Abstract
OBJECTIVE:
The receptor for advanced glycation end products (RAGE) has been proposed to participate in the innate and adaptive immune responses. RAGE can induce production of pro-inflammatory cytokines and chemokines, as well as neutrophil chemotaxis, in a manner that may be suppressed or stimulated by soluble, truncated forms of RAGE including the soluble form of RAGE (sRAGE) and endogenous secretory RAGE (esRAGE). The objective of this study was to determine whether intra-amniotic infection/inflammation (IAI) is associated with changes in the amniotic fluid concentration of sRAGE and esRAGE.
STUDY DESIGN:
Amniotic fluid (AF) was retrieved from patients in the following groups: 1) midtrimester (14–18 weeks of gestation; n=68); 2) term not in labor (n=24); 3) term in labor (n=51); 4) preterm labor and intact membranes (n=124); and 5) preterm PROM (n=80). Intra-amniotic infection and inflammation were defined as the presence of a positive amniotic fluid culture for microorganisms and an AF interleukin-6 concentration ≥2.6 ng/ml, respectively. The AF concentration of sRAGE and esRAGE were determined using specific and sensitive ELISAs which measured total immunoreactive sRAGE and esRAGE, respectively. Patients were matched for gestational age at amniocentesis to compare the AF concentration of sRAGE and esRAGE in patients with and without IAI. Non-parametric statistics were used for analysis and a p<0.05 was considered significant.
RESULTS:
1) Patients at term not in labor had a higher median AF concentrations of sRAGE and esRAGE than those in the mid-trimester (p<0.001 for both comparisons) and those at term in labor (p=0.03 and p=0.04, respectively); 2) patients with preterm labor and intact membranes with intra-amniotic infection/inflammation (IAI) had a higher median AF concentration of sRAGE and esRAGE than those without IAI (p=0.02 and p=0.005, respectively); 3) similarly, patients with preterm PROM with IAI had a higher median AF concentration of sRAGE and esRAGE than those without IAI (p=0.03 and p=0.02, respectively).
CONCLUSION:
Intra-amniotic infection/inflammation is associated with increased amniotic fluid concentration of sRAGE and esRAGE. Changes in the amniotic fluid concentration of sRAGE and esRAGE may represent part of the immune responses to intra-amniotic infection/inflammation.
Keywords: microbial invasion of the amniotic cavity, preterm labor, preterm PROM, MIAC, amniotic fluid, intra-amniotic infection/inflammation, preterm delivery, chorioamnionitis
INTRODUCTION
Intra-amniotic infection/inflammation (IAI) is causally linked to preterm labor/delivery and fetal injury [11, 15, 25, 38, 59, 63, 73, 76, 78, 104, 105]. Microbial invasion of the amniotic cavity (MIAC) is a risk factor for impending preterm delivery [28, 76, 78], spontaneous rupture of membranes [8, 26, 36], clinical chorioamnionitis [78], pulmonary edema while receiving tocolysis [31], short-term neonatal morbidity [3, 24, 78], and long-term sequelae, such as chronic lung disease [23, 105] and cerebral palsy [104, 107]. Accumulating evidence indicates that patients with intra-amniotic inflammation but a negative amniotic fluid culture have a similar outcome to those with a positive amniotic fluid culture [89, 106]. Therefore, the detection of inflammation may be more practical than the detection of infection in patient management. Intra-amniotic inflammation can be detected with tests such as the amniotic fluid white blood cell count [7, 75, 79, 80], concentrations of chemokines [18, 39, 40, 72, 100], cytokines [1, 2, 16, 32, 41, 44, 58, 66, 71, 74, 77, 83], or antimicrobial peptides and proteins [17, 64, 65].
Non-enzymatic protein glycation modifies existing proteins and lipids yielding a heterogenous class of compounds that are collectively termed advanced glycation end products (AGEs) [62, 87, 95, 96]. AGEs have been implicated in the pathogenesis of diabetes, renal failure and aging [84]. RAGE was first identified as a signal transduction receptor for AGEs species [62, 87]. More recently, RAGE has been described as a receptor for pro-inflammatory molecules including S100/calgranulins [33], high mobility group box 1 (HMGB1) [35, 94], amyloid and β-sheet fibrils [9, 46, 88]. Of note, RAGE has recently been proposed to be part of the pattern recognition receptor system and participate in the innate immune response [53]. Further, recent studies illustrate key roles for RAGE in effective T lymphocyte priming in vivo [60].
Interference of the RAGE pathway with soluble RAGE (sRAGE, a truncated isoform of RAGE) can prevent or reduce vascular injury associated with some conditions, presumably by blocking the effect of RAGE [5, 68, 81, 97]. However, recent in vitro studies demonstrated that sRAGE can stimulate the production of pro-inflammatory cytokines including interleukin (IL)-6 and TNF-α, it can produce the chemokine MIP-2 by spleen cells via the NF-κB and Mac-1 pathways [70] as well as induces neutrophil chemotaxis [70]. Recently, a novel splice variant of RAGE mRNA coding for a C-terminally truncated secretory form was identified as endogenous secretory RAGE (esRAGE) [103]. The authors demonstrated that esRAGE is produced by human microvascular endothelial cells and pericytes, and proposed that the endogenous secretory receptor may be present in the circulation and extracellular fluids in the vascular walls [82, 103].
RAGE is expressed in the amnion epithelium, extravillous trophoblast and decidual cells in patients without chorioamnionitis as well as in the neutrophils in the choriodecidua in cases of histologic chorioamnionitis [6]. The objective of this study was to determine whether intra-amniotic infection/inflammation is associated with changes in the amniotic fluid concentration of sRAGE and endogenous secretory RAGE (esRAGE).
STUDY DESIGN
Study population.
Amniotic fluid (AF) was retrieved from patients in the following groups: 1) women in the mid-trimester of pregnancy (14–18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=68); 2) preterm labor and intact membranes (n=124); 3) preterm PROM (n=80); 4) term not in labor (n=24); and 5) term in labor (n=51). Patients with preterm labor and intact membranes and those with preterm PROM with and without intra-amniotic infection/inflammation were matched for gestational age within one week to compare the AF concentration of sRAGE and esRAGE. Inclusion criteria were: 1) singleton gestation; 2) absence of chromosomal or congenital anomalies; and 3) a signed informed consent approved by the Institutional Review Boards of the Sotero del Rio Hospital, Pennsylvania Hospital, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. These samples have been previously used to study the biology of inflammation in amniotic fluid.
Definitions and study procedures.
Intra-amniotic infection was defined as the presence of a positive amniotic fluid culture for microorganisms, and intra-amniotic inflammation as an AF IL-6 concentration ≥2.6 ng/ml [106]. Preterm labor with intact membranes was diagnosed in the presence of regular uterine contractions of at least 3 in 30 minutes and cervical changes at <37 weeks of gestation that requires hospitalization. Rupture of membranes was diagnosed by testing for pooling, nitrazine paper color change, and ferning. Beta-mimetic agents and/or magnesium sulfate were given intravenously for tocolysis, and steroids (betamethasone) were administered between 24 and 34 weeks at the discretion of the attending physician.
Sample collection.
