Abstract
Purpose
There is a general understanding that patient educational interventions for enhancing medication adherence are important. However, their success at improving adherence is debatable. This study aimed to assess the influence of different modes of patient education on medication adherence in patients with rheumatoid arthritis (RA).
Materials and methods
One hundred and twenty RA patients with non-adherence, defined as pill count ≥80% or medication-taking behavior questionnaire for Thai patient ≥23, were randomized by block randomization and assigned in a 1:1 allocation ratio to two study arms: multi-component intervention group or single intervention group. The multi-component intervention group received 30-minute directed counseling and a disease information pamphlet. The single intervention group received only a disease information pamphlet. The primary outcomes were an improvement in an adherence rate measured by pill count after 12 weeks. The Thai Clinical Trial Registry number is TCTR20171207003.
Results
After 12 weeks, the pill count adherence rate increased significantly from baseline in both study groups. In the multi-component intervention group, adherence rate increased from 92.21±14.05 to 97.59±10.07 (P=0.002) and in the single intervention group, it increased from 88.60±19.66 to 92.42±14.27 (P=0.044). However, the mean difference between the multi-component intervention group and the single intervention group was not significant (5.38±12.90 vs 3.18±14.23, P=0.531). Clinical outcomes, including disease activity score 28, EuroQoL-5D, EuroQol visual analog scale, pain score, and physician global assessment were unchanged from baseline in both groups.
Conclusion
Patient education significantly improved adherence. However, there were no differences between single education intervention and multi-component education intervention in improving medication adherence. Provision of a disease information pamphlet with or without directed counseling can equally enhance medication adherence of patients with RA.
Keywords: adherence, rheumatoid arthritis, education, disease pamphlet
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory condition, affecting from 0.5% to 1% of the general population worldwide. The precise causes remain uncertain. A variety of genetic, hormonal, environmental, and infectious agents may contribute to susceptibility and pathogenesis. Inadequately controlled RA leads to serious joint damage, functional disability, morbidity, reduced health-related quality of life, and mortality, which cause a vast economic burden.1,2
Prevalence of RA in Thailand is 0.12%.3 Chronic rheumatic diseases have a substantial negative effect on both the health and quality of life of patients and their caregivers. Moreover, these chronic diseases also have a great influence on society, in terms of health resource utilization, work productivity loss, disability, and death.4 In 2007, the average societal cost of RA in Thailand was 41.1% of a patient’s average annual income, of which, 79.6% accounted for the direct cost of treatment.5
Over the last decade, significant developments have been made in the therapy of RA with the occurrence of novel biological therapies. Several studies have shown that timelier and more aggressive therapy with conventional and biological disease-modifying anti-rheumatic drugs (DMARDs) reduces arthritis symptoms and slows disease progression. However, DMARDs adherence rate in patients with RA is very low, and has varied from 16.4% to 76.9%.6–11 The non-adherence results in higher disease activity, radiographic damage, disability, a lower quality of life, and a higher health care cost.12–14 Multiple factors have been shown to affect medication adherence in patients with RA. However, there are diverging results about the influences of patient and disease characteristics on adherence.7,11,15–33
A number of intervention trials have been performed to improve adherence to medication.9,34–42 These intervention approaches can be categorized into four groups: educational, behavioral, cognitive behavioral, and multi-component interventions.43 Recently, a systematic review determined the impact of different interventions on medication adherence and found that multicomponent interventions had the greatest evidence for improving adherence.36,44 However, their effectiveness at enhancing adherence is still uncertain. The National Institute for Health and Care Excellence guideline in 2009 recommended considering any intervention to improve adherence on an individual basis and to modify the intervention to the actual needs of the patients.45
This study aimed to assess the impact of different modes of patient education on medication adherence in Thai RA patients.
Materials and methods
Participants
Patients who fulfilled the 2010 American College of Rheumatology/European League against Rheumatism criteria for RA were recruited from rheumatology clinic of the Phramongkutklao Hospital from March 2017 to February 2018. Patients were excluded if they were <18 years of age, diagnosed with life-threatening conditions, unable to read Thai, unable to take medication by him/herself, had a high disease activity (disease activity score-28, [DAS28] >5.1), or had a severe mental disorder.
Methods
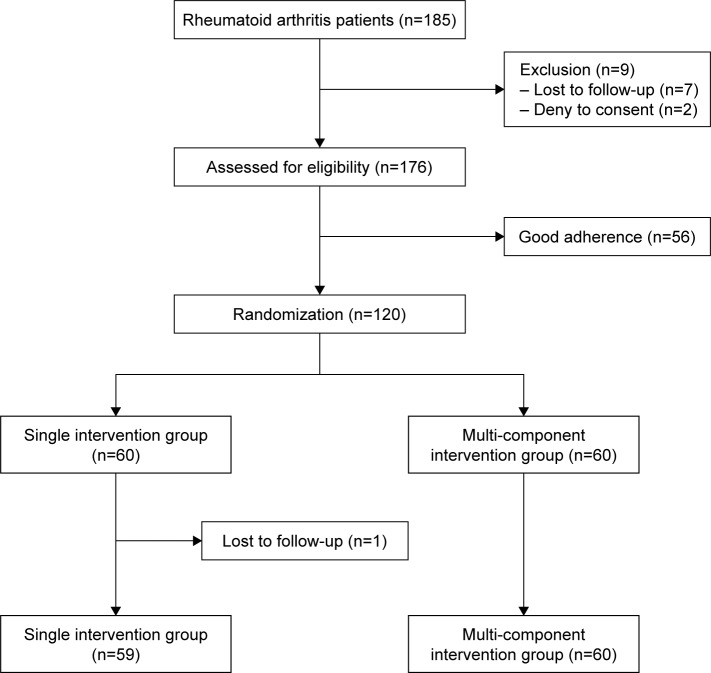
A total of 185 patients with RA were enrolled. Fifty-six patients with good adherence, defined as medication-taking behavior (MTB) questionnaire for the Thai patient >23 or pill count >80%, were excluded. Seven patients could not follow the study protocol and two denied participating. One hundred and twenty patients with non-adherence were randomized by block randomization and allocated in a 1:1 allocation ratio to two study arms: multi-component intervention group or single intervention group. The multi-component intervention group received a 30-minute directed counseling and a disease information pamphlet. The information pamphlet provided brief information on RA disease and drugs commonly used to treat RA, including usage and dosage information. The single intervention group received only a disease information pamphlet. The study flow chart was depicted in Figure 1.
