, both mRNA and protein,[191] with the strongest response in decidua [191] and 4) IL-10 is involved in suppressing TNFα production by human term choriodecidua stimulated by LPS.[192]
Of note, there are conflicting reports on the effects of IL-10 on fetal membranes (amniochorion). Indeed, there is evidence supporting both that IL-10 exerts an anti-inflammatory activity on this target (suppressing the production of LPS-induced TNFα,[193] and that of matrix metalloproteinases,[194,195] as well as the expression of IL-6 and IL-8[196,197] in the amniochorion), as well as evidence that IL-10 induces pro-inflammatory actions (including stimulation of IL-8 production[198]). Thus, the high IL-10 amniotic fluid concentrations in the context of preterm labor, particularly in the presence of IAI, may reflect part of the immune response to a local inflammatory process,
IL-10 may also have a role in regulating prostaglandins (PGs) biosynthesis within gestational tissues, with most of the evidence in support of a down-regulation of PGE2 production including: 1) IL-10 suppresses the LPS stimulated production of PGE2 by human choriodecidua;[192] 2) IL-10 diminishes the basal, as well as the LPS induced, PGE2 production and expression of COX-2 mRNA by human fetal membranes;[199] 4) IL-10 treatment of preterm placental explants significantly inhibits both the intrinsic and the LPS-induced expression of COX-2 and PGE2 release;[184] 5) IL-10 reverses the PGE2 output induced by IL-1β on villous and chorion trophoblasts.[200] In contrast, in another study it has been reported that treatment with IL-10 significantly increased the amnion PGE2 production,[198]
In a previous study, no significant differences in IL-10 amniotic fluid concentrations were reported between women with preterm labor and chorioamnionitis, women with preterm contractions (undelivered within 7 days) and those with preterm labor (delivered within 7 days).[170] Of note, the definition of infection according to clinical rather than the histological criteria, may have contributed to these findings. Some patients with absence of clinical signs of inflammation, in the presence of a subclinical and undetected histological inflammation, may have been classified as “not infection/not inflammation”.
Our findings of higher amniotic fluid concentrations of IL-10 in the presence of infection/inflammation associated preterm birth are consistent with the observations of Greig et al.[171] who reported that patients with intra-amniotic infection (defined in the presence of a positive amniotic fluid culture or, alternatively, chorioamnionitis), have significantly higher amniotic fluid IL-10 concentrations than those of patients in preterm labor without infection (p<0.001). In addition, the Authors[171] reported that IL-10 amniotic fluid concentrations, in the infection-associated preterm labor, were significantly (p=0.014) higher if this event occurred prior the 30th week of gestation than afterward. This finding has been interpreted as an attempt from the mother, or fetus, to prevent preterm birth when the fetal survival rate is low. Greig et al.,[171] however, did not include in his study patients with isolated intra-uterine inflammation in the absence of infection which are, in contrast, included in our study population.
Of note, IL-10 amniotic fluid concentrations in women experiencing an episode of preterm labor have been investigated as a potential tool to predict the interval to delivery. However, no significant differences in IL-10 amniotic fluid concentration (ELISA) have been reported between women with preterm contractions who delivered within 7 days of presentation and those women who delivered after this timeframe.[170]
IL-10 polymorphisms and the risk of preterm birth and neonatal complications:
PTL and PPROM are syndromes which result from genetic and environmental interactions. In particular, it has been hypothesized that the presence of polymorphisms in immunoregulatory genes may influence the susceptibility to PTL and delivery,[67] as well to fetal and neonatal complications.[71] This could be the case for the IL-10 gene, indeed, upon LPS stimulation, there are striking inter-individual differences in IL-10 production, and part of this variability is genetically determined.[201,202] Of interest, the 5’- flanking region of the IL-10 gene contains several polymorphisms including microsatellite polymorphisms upstream the transcription start site, and three single nucleotide polymorphisms (SNPs) at position −1082 (G/A), −819 (C/T) and −592 (C/A). Only three haplotypes are found in Caucasian population (GCC, ACC, and ATA).[201,203–205]
Two haplotypes, in particular, have been object of intense investigation in the context of preterm birth, the IL-10 1082A/−819T/−592A (IL-10 ATA)-haplotype,[67] associated with low IL-10 production[201,205] and the IL-10 −1082G/−819C/−592C (IL-10 GCC)-haplotype which has been associated with a higher protein production.[201,205]
Annels et al.,[67] in a study including 387 white pregnant women, examined the relationship between preterm birth and 22 SNPs in genes encoding cytokines, apoptosis mediators and involved in the host defense. The IL-10 ATA-haplotype was independently associated with early preterm birth (< 29 weeks of gestation) (multivariable odds ratio, 2.1;P=0.4), as was homozygosity for the IL-10 −590C allele (OR, 3.4;P=0.02). In addition, homozygosity for the IL-10 GCC-haplotype was more common in women with PPROM.[67] After Bonferroni correction for multiple comparisons, none of the genetic associations in the study were significant,[67] but this has been possibly attributed to an over-correction.