An amniocentesis was performed for genetic studies or to determine the microbiological state of the amniotic cavity and/or fetal lung maturity. Amniotic fluid was retrieved by transabdominal amniocentesis under sonographic guidance. The fluid was transported to the laboratory in a capped plastic syringe and cultured for aerobic and anaerobic bacteria, as well as genital Mycoplasmas. A white blood cell count, glucose concentration, and Gram stain for microorganisms were performed in amniotic fluid immediately after collection. The results of these analyses were available for patient management. Amniotic fluid not needed for clinical assessment was centrifuged at 700 x g for 10 minutes at 4°C and the supernatant was aliquoted and stored frozen at −70°C until analysis.
sRAGE, esRAGE, Interleukin-6 (IL-6), and matrix metalloproteinase 8 (MMP-8) immunoassays.
Specific and sensitive enzyme-linked immunoassays were used to determine concentrations of sRAGE, esRAGE, IL-6 and MMP-8 in human amniotic fluid. Immunoassay kits for sRAGE, which measures total immunoreactive sRAGE, IL-6 and MMP-8 were obtained from R&D Systems (Minneapolis, MN). Immunoassay kits for esRAGE, which measures the endogenous secretory RAGE, were purchased from B-Bridge International, Inc (Mountain View, CA). All these assays were specifically validated for human AF in our laboratory. Validation included spike and recovery experiments, which produced parallel curves indicating that amniotic fluid constituents did not interfere with antigen-antibody binding in this assay system.
sRAGE:
AF samples were incubated in duplicate wells of the micro titer plates, which have been pre-coated with a monoclonal antibody specific for RAGE (extracellular domain). During this incubation, any sRAGE present in the standards or AF samples is bound by the immobilized antibodies. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for RAGE was added to the wells. Following a wash to remove excess and unbound materials, a substrate solution was added to the wells and color developed in proportion to the amount of RAGE bound in the initial step.
esRAGE:
Standards or AF samples and detection antibody (esRAGE antibody conjugated with horseradish peroxidase) were incubated for 16 hours at 4°C in duplicate wells of the micro titer plates, which have been pre-coated with a monoclonal antibody specific for human esRAGE. During this incubation, any esRAGE present in the standards or AF samples is bound by the immobilized antibodies. After repeated washing and aspiration to remove all unbound substances, a substrate solution was added to the wells and color developed in proportion to the amount of esRAGE bound in the initial step.
The color development was stopped with the addition of an acid solution and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA). The concentrations of sRAGE and esRAGE in AF samples were determined by interpolation from individual standard curves composed of human esRAGE.
The calculated inter-assay coefficients of variation (CVs) for sRAGE, esRAGE, IL-6 and MMP-8 immunoassays in our laboratory were 3.2%, 4.8%, 9% and 4.6%, respectively. Calculated intra-assay CVs for sRAGE, esRAGE, IL-6 and MMP-8 were 4.2%, 2.1%, 7.2% and 3.7%, respectively. The sensitivity was calculated to be 33 pg/ml for sRAGE, 0.028 ng/ml for esRAGE, 2.28 pg/ml for IL-6 and 0.05 ng/ml for MMP-8 assays.
Statistical analysis.
The Shapiro-Wilk test was used to evaluate the distribution of data. Amniotic fluid sRAGE and esRAGE concentrations were not normally distributed. Therefore, Kruskal-Wallis and Mann-Whitney U tests were used for comparison of continuous variables. First, the amniotic fluid concentrations of sRAGE and esRAGE of patients with a normal pregnancy in the midtrimester were compared to that of those at term not in labor in order to determine if the amniotic fluid concentrations of sRAGE and esRAGE change with advancing gestational age. Second, patients not in labor were compared to those in spontaneous labor to test if the amniotic fluid concentrations of sRAGE and esRAGE change in the process of term parturition. Finally, the amniotic fluid concentrations of sRAGE and esRAGE were measured and compared between patients with preterm labor and intact membranes, as well as between those with preterm PROM, with the aim of determining if the amniotic fluid concentrations of sRAGE and esRAGE change with and without the presence of intra-amniotic infection/inflammation. Spearman’s rho was utilized for the analysis of non-parametric correlations. Patients were matched for gestational age at amniocentesis (within one week) to compare the AF concentrations of sRAGE and esRAGE among patients with preterm labor with (n=42) and without intra-amniotic infection/inflammation (IAI) (n=82) as well as among patients with preterm PROM with (n=40) and without IAI (n=40). The statistical package employed was SPSS 12 (SPSS Inc, Chicago, IL). A p value of <0.05 was considered statistically significant.
RESULTS
Table 1 displays the demographic and clinical characteristics of patients in the midtrimester, term no labor and term in labor groups. Tables 2 and 3 display the demographic and clinical characteristics of patients with preterm labor and intact membranes and those with preterm PROM, respectively. There were no differences in the proportion of patients receiving antibiotic therapy at the time of amniocentesis between patients with IAI and those without IAI in both patients with preterm labor and intact membranes and those with preterm PROM.
Table 1.
Demographic and clinical characteristics of patients in the midtrimester, and those at term with and without spontaneous labor
| Midtrimester (n=68) |
Term No labor (n=24) |
Term In labor (n=51) |
P-value* | |
|---|---|---|---|---|
| Maternal age (years) | 36 (24–42) |
28.5 (17–40) |
23 (16–37) |
<0.01 |
| Gestational age at amniocentesis (weeks) | 16 (14–18) |
39.3 (38–42) |
39.2 (37–41.7) |
<0.01 |
| Gestational age at delivery (weeks) | 39 (37–41) |
39.3 (38–42) |
39.2 (37–41.7) |
NS |
| Birthweight (grams) | 3,347 (2,809–4,180) |
3,405 (2,810–4,530) |
3,250 (2,540–4,440) |
NS |
Values are expressed as percentage (number) or median (range).
NS: not significant.
Kruskal-Wallis test
Table 2.
Demographic and clinical characteristics of patients with preterm labor and intact membranes
| No intra-amniotic infection/inflammation (n=82) |
Intra-amniotic infection/inflammation (n=42) | P-value | |
|---|---|---|---|
| Maternal age (years) | 22 (15 – 43) |
23 (15 – 44) |
NS |
| BMI (Kg/m2) | 22.6 (15.9 – 32.9) |
24.2 (17.3 – 32.4) |
NS |
| Gestational age at amniocentesis (weeks) | 31.2 (23.1 – 34.5) |
31.2 (23.2 – 34.5) |
NS |
| Gestational age at delivery (weeks) | 38.3 (28.5 – 41.4) |
32 (23.3 – 39) |
<0.001 |
| Birthweight (grams) | 3,130 (730 – 4,470) |
1,940 (660 – 3,640) |
<0.001 |
| Antibiotic therapy at amniocentesis | 1.2 (1) |
7.1 (3) |
NS |
Values are expressed as percentage (number) or median (range).
BMI: body mass index; NS: not significant.
Table 3.
Demographic and clinical characteristics of patients with preterm PROM
| No intra-amniotic infection/inflammation (n=40) |
Intra-amniotic infection/inflammation (n=40) | P-value | |
|---|---|---|---|
| Maternal age (years) | 25 (15 – 40) |
29.5 (17 – 43) |
NS |
| BMI (Kg/m2) | 23.7 (19.1 – 37.3) |
24.2 (18.1 – 34.7) |
NS |
| Gestational age at amniocentesis (weeks) | 29.5 (23 – 34.3) |
29.5 (22.9 – 34.6) |
NS |
| Gestational age at delivery (weeks) | 32.1 (24.6 – 35.6) |
30.4 (24.9 – 34.7) |
0.004 |
| Birthweight (grams) | 1,800 (740 – 2,670) |
1,620 (740 – 2,600) |
0.02 |
| Antibiotic therapy at amniocentesis | 17.5 (7) |
22.5 (9) |
NS |
Values are expressed as percentage (number) or median (range).