Figure 1.
Patient flow chart.
Baseline demographic and disease characteristics included age, sex, marital status, occupation, salary, year of education, medical insurance, disease duration, tobacco use, alcohol use, comorbidity, rheumatoid factor, anti-citrullinated peptide antibody, dosage and number of DMARDs, and the total number of medicines. All patients were asked to finish a set of standardized self-reporting questionnaires (MTB), patient global assessment (PGA), pain score, EuroQoL-5D (EQ-5D), hospital anxiety and depression scale (HADS), brief illness perception questionnaire (B-IPQ), Montreal cognitive assessment (MoCA), and Thai mental status examination (TMSE). The individual medication was retrieved by querying the electronic medical records combined with patients’ self-report. DAS28 and physician global assessment (PhGA) were assessed by blinded rheumatologists. After 12 weeks, patients completed a second set of questionnaires (MTB, PGA, pain score, EQ-5D, HADS, and B-IPQ) and were assessed by the same blinded rheumatologist. One patient in the single intervention group was lost to follow-up.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and adhered to the principles outlined in the Guideline for Good Clinical Practice International Conference on Harmonization Tripartite Guideline (January 1997). The study protocol was approved by the institutional review board of the Royal Thai army medical department. Written informed consent was obtained from each patient. The Thai Clinical Trial Registry number is TCTR20171207003.
Pill count adherence rate
This objective measure counts the number of drugs that have been taken between two visits. This number would then be compared with the total number of drugs received by the patient to calculate the actual adherence rate. ([Number of dosage units dispensed - number of dosage units which remained]/[prescribed number of dosage unit per day × number of days between two visits]). This study categorized adherence as follows: patients with adherence rates >80% were considered to be good adherents to the medication, and those with adherence rates ≥80% were considered to be non-adherents to the medication.
Self-reported adherence
Adherence was also assessed by using the MTB score. This score has been developed and validated in 1,156 Thai patients. It has clearer and more specific language, lower number of items, and better reliability and validity than the Thai version of the 8-item Morisky Medication Adherence Scale (MMAS) to measure medication adherence of the Thai patients. The MTB-Thai consisted of 6 items (forget to take medicines, not taking medicines at times prescribed, stop taking medicines because of adverse drug reactions, stop taking medicines because of getting better, stop taking medicines for other reasons, and adjust dosage regimens) of which, recall periods were 2 weeks. The highest score was 24. Scores <22 represent “low adherence”, 22–23 suggest “medium adherence”, and equal to 24 indicate “high adherence”.46
Quality of life
The general health status of each patient was determined using the EQ-5D-5L. The EQ-5D-5L consists of 2 scales; the EQ-5D-5L descriptive system and the EQ visual analog scale (EQ-VAS). The descriptive system comprises five dimensions and each dimension has five levels. The EQ-VAS records the patient’s health on a 20 cm vertical VAS.47
Disease activity
Disease activity was assessed by using DAS28, incorporating 28 swollen and tender joint counts, patient’s global health assessment on a 100 mm VAS, and erythrocyte sedimentation rate (mm/hour).48
Anxiety and depression
The HADS was used to measure both anxiety and depression. Each subscale contains seven items, which are rated on a 4-point scale and scored from 0 to 3 with total scores varying from 0 to 21 for each subscale. Scores between 8 and 10 suggest a “possible case”; and 11 and 21 designate a “probable case of anxiety/depression”. These cutoff points have been found to have sensitivity and specificity of 0.8.49,50
Illness perception
The B-IPQ was utilized to evaluate patients’ perceptions of their disease. It comprises 8 items with each level scored from 0 to 10. Five items measure cognitive illness representations: consequences, identity, personal control, timeline, and treatment control; two items gauge emotional representations: concern and emotional responses; and one item assesses illness comprehensibility.51,52
Cognitive assessment
The Thai version of the MoCA-T test is a translated neuropsychiatric test for measuring the mental status of Thai patients. The MoCA-T measures several cognitive domains, including attention, concentration, executive functions, language, orientation to time and place, short-term memory recall task, visuospatial abilities, and working memory. Education attainment was a significant factor correlated with the score of MoCA-T. The compensation by adding one point for subjects with year of education ≤6 was more appropriate in Thai subjects. The score ≤24 of MoCA-T showed the sensitivity and specificity of 0.8.53
The TMSE is another neuropsychiatric test developed for Thais. It consists of six subtests concerning attention, calculation, language, orientation, recall, and registration. The cutoff point for the diagnosis of healthy Thai elderly for TMSE is >23 points. This test is sensitive, reliable, and is an applicable mental status examination for Thai subjects who have various socioeconomic status as well as various levels of education and traditional status.54
Outcome measures
The primary endpoints were differences in adherence rates, measured by pill count and MTB after 12 weeks between multi-component intervention group and single intervention group. The secondary endpoints were the changes in RA disease activity as measured by DAS28, pain score, and PhGA by VAS, EQ-5D-5L questionnaire, Thai-HADS questionnaire, and B-IPQ after 12 weeks between multi-component intervention group and single intervention group.
Sample size calculation
The increase in patient’s adherence to treatment was expected to increase from 55% to 85%. A sample size calculation revealed that a sample of 47 patients in each group was enough to elucidate such difference at 0.05 alpha error and 0.90 power of the test. Sample size calculation was performed using the n4Studies program.55
Statistical analyses
Data were described by standard descriptive statistics. Absolute and relative frequencies were used for categorical variables. Continuous variables were described by mean and range. Randomization was checked by chi-squared test or Fisher’s exact test for categorical variables and independent Student’s t-test for continuous variables. The analysis described was based on an intention-to-treat approach. Significant level was set at P<0.05. All statistical analyses were performed with IBM SPSS Statistics for version 23.
