Abstract
Pre-exposure prophylaxis (PrEP) is a powerful tool that, as part of a comprehensive prevention package, has potential to significantly impact the HIV epidemic. PrEP effectiveness is believed to be dependent on the exposure and efficacy of antiretrovirals at the site of HIV transmission. Clinical trial results as well as modeling and simulation indicate the threshold of adherence required for PrEP efficacy of Truvada may differ between the sites of HIV transmission with less forgiveness for missed doses in women exposed through genital tissue compared to people exposed to colorectal tissue. This suggests a role for local and host factors to influence mucosal pharmacology. Here we review the mucosal pharmacology of antiretrovirals in the female genital tract and explore potential determinants of PrEP efficacy. Host factors such as inflammation, co-infections, hormonal status, and the vaginal microbiome will be explored as well as the role of drug metabolizing enzymes and transporters in regulating local drug exposure. The use of preclinical and early clinical models to predict clinical effectiveness is also discussed.
Keywords: Clinical Pharmacology, Gynecology, HIV/AIDS, Infectious Diseases, Women’s Health, Virology, PrEP, Antiretrovirals, Female Genital Tract
Introduction
Of the approximate 2 million new global HIV infections each year, nearly half are in women1. Exposure by heterosexual vaginal intercourse remains the most significant risk factor for women. The female genital tract (FGT), therefore, is of pertinent interest both for studying HIV transmission events and for targeting prevention interventions.
Drug exposure, and therefore efficacy, can be affected by the complex mucosal environment of the FGT (Figure 1). Extrapolating dose-exposure-response relationships derived from systemic (i.e. blood) drug exposure may not be appropriate due to differences in the expression and activity of drug metabolizing enzymes and transporters (DMET). Furthermore, the FGT is subject to both immunologic and hormonal modulation that may further complicate interactions between drug and virus in this compartment. In this review, we describe what is known about the pharmacology of antiretrovirals in the FGT and highlight areas that require investigation. This review is done in the context of antiretrovirals for pre-exposure prophylaxis (PrEP) for women although some of the principles may apply for other therapeutic areas where local drug efficacy in the FGT is critical for therapeutic success.
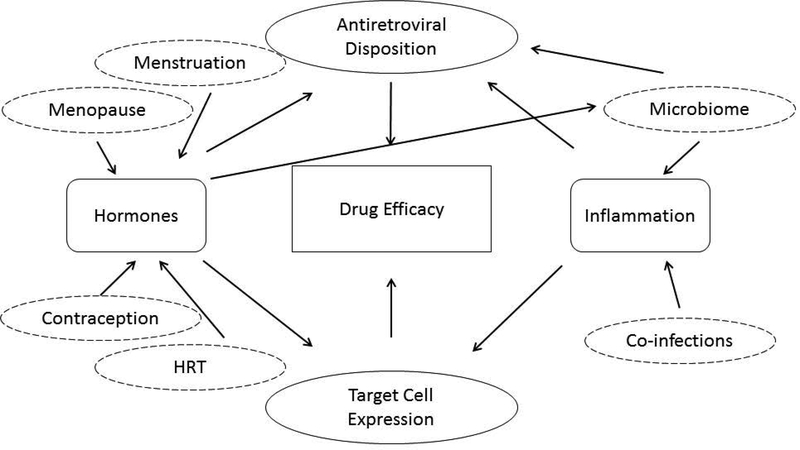
Figure 1: Potential modifiers of antiretroviral efficacy in the female genital tract.
Drug efficacy in the female genital tract is likely dependent on the concentration-response relationship within the mucosal tissue. Depending on the route of drug delivery, concentrations of antiretrovirals in the FGT are highly variable and may be influenced directly or indirectly by hormone and/or inflammation status. In addition, the concentration of HIV target cells can affect risk of HIV acquisition; this may affect the potency of antiretrovirals to prevent HIV infection. HIV target cell expression can also be affected by hormonal and inflammatory regulation. The net effect of these complex and intertwined interactions is difficult to predict.
Heterosexual Transmission and Anatomy of the Female Genital Tract
The FGT is a system with four primary functions: 1) transport of gamete and embryo to the uterus, 2) outflow of uterine shedding and vaginal secretions, 3) birth canal, and 4) protection against microbial infections. Given these primary functions, the distinct sections of the FGT work in coordination under both hormonal and immune regulation. All regions are comprised of a wall of smooth muscle, an inner mucosal lining, and an outer layer of loose supporting tissue. The “upper” genital tract, consisting of the fallopian tubes, uterus, and endocervix the outer layer, is comprised of single-layered columnar epithelium while the “lower” genital tract, comprised of the ectocervix and the vagina is covered with a single layer of stratified, squamous epithelium. Throughout life, morphologic changes occur in response to reproductive hormones (reviewed in Venkatesh et al3). In particular, cervical ectopy, or extension of the columnar epithelium of the endocervix into the ectocervix, is more common in adolescents during puberty due to increased estradiol, and in pregnancy due to hyperplasia induced by reproductive hormones.
The availability of HIV target cells throughout the FGT has been extensively described.4 Studies of SIV transmission in macaques have suggested that the first cells infected in mucosal transmission are resting CD4+ T cells.2 Although CD3+ T cells are expressed throughout the FGT, in contrast to peripheral blood, CD8+ outnumber CD4+,5 perhaps explaining the relatively low efficiency of vaginal HIV acquisition compared to parenteral or colorectal exposures6. Like morphology, cell populations, whether due to hormonal, immunological, or normal aging processes are also dynamic. These are discussed in greater detail below.