BMI: body mass index; NS: not significant.
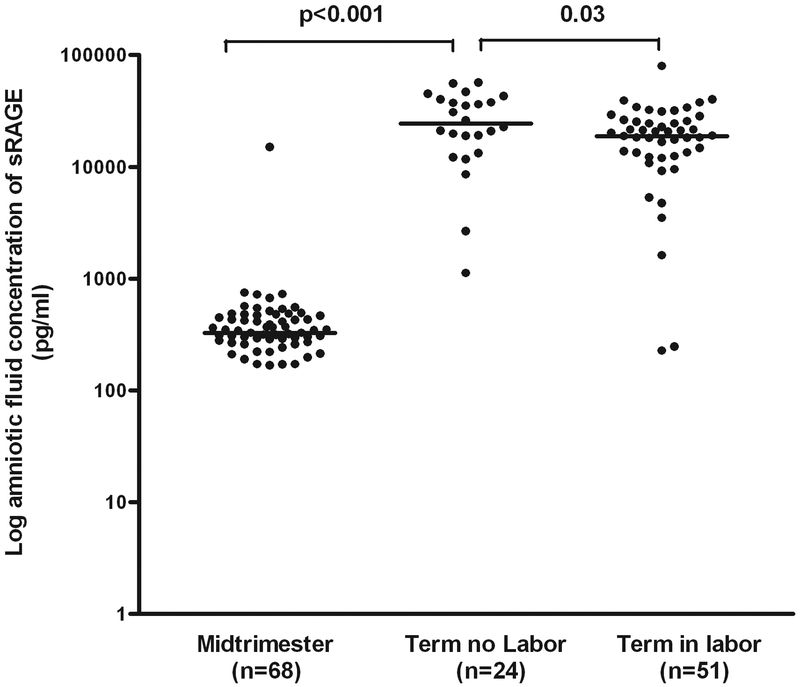
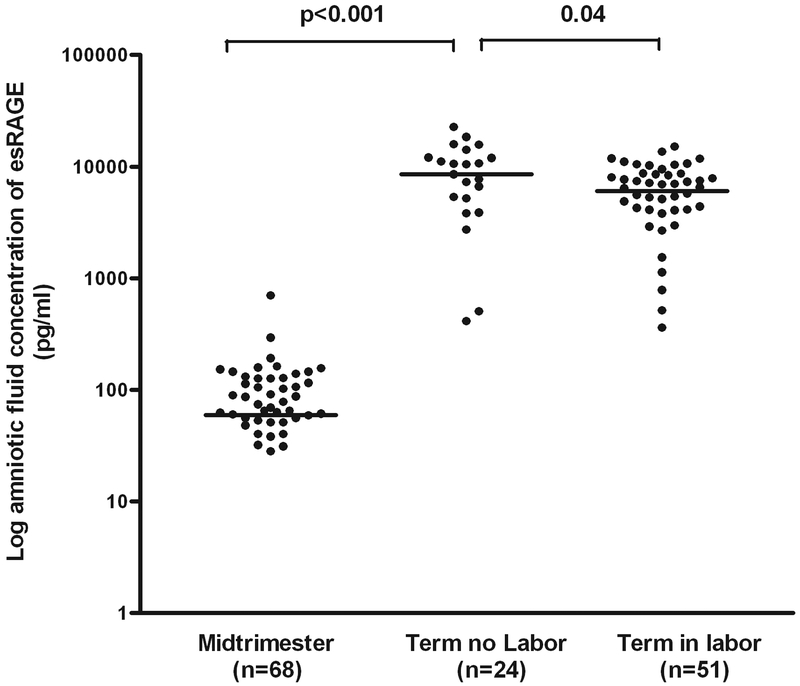
Patients at term not in labor had a higher median AF concentration of sRAGE than those in the mid-trimester (median: 24,346 pg/ml, range: 1,153.1–56,760 vs. median: 327.6 pg/ml, range: 0–15,069; p<0.001) and those at term in labor (median: 24,346 pg/ml, range: 1,153.1–56,760 vs. median: 18,819 pg/ml, range: 0–79,754; p=0.03) (Figure 1). Similarly, the median amniotic fluid concentration of esRAGE was significantly higher in patients at term not in labor than that of those in the mid-trimester (median: 8,520 pg/ml, range: 0–22,628 vs. median: 59.5 pg/ml, range: 0–699; p<0.001) and that of those at term in labor (median: 8,520 pg/ml, range: 0–22,628 vs. median: 6,056 pg/ml, range: 0–15,073; p=0.04) (Figure 2).
Figure 1. Amniotic fluid concentration of sRAGE in normal pregnancies at mid-trimester and in those at term with and without labor.
Patients at term not in labor had a higher median amniotic fluid concentration of sRAGE (median: 24,346 pg/ml, range: 1,153.1–56,760) than those in the mid-trimester (median: 327.6 pg/ml, range: 0–15,069; p<0.001) and those at term in labor (sRAGE, median: 18,819 pg/ml, range: 0–79,754; p=0.03). The logarithmic scale was used to better visualize the midtrimester values.
Figure 2. Amniotic fluid concentration of esRAGE in normal pregnancies at mid-trimester and in those at term with and without labor.
Patients at term not in labor had a higher median amniotic fluid concentration of esRAGE (median: 8,520 pg/ml, range: 0–22,628) than those in the mid-trimester (median: 59.5 pg/ml, range: 0–699; p<0.001) and those at term in labor (median: 6,056 pg/ml, range: 0–15,073; p=0.04). The logarithmic scale was used to better visualize the midtrimester values.