Results
Demographic measures
Patient allocation was well balanced. Baseline age, education, income, and underlying disease were not different between the multi-component intervention group and single intervention group (Table 1). The mean age in the multi-component intervention group was 55.82±11.25 years, whereas it was 57.20±12.24 years in the single intervention group. Females were 83.1% in the multi-component intervention group, whereas they were 85% in the single intervention group. There were no differences in disease duration, duration of treatment, extra-articular manifestations, the presence of autoantibodies, disease activity, number of medications, quality of life, illness perception, or cognitive and mental status between the multi-component intervention group and single intervention group. Percentage of patients with anxiety was greater in multi-component intervention group than in single intervention group (10% vs 0%, P=0.027). The mean disease duration in the multi-component intervention group was 8.29±7.91 years, whereas it was 7.73±6.93 years in the single intervention group. Baseline pill count adherence rates (92.21±14.05 vs 88.60±19.66, P=0.251) and MTB-Thai scores (21.68±1.91 vs 21.61±2.67, P=0.863) were not different (Table 2).
Table 1.
Baseline patient characteristics
| Variables | Single intervention group (N=59) | Multi-component intervention group (N=60) |
|---|---|---|
|
| ||
| Female (%) | 83.1 | 85 |
|
| ||
| Age (years, mean ± SD) | 57.20±12.24 | 55.82±11.25 |
|
| ||
| Education attainment (n, %) | ||
| Under bachelor’s degree | 69.5 | 75 |
| Bachelor’s degree or above | 30.5 | 25 |
|
| ||
| Years of education (years, mean ± SD) | 11.14±4.32 | 10.98±4.12 |
|
| ||
| Employment status (n, %) | ||
| Employed | 64.4 | 56.7 |
| Unemployed | 35.6 | 43.3 |
|
| ||
| Income (10,000 baht/month, mean ± SD) | 1.25±1.36 | 1.49±1.86 |
|
| ||
| Marital status (n, %) | ||
| Single | 15.3 | 10 |
| Married | 59.3 | 70 |
| Divorce | 11.9 | 5 |
| Widow | 13.6 | 15 |
|
| ||
| Social health protection scheme (n, %) | ||
| CSMBS | 49.2 | 56.7 |
| Non-CSMBS | 50.8 | 43.3 |
|
| ||
| Underlying disease (n, %) | ||
| Diabetes mellitus | 5.1 | 10 |
| Hypertension | 37.3 | 36.7 |
| Dyslipidemia | 32.2 | 31.7 |
| Coronary artery disease | 1.7 | 5 |
| Chronic lung disease | 1.7 | 3.3 |
| Chronic kidney disease | 0 | 1.7 |
| Osteoporosis | 27.1 | 18.3 |
|
| ||
| History of alcohol use (n, %) | 30.5 | 28.3 |
|
| ||
| History of smoking (n, %) | 15.3 | 10 |
|
| ||
| Anxiety (n, %) | 0 | 10 |
|
| ||
| Depression (n, %) | 1.7 | 6.7 |
|
| ||
| Cognitive impairment (n, %) | ||
| TMSE | 11.9 | 5 |
| MoCA-T | 62.7 | 53.3 |
|
| ||
| B-IPQ domain (mean ± SD) | ||
| Consequences | 4.03±2.64 | 3.72±2.74 |
| Timeline | 5.58±3.37 | 5.87±3.62 |
| Personal control | 5.76±2.85 | 6.65±2.90 |
| Treatment control | 8.25±2.20 | 8.60±2.25 |
| Identity | 3.97±2.48 | 4.08±2.66 |
| Concern | 4.00±3.17 | 4.03±3.32 |
| Emotional responses | 7.59±2.36 | 7.73±2.82 |
| Illness comprehensibility | 4.15±3.02 | 3.82±3.24 |
Abbreviations: B-IPQ, brief illness perception questionnaire; CSMBS, civil servants’ medical benefit scheme; HADS, hospital anxiety and depression scale; MoCA-T, Thai version of Montreal cognitive assessment; TMSE, Thai mental state examination.
Table 2.
Baseline disease and treatment characteristics
| Variables | Single intervention group (N=59) | Multi-component intervention group (N=60) |
|---|---|---|
|
| ||
| Disease duration (years, mean ± SD) | 7.73±6.93 | 8.29±7.91 |
|
| ||
| Duration of treatment (years, mean ± SD) | 7.14±6.59 | 8.07±7.94 |
|
| ||
| Extra-articular manifestation (%) | 57.6 | 68.3 |
| Sicca | 33.9 | 46.7 |
| Rheumatoid nodule | 28.8 | 41.7 |
| Interstitial lung disease | 0.0 | 6.7 |
| Cervical spine involvement | 6.8 | 3.3 |
|
| ||
| Autoantibodies | ||
| RF positive (N=116) (n, %) | 71.2 | 70.0 |
| ACPA positive (N=92) (n, %) | 54.2 | 56.7 |
|
| ||
| Number of medications (mean ± SD) | 7.47±2.18 | 7.17±2.34 |
|
| ||
| Type and dosage of DMARDs | ||
| MTX use (n, %) | 76.3 | 81.7 |
| MTX dosage (mg/week, mean ± SD) | 12.17±3.94 | 12.14±4.33 |
| SSZ use (n, %) | 62.7 | 65.0 |
| SSZ dosage (g/day, mean ± SD) | 1.92±0.76 | 1.94±0.70 |
| HCQ use (n, %) | 27.1 | 28.3 |
| HCQ dosage (mg/day, mean ± SD) | 179.46±44.26 | 179.82±45.78 |
| CQ use (n, %) | 16.9 | 8.3 |
| CQ dosage (mg/day, mean ± SD) | 219.70±65.09 | 200.00±68.47 |
| LEF use (n, %) | 20.3 | 25.0 |
| LEF dosage (mg/day, mean ± SD) | 12.13±6.44 | 14.19±5.73 |
| Prednisolone use (n, %) | 32.2 | 40.0 |
| Prednisolone dosage (mg/day, mean ± SD) | 5.43±3.11 | 4.18±2.75 |
| Biologics use (n, %) | 1.7 | 1.7 |
|
| ||
| History of adverse drug reaction (n, %) | 20.3 | 33.3 |
|
| ||
| DAS28 (mean ± SD) | 3.24±1.19 | 3.20±0.90 |
|
| ||
| EQ-5D (mean ± SD) | 0.85±0.12 | 0.82±0.17 |
|
| ||
| EQ-VAS (mean ± SD) | 79.66±15.62 | 77.92±20.92 |
|
| ||
| VAS pain (mean ± SD) | 19.14±20.17 | 18.33±21.48 |
|
| ||
| VAS PhGA mm, (mean ± SD) | 14.66±17.29 | 13.90±17.03 |
|
| ||
| Pill count adherence rate (%) | 88.60±19.66 | 92.21±14.05 |
| Good adherencea (n, %) | 16.7 | 27.1 |
|
| ||
| MTB-Thai 0–24, (mean ± SD) | 21.61±2.67 | 21.68±1.91 |
|
| ||
| Good adherenceb (n, %) | 33.3 | 28.8 |
Notes:
Good adherence defined as pill count adherence rate >80%.