Another polymorphism that has been object of investigation particularly in the context of infant risk of inflammatory-related perinatal complications is the IL-10 (−1082 G/A) polymorphism, in which a single nucleotide substitution of adenine for guanine at position −1082, in the promoter region, is associated with a higher production of IL-10.[205] The presence of the IL-10 (−1082 G/A) polymorphism has been proposed to contribute to a reduced inflammatory response in fetuses and infants, thus decreasing the risk for adverse neonatal inflammation-associated outcomes.[71] Premature infants (gestational age at birth <32 weeks) homozygous for the high IL-10 producer (−1082 G/A) allele: 1) were significantly less likely to develop ultrasound-defined periventricular echodensities than their heterozygous and non carrier peers; 2) were less likely to suffer from prematurity associated disorders such as bronchopulmonary dysplasia, high grade retinopathy, cerebral palsy and developmental delay at age 2+ years. It has been proposed that such polymorphism might contribute to a reduced inflammatory response in the fetus and infant, and subsequently decreased the risk for adverse neonatal inflammation-associated outcomes.[71] In another study including 294 patients, however, no major influences of the presence of this polymorphism were reported on prematurity associated diseases.[70]
However, the role of IL-10 polymorphisms in determining the predisposition to preterm birth is however still the subject of debate. Indeed, in a study on the role of SNPs of inflammation-associated genes (IL-10, IL-1, TNFα and TLR-4), no overall differences in genotype and allele frequencies were detected between mothers who delivered preterm and at term.[68] Only in women with clinical chorioamnionitis and carriers of the IL-10(−1082)*G allele, was the risk for delivery before 29 weeks markedly increased (OR 22, 95% confidence interval (CI) 2.5–191).[68]
In another study, no differences in genotype frequencies of IL-10 polymorphisms were detected between women with a history of PTL <37 weeks and women with successful pregnancies.[69] In addition, when multifetal gestations were evaluated, no relationship between the maternal IL-10 genotype and spontaneous preterm birth or PPROM were observed.[206]
A key role for IL-10 in preterm labor:
Endogenous IL-10 synthesis in gestational tissues may be of physiological importance in infection-induced PTL because of its anti-inflammatory role in deactivating macrophages and inhibiting their LPS-induced synthesis of inflammatory cytokines.[63] Evidence in support of a key role for IL-10 includes: 1) LPS administration to IL-10 null mutant mice resulted in an elevation of serum pro-inflammatory cytokines (including TNF-α and IL-6) and lower concentrations of the soluble TNF Receptor II compared to controls;[63] 2) IL-10 null mutant mice are more sensitive to LPS induced PTL,[207] and a 10-fold lower dose of LPS is required to elicit 50% preterm fetal loss when compared to the wild-type mice;[63] 3) surviving fetuses in IL-10 null mice are more likely to display fetal growth restriction;[63] 4) recombinant IL-10 administration, prior to LPS challenge, reduces serum concentrations of pro-inflammatory cytokines and the proportion of fetal loss and preterm delivered fetuses;[63] 5) IL-10 administration rescues pregnancy in LPS treated IL-10 (−/−) mice;[207] 6) co-administration of IL-10 to pregnant rats, either at the time of LPS challenge or 24 hours later, resulted in term deliveries with no differences in litter size and birth weight compared with the controls.[64]
The mechanisms through which IL-10 may delay the onset of preterm labor are still object of debate and may include a reduction of the local production of pro-inflammatory mediators and prostaglandin synthesis. This is supported by the following evidence: 1) IL-10 administration to pregnant rhesus monkeys receiving an intra-amniotic infusion of IL-1β, reduces the IL1-β induced uterine contractility, and is accompanied by a significant reduction in the amniotic fluid leukocyte count, prostaglandins, as well as TNF-α concentrations;[65] 2) IL-10 inhibits the IL-1β and TNFα induced production of PGE-2 by term human placental cells;[208] 3) IL-10 reverses the effects of IL-1β on prostaglandin synthesis and metabolism in purified cultures of villous trophoblast and chorion trophoblast cells from human term placentas.[200]
IL-10 administration: possible implications in the prevention of preterm birth associated complications:
Clinical trials in which IL-10 was administrated systemically to healthy volunteers have demonstrated that, at doses associated with biological activities, this cytokine has minimal side effects,[209–213] including a mild flu-like syndrome,[210] transient mild to moderate neutrophilia, monocytosis, lymphopenia (dramatic reductions of 40–70% in circulating lymphocytes expressing the T cell markers CD2, CD3, and CD7), and a delayed decrease of platelet count.[209,210] Thus, IL-10 has been proposed as a candidate for treatment of bacterial sepsis, and more generally as an effective anti-inflammatory agent[72] and clinical trials have shown that its systemic administration is followed by modest but significant improvement of psoriasis,[214,215] Crohn’s disease,[216] rheumatoid arthritis,[217] and chronic hepatitis C infection.[218]
Infection during pregnancy and the perinatal period has been linked to cerebral white matter damage and cerebral palsy. The reader is referred to the review from Wolfberg et al on the anti-inflammatory and immunomodulatory strategies to protect the perinatal brain.[73] A growing body of evidence suggests that IL-10 administration may modulate the inflammatory response leading to neonatal brain injury in the context of intra-uterine infection/inflammation. Indeed, brains of pups born to infected dams (intrauterine inoculation of E.Coli) treated with IL-10 showed no evidence of severe white matter injury (frank cavitation, massive white matter necrosis) and a reduced rate of mild (evidence of histiocytes and rare apoptotic nuclei) and moderate (small vacuole formation in addition to histiocytes and rare apoptotic nuclei) lesion formation compared to controls not IL-10 treated.[66]
When administered to pregnant rhesus monkeys, this cytokine gains access to the amniotic cavity where it harbors with an half-life of 13.2 hours,[65] which is higher to the half life of 2–4 hours in the circulation of volunteers following subcutaneous administration.[210] The longer half life of IL-10 within the amniotic cavity indicates that the amniotic cavity may provide a depot-like effect prolonging the effectiveness of the treatment.[65] Treatment with IL-10 did not cause a change in the baseline fetal heart rate or in periodic fetal heart rate patterns and variability, as well as no change in maternal and/or fetal arterial pH and PO2.[65]