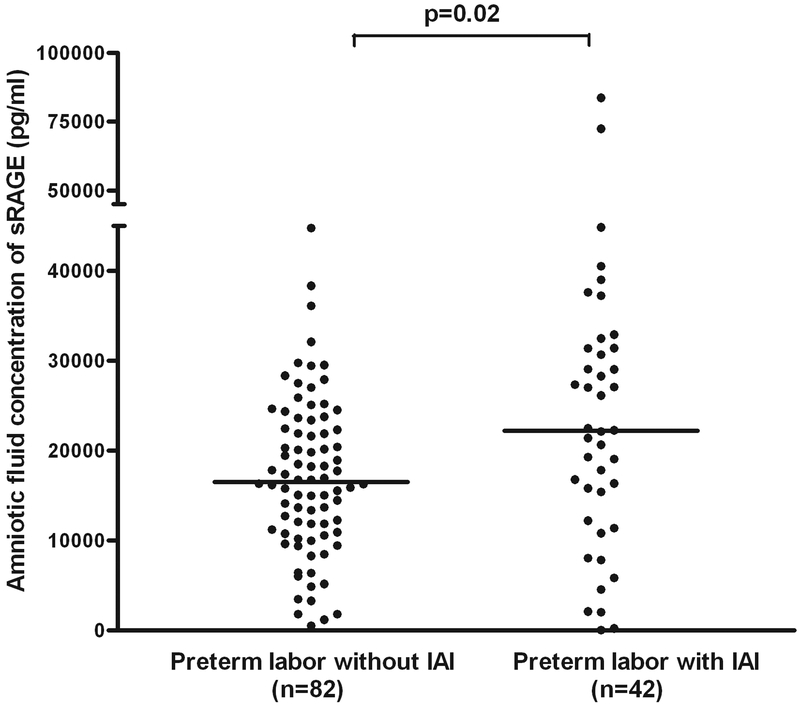
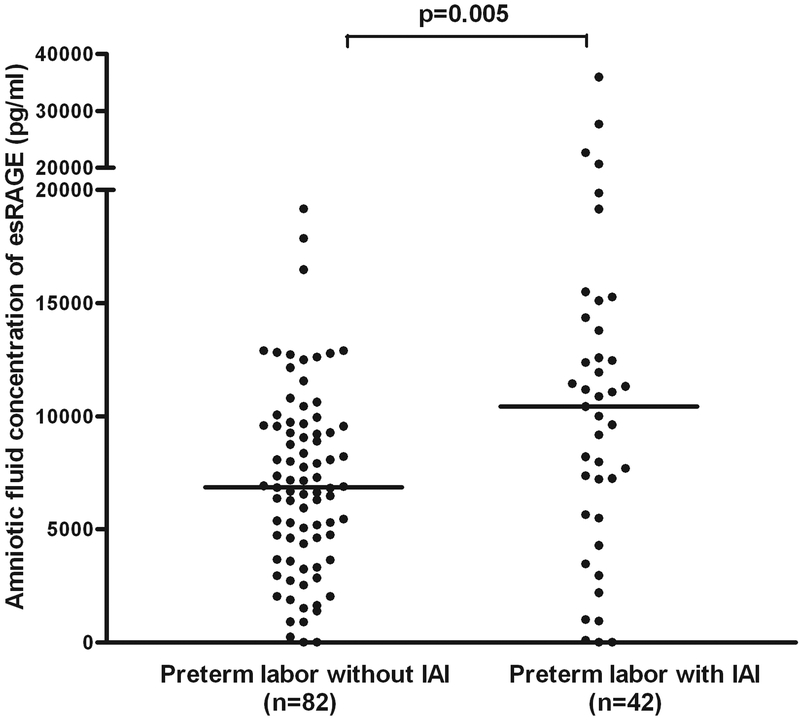
Patients with preterm labor and intact membranes with intra-amniotic infection/inflammation (IAI) had a higher median AF concentration of sRAGE (median: 22,195 pg/ml, range: 0–83,644 vs. median: 16,538 pg/ml, range: 484.5–44,766; p=0.02) and esRAGE (median: 10,420 pg/ml, range: 0–35,958 vs. median: 6,859 pg/ml, range: 0–19,158; p=0.005) than those without IAI (Figures 3 and 4). Among patients with spontaneous preterm labor with intact membranes without IAI included in this study, 76% (62/82) delivered at term and 24% (20/82) delivered preterm. A sub-analysis performed in this subgroup demonstrated that those who delivered preterm had a significantly higher median amniotic fluid concentration of sRAGE (median: 21,391 pg/ml, range: 0–83,644 vs. median: 16,224 pg/ml, range: 1,158–44,766; p=0.02) and esRAGE (median: 8,743 pg/ml, range: 0–35,958 vs. median: 6,859 pg/ml, range: 0–17,854; p=0.03) than those who delivered at term.
Figure 3. Amniotic fluid concentration of sRAGE among women with spontaneous preterm labor and intact membranes.
Patients with preterm labor and intact membranes with intra-amniotic infection/inflammation (IAI) had a higher median AF concentration of sRAGE than those without IAI (median: 22,195 pg/ml, range: 0–83,644 vs. 16,538 pg/ml, range: 484.5–44,766; p=0.02).
Figure 4. Amniotic fluid concentration of esRAGE among women with spontaneous preterm labor and intact membranes.
Patients with preterm labor and intact membranes with intra-amniotic infection/inflammation (IAI) had a higher median AF concentration of esRAGE than those without IAI (median: 10,420 pg/ml, range: 0–35,958 vs. median: 6,859 pg/ml, range: 0–19,158; p=0.005).
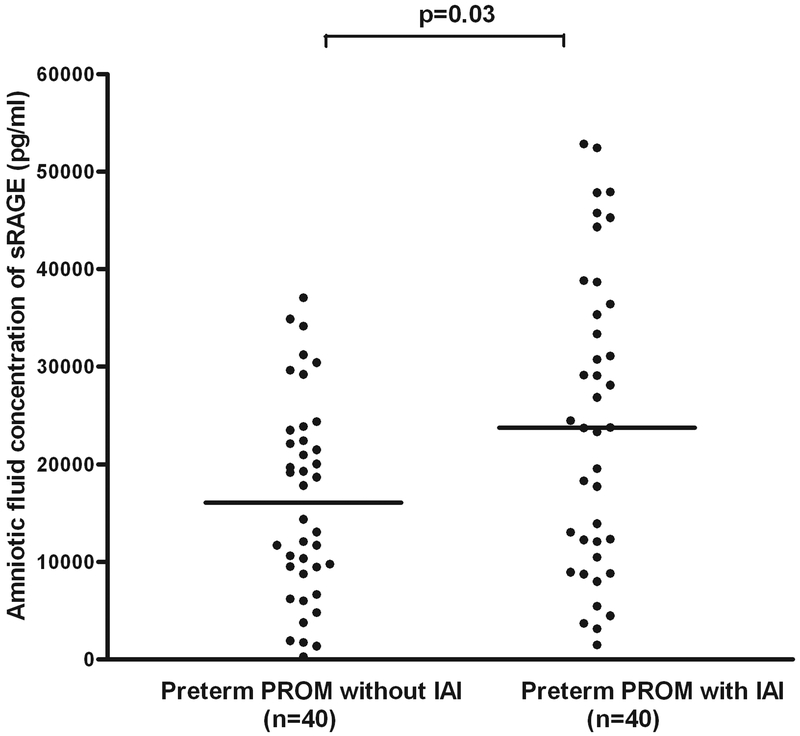
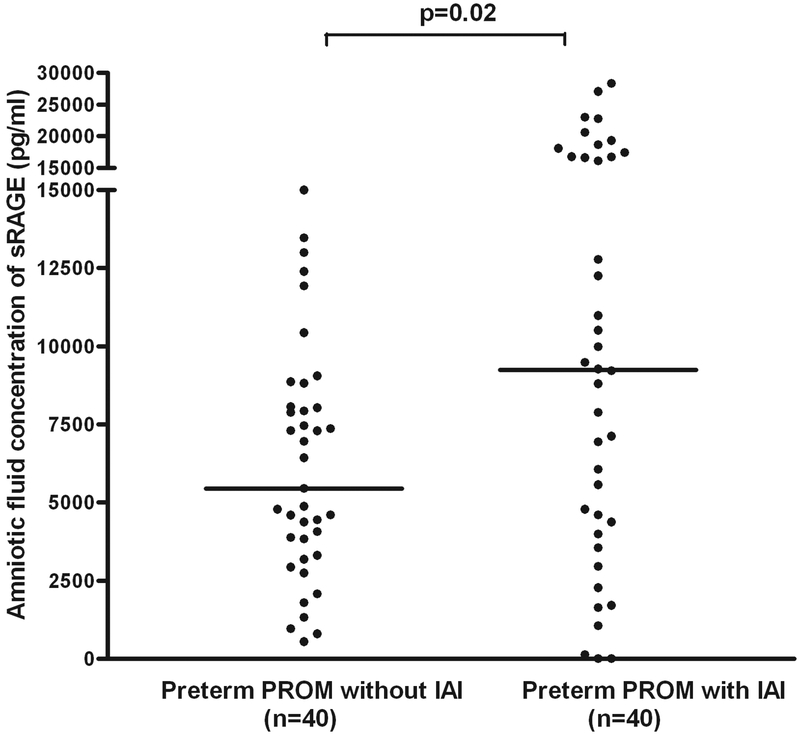
Also, patients with preterm PROM with IAI had a higher median AF concentration of sRAGE (median: 23,730 pg/ml, range: 1,466–52,827 vs. median: 16,077 pg/ml, range: 267–37,070; p=0.03) and esRAGE (median: 9,242 pg/ml, range: 0–28,337 vs. median: 5,451 pg/ml, range: 543–14,996; p=0.02) than those without IAI (Figures 5 and 6). There were 5 patients with gestational diabetes in the group of preterm labor and intact membranes and 3 patients with gestational and pre-gestational diabetes in the preterm PROM group. The results of the study did not change after excluding these patients from the analysis.