Good adherence defined as MTB-Thai ≥22.
Abbreviations: ACPA, anti-citrullinated protein antibody; CQ, chloroquine; EQ-5D, EuroQol 5 dimensions; EQ-VAS, EuroQol visual analog scale; DAS28, disease activity score 28; DMARDs, disease-modifying anti-rheumatic drugs; HCQ, hydroxychloroquine; LEF, leflunomide; MTB-Thai, Medication taking behavior questionnaire for Thai patient; MTX, methotrexate; RF, rheumatoid factor; SSZ, sulfasalazine; VAS pain, visual analog scale for pain; VAS PhGA, visual analog scale for physician global assessment.
Outcome measures
After 12 weeks, pill count adherence rate increased significantly from baseline in both study groups. In the multi-component intervention group adherence rate increased from 92.21±14.05 to 97.59±10.07 (P=0.002) and single intervention group adherence rate increased from 88.60±19.66 to 92.42±14.27 (P=0.044) (Table 3). However, the mean differences between the multi-component intervention group and the single intervention group were not significant (5.38±12.90 vs 3.18±14.23, P=0.531) (Table 4).
Table 3.
Disease parameters at baseline and 12 weeks of the single and multi-component intervention groups
| Variables | Single intervention group (N=59)
|
Multi-component intervention group (N=60)
|
||||
|---|---|---|---|---|---|---|
| At baseline | At 12 weeks | P-value | At baseline | At 12 weeks | P-value | |
|
| ||||||
| Pill count adherence rate (%) | 88.60±19.66 | 92.42±14.27 | 0.044 | 92.21±14.05 | 97.59±10.07 | 0.002 |
| Good adherencea (n, %) | 72.9 | 89.8 | 0.019 | 83.3 | 95 | 0.040 |
|
| ||||||
| Adherence rate per each medication | ||||||
| MTX (%) | 89.76±19.96 | 92.60±15.01 | 0.190 | 92.61±16.05 | 98.37±9.70 | 0.024 |
| SSZ (%) | 87.94±17.27 | 97.74±8.27 | 0.001 | 87.39±19.00 | 97.87±15.87 | 0.008 |
| HCQ (%) | 88.06±35.80 | 87.07±16.00 | 0.893 | 89.38±24.20 | 90.32±24.87 | 0.628 |
| CQ (%) | 95.32±11.80 | 96.19±13.50 | 0.788 | 105.18±4.40 | 103.94±7.53 | 0.585 |
| LEF (%) | 87.95±16.04 | 98.57±15.00 | 0.143 | 100.84±15.78 | 97.18±9.31 | 0.438 |
| Prednisolone (%) | 94.12±13.69 | 94.26±11.91 | 0.975 | 90.95±20.52 | 97.45±7.00 | 0.181 |
|
| ||||||
| MTB score (mean ± SD) | 21.61±2.67 | 22.05±1.91 | 0.251 | 21.68±1.91 | 22.80±1.34 | <0.001 |
| Good adherenceb (n, %) | 71.2 | 71.2 | 1.000 | 66.7 | 85 | 0.020 |
|
| ||||||
| DAS28 (mean ± SD) | 3.24±1.19 | 3.17±1.23 | 0.563 | 3.20±0.90 | 3.01±1.05 | 0.160 |
|
| ||||||
| EQ-5D (mean ± SD) | 0.850±0.123 | 0.825±0.136 | 0.142 | 0.823±0.175 | 0.824±0.177 | 0.945 |
|
| ||||||
| EQ-VAS (mean ± SD) | 79.66±15.62 | 74.64±20.51 | 0.055 | 77.92±20.92 | 76.08±21.35 | 0.577 |
|
| ||||||
| VAS pain (mean ± SD) | 19.41±20.17 | 20.00±23.27 | 0.840 | 18.33±21.48 | 12.58±17.72 | 0.092 |
|
| ||||||
| VAS PhGA (mean ± SD) | 14.66±17.29 | 17.29±20.75 | 0.272 | 13.90±17.03 | 10.12±15.62 | 0.159 |
|
| ||||||
| Anxietyc (n, %) | 0 | 1.7 | 0.317 | 10 | 3.3 | 0.142 |
|
| ||||||
| Depressionc (n, %) | 1.7 | 3.4 | 0.560 | 6.7 | 3.3 | 0.724 |
|
| ||||||
| B-IPQ domain (mean ± SD) | ||||||
| Consequences | 4.03±2.64 | 3.47±2.57 | 0.160 | 3.73±2.77 | 3.54±2.98 | 0.594 |
| Timeline | 5.58±3.37 | 5.47±3.37 | 0.842 | 5.92±3.63. | 6.32±3.47 | 0.423 |
| Personal control | 5.76±2.85 | 6.07±2.77 | 0.501 | 6.66±2.93 | 6.19±3.12 | 0.268 |
| Treatment control | 8.25±2.02 | 7.83±2.08 | 0.173 | 8.64±2.24 | 8.54±2.03 | 0.778 |
| Identity | 3.94±2.48 | 3.80±2.34 | 0.621 | 4.12±2.67 | 3.95±2.63 | 0.621 |
| Concern | 4.00±3.17 | 4.19±2.84 | 0.587 | 4.07±3.34 | 4.08±3.13 | 0.972 |
| Emotional responses | 7.59±2.36 | 7.78±1.90 | 0.551 | 7.75±3.84 | 7.95±2.47 | 0.670 |
| Illness comprehensibility | 4.15±3.02 | 4.44±3.05 | 0.520 | 3.81±3.27 | 3.49±2.94 | 0.329 |
Notes:
Good adherence defined as pill count adherence rate >80%.
Good adherence defined as MTB-Thai ≥22.
Anxiety and depression defined as HADS score >10.