Suppression of macrophage/microglial activation has been proposed as a potential mechanism involved in the protective effect of IL-10 against maternal E.Coli-induced neonatal white matter damage.[219] Alternatively, Sadowsky et al.[65] proposed that the intravenous administration of IL-10, despite its rapid decay, may be important in targeting decidual macrophages.
In conclusion, IL-10 concentration in amniotic fluid is elevated in spontaneous term and preterm labor (with and without infection/inflammation) and intra-amniotic infection/inflammation. This cytokine plays an important role in the control of labor and fetal and maternal inflammation.
Acknowledgment:
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am.J.Obstet.Gynecol 1989; 160:1117–1123. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am.J.Obstet.Gynecol 1992; 167:1041–1045. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am.J.Reprod.Immunol 1992; 27:117–123. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Sepulveda W, Mazor M, Brandt F, Cotton DB, Dinarello CA, Mitchell MD. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am.J.Obstet.Gynecol 1992; 167:863–872. [DOI] [PubMed] [Google Scholar]
- 5.Bry K, Hallman M. Transforming growth factor-beta 2 prevents preterm delivery induced by interleukin-1 alpha and tumor necrosis factor-alpha in the rabbit. Am.J.Obstet.Gynecol 1993; 168:1318–1322. [DOI] [PubMed] [Google Scholar]
- 6.Fidel PL Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am.J.Obstet.Gynecol 1994; 170:1467–1475. [DOI] [PubMed] [Google Scholar]
- 7.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am.J.Obstet.Gynecol 1999; 181:1530–1536. [DOI] [PubMed] [Google Scholar]
- 8.Winkler M, Kemp B, Fischer DC, Maul H, Hlubek M, Rath W. Tissue concentrations of cytokines in the lower uterine segment during preterm parturition. J.Perinat.Med 2001; 29:519–527. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J.Clin.Invest 1990; 85:1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found.Symp 1992; 167:205–220. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am.J.Reprod.Immunol. 1993; 30:167–183. [DOI] [PubMed] [Google Scholar]
- 12.El-Bastawissi AY, Williams MA, Riley DE, Hitti J, Krieger JN. Amniotic fluid interleukin-6 and preterm delivery: a review. Obstet.Gynecol 2000; 95:1056–1064. [PubMed] [Google Scholar]
- 13.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am.J.Obstet.Gynecol 1989; 161:336–341. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am.J.Obstet.Gynecol 1992; 166:1576–1587. [DOI] [PubMed] [Google Scholar]
- 15.Baumann P, Romero R, Berry S, Gomez R, McFarlin B, Araneda H, Cotton DB, Fidel P. Evidence of participation of the soluble tumor necrosis factor receptor I in the host response to intrauterine infection in preterm labor. Am.J.Reprod.Immunol 1993; 30:184–193. [DOI] [PubMed] [Google Scholar]
- 16.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, Romero R. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am.J.Obstet.Gynecol 1999; 181:1142–1148. [DOI] [PubMed] [Google Scholar]
- 17.Fortunato SJ, Menon R, Lombardi SJ. Role of tumor necrosis factor-alpha in the premature rupture of membranes and preterm labor pathways. Am.J.Obstet.Gynecol 2002; 187:1159–1162. [DOI] [PubMed] [Google Scholar]
- 18.Lonergan M, Aponso D, Marvin KW, Helliwell RJ, Sato TA, Mitchell MD, Chaiwaropongsa T, Romero R, Keelan JA. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), TRAIL receptors, and the soluble receptor osteoprotegerin in human gestational membranes and amniotic fluid during pregnancy and labor at term and preterm. J.Clin.Endocrinol.Metab 2003; 88:3835–3844. [DOI] [PubMed] [Google Scholar]
- 19.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am.J.Obstet.Gynecol 2000; 183:1138–1143. [DOI] [PubMed] [Google Scholar]
- 20.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH, Edwin SS. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am.J.Obstet.Gynecol 2000; 182:135–141. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am.J.Obstet.Gynecol 1991; 165:813–820. [DOI] [PubMed] [Google Scholar]
- 22.Cherouny PH, Pankuch GA, Romero R, Botti JJ, Kuhn DC, Demers LM, Appelbaum PC. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am.J.Obstet.Gynecol 1993; 169:1299–1303. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez BE, Ferrer I, Valls C, Borras M, Lailla JM. The value of interleukin-8, interleukin-6 and interleukin-1beta in vaginal wash as predictors of preterm delivery. Gynecol.Obstet.Invest 2005; 59:175–178. [DOI] [PubMed] [Google Scholar]
- 24.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, Adashi EY. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J.Matern.Fetal Neonatal Med 2005; 17:365–373. [DOI] [PubMed] [Google Scholar]
- 25.Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, Branch DW, Silver RM, Adashi EY. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta 2005; 26:661–671. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, Svinarich D, Cotton DB. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am.J.Reprod.Immunol 1994; 32:108–113. [DOI] [PubMed] [Google Scholar]
- 27.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, Yoon BH. A role for the novel cytokine RANTES in pregnancy and parturition. Am.J.Obstet.Gynecol 1999; 181:989–994. [DOI] [PubMed] [Google Scholar]
- 28.Keelan JA, Yang J, Romero RJ, Chaiworapongsa T, Marvin KW, Sato TA, Mitchell MD. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol.Reprod 2004; 70:253–259. [DOI] [PubMed] [Google Scholar]
- 29.Casey ML, Cox SM, Beutler B, Milewich L, MacDonald PC. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. J.Clin.Invest 1989; 83:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liechty KW, Koenig JM, Mitchell MD, Romero R, Christensen RD. Production of interleukin-6 by fetal and maternal cells in vivo during intraamniotic infection and in vitro after stimulation with interleukin-1. Pediatr.Res 1991; 29:1–4. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Mazor M, Manogue K, Oyarzun E, Cerami A. Human decidua: a source of cachectin-tumor necrosis factor. Eur.J.Obstet.Gynecol.Reprod.Biol 1991; 41:123–127. [DOI] [PubMed] [Google Scholar]
- 32.Svinarich DM, Bitonti OM, Araneda H, Romero R, Gonik B. Induction and postranslational expression of G-CSF and RANTES in a first trimester trophoblast cell line by lipopolysaccharide. Am.J.Reprod.Immunol 1996; 36:256–259. [DOI] [PubMed] [Google Scholar]
- 33.Huleihel M, Amash A, Sapir O, Maor E, Levy S, Katz M, Dukler D, Myatt L, Holcberg G. Lipopolysaccharide induces the expression of interleukin-1alpha distinctly in different compartments of term and preterm human placentae. Eur.Cytokine Netw 2004; 15:30–36. [PubMed] [Google Scholar]
- 34.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am.J.Obstet.Gynecol 1993; 169:805–816. [DOI] [PubMed] [Google Scholar]