Figure 5. Amniotic fluid concentration of sRAGE in women with preterm prelabor rupture of the membranes (preterm PROM).
Patients with preterm PROM with intra-amniotic infection/inflammation (IAI) had a higher median AF concentration of sRAGE than those without IAI (median: 23,730 pg/ml, range: 1,466–52,827 vs. median: 16,077 pg/ml, range: 267–37,070; p=0.03).
Figure 6. Amniotic fluid concentration of sRAGE in women with preterm prelabor rupture of the membranes (preterm PROM).
Patients with preterm PROM with intra-amniotic infection/inflammation (IAI) had a higher median AF concentration of esRAGE than those without IAI (median: 9,242 pg/ml, range: 0–28,337 vs. median: 5,451 pg/ml, range: 543–14,996; p=0.02).
Among patients with preterm labor and intact membranes, there was a significant correlation only between the AF concentration of MMP-8 and sRAGE (r=0.19; p=0.04), and almost significant correlation between esRAGE and MMP-8 (r=0.18; p=0.053), but not between the AF concentrations of sRAGE (r=0.13; p=0.2) or esRAGE (r=0.12; p=0.2) and IL-6.
DISCUSSION
Principal findings of the study.
The amniotic fluid concentrations of sRAGE and esRAGE: 1) significantly increase with gestational age; 2) are significantly lower in patients with spontaneous labor at term compared to that of those not in labor; and 3) intra-amniotic infection/inflammation is associated with increased amniotic fluid concentrations of sRAGE and esRAGE. These changes may represent part of the innate immune response to intra-amniotic infection/inflammation.
The biology of RAGE, sRAGE and esRAGE.
RAGE is a member of the immunoglobulin receptor superfamily that is composed of 332-ammino acid extracellular component consisting of 2 “C”-type domains preceded by 1 “V”-type immunoglobulin-like domain [45, 88, 102]. RAGE has a single trans-membrane domain followed by a highly charged 43-amnino acid cytosolic tail [88]. The extracellular domain is essential for ligand binding and the cytosolic tail is central to RAGE-induced intracellular signaling [62, 85, 87, 88]. The human RAGE gene is on chromosome 6 in the major histocompatibility complex [92]. Nuclear binding factor (NF)κB sites, an interferon-γ response element, and a NF-interleukin-6 DNA binding motif are located on the RAGE promote [51]. Thus, NF-κB sites control, in part, cellular expression of RAGE, linking RAGE to the inflammatory response [51]. In adults, RAGE is expressed in multiple cell types, such as neuronal cells and microglia [21, 35, 50, 55, 56, 99, 101], endothelium [4], vascular smooth muscle [4], monocyte-derived macrophages [4], cardiac myocytes [4], hepatocytes [19], podocytes [99], and lung alveolar epithelial cells [90].
Several truncated forms of the RAGE have been described [12, 13, 57, 67, 103]. These isoforms may arise from alternative splicing of the pre-mRNA of RAGE [103]. The mRNA of the C-truncated isoform of RAGE does not contain the exon 10 sequence that encodes the transmembrane-spanning domain [103]. This isoform has been proposed to be secreted extracellularly as endogenous secretory RAGE (esRAGE) and can be detected in human sera [103]. However, Geroldi et al. [22] proposed that esRAGE may not represent the entire pool of soluble RAGE (sRAGE) that is present in the serum, because it remains possible that some RAGE isoforms may result from proteolytic cleavage from the native membranous receptor by enzymes such as MMPs [37].
Soluble isoforms of RAGE have been proposed to play an antagonistic role with RAGE by competing with cell surface RAGE for ligand-binding [22]. Evidence in support of this view includes: 1) administration of sRAGE attenuated alveolar bone loss and decreased the concentration of MMP-9, MMP-2, and TNF-α in gingival tissue extracts in an animal model of periodontitis induced by Porphyromonas gingivalis [47]; 2) the administration of sRAGE to diabetic rats blocked vascular leakage in the intestine, skin and kidney [97]; 3) the administration of sRAGE to apolipoprotein E null mice, who develop spontaneous hypercholesterolemia and atherosclerosis, had a dose-dependent decrease in atherosclerosis area and complexity compared to vehicle-treated mice [68]; 4) the administration of sRAGE to hyperglycemic rats in whom neointimal expansion triggered by acute vessel injury reduced the smooth muscle cell numbers and the degree of matrix encompassed within the neointima [81]. This decoy function of sRAGE has been proposed to provide a regulatory negative feedback mechanism to modulate the activity of RAGE [22]. More recently, in vitro studies have suggested that sRAGE may have biological effects other than acting as a decoy to trap cell surface-bound RAGE ligands [70]. The authors reported that: 1) sRAGE dose-dependently induced the production of pro-inflammatory cytokines including IL-6 and TNF-α, as well as the production of chemokine MIP-2 by spleen cells via the NF-κB and Mac-1 pathways; and that 2) sRAGE induces neuthrophil chemotaxis [70]. The authors concluded that sRAGE acts as an important proinflammatory and chemotactic molecule [70]. However, a key limitation of the latter experiments is that they were restricted to the in vitro setting.
RAGE and the immune response.
Lin [53] proposed that RAGE is a pattern recognition receptor that may use signaling mechanisms parallel to Toll-like receptors during the innate immune response. Evidence in support of this view includes: 1) RAGE may recognize its ligands through their shared three dimensional structure [53]; 2) RAGE is expressed in phagocytes such as macrophages [4], monocytes [4], and astrocytes [69]; 3) binding of AGEs and other endogenous ligands to RAGE activates the NF-κB pathway [53]; moreover, blocking access of the ligand to RAGE (with excess sRAGE or anti-RAGE) or inhibition of RAGE signaling, suppressed NF-κB activation in mononuclear phagocytes [33]; 4) RAGE-ligand interaction activates mitogen-activated protein kinase (MAPK) family members such as Jun-N-terminal kinase (JNK), p38 and extracellular signal regulated kinase (ERK) [61, 91, 94]; and 5) RAGE null mice (RAGE−/−) are protected from the lethal effects of septic shock caused by cecal ligation and puncture, an effect that is largely dependent on the innate immune response [52]. Recent studies have illustrated definitive roles for RAGE in effective T lymphocyte priming in vitro and in vivo, thereby establishing that RAGE participates in adaptive immune responses as well. Collectively, this evidence supports the role of RAGE signaling in innate and adaptive immune responses [53].