Abbreviations: B-IPQ, brief illness perception questionnaire; CQ, chloroquine; DAS28, disease activity score 28; EQ-5D, EuroQol 5 dimensions; EQ-VAS, EuroQol visual analog scale; HADS, hospital anxiety and depression scale; HCQ, hydroxychloroquine; LEF, leflunomide; MTB-Thai, medication taking behavior questionnaire for Thai patient; MTX, methotrexate; SSZ, sulfasalazine; VAS pain, visual analog scale for pain; VAS PhGA, visual analog scale for physician global assessment.
Table 4.
Mean changes in disease parameters from baseline until the 12-week follow-up in both single and multi-component intervention groups
| Variables | Single intervention group (N=59)
|
Multi-component intervention group (N=60)
|
P-value |
|---|---|---|---|
| Mean difference | Mean difference | ||
|
| |||
| Pill count adherence rate (%) | 3.81±14.23 | 5.38±12.90 | 0.531 |
|
| |||
| Adherence rate per each medication | |||
| MTX (%) | 2.83±13.45 | 5.76±16.78 | 0.379 |
| SSZ (%) | 9.80±16.64 | 10.48±23.05 | 0.887 |
| HCQ (%) | −0.99±28.73 | 0.94±7.84 | 0.792 |
| CQ (%) | 0.87±9.88 | −1.24±4.68 | 0.662 |
| LEF (%) | 10.62±18.20 | −3.66±15.74 | 0.078 |
| Prednisolone (%) | 0.14±17.59 | 6.50±21.47 | 0.342 |
|
| |||
| MTB score (mean ± SD) | 0.44±2.92 | 1.12±2.22 | 0.157 |
|
| |||
| DAS28 (mean ± SD) | −0.07±0.89 | −0.20±1.06 | 0.476 |
|
| |||
| EQ-5D (mean ± SD) | −0.025±0.131 | 0.002±0.173 | 0.341 |
|
| |||
| EQ-VAS (mean ± SD) | −5.02±19.71 | −1.83±25.31 | 0.446 |
|
| |||
| VAS pain (mean ± SD) | 0.59±22.52 | −5.75±25.99 | 0.158 |
|
| |||
| VAS PhGA (mean ± SD) | 2.63±18.20 | −3.78±20.56 | 0.074 |
|
| |||
| B-IPQ domain (mean ± SD) | |||
| Consequences | −0.56±3.02 | −0.19±2.67 | 0.479 |
| Timeline | −0.10±3.90 | 0.41±3.87 | 0.478 |
| Personal control | 0.31±3.46 | −0.47±3.26 | 0.210 |
| Treatment control | −0.42±2.36 | −0.10±2.76 | 0.479 |
| Identity | −0.17±2.62 | −0.17±2.62 | 1.000 |
| Concern | 019±2.62 | 0.02±3.68 | 0.774 |
| Emotional responses | 0.19±2.39 | 0.20±3.65 | 0.976 |
| Illness comprehensibility | 0.29±3.42 | −0.32±2.52 | 0.272 |
Abbreviations: B-IPQ, brief illness perception questionnaire; CQ, chloroquine; DAS28, disease activity score 28; EQ-5D, EuroQol 5 dimensions; EQ-VAS, EuroQol visual analog scale; HCQ, hydroxychloroquine; LEF, leflunomide; MTB-Thai, medication taking behavior questionnaire for Thai patient; MTX, methotrexate; SSZ, sulfasalazine; VAS pain, visual analog scale for pain; VAS PhGA, visual analog scale for physician global assessment.
Sulfasalazine had the lowest adherence rate among other DMARDs. After 12 weeks, adherence rate of sulfasalazine use increased in both groups. In the multi-component intervention group, adherence rate increased from 87.39±19 to 97.87±15.87 (P=0.008) and in the single intervention group, it increased from 87.94±17.27 to 97.74±8.27 (P=0.001). However, the mean differences between both groups were not significant 10.48±23.05 vs 9.80±16.64, (P=0.887) Adherence rate of methotrexate use improved only in the multi-component intervention group 5.76±16.78, (P=0.024). The other drugs’ adherences were not significantly changed (Table 3).
MTB score improved in the multi-component intervention group (1.12±2.22, P<0.001), but not in the single intervention group (0.44±2.92, P=0.0251). DAS28, EQ-5D, EQ-VAS, pain, and PhGA were unchanged from baseline in both groups. There was no difference in the proportion of patients who had anxiety or depression. Illness perceptions remained unchanged (Tables 3 and 4).
Discussion
During a 12-week period, adherence rate measured by pill count in both groups (single intervention and multi-component intervention) were significantly improved. However, MTB score improved only in multi-component intervention group. Both pill count and MTB score are indirect measurements of adherence.31 The result of pill count method is more reliable than MTB score because MTB score is a questionnaire that patients report by themselves. The data suggested that patient education could improve medication adherence. However, there were no differences between single intervention and multi-component intervention groups in improving medication adherence.
A number of intervention trials have been conducted to enhance adherence to treatment in patients with immune-mediated inflammatory disorders, including information about disease,41 medication reminders using pillbox or mobile phone,56 and motivational interview.57 A recent systemic analysis revealed that multi-component interventions showed the greatest evidence for promoting adherence in patients.36 However, this systematic analysis found a high level of heterogeneity in study methods as well as little consistency in their conclusions,9,40,58,59 which does not allow us to draw clear assumptions about the interventions intended to improve medication adherence.
A British randomized controlled study of RA patients revealed that patient education was correlated with adherence.41 One hundred active RA patients were randomized to an intervention group, which received seven 30-minute one-on-one sessions with a rheumatology nurse directed at improving self-efficacy, or a control group, which received standard treatment (providing a drug information pamphlet). After 6 months, those in the intervention group were more adherent to drugs. In contrast, a less intensive intervention involving two pharmacist-led motivational interviewing group sessions failed to improve DMARDs adherence more than providing information regarding their medications.60
Our study showed no differences between single intervention and multi-component interventions in improving medication adherence. This implied that educational interventions, which deliver instructive information alone, may lead to improved medication adherence. However, the 30-minute directed counseling with provision of brochures did not provide any add-on benefit. One explanation for the imperfect effect of these educational interventions may be that provision of information presumes the patient lacks understanding of their disease and medications. This attributes a passive role to the patient and fails to reflect pre-existing “lay beliefs” acquired from other sources, for example, the newspapers, social media, or friends and family.18 A successful educational intervention may require participation from both the physician and patient. Admittedly, some patients may wish to entrust most of the decision making to their treating physician. It is, therefore, crucial to determine how involved the patient wishes to be in constructing the management plan.61 As soon as this is established, a proper educational intervention could involve intertwining biomedical information into pre-existing lay belief systems.18
Although education interventions in this study improved adherence to DMARD treatment, as in other studies, this boosted adherence did not produce considerable additional beneficial clinical outcome.37,62,63 Possibly, the composite index for measuring disease activity and quality of life might not have been a sensitive indicator to detect trivial changes between the two groups.