- 35.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am.J.Obstet.Gynecol 1995; 172:960–970. [DOI] [PubMed] [Google Scholar]
- 36.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am.J.Obstet.Gynecol 1995; 173:606–612. [DOI] [PubMed] [Google Scholar]
- 37.Kara M, Ozden S, Arioglu P, Cetin A. The significance of amniotic fluid interleukin-6 levels in preterm labour. Aust.N.Z.J.Obstet.Gynaecol 1998; 38:403–406. [DOI] [PubMed] [Google Scholar]
- 38.Baud O, Emilie D, Pelletier E, Lacaze-Masmonteil T, Zupan V, Fernandez H, Dehan M, Frydman R, Ville Y. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br.J.Obstet.Gynaecol 1999; 106:72–77. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Mazor M, Wu YK, Avila C, Oyarzun E, Mitchell MD. Bacterial endotoxin and tumor necrosis factor stimulate prostaglandin production by human decidua. Prostaglandins Leukot.Essent.Fatty Acids 1989; 37:183–186. [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins 1989; 37:13–22. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell MD, Edwin S, Romero RJ. Prostaglandin biosynthesis by human decidual cells: effects of inflammatory mediators. Prostaglandins Leukot.Essent.Fatty Acids 1990; 41:35–38. [DOI] [PubMed] [Google Scholar]
- 42.Hertelendy F, Romero R, Molnar M, Todd H, Baldassare JJ. Cytokine-initiated signal transduction in human myometrial cells. Am.J.Reprod.Immunol 1993; 30:49–57. [DOI] [PubMed] [Google Scholar]
- 43.Molnar M, Romero R, Hertelendy F. Interleukin-1 and tumor necrosis factor stimulate arachidonic acid release and phospholipid metabolism in human myometrial cells. Am.J.Obstet.Gynecol 1993; 169:825–829. [DOI] [PubMed] [Google Scholar]
- 44.Eykholt RL, Hansen WR, Potter S, Marvin KW, Mitchell MD. Cytokines regulate prostaglandin H synthase-1 transcription in human amnion-derived cells. Prostaglandins Leukot.Essent.Fatty Acids 1999; 61:323–329. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell MD, Romero RJ, Edwin SS, Trautman MS. Prostaglandins and parturition. Reprod.Fertil.Dev. 1995; 7:623–632. [DOI] [PubMed] [Google Scholar]
- 46.Rauk PN, Chiao JP. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am.J.Reprod.Immunol 2000; 43:152–159. [DOI] [PubMed] [Google Scholar]
- 47.Hertelendy F, Molnar M, Romero R. Interferon gamma antagonizes interleukin-1beta-induced cyclooxygenase-2 expression and prostaglandin E(2) production in human myometrial cells. J.Soc.Gynecol.Investig 2002; 9:215–219. [PubMed] [Google Scholar]
- 48.Hertelendy F, Rastogi P, Molnar M, Romero R. Interleukin-1beta-induced prostaglandin E2 production in human myometrial cells: role of a pertussis toxin-sensitive component. Am.J.Reprod.Immunol 2001; 45:142–147. [DOI] [PubMed] [Google Scholar]
- 49.Sooranna SR, Engineer N, Loudon JA, Terzidou V, Bennett PR, Johnson MR. The mitogen-activated protein kinase dependent expression of prostaglandin H synthase-2 and interleukin-8 messenger ribonucleic acid by myometrial cells: the differential effect of stretch and interleukin-1{beta}. J.Clin.Endocrinol.Metab 2005; 90:3517–3527. [DOI] [PubMed] [Google Scholar]
- 50.Zaragoza DB, Wilson RR, Mitchell BF, Olson DM. The interleukin 1beta-induced expression of human prostaglandin F2alpha receptor messenger RNA in human myometrial-derived ULTR cells requires the transcription factor, NFkappaB. Biol.Reprod 2006; 75:697–704. [DOI] [PubMed] [Google Scholar]
- 51.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am.J.Obstet.Gynecol 1991; 165:969–971. [DOI] [PubMed] [Google Scholar]
- 52.Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J.Soc.Gynecol.Investig 1996; 3:121–126. [DOI] [PubMed] [Google Scholar]
- 53.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am.J.Obstet.Gynecol 2006; 195:1578–1589. [DOI] [PubMed] [Google Scholar]
- 54.Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu.Rev.Immunol 2001; 19:683–765. [DOI] [PubMed] [Google Scholar]
- 55.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J.Immunol 1993; 150:353–360. [PubMed] [Google Scholar]
- 56.Benjamin D, Knobloch TJ, Dayton MA. Human B-cell interleukin-10: B-cell lines derived from patients with acquired immunodeficiency syndrome and Burkitt’s lymphoma constitutively secrete large quantities of interleukin-10. Blood 1992; 80:1289–1298. [PubMed] [Google Scholar]
- 57.de Waal MR, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J.Exp.Med 1991; 174:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boonstra A, Rajsbaum R, Holman M, Marques R, Asselin-Paturel C, Pereira JP, Bates EE, Akira S, Vieira P, Liu YJ, et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J.Immunol 2006; 177:7551–7558. [DOI] [PubMed] [Google Scholar]
- 59.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J.Immunol 2000; 164:5721–5728. [DOI] [PubMed] [Google Scholar]
- 60.Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J.Exp.Med 1996; 184:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ekerfelt C, Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J. Spontaneous secretion of interleukin-4, interleukin-10 and interferon-gamma by first trimester decidual mononuclear cells. Am.J.Reprod.Immunol 2002; 47:159–166. [DOI] [PubMed] [Google Scholar]
- 62.Gustafsson C, Hummerdal P, Matthiesen L, Berg G, Ekerfelt C, Ernerudh J. Cytokine secretion in decidual mononuclear cells from term human pregnancy with or without labour: ELISPOT detection of IFN-gamma, IL-4, IL-10, TGF-beta and TNF-alpha. J.Reprod.Immunol 2006; 71:41–56. [DOI] [PubMed] [Google Scholar]
- 63.Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J.Immunol. 2006; 177:4888–4896. [DOI] [PubMed] [Google Scholar]
- 64.Terrone DA, Rinehart BK, Granger JP, Barrilleaux PS, Martin JN Jr., Bennett WA. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet.Gynecol 2001; 98:476–480. [DOI] [PubMed] [Google Scholar]
- 65.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am.J.Obstet.Gynecol 2003; 188:252–263. [DOI] [PubMed] [Google Scholar]
- 66.Rodts-Palenik S, Wyatt-Ashmead J, Pang Y, Thigpen B, Cai Z, Rhodes P, Martin JN, Granger J, Bennett WA. Maternal infection-induced white matter injury is reduced by treatment with interleukin-10. Am.J.Obstet.Gynecol 2004; 191:1387–1392. [DOI] [PubMed] [Google Scholar]
- 67.Annells MF, Hart PH, Mullighan CG, Heatley SL, Robinson JS, Bardy P, McDonald HM. Interleukins-1, −4, −6, −10, tumor necrosis factor, transforming growth factor-beta, FAS, and mannose-binding protein C gene polymorphisms in Australian women: Risk of preterm birth. Am.J.Obstet.Gynecol 2004; 191:2056–2067. [DOI] [PubMed] [Google Scholar]
- 68.Kerk J, Dordelmann M, Bartels DB, Brinkhaus MJ, Dammann CE, Dork T, Dammann O. Multiplex measurement of cytokine/receptor gene polymorphisms and interaction between interleukin-10 (−1082) genotype and chorioamnionitis in extreme preterm delivery. J.Soc.Gynecol.Investig 2006; 13:350–356. [DOI] [PubMed] [Google Scholar]