Possible sources of sRAGE in the amniotic cavity.
The observations that RAGE is expressed in the amnion epithelium, extravillous trophoblast and decidual cells in patients without chorioamnionitis as well as in the neutrophils in the choriodecidua of patients with histologic chorioamnionitis [6] suggest that these cells may contribute to the increased amniotic fluid concentration of sRAGE in patients with IAI. Since the chorioamniotic membranes are considered fetal tissues, it is possible that the fetus may contribute to the increased AF concentration of sRAGE in the amniotic fluid in the same manner that the fetus may participate in the innate immune response by increasing the mRNA expression of Surfactant protein-A (SP-A) in the chorioamniotic membranes during histologic chorioamnionitis [29]. SP-A is produced by the fetal lung, participates in innate immunity, and has been proposed to play a role in the initiation of parturition in mice [10].
Changes in the AF concentration of sRAGE and esRAGE with advancing gestational age and spontaneous labor at term in normal pregnancies may represent changes in the innate immunity in preparation for delivery. Indeed, among women with a normal pregnancy, there was a dramatic increase in the amniotic fluid concentration of sRAGE and esRAGE between those in the midtrimester and those at term in the absence of labor (74-fold and 143-fold, respectively). However, there was a significantly decrease in the amniotic fluid concentration of sRAGE and esRAGE in the presence of spontaneous labor at term. These results may be unexpected since parturition has been proposed to be an inflammatory process[27, 30, 43] and compelling evidence suggests that RAGE has pro-inflammatory properties. One interpretation of the findings may be that both sRAGE and esRAGE may be consumed by an over expression of RAGE ligands during labor. It is possible that sRAGE and esRAGE may play an antagonistic role with RAGE by competing with cell surface RAGE for ligand-binding. This decoy function of sRAGE has been proposed to provide a regulatory negative feedback mechanism to modulate the activity of RAGE. Moreover, although a significant correlation was found between the AF concentration of MMP-8 and sRAGE, and almost also between esRAGE and MMP-8 (p=0.053), there was not a significant relationship between the AF concentrations of sRAGE or esRAGE and IL-6 in patients with spontaneous preterm labor with intact membranes.
Possible roles of sRAGE and esRAGE in the amniotic cavity during intra-amniotic infection/inflammation.
To the extent that RAGE represent a novel pattern recognition receptor [53], it is possible that increased amniotic fluid concentrations of sRAGE and esRAGE may participate in the immune response to intra-amniotic infection. Thus both sRAGE and esRAGE may be added to the list of molecules involved in the innate immune response to intra-amniotic infection/inflammation including chemokines [18, 39, 40, 72, 100], cytokines [1, 2, 16, 32, 41, 44, 58, 66, 71, 74, 77, 83], or antimicrobial peptides and proteins [17, 64, 65]. Alternatively, increased AF concentration of sRAGE and esRAGE may represent additional biomarkers of inflammation during IAI.
RAGE mediates the increased secretion of IL-6 and TNF-α in cultured astrocytes induced by S100B [69]. S100B is a calcium binding protein that can stimulate the expression of proinflammatory cytokines and induce neural apoptosis [69]. Since the AF concentration of S100B is increased in patients with intra-amniotic infection/inflammation [20], it is also possible that increased AF concentration of sRAGE may modulate the pro-inflammatory effects of S100B during IAI.
Conclusions.
The AF concentration of sRAGE and esRAGE increases dramatically with advancing gestational age and decreases with spontaneous labor at term. The observation that AF concentration of sRAGE and esRAGE increases with intra-amniotic infection/inflammation suggests that these soluble isoforms of the receptor for advanced glycation end products may participate in the innate immune response to intra-amniotic infection/inflammation.
Acknowledgment:
This research was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Disclosure: The authors report no conflicts of interest.
Reference List
- 1.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am. J Obstet Gynecol. 1999;181:989–994. [DOI] [PubMed] [Google Scholar]
- 2.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH, et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am. J Obstet Gynecol. 2000;182:135–141. [DOI] [PubMed] [Google Scholar]
- 3.Belady PH, Farkouh LJ,Gibbs RS. Intra-amniotic infection and premature rupture of the membranes. Clin. Perinatol 1997;24:43–57. [PubMed] [Google Scholar]
- 4.Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–1712. [PMC free article] [PubMed] [Google Scholar]
- 5.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–2835. [DOI] [PubMed] [Google Scholar]
- 6.Buhimschi IA, Zhao G, Pettker CM, Bahtiyar MO, Magloire LK, Thung S, et al. The receptor for advanced glycation end products (RAGE) system in women with intraamniotic infection and inflammation. Am J Obstet Gynecol. 2007;196:181–13. [DOI] [PubMed] [Google Scholar]
- 7.Carroll SG, Philpott-Howard J,Nicolaides KH. Amniotic fluid gram stain and leukocyte count in the prediction of intrauterine infection in preterm prelabour amniorrhexis. Fetal Diagn. Ther 1996;11:1–5. [DOI] [PubMed] [Google Scholar]
- 8.Cassell GH, Davis RO, Waites KB, Brown MB, Marriott PA, Stagno S, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm. Dis 1983;10:294–302. [PubMed] [Google Scholar]
- 9.Chavakis T, Bierhaus A, Al Fakhri N, Schneider D, Witte S, Linn T, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp. Med 2003;198:1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condon JC, Jeyasuria P, Faust JM,Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc. Natl. Acad. Sci. U. S. A 2004;101:4978–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dammann O,Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr. Res 1997;42:1–8. [DOI] [PubMed] [Google Scholar]
- 12.Ding Q,Keller JN. Evaluation of rage isoforms, ligands, and signaling in the brain. Biochim. Biophys. Acta 2005;1746:18–27. [DOI] [PubMed] [Google Scholar]
- 13.Ding Q,Keller JN. Splice variants of the receptor for advanced glycosylation end products (RAGE) in human brain. Neurosci. Lett 2005;373:67–72. [DOI] [PubMed] [Google Scholar]
- 14.Drinda S, Franke S, Ruster M, Petrow P, Pullig O, Stein G, et al. Identification of the receptor for advanced glycation end products in synovial tissue of patients with rheumatoid arthritis. Rheumatol. Int 2005;25:411–413. [DOI] [PubMed] [Google Scholar]
- 15.Elovitz MA, Mrinalini C,Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr. Res 2006;59:50–55. [DOI] [PubMed] [Google Scholar]
- 16.Emerson GA, Bry K, Hallman M, Jobe AH, Wada N, Ervin MG, et al. Intra-amniotic interleukin-1 alpha treatment alters postnatal adaptation in premature lambs. Biol. Neonate 1997;72:370–379. [DOI] [PubMed] [Google Scholar]
- 17.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern. Fetal Neonatal Med. 2003;13:2–21. [DOI] [PubMed] [Google Scholar]
- 18.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern. Fetal Neonatal Med. 2005;17:365–373. [DOI] [PubMed] [Google Scholar]
- 19.Fehrenbach H, Weiskirchen R, Kasper M,Gressner AM. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology. 2001;34:943–952. [DOI] [PubMed] [Google Scholar]