Limitations
This study had several limitations. First, patient’s adherence is challenging to measure and all techniques have downsides. Both self-reported questionnaire and pill count methods tend to overestimate adherence behavior and generally have low sensitivity.64,65 Second, a 12-week study period may perhaps be too short to affect the clinical outcome. A longer time period might be needed to demonstrate maximal efficacy. Third, nearly one-fourth of patients in this trial reported a perception of having experienced adverse drug reactions. This perception of medication side effects may affect their adherence. Finally, this study did not assess patients’ health literacy. Patients require sufficient literacy to comprehend medication instructions and calculate accurate medication doses to be taken for the suitable duration.66,67 Poor health literacy is linked to a range of unfavorable health outcomes possibly due to improper self-care, incomplete health responsibility, and under-utilization of available healthcare resources. Methods to augment health literacy, rather than providing disease information might be valuable in enhancing medication adherence.
Conclusion
Patient education significantly improves adherence. However, in this study, there were no differences between single education intervention and multi-component education intervention in improving medication adherence. Provision of disease information pamphlet with or without directed counseling can equally improve medication adherence of patients with RA.
Data sharing statement
Individual participant data that underlie the results reported in this article, after deidentification, information pamphlet, and study protocol, are available on request from the corresponding author after article publication.
Acknowledgments
This study was supported by a research grant from the Thai Rheumatism Association. This manuscript was verified to be correct English by Stephen John Pinder, a native speaker experienced in medical English.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Harris ED. Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 2.Erickson AR, Cannella AC, Mikuls TR. Clinical Features of Rheumatoid Arthritis. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell JR, editors. Kelley and Firestein’s Textbook of Rheumatology. 10 ed. Philadelphia: Elservier, Inc.; 2016. pp. 1167–1168. [Google Scholar]
- 3.Chaiamnuay P, Darmawan J, Muirden KD, Assawatanabodee P. Epidemiology of rheumatic disease in rural Thailand: a WHO-ILAR COPCORD study. Community oriented programme for the control of rheumatic disease. J Rheumatol. 1998;25(7):1382–1387. [PubMed] [Google Scholar]
- 4.Louthrenoo W. An insight into rheumatology in Thailand. Nat Rev Rheumatol. 2015;11(1):55–61. doi: 10.1038/nrrheum.2014.142. [DOI] [PubMed] [Google Scholar]
- 5.Osiri M, Maetzel A, Tugwell P. The economic burden of rheumatoid arthritis in a developing nation: results from a one-year prospective cohort study in Thailand. J Rheumatol. 2007;34(1):57–63. [PubMed] [Google Scholar]
- 6.Xia Y, Yin R, Fu T, et al. Treatment adherence to disease-modifying antirheumatic drugs in Chinese patients with rheumatoid arthritis. Patient Prefer Adherence. 2016;10:735–742. doi: 10.2147/PPA.S98034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prudente LR, Diniz JS, Ferreira TX, et al. Medication adherence in patients in treatment for rheumatoid arthritis and systemic lupus erythematosus in a university hospital in Brazil. Patient Prefer Adherence. 2016;10:863–870. doi: 10.2147/PPA.S79451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar K, Raza K, Nightingale P, et al. Determinants of adherence to disease modifying anti-rheumatic drugs in White British and South Asian patients with rheumatoid arthritis: a cross sectional study. BMC Musculoskelet Disord. 2015;16(1):396. doi: 10.1186/s12891-015-0831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Bemt BJ, den Broeder AA, van den Hoogen FH, et al. Making the rheumatologist aware of patients’ non-adherence does not improve medication adherence in patients with rheumatoid arthritis. Scand J Rheumatol. 2011;40(3):192–196. doi: 10.3109/03009742.2010.517214. [DOI] [PubMed] [Google Scholar]
- 10.Scheiman-Elazary A, Duan L, Shourt C, et al. The rate of adherence to antiarthritis medications and associated factors among patients with rheumatoid arthritis: a systematic literature review and metaanalysis. J Rheumatol. 2016;43(3):512–523. doi: 10.3899/jrheum.141371. [DOI] [PubMed] [Google Scholar]
- 11.Wong PK. Medication adherence in patients with rheumatoid arthritis: why do patients not take what we prescribe? Rheumatol Int. 2016;36(11):1535–1542. doi: 10.1007/s00296-016-3566-4. [DOI] [PubMed] [Google Scholar]
- 12.De Vera MA, Mailman J, Galo JS. Economics of non-adherence to biologic therapies in rheumatoid arthritis. Curr Rheumatol Rep. 2014;16(11):460. doi: 10.1007/s11926-014-0460-5. [DOI] [PubMed] [Google Scholar]
- 13.Hovstadius B, Petersson G. Non-adherence to drug therapy and drug acquisition costs in a national population – a patient-based register study. BMC Health Serv Res. 2011;11(1):326. doi: 10.1186/1472-6963-11-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual-Ramos V, Contreras-Yáñez I, Villa AR, Cabiedes J, Rull-Gabayet M. Medication persistence over 2 years of follow-up in a cohort of early rheumatoid arthritis patients: associated factors and relationship with disease activity and with disability. Arthritis Res Ther. 2009;11(1):R26. doi: 10.1186/ar2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandstetter S, Riedelbeck G, Steinmann M, Loss J, Ehrenstein B, Apfelbacher C. Depression moderates the associations between beliefs about medicines and medication adherence in patients with rheumatoid arthritis: Cross-sectional study. J Health Psychol. 2018;23(9):1185–1195. doi: 10.1177/1359105316646440. [DOI] [PubMed] [Google Scholar]