- 69.Mattar R, de Souza E, Daher S. Preterm delivery and cytokine gene polymorphisms. J.Reprod.Med 2006; 51:317–320. [PubMed] [Google Scholar]
- 70.Yanamandra K, Boggs P, Loggins J, Baier RJ. Interleukin-10 −1082 G/A polymorphism and risk of death or bronchopulmonary dysplasia in ventilated very low birth weight infants. Pediatr.Pulmonol 2005; 39:426–432. [DOI] [PubMed] [Google Scholar]
- 71.Dordelmann M, Kerk J, Dressler F, Brinkhaus MJ, Bartels DB, Dammann CE, Dork T, Dammann O. Interleukin-10 high producer allele and ultrasound-defined periventricular white matter abnormalities in preterm infants: a preliminary study. Neuropediatrics 2006; 37:130–136. [DOI] [PubMed] [Google Scholar]
- 72.Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J.Exp.Med 1993; 177:1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolfberg AJ, Dammann O, Gressens P. Anti-inflammatory and immunomodulatory strategies to protect the perinatal brain. Semin.Fetal Neonatal Med 2007; 12:296–302. [DOI] [PubMed] [Google Scholar]
- 74.Mosmann TR, Schumacher JH, Fiorentino DF, Leverah J, Moore KW, Bond MW. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J.Immunol 1990; 145:2938–2945. [PubMed] [Google Scholar]
- 75.Mosmann TR. Interleukin-10. 1994; Second:223–237. [Google Scholar]
- 76.Kim JM, Brannan CI, Copeland NG, Jenkins NA, Khan TA, Moore KW. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes. J.Immunol 1992; 148:3618–3623. [PubMed] [Google Scholar]
- 77.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J.Exp.Med 1989; 170:2081–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 1990; 248:1230–1234. [DOI] [PubMed] [Google Scholar]
- 79.Vieira P, de Waal-Malefyt R, Dang MN, Johnson KE, Kastelein R, Fiorentino DF, deVries JE, Roncarolo MG, Mosmann TR, Moore KW. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc.Natl.Acad.Sci.U.S.A 1991; 88:1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore KW, O’Garra A, de Waal MR, Vieira P, Mosmann TR. Interleukin-10. Annu.Rev.Immunol 1993; 11:165–190. [DOI] [PubMed] [Google Scholar]
- 81.Fickenscher H, Hor S, Kupers H, Knappe A, Wittmann S, Sticht H. The interleukin-10 family of cytokines. Trends Immunol 2002; 23:89–96. [DOI] [PubMed] [Google Scholar]
- 82.Oral HB, Kotenko SV, Yilmaz M, Mani O, Zumkehr J, Blaser K, Akdis CA, Akdis M. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur.J.Immunol 2006; 36:380–388. [DOI] [PubMed] [Google Scholar]
- 83.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr.Opin.Pharmacol 2006; 6:379–386. [DOI] [PubMed] [Google Scholar]
- 84.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J.Immunol 1986; 136:2348–2357. [PubMed] [Google Scholar]
- 85.Parish CR. The relationship between humoral and cell-mediated immunity. Transplant.Rev 1972; 13:35–66. [DOI] [PubMed] [Google Scholar]
- 86.Katsura Y. Cell-mediated and humoral immune responses in mice. III. Dynamic balance between delayed-type hypersensitivity and antibody response. Immunology 1977; 32:227–235. [PMC free article] [PubMed] [Google Scholar]
- 87.Del Prete G, De Carli M, Mastromauro C, Biagiotti R, Macchia D, Falagiani P, Ricci M, Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J.Clin.Invest 1991; 88:346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J.Exp.Med 2007; 204:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J.Exp.Med 2007; 204:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Garra A, Stapleton G, Dhar V, Pearce M, Schumacher J, Rugo H, Barbis D, Stall A, Cupp J, Moore K, et al. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int.Immunol 1990; 2:821–832. [DOI] [PubMed] [Google Scholar]
- 91.O’Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur.J.Immunol 1992; 22:711–717. [DOI] [PubMed] [Google Scholar]
- 92.Burdin N, Peronne C, Banchereau J, Rousset F. Epstein-Barr virus transformation induces B lymphocytes to produce human interleukin 10. J.Exp.Med 1993; 177:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benjamin D, Park CD, Sharma V. Human B cell interleukin 10. Leuk.Lymphoma 1994; 12:205–210. [DOI] [PubMed] [Google Scholar]
- 94.Edwards AD, Manickasingham SP, Sporri R, Diebold SS, Schulz O, Sher A, Kaisho T, Akira S, Reis e Sousa. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J.Immunol 2002; 169:3652–3660. [DOI] [PubMed] [Google Scholar]
- 95.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J.Exp.Med 2002; 195:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J.Immunol 2004; 172:4733–4743. [DOI] [PubMed] [Google Scholar]
- 97.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997; 389:737–742. [DOI] [PubMed] [Google Scholar]
- 98.Pontoux C, Banz A, Papiernik M. Natural CD4 CD25(+) regulatory T cells control the burst of superantigen-induced cytokine production: the role of IL-10. Int.Immunol 2002; 14:233–239. [DOI] [PubMed] [Google Scholar]
- 99.O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat.Med 2004; 10:801–805. [DOI] [PubMed] [Google Scholar]
- 100.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J.Immunol 2006; 177:5852–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity 2006; 25:941–952. [DOI] [PubMed] [Google Scholar]
- 102.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol.Rev 2006; 212:28–50. [DOI] [PubMed] [Google Scholar]
- 103.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol.Rev 2001; 182:18–32. [DOI] [PubMed] [Google Scholar]
- 104.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat.Rev.Immunol 2002; 2:389–400. [DOI] [PubMed] [Google Scholar]
- 105.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J.Exp.Med 2003; 197:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat.Immunol 2001; 2:816–822. [DOI] [PubMed] [Google Scholar]
- 107.Cobbold SP, Graca L, Lin CY, Adams E, Waldmann H. Regulatory T cells in the induction and maintenance of peripheral transplantation tolerance. Transpl.Int 2003; 16:66–75. [DOI] [PubMed] [Google Scholar]
- 108.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J.Exp.Med 2002; 195:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buer J, Lanoue A, Franzke A, Garcia C, von Boehmer H, Sarukhan A. Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II-restricted T cells anergized in vivo. J.Exp.Med 1998; 187:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4(+)CD25(+) T cells. J.Autoimmun 2001; 16:115–123. [DOI] [PubMed] [Google Scholar]
- 111.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int.Immunol 1999; 11:1625–1634. [DOI] [PubMed] [Google Scholar]
- 112.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat.Med 2002; 8:1024–1032. [DOI] [PubMed] [Google Scholar]
- 113.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int.Immunol 2004; 16:249–256. [DOI] [PubMed] [Google Scholar]
- 114.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J.Exp.Med 1999; 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hsu DH, de Waal MR, Fiorentino DF, Dang MN, Vieira P, de Vries J, Spits H, Mosmann TR, Moore KW. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science 1990; 250:830–832. [DOI] [PubMed] [Google Scholar]