- 20.Friel LA, Romero R, Edwin S, Kae NJ, Gomez R, Chaiworapongsa T, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat. Med 2007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasic-Milenkovic J, Loske C,Munch G. Advanced glycation endproducts cause lipid peroxidation in the human neuronal cell line SH-SY5Y. J Alzheimers. Dis 2003;5:25–30. [DOI] [PubMed] [Google Scholar]
- 22.Geroldi D, Falcone C,Emanuele E. Soluble receptor for advanced glycation end products: from disease marker to potential therapeutic target. Curr. Med. Chem 2006;13:1971–1978. [DOI] [PubMed] [Google Scholar]
- 23.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur. J. Obstet. Gynecol. Reprod. Biol 1998;78:5–10. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs RS, Dinsmoor MJ, Newton ER,Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol. 1988;72:823–828. [DOI] [PubMed] [Google Scholar]
- 25.Goncalves LF, Chaiworapongsa T,Romero R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev 2002;8:3–13. [DOI] [PubMed] [Google Scholar]
- 26.Gray DJ, Robinson HB, Malone J,Thomson RB, Jr. Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat. Diagn 1992;12:111–117. [DOI] [PubMed] [Google Scholar]
- 27.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J. Obstet. Gynecol 2006;195:394–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hameed C, Tejani N, Verma UL,Archbald F. Silent chorioamnionitis as a cause of preterm labor refractory to tocolytic therapy. Am. J Obstet Gynecol. 1984;149:726–730. [DOI] [PubMed] [Google Scholar]
- 29.Han YM, Romero R, Kim YM, Kim JS, Richani K, Friel LA, et al. Surfactant protein-A mRNA expression by human fetal membranes is increased in histological chorioamnionitis but not in spontaneous labour at term. J Pathol. 2007;211:489–496. [DOI] [PubMed] [Google Scholar]
- 30.Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–786. [DOI] [PubMed] [Google Scholar]
- 31.Hatjis CG,Swain M. Systemic tocolysis for premature labor is associated with an increased incidence of pulmonary edema in the presence of maternal infection. Am. J. Obstet. Gynecol 1988;159:723–728. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman DR, Romero R,Johnston JM. Detection of platelet-activating factor in amniotic fluid of complicated pregnancies. Am. J Obstet Gynecol. 1990;162:525–528. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002;3:123–135. [DOI] [PubMed] [Google Scholar]
- 35.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol. Chem 1995;270:25752–25761. [DOI] [PubMed] [Google Scholar]
- 36.Horowitz S, Mazor M, Romero R, Horowitz J,Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod. Med 1995;40:375–379. [PubMed] [Google Scholar]
- 37.Hudson BI, Harja E, Moser B,Schmidt AM. Soluble levels of receptor for advanced glycation endproducts (sRAGE) and coronary artery disease: the next C-reactive protein? Arterioscler. Thromb. Vasc. Biol 2005;25:879–882. [DOI] [PubMed] [Google Scholar]
- 38.Jacobsson B,Hagberg G. Antenatal risk factors for cerebral palsy. Best. Pract. Res. Clin. Obstet Gynaecol. 2004;18:425–436. [DOI] [PubMed] [Google Scholar]
- 39.Jacobsson B, Holst RM, Andersson B,Hagberg H. Monocyte chemotactic protein-2 and −3 in amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation and preterm delivery. Acta Obstet Gynecol. Scand 2005;84:566–571. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsson B, Holst RM, Wennerholm UB, Andersson B, Lilja H,Hagberg H. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am. J Obstet Gynecol. 2003;189:1161–1167. [DOI] [PubMed] [Google Scholar]
- 41.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Wennerholm UB, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol. Scand 2003;82:120–128. [DOI] [PubMed] [Google Scholar]
- 42.Katz J, Bhattacharyya I, Farkhondeh-Kish F, Perez FM, Caudle RM,Heft MW. Expression of the receptor of advanced glycation end products in gingival tissues of type 2 diabetes patients with chronic periodontal disease: a study utilizing immunohistochemistry and RT-PCR. J Clin. Periodontol 2005;32:40–44. [DOI] [PubMed] [Google Scholar]
- 43.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM,Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J. Obstet. Gynecol 1999;181:1530–1536. [DOI] [PubMed] [Google Scholar]
- 44.Keelan JA, Yang J, Romero RJ, Chaiworapongsa T, Marvin KW, Sato TA, et al. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol. Reprod 2004;70:253–259. [DOI] [PubMed] [Google Scholar]
- 45.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du YS, et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol. Chem 1999;274:31740–31749. [DOI] [PubMed] [Google Scholar]
- 46.Krieger M,Stern DM. Series introduction: multiligand receptors and human disease. J Clin. Invest 2001;108:645–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalla E, Lamster IB, Feit M, Huang L, Spessot A, Qu W, et al. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J Clin. Invest 2000;105:1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lalla E, Lamster IB,Schmidt AM. Enhanced interaction of advanced glycation end products with their cellular receptor RAGE: implications for the pathogenesis of accelerated periodontal disease in diabetes. Ann Periodontol. 1998;3:13–19. [DOI] [PubMed] [Google Scholar]
- 49.Lalla E, Lamster IB, Stern DM,Schmidt AM. Receptor for advanced glycation end products, inflammation, and accelerated periodontal disease in diabetes: mechanisms and insights into therapeutic modalities. Ann Periodontol. 2001;6:113–118. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Qu X,Schmidt AM. Sp1-binding elements in the promoter of RAGE are essential for amphoterin-mediated gene expression in cultured neuroblastoma cells. J Biol. Chem 1998;273:30870–30878. [DOI] [PubMed] [Google Scholar]
- 51.Li J,Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol. Chem 1997;272:16498–16506. [DOI] [PubMed] [Google Scholar]
- 52.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin. Invest 2004;113:1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin L RAGE on the Toll Road? Cell Mol. Immunol 2006;3:351–358. [PubMed] [Google Scholar]
- 54.Lu C, He JC, Cai W, Liu H, Zhu L,Vlassara H. Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc. Natl. Acad. Sci. U. S. A 2004;101:11767–11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp. Neurol 2001;171:29–45. [DOI] [PubMed] [Google Scholar]
- 56.Ma L, Carter RJ, Morton AJ,Nicholson LF. RAGE is expressed in pyramidal cells of the hippocampus following moderate hypoxic-ischemic brain injury in rats. Brain Res. 2003;966:167–174. [DOI] [PubMed] [Google Scholar]
- 57.Malherbe P, Richards JG, Gaillard H, Thompson A, Diener C, Schuler A, et al. cDNA cloning of a novel secreted isoform of the human receptor for advanced glycation end products and characterization of cells co-expressing cell-surface scavenger receptors and Swedish mutant amyloid precursor protein. Brain Res. Mol. Brain Res. 1999;71:159–170. [DOI] [PubMed] [Google Scholar]
- 58.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am. J Obstet Gynecol. 1999;181:1142–1148. [DOI] [PubMed] [Google Scholar]
- 59.Mays J, Verma U, Klein S,Tejani N. Acute appendicitis in pregnancy and the occurrence of major intraventricular hemorrhage and periventricular leukomalacia. Obstet Gynecol. 1995;86:650–652. [DOI] [PubMed] [Google Scholar]
- 60.Moser B, Desai DD, Downie MP, Chen Y, Yan SF, Herold K, et al. Receptor for advanced glycation end products expression on T cells contributes to antigen-specific cellular expansion in vivo. J. Immunol 2007;179:8051–8058. [DOI] [PubMed] [Google Scholar]