This diverse and dynamic environment influences the susceptibility to HIV infection and may influence how PrEP is targeted to women. To establish infection, the HIV virion must first cross the mucosal barrier. The endocervix and the cervical transformation zone have been primary focus due to the less robust barrier function of the columnar epithelium of the upper FGT. In addition, the large surface area of the uterine endometrium, and the presence of HIV target cells, do not preclude this compartment from being at risk, as indicated in a macaque study which found ovarian and endometrial infected cells following vaginal SIV challenge7. In contrast, the vulnerability of the lower genital tract tissues such as ectocervix and vagina has been largely ascribed to breaches in the epithelium from trauma or ulcerative infections. Shen et al has observed that, even with intact epithelium, dendritic cells, in their role as an antigen presenting cell, may play a role in the trafficking of virus across the membrane, suggesting another means by which the lower FGT may be susceptible to HIV infection8. One human study which used simulated vaginal intercourse showed distribution of cell-free and cell-associated HIV surrogates was limited to the vaginal lumen with none detected in the uterus.9
Therefore, given that vulnerabilities to HIV infection exist throughout the entire FGT, as described in Figure 2, prevention interventions should provide adequate coverage for all compartments along the FGT. Drug exposure in the FGT has been extensively reviewed previously10. Overall, drug exposure in the FGT is highly variable, even between drugs with similar structures and physiochemical properties such as lipophilicity and protein binding, making exposure difficult to predict.11 Differences in drug exposure between different compartments of the FGT, however, have not been well explored. The majority of studies have focused on drug disposition in the lower FGT, due to accessibility of sampling. In one small study, Rahagandale et al looked at drug concentrations of tenofovir and emtricitabine in endometrial biopsies collected from endometrial pipelle in HIV positive women; they found concentrations comparable to or slightly higher than concentrations reported for lower female genital tissues.12 This limited data provide some confidence that, for at least these compounds, if sufficient exposures are achieved in the lower FGT, the upper FGT should be similarly covered. 1313 Within each tissue compartment, differences between cell types may also play a role. Although highly variable, some reports suggest TFVdp kinetics differ across cell types within cervical tissue. Louissaint et al estimated median (IQR) TFVdp half-lives of 34 (21, 40), 82 (43, 89), and 60 (52, 72) when measured by cervical tissue homogenate, total disaggregated cells from a cervical biopsy, and isolated cervical CD4 cells, respectively.14 More research in this area is needed but is limited due to the low number of cells in a typical biopsy and assay limitations for quantification. Concentrations in vaginal CD4+ cells correlated well vaginal tissue homogenate (r=0.74,p<0.01) suggesting homogenates may be appropriate surrogates for exposures in target cells.14
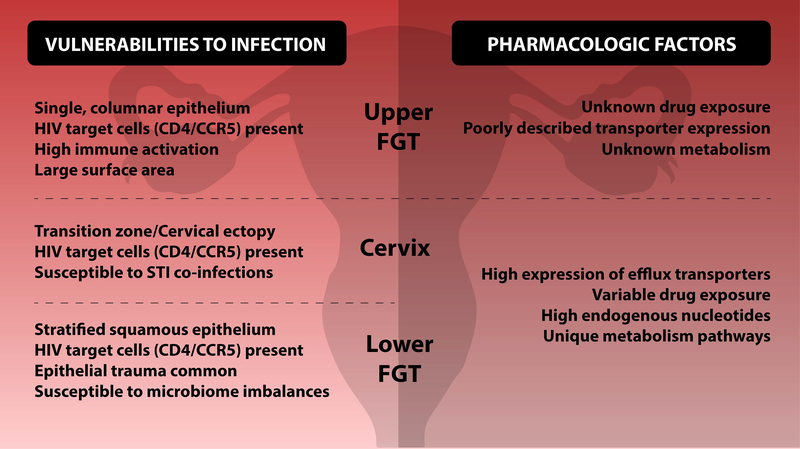
Figure 2: Vulnerabilities to HIV along the female genital tract.
The upper female genital tract, fallopian tubes, uterus, and endocervix are comprised of a single layer of columnar epithelium. HIV target cells are present throughout these tissues and, following sexual intercourse, semen can be transported to the uterus and the oocytes. Coupled with the large surface, these factors indicate that HIV prevention efforts should consider the upper genital tract as a site vulnerable to HIV infection. Less is known about the pharmacologic factors related to the upper female genital tract such as drug exposure, transporter expression, and local mechanisms of metabolism.</p/>The lower female genital tract, ectocervix and vagina is comprised of multiple layers of squamous epithelium. Differences in pharmacologic factors between the cervix and lower FGT have not been well defined so these are displayed together Epithelial breaches from trauma, particularly during sexual intercourse are not uncommon. Cervical ectopy, common in pregnant women or women on hormonal contraception, may increase HIV risk since it increases surface area of endocervical columnar epithelium. HIV target cells are present throughout the lower female genital tract. As the site for many STIs, tissues are susceptible to inflammatory conditions which may alter HIV risk and expression of target cells. Changes in microflora in the vaginal tract can affect HIV susceptibility as well as interactions with local drug concentrations. High expression of efflux transporters in vaginal epithelium may also contribute to low drug exposure in vaginal tissue.. In addition, high concentrations of endogenous nucleotides in cervical and vaginal tissue have been documented and may necessitate higher exposures to nucleotide-based PrEP interventions.
Role of Drug Metabolizing Enzymes and Transporters (DMET) in FGT
The role of ARVS as pre-exposure prophylaxis is complicated by the interplay that a variety of drug metabolizing enzymes and transporters play. Several ARVs currently used in practice are substrates for these transporters and enzymes making them an interest of current research.15,16 Included in this group are cytochrome P450 enzymes, ATP-binding cassette (ABC) efflux transporters and solute carrier (SLC) uptake transporters to name a few. The expression of several of these DMET classes has been identified in the FGT17–19 and therefore their activity may influence achieving proper drug accumulation required for PrEP.
The ABC superfamily includes P-glycoprotein (P-gp), Breast Cancer Resistant Protein (BCRP) and Multidrug Resistance-Associated Proteins (MRPs).15,16,20,21Several efflux transporters, including P-gp, MRP4, and BCRP are expressed at the protein level in the FGT and therefore may have an effective on drug distribution in this tissue compartment.17,19 For example, tenofovir, one of the agents in the currently approved PrEP product, is a substrate for MRP4.22 The presence of high MRP4 in vaginal epithelium compared to colorectal epithelium at both genetic and protein level, 17 may account for the increased accumulation of tenofovir in the colorectal compared to cervicovaginal mucosa.13 This is consistent with functional studies performed in a mouse model that found MRP4 inhibitors significantly affected the disposition of tenofovir gel in genital tissues.23 Similarly, the presence of P-gp in vaginal mucosa may indicate difficulty in reaching appropriate drug concentration within the FGT as several ARVs being considered for PrEP, including maraviroc, darunavir, raltegravir, and cabotegravir, have been identified substrates of P-gp15,24. With the exception of cabotegravir, these drugs appear to penetrate well to the FGT although, exposures are consistently lower than in colorectal tissue25,26, consistent with higher P-gp expression observed in vaginal tissue over colorectal tissue at mRNA level.17 In addition to P-gp, cabotegravir is has also been identified as a BCRP substrate, however due to its high membrane permeability, the effect of transporters on cabotegravir transport may be clinically insignificant. 24 As target concentrations required for protection in the FGT have not been defined for the majority of these drugs, more research is needed to determine whether modulation of drug transporters could influence local tissue concentrations to a degree that plays a significant role in drug efficacy.
Within the Solute Carrier (SLC) superfamily, of most interest to HIV therapy are the organic anion-transporting polypeptides (OATPs), organic anion/cation transporters (OATs/OCTs), and the equilibrative/concentrative nucleoside transporters (ENTs, CNTs). The role of OATP transporters on ARV disposition has been mainly studied in other organs15; to date no OATPs have been found to be expressed in the FGT.17,27 Tenofovir can be transported by OAT transporters.28 Both Nicol and Zhou found only low or moderate levels of mRNA for OAT transporters in human ectocervix and vagina relative to human liver.17,18 Through further immunohistochemical staining, Nicol et al. further found no protein expression of OAT1 in human vagina or ectocervix, suggesting this transporter is unlikely to have a significant role in drug distribution in the FGT.17 NRTIs are readily transported by the CNT and ENT subfamily due to the transporters’ affinity to nucleoside analogues.These transporters are ubiquitously expressed throughout the body,29 including in the FGT although only the mRNA level has been assessed in this compartment.18 Therefore, CNTs and ENTs may have the potential to influence local ARV distribution; however their role in this compartment has never been directly studied and further proteomic and functional data are needed.