- 116.Rode HJ, Janssen W, Rosen-Wolff A, Bugert JJ, Thein P, Becker Y, Darai G. The genome of equine herpesvirus type 2 harbors an interleukin 10 (IL-10)-like gene. Virus Genes 1993; 7:111–116. [DOI] [PubMed] [Google Scholar]
- 117.Dudley DJ, Edwin SS, Dangerfield A, Jackson K, Trautman MS. Regulation of decidual cell and chorion cell production of interleukin-10 by purified bacterial products. Am.J.Reprod.Immunol 1997; 38:246–251. [DOI] [PubMed] [Google Scholar]
- 118.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol.Hum.Reprod 2002; 8:399–408. [DOI] [PubMed] [Google Scholar]
- 119.Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin.Exp.Immunol 2003; 131:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am.J.Reprod.Immunol 2003; 50:444–452. [DOI] [PubMed] [Google Scholar]
- 121.Vigano P, Gaffuri B, Somigliana E, Infantino M, Vignali M, Di Blasio AM. Interleukin-10 is produced by human uterine natural killer cells but does not affect their production of interferon-gamma. Mol.Hum.Reprod 2001; 7:971–977. [DOI] [PubMed] [Google Scholar]
- 122.Krasnow JS, Tollerud DJ, Naus G, DeLoia JA. Endometrial Th2 cytokine expression throughout the menstrual cycle and early pregnancy. Hum.Reprod 1996; 11:1747–1754. [DOI] [PubMed] [Google Scholar]
- 123.Trinchieri G Interleukin-10 production by effector T cells: Th1 cells show self control. J.Exp.Med 2007; 204:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jankovic D, Trinchieri G. IL-10 or not IL-10: that is the question. Nat.Immunol 2007; 8:1281–1283. [DOI] [PubMed] [Google Scholar]
- 125.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat.Immunol 2007; 8:1372–1379. [DOI] [PubMed] [Google Scholar]
- 126.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat.Immunol 2007; 8:1380–1389. [DOI] [PubMed] [Google Scholar]
- 127.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat.Immunol 2007; 8:1363–1371. [DOI] [PubMed] [Google Scholar]
- 128.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat.Immunol 2007; 8:1390–1397. [DOI] [PubMed] [Google Scholar]
- 129.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha . J.Immunol 1996; 157:798–805. [PubMed] [Google Scholar]
- 130.Hunter CA, Ellis-Neyes LA, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo FG. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J.Immunol 1997; 158:3311–3316. [PubMed] [Google Scholar]
- 131.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect.Immun 1999; 67:4435–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deckert M, Soltek S, Geginat G, Lutjen S, Montesinos-Rongen M, Hof H, Schluter D. Endogenous interleukin-10 is required for prevention of a hyperinflammatory intracerebral immune response in Listeria monocytogenes meningoencephalitis. Infect.Immun 2001; 69:4561–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol 2007; 28:378–384. [DOI] [PubMed] [Google Scholar]
- 134.Lilic D, Gravenor I, Robson N, Lammas DA, Drysdale P, Calvert JE, Cant AJ, Abinun M. Deregulated production of protective cytokines in response to Candida albicans infection in patients with chronic mucocutaneous candidiasis. Infect.Immun 2003; 71:5690–5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fornari MC, Bava AJ, Guereno MT, Berardi VE, Silaf MR, Negroni R, Diez RA. High serum interleukin-10 and tumor necrosis factor alpha levels in chronic paracoccidioidomycosis. Clin.Diagn.Lab Immunol 2001; 8:1036–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Benard G, Romano CC, Cacere CR, Juvenale M, Mendes-Giannini MJ, Duarte AJ. Imbalance of IL-2, IFN-gamma and IL-10 secretion in the immunosuppression associated with human paracoccidioidomycosis. Cytokine 2001; 13:248–252. [DOI] [PubMed] [Google Scholar]
- 137.Romano CC, Mendes-Giannini MJ, Duarte AJ, Benard G. IL-12 and neutralization of endogenous IL-10 revert the in vitro antigen-specific cellular immunosuppression of paracoccidioidomycosis patients. Cytokine 2002; 18:149–157. [DOI] [PubMed] [Google Scholar]
- 138.Romano CC, Mendes-Giannini MJ, Duarte AJ, Benard G. The role of interleukin-10 in the differential expression of interleukin-12p70 and its beta2 receptor on patients with active or treated paracoccidioidomycosis and healthy infected subjects. Clin.Immunol 2005; 114:86–94. [DOI] [PubMed] [Google Scholar]
- 139.Pagliari C, Fernandes ER, Guedes F, Alves C, Sotto MN. Role of mast cells as IL-10 producing cells in paracoccidioidomycosis skin lesions. Mycopathologia 2006; 162:331–335. [DOI] [PubMed] [Google Scholar]
- 140.Costa DL, Dias-Melicio LA, Acorci MJ, Bordon AP, Tavian EG, Peracoli MT, Soares AM. Effect of interleukin-10 on the Paracoccidioides brasiliensis killing by gamma-interferon activated human neutrophils. Microbiol.Immunol 2007; 51:73–80. [DOI] [PubMed] [Google Scholar]
- 141.Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J.Clin.Invest 1995; 96:2339–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lang R, Rutschman RL, Greaves DR, Murray PJ. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J.Immunol 2002; 168:3402–3411. [DOI] [PubMed] [Google Scholar]
- 143.Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, Muller W. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J.Exp.Med 2004; 200:1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat.Med 2007;13:1450–1457. [DOI] [PubMed] [Google Scholar]
- 145.Camby I, Le Mercier M., Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology 2006; 16:137R–157R. [DOI] [PubMed] [Google Scholar]
- 146.van der Leij J, van den Berg A, Blokzijl T, Harms G, van Goor H., Zwiers P, van Weeghel R, Poppema S, Visser L Dimeric galectin-1 induces IL-10 production in T-lymphocytes: an important tool in the regulation of the immune response. J.Pathol 2004; 204:511–518. [DOI] [PubMed] [Google Scholar]
- 147.van der Leij J, van den Berg A, Harms G, Eschbach H, Vos H, Zwiers P, van Weeghel R, Groen H, Poppema S, Visser L. Strongly enhanced IL-10 production using stable galectin-1 homodimers. Mol.Immunol 2007; 44:506–513. [DOI] [PubMed] [Google Scholar]
- 148.Bevan BH, Kilpatrick DC, Liston WA, Hirabayashi J, Kasai K. Immunohistochemical localization of a beta-D-galactoside-binding lectin at the human maternofetal interface. Histochem.J 1994; 26:582–586. [DOI] [PubMed] [Google Scholar]
- 149.Than NG, Erez O, Wildman DE, Tarca AL, Edwin SS, Abbas A, Hotra J, Kusanovic JP, Gotsch F, Hassan SS, Espinoza J, Papp Z, Romero R. Severe preeclampsia is characterized by increased placental expression of galectin-1. J Matern Fetal Neonatal Med 2008;21:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Walzel H, Neels P, Bremer H, Kohler H, Raab N, Barten M, Brock J. Immunohistochemical and glycohistochemical localization of the beta-galactoside-binding S-type lectin in human placenta. Acta Histochem 1995; 97:33–42. [DOI] [PubMed] [Google Scholar]