- 61.Nah SS, Choi IY, Yoo B, Kim YG, Moon HB,Lee CK. Advanced glycation end products increases matrix metalloproteinase-1, −3, and −13, and TNF-alpha in human osteoarthritic chondrocytes. FEBS Lett. 2007;581:1928–1932. [DOI] [PubMed] [Google Scholar]
- 62.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol. Chem 1992;267:14998–15004. [PubMed] [Google Scholar]
- 63.Nelson KB. The neurologically impaired child and alleged malpractice at birth. Neurol. Clin 1999;17:283–293. [DOI] [PubMed] [Google Scholar]
- 64.Otsuki K, Yoda A, Saito H, Mitsuhashi Y, Toma Y, Shimizu Y, et al. Amniotic fluid lactoferrin in intrauterine infection. Placenta. 1999;20:175–179. [DOI] [PubMed] [Google Scholar]
- 65.Pacora P, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH, et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am. J Obstet Gynecol. 2000;183:904–910. [DOI] [PubMed] [Google Scholar]
- 66.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, et al. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am. J Obstet Gynecol. 2000;183:1138–1143. [DOI] [PubMed] [Google Scholar]
- 67.Park IH, Yeon SI, Youn JH, Choi JE, Sasaki N, Choi IH, et al. Expression of a novel secreted splice variant of the receptor for advanced glycation end products (RAGE) in human brain astrocytes and peripheral blood mononuclear cells. Mol. Immunol 2004;40:1203–1211. [DOI] [PubMed] [Google Scholar]
- 68.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr., Chow WS, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med 1998;4:1025–1031. [DOI] [PubMed] [Google Scholar]
- 69.Ponath G, Schettler C, Kaestner F, Voigt B, Wentker D, Arolt V, et al. Autocrine S100B effects on astrocytes are mediated via RAGE. J Neuroimmunol. 2007;184:214–222. [DOI] [PubMed] [Google Scholar]
- 70.Pullerits R, Brisslert M, Jonsson IM,Tarkowski A. Soluble receptor for advanced glycation end products triggers a proinflammatory cytokine cascade via beta2 integrin Mac-1. Arthritis Rheum. 2006;54:3898–3907. [DOI] [PubMed] [Google Scholar]
- 71.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am. J Obstet Gynecol. 1989;160:1117–1123. [DOI] [PubMed] [Google Scholar]
- 72.Romero R, Ceska M, Avila C, Mazor M, Behnke E,Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am. J Obstet Gynecol. 1991;165:813–820. [DOI] [PubMed] [Google Scholar]
- 73.Romero R, Espinoza J, Chaiworapongsa T,Kalache K. Infection and prematurity and the role of preventive strategies. Semin. Neonatol 2002;7:259–274. [DOI] [PubMed] [Google Scholar]
- 74.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am. J Obstet Gynecol. 1989;161:336–341. [DOI] [PubMed] [Google Scholar]
- 75.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am. J Obstet Gynecol. 1991;165:821–830. [DOI] [PubMed] [Google Scholar]
- 76.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am. J Obstet Gynecol. 1988;159:661–666. [DOI] [PubMed] [Google Scholar]
- 77.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC,Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found. Symp 1992;167:205–220. [DOI] [PubMed] [Google Scholar]
- 78.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am. J Obstet Gynecol. 1989;161:817–824. [DOI] [PubMed] [Google Scholar]
- 79.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am. J Obstet Gynecol. 1993;169:805–816. [DOI] [PubMed] [Google Scholar]
- 80.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am. J Obstet Gynecol. 1993;169:839–851. [DOI] [PubMed] [Google Scholar]
- 81.Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin. Invest 2003;111:959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakurai S, Yonekura H, Yamamoto Y, Watanabe T, Tanaka N, Li H, et al. The AGE-RAGE system and diabetic nephropathy. J. Am. Soc. Nephrol 2003;14:S259–S263. [DOI] [PubMed] [Google Scholar]
- 83.Santhanam U, Avila C, Romero R, Viguet H, Ida N, Sakurai S, et al. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine. 1991;3:155–163. [DOI] [PubMed] [Google Scholar]
- 84.Schleicher ED, Wagner E,Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin. Invest 1997;99:457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmidt AM, Hori O, Brett J, Yan SD, Wautier JL,Stern D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler. Thromb 1994;14:1521–1528. [DOI] [PubMed] [Google Scholar]
- 86.Schmidt AM,Stern D. Atherosclerosis and diabetes: the RAGE connection. Curr. Atheroscler. Rep 2000;2:430–436. [DOI] [PubMed] [Google Scholar]
- 87.Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol. Chem 1992;267:14987–14997. [PubMed] [Google Scholar]
- 88.Schmidt AM, Yan SD, Yan SF,Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin. Invest 2001;108:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am. J Obstet Gynecol. 2004;191:1339–1345. [DOI] [PubMed] [Google Scholar]
- 90.Shirasawa M, Fujiwara N, Hirabayashi S, Ohno H, Iida J, Makita K, et al. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells. 2004;9:165–174. [DOI] [PubMed] [Google Scholar]
- 91.Stern D, Yan SD, Yan SF,Schmidt AM. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv. Drug Deliv. Rev 2002;54:1615–1625. [DOI] [PubMed] [Google Scholar]
- 92.Sugaya K, Fukagawa T, Matsumoto K, Mita K, Takahashi E, Ando A, et al. Three genes in the human MHC class III region near the junction with the class II: gene for receptor of advanced glycosylation end products, PBX2 homeobox gene and a notch homolog, human counterpart of mouse mammary tumor gene int-3. Genomics. 1994;23:408–419. [DOI] [PubMed] [Google Scholar]
- 93.Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M,Makino H. Increased expression of receptor for advanced glycation end products by synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2006;54:97–104. [DOI] [PubMed] [Google Scholar]
- 94.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. [DOI] [PubMed] [Google Scholar]
- 95.Wautier JL,Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ. Res 2004;95:233–238. [DOI] [PubMed] [Google Scholar]
- 96.Wautier JL, Wautier MP, Schmidt AM, Anderson GM, Hori O, Zoukourian C, et al. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: a link between surface-associated AGEs and diabetic complications. Proc. Natl. Acad. Sci. U. S. A 1994;91:7742–7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, et al. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin. Invest 1996;97:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wendt T, Harja E, Bucciarelli L, Qu W, Lu Y, Rong LL, et al. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis. 2006;185:70–77. [DOI] [PubMed] [Google Scholar]
- 99.Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162:1123–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Witt A, Berger A, Gruber CJ, Petricevic L, Apfalter P,Husslein P. IL-8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. J Perinat. Med 2005;33:22–26. [DOI] [PubMed] [Google Scholar]
- 101.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. [DOI] [PubMed] [Google Scholar]
- 102.Yan SF, Ramasamy R, Naka Y,Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ. Res 2003;93:1159–1169. [DOI] [PubMed] [Google Scholar]
- 103.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J 2003;370:1097–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am. J. Obstet. Gynecol 1997;177:19–26. [DOI] [PubMed] [Google Scholar]
- 105.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am. J. Obstet. Gynecol 1999;181:773–779. [DOI] [PubMed] [Google Scholar]
- 106.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J Obstet Gynecol. 2001;185:1130–1136. [DOI] [PubMed] [Google Scholar]
- 107.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am. J. Obstet. Gynecol 2000;182:675–681. [DOI] [PubMed] [Google Scholar]