The cytochrome P450 superfamily of enzymes has long been known to account for the phase I metabolism of many clinically used drugs, including ARVs. CYP enzymes are highly expressed in the tissues of the liver accounting for much of the metabolism of xenobiotic compounds in the body. It is currently unknown whether topically applied vaginal microbicides, such as ones used in PrEP, are metabolized locally in mucosal tissues. Research performed by To et al., identified higher protein expression in vaginal tissues of CYP2B6, −2C19, and 3A4 in the FGT compared to colorectal tissues.30 They also measured, ex vivo, the metabolism of maraviroc and dapivirine in vaginal and colorectal explants. Lower levels of monoxygenated metabolites of maraviroc were found in vaginal tissues compared to colorectal while higher concentrations were found for dapivirine suggesting differential metabolism pathways between tissue compartments. This is consistent with in vivo findings. Following oral administration of maraviroc, Dumond et al reported steady-state AUC in vaginal tissue is 1.9 fold higher than blood plasma AUC.31 This is in contrast to Brown et al where colorectal tissue exposures 27-fold higher than blood plasma were reported.32 In contrast to To et al, using RT-PCR Zhou et al., did not detect in human ectocervix many major isoenzymes involved in phase I metabolism of xenobiotic compounds, notably CYP3A4.18 Notably, Zhou did not look for expression of these enzymes in vaginal tissue (only cervix) which may in part explain the discrepant findings with To et al.
Phosphorylating enzymes and nucleotidases may also be important in the success of nucleotide/side-based PrEP. The only currently approved PrEP therapy is a combination two nucleotide/side analogues, tenofovir and emtricitabine. Both of these compounds must undergo multiple phosphorylation steps by intracellular kinases to become pharmacologically active. While current research on the activation of TFV in the FGT is lacking, a study carried out by Lade et al. suggested several kinases, notably adenylate kinase 2, (AK2), pyruvate kinase muscle, (PKM) pyruvate kinase liver/red blood cell (PKLR), were responsible for the transformation of TFV to the active metabolite of tenofovir diphosphate (TFVdp) in PBMC and FGT biopsies, differing from the kinase activity observed in the colorectal tissues which relied on AK2 and creatinine kinase muscle (CKM)33. Genetic diversity in these enzymes, as well as those responsible for FTC activation, may also impact local drug disposition, but due to low levels of polymorphisms in these specific kinases the overall impact at the population level may be low.34 Further research must be conducted to gain a clearer picture of these mechanisms and their connection to the success of PrEP therapy.
Endogenous concentrations of molecules dATP and dCTP may be another factor influencing nucleotide/side-based PrEP in the FGT. The phosphorylated metabolites of nucletotide/side analogues actively compete against these endogenous nucleotides to be incorporated into the HIV DNA by HIV-1 reverse transcriptase, an event that blocks further replication.35 A study by Cottrell et al., identified that the amount of endogenous nucleotides was 7–11 higher in the FGT compared to colorectal tissues.36 These higher concentrations may indicate a need for higher exposures of nucleotide/side PrEP agents to outcompete the endogenous substrates for incorporation into the proviral DNA strand. Use of the molar ratio between nucleotide metabolites and endogenous substrates in modeling studies suggests alignment with real-world observations of TDF/FTC PrEP efficacy (see Population PK/PD modeling).
Role of Inflammation and co-infections in regulating drug efficacy in the female genital tract
Inflammation in female genital tissues is not uncommon, especially in the setting of sexually transmitted infections (STIs).37 Unprotected sexual intercourse is a risk factor for both HIV acquisition as well as other STIs. This is demonstrated by the high prevalence of STIs in the at-risk female participants of the PrEP studies.38–40 Much research has looked into the role that inflammation plays in susceptibility to HIV infection.41,42 The presence of inflammation may facilitate HIV transmission by recruitment or increased expression of HIV target cells, CD4+ CCR5+ T cells43. It is not known, however, whether increased susceptibility translates to changes in drug potency. McKinnon et al and colleagues examined cytokine profiles from cervicovaginal fluid from 774 women in the CAPRISA 004 study and found that in women with genital inflammation (defined as elevated concentrations of ≥ 3 proinflammatory cytokines), tenofovir gel resulted in 3% (95% CI −104–54%) protection against HIV infection compared to 57% (95% CI 7–80%) in women with no genital inflammation.44 Importantly, this lack of tenofovir gel efficacy was observed even among women considered highly adherent to therapy where protection was −10% (95% CI −184–57%). The mechanism of this undermining of PrEP efficacy is not fully known and it is not clear whether a similar effect would be expected from oral or systemically delivered tenofovir or from the vaginal ring dapivirine. Higher rates of genital inflammation in young (<25 years old) women42 may have contributed to the age-dependent effect observed in the vaginal ring trials, although adherence was clearly a large driver of this discrepancy.45,46
The potency of antiretrovirals relative to the expression of HIV target cells is likely dependent on the mechanism of action for each respective drug class. For competitive inhibitors such as the direct CCR5 antagonist maraviroc, an increase in CCR5 expression could conceivably require more drug coverage to occupy receptors47. The competing endogenous nucleotides are thought to be more expressed in “activated” cells and therefore, it is conceivable in states of inflammation, where immune cells become activated and are more numerous, more drug would be required to outcompete the endogenous nucleotides. For other antiretrovirals that target the virus directly (integrase inhibitors, protease inhibitors, non-nucleotide reverse transcriptase inhibitors), the number of cell targets may be less predictive. Inflammation may, however, facilitate viral replication (and therefore number of viral particles) as increased HIV shedding in the genital tract has been correlated to increased inflammation in the presence of co-infections.48
Systemic inflammation can alter drug metabolism by regulating the expression and activity of drug metabolizing enzymes and transporters, leading to altered pharmacokinetics.49,50 The majority of data available is derived from in vitro or animal studies with limited data from human tissues. Generally, pro-inflammatory cytokines seem to have a repressive effect on drug transporter expression and function, although there are some exceptions. For example, a decrease in P-gp expression has been observed in inflamed intestinal epithelium.51 This may explain why patients with inflammatory bowel disease have nearly five-fold increase in plasma exposure to P-gp substrates, propranolol and oxeprenol.52 In some cases, the net effect of inflammation on drug disposition is less clear. For example IL-6 leads to a reduction in expression while TNF alpha, although also a pro-inflammatory cytokine, leads to an expression induction. The knowledge of how most “inflammatory” disease states affect expression of drug transporters is largely limited to preclinical rodent models, which due to differences in gene homology make translation to humans difficult, or in vitro cell lines. Examples of how “local” inflammation may affect local expression of transporters in humans includes changes in hepatic expression of transporters in HCV, changes in expression of renal expression in glomerular nephritis, and changes at the BBB in neuroinflammation.53 None of these are directly comparable to the setting of co-infections within the same tissue (mucosal epithelium) such as occurs in STIs and directed studies are needed to fully understand the effect of STIs on local transporter expression and function within the FGT and to establish clinical relevance.
Although participants in PrEP studies have displayed a high prevalence of STIs at enrollment, per protocol these STIs were soon treated and likely resolved.54 Post-hoc analyses of these studies did not identify any significant differences in PrEP efficacy between users with and without STI co-infection.54,39,40,38,54 While no prospective clinical trials can directly address the influence of ongoing STI on PrEP efficacy, investigations in macaque models have shown in vivo proof of concept that STIs may decrease PrEP efficacy through pharmacologic mechanisms. CDC investigators found that daily oral TDF + FTC protected 67% (4 of 6) macaques inoculated with chlamydia and trichomoniasis from HIV infection compared to 100% (6 of 6) macaques with no genital co-infections 55,56, suggesting a modest decrease in PrEP efficacy in the presence of these STIs. Endogenous nucleotides dATP and dCTP measured in vaginal tissue homogenates were about ½ log higher in monkeys following STI infection compared to measurements obtained before STI infection. Interestingly, STIs appeared to significantly decrease emtricitabine triphosphate (FTCtp) concentrations, but not TFVdp concentrations. Taken together, these data are consistent with the concept that higher endogenous nucleotides, coupled with lower intracellular drug exposure, may contribute to decreased efficacy of nucleotide analogs in the setting of active co-infections.