- 151.Maquoi E, van den Brule FA, Castronovo V, Foidart JM. Changes in the distribution pattern of galectin-1 and galectin-3 in human placenta correlates with the differentiation pathways of trophoblasts. Placenta 1997; 18:433–439. [DOI] [PubMed] [Google Scholar]
- 152.Vicovac L, Jankovic M, Cuperlovic M. Galectin-1 and −3 in cells of the first trimester placental bed. Hum.Reprod 1998; 13:730–735. [DOI] [PubMed] [Google Scholar]
- 153.Than NG, Erez O, Wildman DE, Edwin S, Masaki-Tovi S, Gotsch F, Cushenberry E, Pineles B, Montenegro D, Espinoza J, et al. Preterm labor and preterm PROM are characterized by an increase in maternal serum concentration of galectin-1. Reproductive Sciences 2007;90A. [Google Scholar]
- 154.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J.Immunol 1991; 146:3444–3451. [PubMed] [Google Scholar]
- 155.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J.Immunol 1991; 147:3815–3822. [PubMed] [Google Scholar]
- 156.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J.Exp.Med 1991; 174:1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J.Immunol 1993; 151:1224–1234. [PubMed] [Google Scholar]
- 158.Willems F, Marchant A, Delville JP, Gerard C, Delvaux A, Velu T, de BM, Goldman M. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur.J.Immunol 1994; 24:1007–1009. [DOI] [PubMed] [Google Scholar]
- 159.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage-colony-stimulating factor. Eur.J.Immunol 1997; 27:756–762. [DOI] [PubMed] [Google Scholar]
- 160.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur.J.Immunol 1998; 28:359–369. [DOI] [PubMed] [Google Scholar]
- 161.Morel AS, Quaratino S, Douek DC, Londei M. Split activity of interleukin-10 on antigen capture and antigen presentation by human dendritic cells: definition of a maturative step. Eur.J.Immunol 1997; 27:26–34. [DOI] [PubMed] [Google Scholar]
- 162.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J.Exp.Med 1996; 184:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Trinchieri G Regulatory role of T cells producing both interferon gamma and interleukin 10 in persistent infection. J.Exp.Med 2001; 194:F53–F57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hsu DH, Moore KW, Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int.Immunol 1992; 4:563–569. [DOI] [PubMed] [Google Scholar]
- 165.Thompson-Snipes L, Dhar V, Bond MW, Mosmann TR, Moore KW, Rennick DM. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J.Exp.Med 1991; 173:507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ghildyal N, McNeil HP, Gurish MF, Austen KF, Stevens RL. Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J.Biol.Chem 1992; 267:8473–8477. [PubMed] [Google Scholar]
- 167.Ghildyal N, McNeil HP, Stechschulte S, Austen KF, Silberstein D, Gurish MF, Somerville LL, Stevens RL. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J.Immunol 1992; 149:2123–2129. [PubMed] [Google Scholar]
- 168.Madsen KL, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology 1997; 113:151–159. [DOI] [PubMed] [Google Scholar]
- 169.Heyborne KD, McGregor JA, Henry G, Witkin SS, Abrams JS. Interleukin-10 in amniotic fluid at mid-trimester: immune activation and suppression in relation to fetal growth. Am.J.Obstet.Gynecol 1994; 171:55–59. [DOI] [PubMed] [Google Scholar]
- 170.Dudley DJ, Hunter C, Mitchell MD, Varner MW. Amniotic fluid interleukin-10 (IL-10) concentrations during pregnancy and with labor. J.Reprod.Immunol 1997; 33:147–156. [DOI] [PubMed] [Google Scholar]
- 171.Greig PC, Herbert WN, Robinette BL, Teot LA. Amniotic fluid interleukin-10 concentrations increase through pregnancy and are elevated in patients with preterm labor associated with intrauterine infection. Am.J.Obstet.Gynecol 1995; 173:1223–1227. [DOI] [PubMed] [Google Scholar]
- 172.Holmes VA, Wallace JM, Gilmore WS, McFaul P, Alexander HD. Plasma levels of the immunomodulatory cytokine interleukin-10 during normal human pregnancy: a longitudinal study. Cytokine 2003; 21:265–269. [DOI] [PubMed] [Google Scholar]
- 173.van der Weiden RM, Janssen JW, Stegeman M. Systemic interleukin-10 concentrations do not alter between the first and second trimester in normal pregnancy. Arch.Gynecol.Obstet 2005; 273:150–151. [DOI] [PubMed] [Google Scholar]
- 174.Enk AH, Katz SI. Identification and induction of keratinocyte-derived IL-10. J.Immunol 1992; 149:92–95. [PubMed] [Google Scholar]
- 175.Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J.Immunol 1992; 149:3865–3871. [PubMed] [Google Scholar]
- 176.Kimatrai M, Blanco O, Munoz-Fernandez R, Tirado I, Martin F, Abadia-Molina AC, Olivares EG. Contractile activity of human decidual stromal cells. II. Effect of interleukin-10. J.Clin.Endocrinol.Metab 2005; 90:6126–6130. [DOI] [PubMed] [Google Scholar]
- 177.Apuzzio J, Chan Y, Al-Khan A, Illsley N, Kim PL, Vonhaggen S. Second-trimester amniotic fluid interleukin-10 concentration predicts preterm delivery. J.Matern.Fetal Neonatal Med 2004; 15:313–317. [DOI] [PubMed] [Google Scholar]
- 178.Peltier MR. Immunology of term and preterm labor. Reprod.Biol.Endocrinol 2003; 1:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin.Fetal Neonatal Med 2006; 11:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction 2005; 130:569–581. [DOI] [PubMed] [Google Scholar]
- 181.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin.Reprod.Med 2007; 25:21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am.J.Obstet.Gynecol 2006; 195:394:e1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jones CA, Finlay-Jones JJ, Hart PH. Type-1 and type-2 cytokines in human late-gestation decidual tissue. Biol.Reprod 1997; 57:303–311. [DOI] [PubMed] [Google Scholar]
- 184.Hanna N, Bonifacio L, Weinberger B, Reddy P, Murphy S, Romero R, Sharma S. Evidence for interleukin-10-mediated inhibition of cyclo- oxygenase-2 expression and prostaglandin production in preterm human placenta. Am.J.Reprod.Immunol 2006; 55:19–27. [DOI] [PubMed] [Google Scholar]
- 185.Simpson KL, Keelan JA, Mitchell MD. Labor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodecidua. J.Clin.Endocrinol.Metab 1998; 83:4332–4337. [DOI] [PubMed] [Google Scholar]
- 186.Vives A, Balasch J, Yague J, Quinto L, Ordi J, Vanrell JA. Type-1 and type-2 cytokines in human decidual tissue and trophoblasts from normal and abnormal pregnancies detected by reverse transcriptase polymerase chain reaction (RT-PCR). Am.J.Reprod.Immunol 1999; 42:361–368. [DOI] [PubMed] [Google Scholar]
- 187.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, Athanassiadis AP, Mitchell MD. Amniotic fluid interleukin-1 in spontaneous labor at term. J.Reprod.Med 1990; 35:235–238. [PubMed] [Google Scholar]
- 188.Chan EC, Fraser S, Yin S, Yeo G, Kwek K, Fairclough RJ, Smith R. Human myometrial genes are differentially expressed in labor: a suppression subtractive hybridization study. J.Clin.Endocrinol.Metab 2002; 87:2435–2441. [DOI] [PubMed] [Google Scholar]