- 16.Harnett J, Wiederkehr D, Gerber R, Gruben D, Bourret J, Koenig A. Primary nonadherence, associated clinical outcomes, and health care resource use among patients with rheumatoid arthritis prescribed treatment with injectable biologic disease-modifying antirheumatic drugs. J Manag Care Spec Pharm. 2016;22(3):209–218. doi: 10.18553/jmcp.2016.22.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrold LR, Briesacher BA, Peterson DAN, et al. Cost-related medication nonadherence in older patients with rheumatoid arthritis. J Rheumatol. 2013;40(2):137–143. doi: 10.3899/jrheum.120441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joplin S, van der Zwan R, Joshua F, Wong PK. Medication adherence in patients with rheumatoid arthritis: the effect of patient education, health literacy, and musculoskeletal ultrasound. BioMed Res Int. 2015;2015:150658. doi: 10.1155/2015/150658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Cui Y, Yin R, et al. Medication adherence has an impact on disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Patient Prefer Adherence. 2017;11:1343–1356. doi: 10.2147/PPA.S140457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan C, McBeth J, Cordingley L, et al. The influence of behavioural and psychological factors on medication adherence over time in rheumatoid arthritis patients: a study in the biologics era. Rheumatology. 2015;54(10):1780–1791. doi: 10.1093/rheumatology/kev105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauscher V, Englbrecht M, van der Heijde D, Schett G, Hueber AJ. High degree of nonadherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. J Rheumatol. 2015;42(3):386–390. doi: 10.3899/jrheum.140982. [DOI] [PubMed] [Google Scholar]
- 22.Salt E, Frazier SK. Predictors of medication adherence in patients with rheumatoid arthritis. Drug Dev Res. 2011;72(8):756–763. doi: 10.1002/ddr.20484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salt E, Hall L, Peden AR, Home R. Psychometric properties of three medication adherence scales in patients with rheumatoid arthritis. J Nurs Meas. 2012;20(1):59–72. doi: 10.1891/1061-3749.20.1.59. [DOI] [PubMed] [Google Scholar]
- 24.Santoleri F, Sorice P, Lasala R, Rizzo RC, Costantini A. Medication adherence and persistence in the treatment of rheumatoid arthritis with adalimumab and etanercept. Six years of analysis. J Med Econ. 2014;17(5):320–325. doi: 10.3111/13696998.2014.902844. [DOI] [PubMed] [Google Scholar]
- 25.Shetty R, Reddy K, Inam S, Khera K. Impact of medication adherence by using Indian version compliance questionnaire rheumatology (Cqr) and medication adherence report scale (Mars) tools on quality of life of patients with rheumatoid arthritis. Value Health. 2014;17(7):A385. doi: 10.1016/j.jval.2014.08.2639. [DOI] [PubMed] [Google Scholar]
- 26.Spruill TM, Ogedegbe G, Harrold LR, et al. Association of medication beliefs and self-efficacy with adherence in urban Hispanic and African-American rheumatoid arthritis patients. Ann Rheum Dis. 2014;73(1):317–318. doi: 10.1136/annrheumdis-2013-203560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh YS, Cheon YH, Kim HO, et al. Medication nonadherence in Korean patients with rheumatoid arthritis: the importance of belief about medication and illness perception. The Korean J Intern Med. 2018;33(1):203–210. doi: 10.3904/kjim.2015.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treharne GJ, Lyons AC, Hale ED, Douglas KM, Kitas GD. Predictors of medication adherence in people with rheumatoid arthritis: studies are necessary but non-validated measures of medication adherence are of concern. Rheumatology (Oxford) 2005;44(10):1330. doi: 10.1093/rheumatology/kei001. [DOI] [PubMed] [Google Scholar]
- 29.Tuncay R, Eksioglu E, Cakir B, Gurcay E, Cakci A. Factors affecting drug treatment compliance in patients with rheumatoid arthritis. Rheumatol Int. 2007;27(8):743–746. doi: 10.1007/s00296-006-0299-9. [DOI] [PubMed] [Google Scholar]
- 30.van den Bemt BJ, van den Hoogen FH, Benraad B, Hekster YA, van Riel PL, van Lankveld W. Adherence rates and associations with non-adherence in patients with rheumatoid arthritis using disease modifying antirheumatic drugs. J Rheumatol. 2009;36(10):2164–2170. doi: 10.3899/jrheum.081204. [DOI] [PubMed] [Google Scholar]
- 31.van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8(4):337–351. doi: 10.1586/eci.12.23. [DOI] [PubMed] [Google Scholar]
- 32.Viller F, Guillemin F, Briancon S, Moum T, Suurmeijer T, van den Heuvel W. Compliance to drug treatment of patients with rheumatoid arthritis: a 3 year longitudinal study. J Rheumatol. 1999;26(10):2114–2122. [PubMed] [Google Scholar]
- 33.Zwikker HE, van Dulmen S, den Broeder AA, van den Bemt BJ, van den Ende CH. Perceived need to take medication is associated with medication non-adherence in patients with rheumatoid arthritis. Patient Prefer Adherence. 2014;8:1635–1645. doi: 10.2147/PPA.S66849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruera S, Barbo AG, Lopez-Olivo MA. Use of medication reminders in patients with rheumatoid arthritis. Rheumatol Int. 2016;36(11):1543–1548. doi: 10.1007/s00296-016-3558-4. [DOI] [PubMed] [Google Scholar]
- 35.Barton JL, Trupin L, Schillinger D, et al. Use of low-literacy decision aid to enhance knowledge and reduce decisional conflict among a diverse population of adults with rheumatoid arthritis: results of a pilot study. Arthritis Care Res. 2016;68(7):889–898. doi: 10.1002/acr.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Depont F, Berenbaum F, Filippi J, et al. Interventions to improve adherence in patients with immune-mediated inflammatory disorders: a systematic review. PLoS One. 2015;10(12):e0145076. doi: 10.1371/journal.pone.0145076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill J, Bird H, Johnson S. Effect of patient education on adherence to drug treatment for rheumatoid arthritis: a randomised controlled trial. Ann Rheum Dis. 2001;60(9):869–875. [PMC free article] [PubMed] [Google Scholar]
- 38.Evers AW, Kraaimaat FW, van Riel PL, de Jong AJ. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain. 2002;100(1–2):141–153. doi: 10.1016/s0304-3959(02)00274-9. [DOI] [PubMed] [Google Scholar]