A similar study was performed in macaques to assess the effect of these STIs on the efficacy of TFV gel.57 In contrast to the oral TDF regimen, 1% tenofovir gel, when applied 30 minutes before SHIV exposure, protected all six monkeys from 16 weekly SHIV inoculations. When the gel was applied 3 days before inoculation however, a regimen that was previously shown to provide 74% efficacy in macaques 58, in the presence of STI co-infection this protection fell to 60% (p=0.07), suggesting little to no impact of these STIs on the efficacy of topically delivered TFV. TFVdp measured in vaginal homogenates 2 hours post dose were 11-fold higher in STI-infected animals, although the difference was not statistically significant in this small sample size (n=4/group), this trend was consistent even up to 3 days after a single dose of 1% TFV gel. These findings are in contrast to the findings with oral TDF/FTC and suggest STIs modulation on TFVdp and FTCtp may differ based on route of administration.
Role of the vaginal microbiome in regulating drug efficacy in the female genital tract
The microflora of a healthy FGT is colonized predominantly by Lactobacillus. Bacterial vaginosis (BV), defined as an overgrowth of atypical bacteria relative to Lactobacillus, is one of the most prevalent disorders related to a microbial imbalance in the FGT. BV, although often asymptomatic, can lead to increased risk of HIV acquisition through increased inflammation and target cell recruitment, as well as direct compromise of the vaginal epithelium and reduced barrier function.59 The indication that BV, or altered microbial communities, could also undermine PrEP efficacy was first described in a retrospective analysis of the CAPRISA 004 study which reported a 3-fold higher efficacy of tenofovir gel in women who were Lactobacillus dominant compared to women with high amounts of G.vaginalis.60 To further investigate the mechanism for this, the group performed a series of experiments that showed Gardenerella in vitro cleaves tenofovir to adenine, its inactive metabolite. It cannot be ruled out that that altered drug exposure is achieved via indirect mechanism as dysbiosis can also be associated with elevated inflammation and lower pH. These findings were confirmed by retrospective data from FAME04, a phase 1 study to assess the safety of 1% tenofovir gel and 10 mg and 40 mg tenofovir film.61 In this study, Day 1 and Day 7 doses were directly observed providing some objective control for adherence. Higher concentrations of G. vaginalis were associated with lower tenofovir concentrations in both CVF and plasma, in addition to lower TFVdp concentrations in cervical tissue. Other markers of BV including Nugent score and Atropium vaginae were also associated with lower tenofovir and TFVdp concentrations. In contrast, neither genital nor plasma concentrations of dapivirine following film or gel use in FAME04 were effected by microbial populations or Nugent score.62
While these data suggest that vaginal microbiome can modulate efficacy of locally applied tenofovir, the effect of the microbiome following oral/systemic dosing is less clear. A post-hoc analysis of the Partners PrEP study concluded that, even among women with Lactobacillus non-dominant flora, oral TDF+FTC still provided significant protection with a 68% reduction in HIV infections among women with detectable G. vaginalis or Bacteriodes.63 However, the rate of infections in the Lactobacillus non-dominant group was nearly double that of the Lactobacillus dominant. The findings from the Partners PrEP study lend confidence that women with Lactobacillus non-dominant vaginal flora still receive benefit from TDF+FTC, but whether they are at greater risk from drug failure with imperfect adherence requires further study. Another study examined the effect of the microbiome to modulate antiretroviral exposure in FGT by quantifying drug from cervicovaginal lavages of HIV positive women who were virally suppressed without clinical symptoms of BV or other STIs.64 This study reported exposures were significantly reduced by 3–5 fold (TFV), 1–2 fold (FTC), or 2-fold (ATV) in “low and high diversity” compared to “intermediate diversity”. The reason for this bimodal effect and its clinical implications are unclear but further study of the interactions between host flora and drug efficacy are warranted.
Role of sex hormones in regulating drug efficacy in the female genital tract
The FGT is subject to a variety of anatomical changes and hormonal fluctuations from birth to menopause; and the implications of these changes on HIV infectability and prevention research have been reviewed in detail elsewhere.3 Herein the focus will be to review available data on the pharmacologic effects of the dynamic hormone profile of the female reproductive cycle across a woman’s lifespan as well as those effects of exogenously administered female sex hormones.
Female Reproductive Cycle
Wira, et. al. first described a window of vulnerability postulating that the delicate balance between immune protection and reproductive capability would result in a hormone mediated period of reduced immune function and heightened HIV infectability within the normal female reproductive cycle.65 In this context, understanding the potential modulatory effects of hormones on PrEP efficacy is of utmost importance in ensuring maximal protection especially during this window of vulnerability.
It has been well established that estradiol and progesterone heavily regulate immune function within the FGT.66,67 Additionally, these hormones have been shown to mediate enzymes and transporters, which could lead to altered pharmacology during times of high or low hormone exposure. For instance, in cell culture, treatment with estradiol and (to a lesser extent) progesterone increased the expression and function of the efflux transporter, p-glycoprotein (P-gp).68 Wira et al also demonstrated ex vivo treatment with estradiol increases biological activity of 5’nucleotidases (5’NT; a class of enzymes involving in maintaining nucleotide balance) in fibroblasts and epithelial cells isolated from female reproductive tissue but were unable to detect any enzymatic activity in peripheral blood lymphocytes.69 These enzymes dephosphorylate adenosine monophosphate to adenosine (a precursor molecule in the dATP salvage pathway) and may also dephosphorylate tenofovir.70 Therefore this increased activity could reduce the concentrations of the phosphorylated drug moiety (and ultimately TFVdp) while increasing concentrations of its competitive substrate (dATP). Alternatively in vitro treatment with estradiol has been shown to increase activity of creatinine kinases in human bone cells71, which could theoretically increase phosphorylation of tenofovir into its active form.72 In cell culture experiments, TFVdp formation was reduced by estradiol treatment only when co-administered with progesterone within CD4+cells isolated from the FGT but not the blood.73 These data suggest tissue specific hormonal modulation of these active metabolites is possible and may provide a pharmacologic explanation of the wide degree of between tissue variability observed in TFVdp/FTCtp concentrations. Importantly, all of these in vitro experiments used estradiol doses ranging from 2730 to 13,600 pg/mL. Since these concentrations are 6–30 times higher than typical peak serum estradiol concentrations (400pg/mL)74, these findings may be limited to the particular experimental system and careful in vivo study of estradiol’s effect on TFVdp and dATP is needed.
Investigators have aimed to understand whether hormonal fluctuation across the normal female reproductive cycle impacts local FGT pharmacokinetics in vivo. One small study exploring these effects on raltegravir concentrations in cervical tissue biopsies collected from 9 healthy volunteers taking 400mg twice daily demonstrated no association between trough (C12hr) concentration and menstrual cycle phase (proliferative/late follicular vs luteal) but did observe higher between subject variability among samples collected during the proliferative phase.75 While not statistically significant, one clinical study exploring menstrual cycle effect on atazanavir/ritonavir+TDF/FTC PK/PD, reported that 59% of cervicovaginal lavage samples collected during the follicular phase had detectable HIV RNA and DNA compared to 41% of those collected during the luteal phase despite consistent antiretroviral concentrations in the cervicovaginal fluid (CVF) across the menstrual cycle.76 Importantly, both of these studies only measured the parent drug concentrations of TDF + FTC and not the active metabolites (TFVdp/FTCtp). Previous reports have shown that CVF TFV and FTC concentrations are poorly predictive of TFVdp and FTCtp concentrations in FGT tissues (r2=0.20–0.23).77 Thus a characterization of active metabolites concentrations in these tissues across the menstrual cycle is needed to understand whether PrEP pharmacokinetics are modulated by natural hormonal fluctuations across the menstrual cycle.