- 189.Bisits AM, Smith R, Mesiano S, Yeo G, Kwek K, MacIntyre D, Chan EC. Inflammatory aetiology of human myometrial activation tested using directed graphs. PLoS.Comput.Biol 2005; 1:132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bell MJ, Hallenbeck JM, Gallo V. Determining the fetal inflammatory response in an experimental model of intrauterine inflammation in rats. Pediatr.Res 2004; 56:541–546. [DOI] [PubMed] [Google Scholar]
- 191.Trautman MS, Collmer D, Edwin SS, White W, Mitchell MD, Dudley DJ. Expression of interleukin-10 in human gestational tissues. J.Soc.Gynecol.Investig 1997; 4:247–253. [PubMed] [Google Scholar]
- 192.Sato TA, Keelan JA, Mitchell MD. Critical paracrine interactions between TNF-alpha and IL-10 regulate lipopolysaccharide-stimulated human choriodecidual cytokine and prostaglandin E2 production. J.Immunol 2003; 170:158–166. [DOI] [PubMed] [Google Scholar]
- 193.Fortunato SJ, Menon R, Lombardi SJ. Interleukin-10 and transforming growth factor-beta inhibit amniochorion tumor necrosis factor-alpha production by contrasting mechanisms of action: therapeutic implications in prematurity. Am.J.Obstet.Gynecol 1997; 177:803–809. [DOI] [PubMed] [Google Scholar]
- 194.Fortunato SJ, Menon R, Lombardi SJ, LaFleur B. Interleukin-10 inhibition of gelatinases in fetal membranes: therapeutic implications in preterm premature rupture of membranes. Obstet.Gynecol 2001; 98:284–288. [DOI] [PubMed] [Google Scholar]
- 195.Roth I, Fisher SJ. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev.Biol 1999; 205:194–204. [DOI] [PubMed] [Google Scholar]
- 196.Fortunato SJ, Menon R, Swan KF, Lombardi SJ. Interleukin-10 inhibition of interleukin-6 in human amniochorionic membrane: transcriptional regulation. Am.J.Obstet.Gynecol 1996; 175:1057–1065. [DOI] [PubMed] [Google Scholar]
- 197.Fortunato SJ, Menon R, Lombardi SJ. The effect of transforming growth factor and interleukin-10 on interleukin-8 release by human amniochorion may regulate histologic chorioamnionitis. Am.J.Obstet.Gynecol 1998; 179:794–799. [DOI] [PubMed] [Google Scholar]
- 198.Mitchell MD, Simpson KL, Keelan JA. Paradoxical proinflammatory actions of interleukin-10 in human amnion: potential roles in term and preterm labour. J.Clin.Endocrinol.Metab 2004; 89:4149–4152. [DOI] [PubMed] [Google Scholar]
- 199.Brown NL, Alvi SA, Elder MG, Bennett PR, Sullivan MH. The regulation of prostaglandin output from term intact fetal membranes by anti-inflammatory cytokines. Immunology 2000; 99:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pomini F, Caruso A, Challis JR. Interleukin-10 modifies the effects of interleukin-1beta and tumor necrosis factor-alpha on the activity and expression of prostaglandin H synthase-2 and the NAD+-dependent 15-hydroxyprostaglandin dehydrogenase in cultured term human villous trophoblast and chorion trophoblast cells. J.Clin.Endocrinol.Metab 1999; 84:4645–4651. [DOI] [PubMed] [Google Scholar]
- 201.Reuss E, Fimmers R, Kruger A, Becker C, Rittner C, Hohler T. Differential regulation of interleukin-10 production by genetic and environmental factors--a twin study. Genes Immun 2002; 3:407–413. [DOI] [PubMed] [Google Scholar]
- 202.Westendorp RG, Langermans JA, Huizinga TW, Verweij CL, Sturk A. Genetic influence on cytokine production in meningococcal disease. Lancet 1997; 349:1912–1913. [DOI] [PubMed] [Google Scholar]
- 203.Eskdale J, Gallagher G. A polymorphic dinucleotide repeat in the human IL-10 promoter. Immunogenetics 1995; 42:444–445. [DOI] [PubMed] [Google Scholar]
- 204.Eskdale J, Kube D, Gallagher G. A second polymorphic dinucleotide repeat in the 5’ flanking region of the human IL-10 gene. Immunogenetics 1996; 45:82–83. [DOI] [PubMed] [Google Scholar]
- 205.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur.J.Immunogenet 1997; 24:1–8. [DOI] [PubMed] [Google Scholar]
- 206.Kalish RB, Vardhana S, Gupta M, Perni SC, Witkin SS. Interleukin-4 and −10 gene polymorphisms and spontaneous preterm birth in multifetal gestations. Am.J.Obstet.Gynecol. 2004; 190:702–706. [DOI] [PubMed] [Google Scholar]
- 207.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J.Immunol 2005; 175:4084–4090. [DOI] [PubMed] [Google Scholar]
- 208.Goodwin VJ, Sato TA, Mitchell MD, Keelan JA. Anti-inflammatory effects of interleukin-4, interleukin-10, and transforming growth factor-beta on human placental cells in vitro. Am.J.Reprod.Immunol 1998; 40:319–325. [DOI] [PubMed] [Google Scholar]
- 209.Chernoff AE, Granowitz EV, Shapiro L, Vannier E, Lonnemann G, Angel JB, Kennedy JS, Rabson AR, Wolff SM, Dinarello CA. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J.Immunol 1995; 154:5492–5499. [PubMed] [Google Scholar]
- 210.Huhn RD, Radwanski E, Gallo J, Affrime MB, Sabo R, Gonyo G, Monge A, Cutler DL. Pharmacodynamics of subcutaneous recombinant human interleukin-10 in healthy volunteers. Clin.Pharmacol.Ther 1997; 62:171–180. [DOI] [PubMed] [Google Scholar]
- 211.Pajkrt D, Camoglio L, Tiel-van Buul MC, de Bruin K, Cutler DL, Affrime MB, Rikken G, van der Poll T, ten Cate JW, van Deventer SJ. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: effect of timing of recombinant human IL-10 administration. J.Immunol 1997; 158:3971–3977. [PubMed] [Google Scholar]
- 212.Chakraborty A, Blum RA, Mis SM, Cutler DL, Jusko WJ. Pharmacokinetic and adrenal interactions of IL-10 and prednisone in healthy volunteers. J.Clin.Pharmacol 1999; 39:624–635. [DOI] [PubMed] [Google Scholar]
- 213.Lauw FN, Pajkrt D, Hack CE, Kurimoto M, van Deventer SJ, van der Poll T. Proinflammatory effects of IL-10 during human endotoxemia. J.Immunol 2000; 165:2783–2789. [DOI] [PubMed] [Google Scholar]
- 214.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, Volk HD, Docke WD. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J.Clin.Invest 1998; 101:783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McInnes IB, Illei GG, Danning CL, Yarboro CH, Crane M, Kuroiwa T, Schlimgen R, Lee E, Foster B, Flemming D, et al. IL-10 improves skin disease and modulates endothelial activation and leukocyte effector function in patients with psoriatic arthritis. J.Immunol 2001; 167:4075–4082. [DOI] [PubMed] [Google Scholar]
- 216.Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, Jacyna M, Lashner BA, Gangl A, Rutgeerts P, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology 2000; 119:1461–1472. [DOI] [PubMed] [Google Scholar]
- 217.Keystone E, Wherry J, Grint P. IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum.Dis.Clin.North Am 1998; 24:629–639. [DOI] [PubMed] [Google Scholar]
- 218.Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology 2000; 118:655–660. [DOI] [PubMed] [Google Scholar]
- 219.Pang Y, Rodts-Palenik S, Cai Z, Bennett WA, Rhodes PG. Suppression of glial activation is involved in the protection of IL-10 on maternal E. coli induced neonatal white matter injury. Brain Res.Dev.Brain Res. 2005; 157:141–149. [DOI] [PubMed] [Google Scholar]