- 39.El Miedany Y, El Gaafary M, Palmer D. Assessment of the utility of visual feedback in the treatment of early rheumatoid arthritis patients: a pilot study. Rheumatol Int. 2012;32(10):3061–3068. doi: 10.1007/s00296-011-2098-1. [DOI] [PubMed] [Google Scholar]
- 40.El Miedany Y, El Gaafary M, El Arousy N, Ahmed I, Youssef S, Palmer D. Arthritis education: the integration of patient-reported outcome measures and patient self-management. Clin Exp Rheumatol. 2012;30(6):899–904. [PubMed] [Google Scholar]
- 41.Homer D, Nightingale P, Jobanputra P. Providing patients with information about disease-modifying anti-rheumatic drugs: individually or in groups? A pilot randomized controlled trial comparing adherence and satisfaction. Musculoskeletal Care. 2009;7(2):78–92. doi: 10.1002/msc.141. [DOI] [PubMed] [Google Scholar]
- 42.Brus HL, van de Laar MA, Taal E, Rasker JJ, Wiegman O. Effects of patient education on compliance with basic treatment regimens and health in recent onset active rheumatoid arthritis. Ann Rheum Dis. 1998;57(3):146–151. doi: 10.1136/ard.57.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenley RN, Kunz JH, Walter J, Hommel KA. Practical strategies for enhancing adherence to treatment regimen in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(7):1534–1545. doi: 10.1097/MIB.0b013e3182813482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36(8):1138–1161. doi: 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 45.National Collaborating Centre for Primary Care Medicines Adherence: Involving Patients in Decisions About Prescribed Medicines and Supporting Adherence. 2009. [AccessedOctober 25, 2018]. Available from: https://www.nice.org.uk/guidance/cg76. [PubMed]
- 46.Sakthong P, Sonsa-Ardjit N, Sukarnjanaset P, Munpan W, Suksanga P. Development and psychometric testing of the medication taking behavior tool in Thai patients. Int J Clin Pharm. 2016;38(2):438–445. doi: 10.1007/s11096-016-0275-8. [DOI] [PubMed] [Google Scholar]
- 47.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prevoo MLL, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LBA, van Riel PLCM. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 49.Nilchaikovit T, Lotrakul M, Phisansuthideth U. Development of Thai version of hospital anxiety and depression scale in cancer patients. J Psychiatr Assoc Thailand. 1996;41(1):18–30. [Google Scholar]
- 50.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 51.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Sowattanangoon N. Brief Illness Perception Scale (Thai version) 2008. [Accessed October 25, 2018]. Available from: http://www.uib.no/ipq/html/b-ipq.html.
- 53.Tangwongchai S, Phanasathit M, Charernboon T, et al. The validity of Thai version of the Montreal Cognitive Assessment (MoCA-T) [Accessed January 3, 2019.];Dement Neuropsychol. 2009 3(2):172. Available from: http://demneuropsy.com.br/imageBank/pdf/dnv03n02a11.pdf. [Google Scholar]
- 54.Train The Brain Forum Committee Thai Mental State Examination (TMSE) Siriraj Med J. 1993;45(6):1695–1703. [Google Scholar]
- 55.Ngamjarus C. n4Studies: Sample Size Calculation for an Epidemiological Study on a Smart Device. Siriraj Med J. 2016;68(3):160–170. [Google Scholar]
- 56.Moshkovska T, Stone MA, Smith RM, Bankart J, Baker R, Mayberry JF. Impact of a tailored patient preference intervention in adherence to 5-aminosalicylic acid medication in ulcerative colitis: results from an exploratory randomized controlled trial. Inflamm Bowel Dis. 2011;17(9):1874–1881. doi: 10.1002/ibd.21570. [DOI] [PubMed] [Google Scholar]
- 57.Cook PF, Emiliozzi S, El-Hajj D, McCabe MM. Telephone nurse counseling for medication adherence in ulcerative colitis: a preliminary study. Patient Educ Couns. 2010;81(2):182–186. doi: 10.1016/j.pec.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Elkjaer M, Shuhaibar M, Burisch J, et al. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided ‘constant-care’ approach. Gut. 2010;59(12):1652–1661. doi: 10.1136/gut.2010.220160. [DOI] [PubMed] [Google Scholar]
- 59.Moss AC, Chaudhary N, Tukey M, et al. Impact of a patient-support program on mesalamine adherence in patients with ulcerative colitis – a prospective study. J Crohns Colitis. 2010;4(2):171–175. doi: 10.1016/j.crohns.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Zwikker HE, van den Ende CH, van Lankveld WG, et al. Effectiveness of a group-based intervention to change medication beliefs and improve medication adherence in patients with rheumatoid arthritis: a randomized controlled trial. Patient Educ Couns. 2014;94(3):356–361. doi: 10.1016/j.pec.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Treharne GJ, Lyons AC, Hale ED, Douglas KM, Kitas GD. ‘Compliance’ is futile but is ‘concordance’ between rheumatology patients and health professionals attainable? Rheumatology. 2006;45(1):1–5. doi: 10.1093/rheumatology/kei223. [DOI] [PubMed] [Google Scholar]
- 62.Peveler R, George C, Kinmonth AL, Campbell M, Thompson C. Effect of antidepressant drug counselling and information leaflets on adherence to drug treatment in primary care: randomised controlled trial. BMJ. 1999;319(7210):612–615. doi: 10.1136/bmj.319.7210.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helliwell PS, O’Hara M, Holdsworth J, Hesselden A, King T, Evans P. A 12-month randomized controlled trial of patient education on radiographic changes and quality of life in early rheumatoid arthritis. Rheumatology. 1999;38(4):303–308. doi: 10.1093/rheumatology/38.4.303. [DOI] [PubMed] [Google Scholar]
- 64.Dunbar J, Dunning EJ, Dwyer K. Compliance measurement with arthritis regimen. Arthritis Care Res. 1989;2(3):A8–A16. doi: 10.1002/anr.1790020309. [DOI] [PubMed] [Google Scholar]
- 65.Pullar T. Compliance with drug therapy. Br J Clin Pharmacol. 1991;32(5):535–539. doi: 10.1111/j.1365-2125.1991.tb03948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miles S, Davis T. Patients who can’t read. Implications for the health care system. JAMA. 1995;274(21):1719–1720. [PubMed] [Google Scholar]
- 67.Health literacy: report of the Council on Scientific Affairs Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. JAMA. 1999;281(6):552–557. [PubMed] [Google Scholar]