Menopause
Menopause is characterized by estradiol and progesterone levels that at or lower than those of prepubescent girls. The role of menopause status on TDF + FTC pharmacology is not well understood, and has been primarily studied in the context of small observational cohort studies. Patterson et al reported 2–6 fold higher TFV plasma exposure in the blood and CVF of post vs premenopausal women at steady state. However, their small cohort of HIV infected women (n=12) was primarily African American (83%) and the impact of potential covariates such as creatinine clearance and age were not reported.78 Subsequently, a larger, primarily white (n=22, 84%) cohort of HIV infected women demonstrated no difference in TFV plasma trough concentrations in post vs premenopausal women [mean (SD)=119(64) vs 121(98)ng/ml, respectively].79 Using multiple regression analysis, these investigators found serum creatinine clearance (r=0.4, p=0.04) and body weight (r= −0.43, p=0.21) but not menopause (r= −0.049, p=0.85) were independently associated with TFV plasma trough concentrations. These between study discrepancies could arise from differences in racial representation of the study cohorts. Estradiol concentrations across the menstrual cycle are higher in African American vs white pre-menopausal women.80 Thus changes in estradiol exposure during the menopausal transition may be more pronounced among African American women. Shen et al. demonstrated that estradiol acts as a signal for epithelial cells from the FGT but not CD4 cells to increase phosphorylation of TFV to its active form TFVdp.73 Therefore, in response to high estradiol concentrations among pre-menopausal women these cells may act as a TFV sink – an effect that may be more pronounced between pre- and post-menopausal African American women.
Nicol et al sought to understand the role of menopause status in activation of TFVdp and FTCtp and found 9-fold lower concentrations of TFVdp in ectocervical tissue biopsies treated ex vivo with tenofovir (p=0.007). A significant relationship between age and TFVdp phosphorylation was also noted across the cohort but not within pre and postmenopausal groups.81 The authors did not separate HIV target cells for drug measurement so it is not known whether the overall lower TFVdp concentrations were due to less total number of epithelial cells (which may phosphorylate TFV more efficiently)73 per mg total tissue from epithelial thinning of postmenopausal tissues.
Exogenous Hormone Administration
Exogenous derivatives of estrogen and progesterone are frequently used to suppress ovulation for contraceptive purposes in premenopausal women, to treat symptoms such as hot flashes during the menopause transition, or to achieve feminization in transgender women. Of these exogenous hormone therapies, systemic pharmacokinetic interactions between hormonal contraception and antiretrovirals have been best studied and reviewed in detail elsewhere.82,83 Our review will focus on hormonal contraceptive’s effect on PrEP pharmacology within the FGT. Clinical and animal data suggest that hormonal contraception in the form of DMPA (which suppresses estradiol) does not render PrEP ineffective. A secondary analysis of data from the Partners PrEP randomized controlled trial of TDF + FTC for PrEP demonstrated 65 vs 76% PrEP efficacy among women who used DMPA vs no hormonal contraception.84 Furthermore, 100% of rhesus macaques treated with DMPA to mimic hormonal contraception and TDF + FTC PrEP remained uninfected after 20 vaginal challenges with SHIV.85 However, in vitro analysis of CD4 cells isolated from the FGT and treated DMPA, levonorgestrel, or norethisterone revealed reduced formation of TFVdp and its anti-HIV activity in the DMPA treatment group following TFV treatment but not for a similar prodrug, tenofovir alafenamide (TAF).86 One exploratory analysis of data from the MTN001 trial (a 7-site, open label, 3 period crossover PK study of oral and topical dosing) found that using any type of hormonal contraceptives (administered as oral or injectable) was associated with a 27–73% decrease in TFVdp concentrations in peripheral blood mononuclear cells (PBMCs) from HIV uninfected women.87 However, the post hoc nature of this analysis precluded controlling for potential confounders such as geographical differences in hormonal contraceptive prescribing patterns and adherence rates among the 4 US and 3 African sites. It remains to be determined whether these pharmacokinetic differences might mean that women on hormonal contraception have less forgiveness for missed PrEP doses.
Serum concentrations of estradiol in postmenopausal women using estrogen/progesterone hormone replacement therapy match those of premenopausal women during the proliferative (i.e. late follicular) phase and are 15–19 fold higher than those of postmenopausal women not on HRT.88 Yet, the effects of exogenously administered hormone replacement therapy have not been studied in HIV infected women taking TDF + FTC based antiretroviral therapy or uninfected women taking PrEP.
Despite a disproportionate HIV prevalence among transgender women (up to 50% among African American transgender women),89 PrEP clinical trials and demonstration projects to date have excluded or failed to achieve a meaningful representation of transgender women in study populations.90 Estradiol concentrations are even higher (1.3–2 fold) in transgender women being treated with hormone therapy for feminization [median (IQR)= 258 (812) pg/ml] compared to premenopausal women during the proliferative phase (15–350 pg/ml).91 In the open label extension of the iPrEx trial (a randomized clinical trial exploring PrEP efficacy among men who have sex with men and transgender women) these women were found to have lower concentrations of TFVdp in dried blood spots with an adjusted odds ratio of 0.72 (95% CI= 0.55–0.94) compared to the reference population.92 However, it could not be determined whether this finding was secondary to lower adherence or pharmacokinetic differences among the population. Whether these potential differences are more pronounced in mucosal tissues associated with HIV transmission (potentially including the vaginal vault constructed during vaginoplasty) remains to be determined. Multiple clinical trials (NCT#02983110, 03060785, and 03270969) are currently enrolling participants to assess whether a pharmacologic interaction exists in transgender women on hormone therapy for feminization.
Models to predict efficacy in the FGT
Preclinical (Humanized mice/Macaques)
Macaque Models
The attributes and limitations of non-human primate and humanized mice animal models used to explore PrEP PK/PD relationships been reviewed in detail elsewhere.93,94 This review will focus on describing the resulting PrEP PK/PD data rather than details of the animal models.
Proof of concept for HIV chemoprophylaxis was first established in animal models in the late 1990s when a study observed 100% protection among macaques given 20mg/kg subcutaneous tenofovir within 24 hours of a single intravenous challenge of simian immunodeficiency virus (SIV) and continued daily for 28 days following.95 Subsequently, the importance of initiation and duration were established in this same model showing that tenofovir’s protective effects could be reduced by >50% by initiating the drug >24 hours post challenge or reducing its duration to 10 days.96 Importantly, these initial studies utilized an unlabeled dosing formulation and failed to characterize pharmacokinetics making it difficult to assess the clinical relevance of the resulting data. Pharmacokinetics in the blood following oral TDF + FTC dosing have since been fully characterized in macaques and doses of 22 and 20mg/kg, respectively have been shown to result in parent (TFV/FTC) plasma and metabolite (TFVdp/FTCtp) PBMC concentrations consistent with those observed in humans given treatment doses.97 The local FGT PK has also been characterized in macaques for these human equivalent doses which achieve TFVdp and FTCtp concentrations ranging from 4–5 and 145–179 fmol/mg of vaginal tissue, respectively at 24 hours following a single dose. This concentration range is within the realm of clinical observations in women 24 hours after taking a single 300/200mg TDF/FTC dose which were 3.5 and 234 fmol/mg of vaginal tissue for TFVdp and FTCtp, respectively.36
Subsequently, event driven administration with these human equivalent TDF+ FTC doses (given up to 72 hours prior and 2 hours post challenge) was found to protect up to 100% of macaques given up to 18 once weekly vaginal SHIV challenge.56 Using this same dosing model investigators also established high levels of protection was sustained when challenging with an FTC resistant (SHIV162M184V) but not TDF resistant (SHIV162K65R) virus resulting in 0% and 66% of macaques becoming infected, respectively.98,99 While these latter studies explored a rectal challenge, they do provide encouraging preclinical data that TDF + FTC efficacy may be retained in the FGT in the event of a FTC resistant transmitted founder virus.
The long acting injectable, cabotegravir, has been studied for PrEP in a pigtailed macaque model where animals were given three 50mg/kg once monthly injections starting one week prior to biweekly low titer SHIV162P intravaginal challenges.100 While plasma cabotegravir concentrations remained ~1.5 log10 above the protein adjusted IC90 (166ng/ml) for the duration of the 12 week challenge, cervical and vaginal tissue concentrations fell below that target between 21 and 28 days post injection. Despite this seeming lapse of protective exposure, 100% of the animals remained uninfected over the course of the 12 weeks challenge. Alternatively, only 75% of Indian rhesus macaques remained uninfected across a total of 3 high titer intravaginal challenges when dosed with 50mg/kg monthly injection 1 week prior to the first and second challenge.101 In this study while plasma concentrations remained ~1log10 above the IC90, cervical concentrations fell below within 2 weeks after the initial injection and within 1 week after the second injection. Investigators postulated that the lower than expected concentrations may have resulted from a drug interaction between Depo-Provera (required for the vaginal SHIV challenge model) and cabotegravir; but whether the lower efficacy was due to reduced cabotegravir exposure in the FGT or the use of a high titer viral challenge is uncertain.
Humanized Mice Models
More recently humanized mouse models have also been developed for PrEP. While these models are advantageous for exploring transmitted founder viruses, the low blood volume of these small mammals makes it challenging to intensively characterize PK. Daily intraperitoneal dosing of TDF/FTC 5.2/3.5mg starting 48 hours prior to a single intravaginal challenge with HIV1JRcsf resulted in 100% protection among BLT humanized mice.102 However, pharmacokinetic characterization of this dosing strategy was not preformed making it difficult to assess the clinical applicability of the efficacy data. In a Rag-hu humanized mouse model, allometric scaling of the entry inhibitor, maraviroc, and the integrase strand transfer inhibitor, raltegravir, was used to predict a human equivalent dose of 62 and 164mg/kg, respectively (allometric scaling factor = 12.3). Daily oral gavage using these human equivalent doses starting 4 days prior to a single intravaginal challenge with HIV1BAL resulted in 100% protection.103 Subsequent PK evaluation confirmed that this human equivalent dose of MVC achieved vaginal tissues exposure (as measured by AUC) that was within 1.5 fold of clinical observations.104 However, vaginal concentrations of raltegravir in Rag-hu mice dosed with 164mg/kg were ~1 log10 higher than median concentrations reported in HIV uninfected women dosed to steady state with raltegravir 400mg twice daily.75,104 Thus it is possible that this Rag-hu mouse model may be an overestimation of raltegravir efficacy. Finally, a novel long acting formulation of the integrase inhibitor RTG has also been tested for PrEP in BLT humanized, where a single subcutaneous dose resulted in 100% protection from two high-dose HIV vaginal challenges 1 week and 4 weeks after drug administration.105
Explant studies and dose-challenge
Human tissue explants have long been used to study HIV infection and transmission. In contrast to other ex vivo or in vitro approaches, the explant model allows for the tissue to remain intact, providing the opportunity to study the influence of tissue architecture and cellular composition on HIV infection events. While the first uses of the mucosal tissue explant model were to study HIV transmission, more recently, the explant model has been used in the development of PrEP candidates. While colorectal and cervical/vaginal explants have both studied, for the purpose of this review we will focus on cervicovaginal.
In preclinical development, the explant model has been used to verify activity against PrEP candidates in mucosal tissues. Here, explant tissues are exposed to drugs of interest ex vivo prior to being exposed to viral inoculum. Because this approach typically uses surgical discard or cadaver tissue rather than biopsies, the amount of tissue is typically not a limiting factor which allows for the examination of several doses from the same donor- lending itself to the possibility for dose response curves and establishment of IC50s. The explant model has shown several PrEP candidates have preventive capacity in FGT including tenofovir, dapivirine, rilpivirine, and maraviroc as protective in blocking HIV infection in FGT106–112.
Because the incidence of HIV, even in high-risk populations would require a large sample size to statistically establish a dose-response relationship, the use of an ex-vivo efficacy surrogate in early drug development is appealing. The “dose-challenge” model, an adaption of the explant model has been employed as a means to provide a surrogate for efficacy in early phase trials. In these studies, cervicovaginal biopsies are obtained from HIV-negative volunteers post-dose while pre-dose cervicovaginal biopsies can be used as donor-matched controls. These biopsies are then exposed to HIV ex vivo to determine protection against HIV. This ex vivo surrogate endpoint allows some estimate of efficacy in early phase clinical trials.
The ability of the explant model to define target concentrations has never been robustly confirmed and many have questioned whether a scaling factor is needed. 106Many of the explant models evaluating NRTIs did not quantify intracellular metabolites, making comparisons to clinical observations difficult due to difference in phosphorylation efficiency in vitro compared to in vivo. However, models that directly measure concentrations of the active metabolite show promise. Nicol et al predicted a TFVdp EC50 in vaginal tissue of 716 fmol/mg.106 This was similar to predicted efficacy from a polarized ectocervical model which found complete inhibition in tissues with TFVdp ranging from ~300–2400 fmol/mg (although these explants were also dosed with UC781).107 Given steady state concentrations of TFVdp in genital cells from cervical brush following oral dosing are equivalent to ~555 fmol/mg (111 fmol/106 cells)113, this would suggest partial efficacy from oral tenofovir, consistent with 71% efficacy seen with TDF in women in Partners PrEP (Table 1).38 Similarly, pharmacokinetic analysis from CAPRISA samples found CVF concentrations of 1000 ng/mL (equivalent to 500 fmol/mg) were associated with 76% protection against HIV.114 Although a variety of other antiretrovirals have been tested in the explant system, the only other compound with clinical outcomes data is dapivirine, an NNRTI that was formulated into a vaginal ring and reduced infections by 29–37% in Phase 3 trials (Table 1). Initial cervical explant models predicted dapivirine was 100% protective at 0.1 uM.109 Dose challenge studies performed in a Phase 2 studies of DPV/MVC rings estimated a DPV EC50 of 0.3 μM.115 Vaginal tissue concentrations after 28 days of ring use were ~ 1.5 μM suggesting the models slightly overpredicted clinical effectiveness.116 This may, in part, have been due to imperfect adherence in these trials.45,46 These data give some confidence to the utility of explant models to identify target concentrations but whether this will be applicable to all antiretroviral classes is not clear.
Table 1:
Clinical studies evaluating antiretroviral efficacy for prevention in the female genital tract
| Intervention | Estimated tissue exposure if perfect adherence^ | Clinical Study | Estimated adherence (% plasma (or swabs) with drug detected)~ | Observed Efficacy HR (95% CI) |
|---|---|---|---|---|
| TFV 1% gel precoitally | n/a | CAPRISA 004118 | n/a | 0.61 (0.4–0.94) |
| FACTS001120 | n/a | 1.0 (0.7–1.4) | ||
| TFV 1% gel daily | 1.8 μM TFVdp119 | VOICE40 | (49%) | 0.85 (0.61–1.21) |
| Oral TDF 300mg daily | 0.56 μM TFVdp113 | Partners PrEP38 | 80% | 0.29 (0.13–0.63)* |
| VOICE40 | 30% | 1.49 (0.97–2.29) | ||
| Oral TDF/FTC 300/200 mg daily | 0.56 μM TFVdp113 35 μM FTCtp113 |
Partners PrEP38 | 79% | 0.34 (0.16–0.72)* |
| FemPREP39 | 35% | 0.94 (0.59–1.52) | ||
| VOICE40 | 29% | 1.04 (0.73–1.49) | ||
| TDF2121 | 79% | 0.51 (0.19–1.22)* | ||
| DPV ring 25 mg monthly |
1.52 μM DPV83 | ASPIRE45 | 82% | 0.73 (0.54–0.99) |
| RING46 | 84% | 0.69 (0.49–0.99) | ||
| Oral MVC 300 mg daily | 1.65 μM MVC31 | HPTN 069122 | 0% incidence+ | |
| Oral MVC/TDF 300/300 mg daily | 1.65 μM MVC31 0.56 μM TFVdp113 |
HPTN 069122 | 0% incidence+ | |
| Oral MVC/FTC 300/200 mg daily | 1.65 μM MVC31 35 μM FTCtp113 |
HPTN 069122 | 0% incidence+ | |
| Cabotegravir long-active injectable 600 mg IM q4 weeks × 2, then q8weeks | 0.45–6 μM CAB#26 | HPTN 084 | Phase III study launched Fall of 2017 with plans to enroll 3200 women |
CAB=cabotegravir; CI=confidence interval; DPV=dapivirine; FTC=emtricitabine; HR=hazard ratio; IM=intramuscular; MVC=maraviroc; TDF=tenofovir disoproxil fumarate; TFV=tenofovir; TFVdp= tenofovir diphosphate
n/a= not applicable for studies with on-demand dosing
All values converted to μM for comparison; assumed volume of 106 mononuclear cells =0.2 μL123 and the density of tissue is 1g/mL
FemPrEP cutoff of TFV 10 ng/mL; Partners PrEP, VOICE, and TDF2 cutoff of TFV 0.3 ng/mL; ASPIRE and RING study cutoff 95 pg/mL DPV
subgroup analysis in women only
Phase 2 data not powered for efficacy (no incidence in 188 women enrolled in study)
Extrapolated from 400 mg IM dose assuming dose proportionality
Population PK/PD Modeling
A recent investigation of PrEP pharmacodynamics in TZMbl cells as well as CD4 cells isolated from the blood quantified the exposure response relationship for preventing HIV-1JRcsf infection using exposure variables of active metabolite (TFVdp and FTCtp) concentrations or the ratio of active metabolites to their competing endogenous nucleotides (TFVdp:dATP and FTCtp:dCTP).36 Interestingly, this study found the difference in calculated EC90 between the cellular models was reduced by 70% when the active metabolite exposure variable was normalized for endogenous nucleotide concentration. An interaction surface term was also in this study to quantify contribution of TDF + FTC combination therapy in the predicted exposure response. These EC90 efficacy targets and interaction term were then linked to a population PK model simulating these ratios in mucosal tissues of women given dosing scenarios of once weekly to daily dosing. In the FGT 7 daily doses were required for 100% of the simulated population to achieve this efficacy target and declined to ~80% when the simulated population was ~50% adherent. Alternatively, only 2–3 doses per week were required for 100% of the simulated population to achieve target exposure in the lower gastrointestinal tract.
The Partners PrEP randomized controlled trial demonstrated 66% efficacy of TDF + FTC among heterosexual women in stable serodiscordant relationships.38 Using plasma drug concentrations from women enrolled in this trial, Donnel et al demonstrated 92% protection among women taking 7 doses per week (confirmatory of these population PK/PD model predictions); but uniformly high adherence rates constrained their ability to make predictions for less consistent117 Conversely, futility due to uniformly low adherence rates among women enrolled in the FEM-PrEP and VOICE trials39,40 also precluded identifying protection in the FGT with less than perfect adherence. Given the challenges of the available clinical data, modeling exercises such as the one described above represent an opportunity to predict effectiveness for “real world” drug taking behavior and design user specific adherence messaging. Additionally, these models can be used in advance of clinical trials to distinguish and prioritize investigational agents with more forgiveness for missed doses.
Paramount to employing this approach is selecting the right PD target. The lack of PD surrogates (e.g. viral load, cholesterol, blood pressure) for PrEP make identification of clinical PD targets difficult and necessitate relying on models. Garnering scientific consensus of the most translatable model (e.g. animals, explants, isolated cells) for this derivation represents a major opportunity for the field. Additionally, while vulnerabilities to HIV infection exist throughout the entire FGT, the exact anatomical level of PrEP action (i.e. squamous epithelium, regional lymph nodes) is still uncertain. Partial efficacy of TFV gel118, which achieves TFVdp concentrations in vaginal tissue biopsies that are 130 fold higher compared to oral dosing119 suggest superficial coverage of the epidermis alone is inadequate. Thus PK/PD models incorporating information about compartmental PK may not generalize across dosing modalities.
Conclusion
More prevention options are needed to curb the HIV epidemic in women. Table 1 summarizes the clinical trials that have been performed in women that provided an efficacy endpoint. Many novel and innovative approaches in the pipeline for PrEP have been designed to overcome the limitations of adherence requirements for oral daily PrEP. However lessons learned from the work reviewed above highlight the importance of pharmacology as a critical consideration in the success of any PrEP product. The complex environment of the FGT can be vulnerable to changes in hormones, inflammation, and microbiome; development of future PrEP interventions will need to ensure efficacy against a spectrum of potential conditions. Models in preclinical or early clinical development may have utility in assessing these conditions. Animal models have been improved since early models by targeting dosing strategies intended to mimic expected human exposure but are not yet able to fully replicate compartmental PK in part because of between species differences in pharmacologic effectors. Thus these models are most useful for establishing proof of concept of efficacy rather than predicting effectiveness on a population level. Explant models using human tissues may be more useful in identifying specific target concentrations when concentrations of active drug are measured tissue in are available. Further investigation of potential PrEP effectors such as hormones, co-infections, and microbial communities is needed; and these studies should be thoughtfully designed to mimic clinical conditions and provide maximally informative data regarding clinical significance for current and future PrEP interventions.
Acknowledgements
The authors would like to thank Derek Nicol for assistance with creation of Figure 2.
Footnotes
Disclosures: No disclosures to report
References
- [1].Joint United Nations Programme on HIV/AIDS. Global AIDS Update 2016. Published 31May2016. Available at http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016. Accessed 7Aug2016. [PubMed]
- [2].Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–23. [DOI] [PubMed] [Google Scholar]
- [3].Venkatesh KK, Cu-Uvin S. Anatomic and hormonal changes in the female reproductive tract immune environment during the life cycle: implications for HIV/STI prevention research. Am J Reprod Immunol. 2014;71:495–504. [DOI] [PubMed] [Google Scholar]
- [4].Lee SK, Kim CJ, Kim DJ, Kang JH. Immune cells in the female reproductive tract. Immune Netw. 2015;15:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999; 96: 272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stieh DJ, Maric D, Kelley ZL et al. Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog. 2014;10:e1004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shen R, Kappes JC, Smythies LE, Richter HE, Novak L, Smith PD. Vaginal myeloid dendritic cells transmit founder HIV-1. J Virol. 2014;88:7683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Louissaint NA, Fuchs EJ, Bakshi RP et al. Distribution of cell-free and cell-associated HIV surrogates in the female genital tract after simulatd vaginal intercourse. J Infect Dis. 2012; 205:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cottrell ML, Srinivas N, Kashuba AD. Pharmacokinetics of antiretrovirals in mucosal tissue. Expert Opin Drug Metab Toxicol. 2015:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nicol MR, Kashuba AD. Pharmacologic opportunities for HIV prevention. Clin Pharmacol Therapeut. 2010;88:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rahangdale L, De Paris K, Kashuba AD, et al. Immunologic, virologic, and pharmacologic characterization of the female upper genital tract in HIV-infected women. J Acquir Immune Defic Syndr. 2015;68:420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Louissant NA, Cao YJ, Skipper PK et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013;29:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kis O, Robillard K, Chan GN, Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2010;31:22–35. [DOI] [PubMed] [Google Scholar]
- [16].Michaud V, Bar-Magen T, Turgeon J, Flockhart D, Desta Z, Wainberg MA. The dual role of pharmacogenetics in HIV treatment: mutations and polymorphisms regulating antiretroviral drug resistance and disposition. Pharmacol Rev. 2012;64:803–33. [DOI] [PubMed] [Google Scholar]
- [17].Nicol MR, Fedoriw Y, Mathews M, et al. Expression of six drug transporters in vaginal, cervical, and colorectal tissues: Implications for drug disposition in HIV prevention. J Clin Pharmacol. 2014;54:574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou T, Hu M, Cost M, Poloyac S, Rohan L. Short communication: expression of transporters and metabolizing enzymes in the female lower genital tract: implications for microbicide research. AIDS Res Hum Retroviruses. 2013;29:1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhou T, Hu M, Pearlman A, Patton D, Rohan L. Expression and localization of p-glycoprotein, multidrug resistance protein 4, and breast cancer resistance protein in the female lower genital tract of human and pigtailed macaque. AIDS Res Hum Retroviruses. 2014;30:1106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Giacomini KM, Huang SM, Tweedie DJ, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hu M, Patel SK, Zhou T, Rohan LC. Drug Transporters in Tissues and Cells Relevant to Sexual Transmission of HIV: Implications for Drug Delivery. J Control Release. 2015;219:681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50:3297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou T, Hu M, Pearlman A, Rohan LC. Expression, regulation, and function of drug transporters in cervicovaginal tissues of a mouse model used for microbicide testing. Biochem Pharmacol. 2016;116:162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reese MJ, Bowers GD, Humphreys JE, et al. Drug interaction profile of the HIV integrase inhibitor cabotegravir: assessment from in vitro studies and a clinical investigation with midazolam. Xenobiotica. 2016;46:445–56. [DOI] [PubMed] [Google Scholar]
- [25].Trezza CR, Kashuba AD. Pharmacokinetics of antiretrovirals in genital secretions and anatomic sites of HIV transmission: implications for HIV prevention. Clin Pharmacokinet. 2014;53:611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Spreen W, Ford SL, Chen S, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014;67:481–6. [DOI] [PubMed] [Google Scholar]
- [27].Hijazi K, Cuppone AM, Smith K, et al. Expression of Genes for Drug Transporters in the Human Female Genital Tract and Modulatory Effect of Antiretroviral Drugs. PloS One. 2015;10:e0131405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cihlar T, Ho ES, Lin DC, Mulato AS. Human renal organic anion transporter (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides, Nucleotides Nucleic Acids. 2001;20:641–8. [DOI] [PubMed] [Google Scholar]
- [29].Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab. 2004;5:63–84. [DOI] [PubMed] [Google Scholar]
- [30].To EE, Hendrix CW, Bumpus NN. Dissimilarities in the metabolism of antiretroviral drugs used in HIV pre-exposure prophylaxis in colon and vagina tissues. Biochem Pharmacol. 2013;86:979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dumond JB, Patterson KB, Pecha AL, et al. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brown KC, Patterson KB, Malone SA, et al. Single and multiple dose pharmacokinetics of maraviroc in saliva, semen, and rectal tissue of healthy HIV-negative men. J Infect Dis. 2011;203:1484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lade JM, To EE, Hendrix CW, Bumpus NN. Discovery of Genetic Variants of the Kinases That Activate Tenofovir in a Compartment-specific Manner. EBioMedicine. 2015;2:1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Figueroa D, Madeen E, Tillotson J, et al. Genetic Variation of the Kinases that Phosphorylate Tenofovir and Emtricitabine in Peripheral Blood Mononuclear Cells. AIDS Res Hum Retroviruses. 2018. doi: 10.1089/AID.2017.0243. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schinazi RF, Hernandez-Santiago BI, Hurwitz SJ. Pharmacology of current and promising nucleosides for the treatment of human immunodeficiency viruses. Antivir Res. 2006;71:322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cottrell ML, Yang KH, Prince HM, et al. A Translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Masson L, Mlisana K, Little F, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect. 2014;90:580–7. [DOI] [PubMed] [Google Scholar]
- [38].Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV Infection among African women. N Engl J Med. 2015;372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microb. 2004;2:33–42. [DOI] [PubMed] [Google Scholar]
- [42].Masson L, Passmore JA, Liebenberg LJ, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis. 2015;61:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McKinnon LR, Liebenberg LJ, Yende-Zuma N, et al. Genital inflammation undermines the effectiveness of tenofovir gel in preventing HIV acquisition in women. Nat Med. 2018. doi: 10.1038/nm.4506. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375:2122–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med.. 2016;375:2133–43. [DOI] [PubMed] [Google Scholar]
- [47].Fatkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AI, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11:1170–2. [DOI] [PubMed] [Google Scholar]
- [48].Fastring DR, Amedee A, Gatski M, Clark RA, Mena LA, Levison J, et al. Co-occurrence of Trichomonas vaginalis and bacterial vaginosis and vaginal shedding of HIV-1 RNA. Sex Transm Dis. 2014;41:173–9. [DOI] [PubMed] [Google Scholar]
- [49].Cressman AM, Petrovic V, Piquette-Miller M. Inflammation-mediated changes in drug transporter expression/activity: implications for therapeutic drug response. Exp rev Clin Pharmacol. 2012;5:69–89. [DOI] [PubMed] [Google Scholar]
- [50].Renton KW. Regulation of drug metabolism and disposition during inflammation and infection. Exp Opin Drug Metab Toxicol. 2005;1:629–40. [DOI] [PubMed] [Google Scholar]
- [51].Blokzijl H, Vander Borght S, et al. Decreased P-glycoprotein (P-gp/MDR1) expression in inflamed human intestinal epithelium is independent of PXR protein levels. Inflammat Bowel Dis. 2007;13:710–20. [DOI] [PubMed] [Google Scholar]
- [52].Schneider RE, Babb J, Bishop H, Mitchard M, Hoare AM. Plasma levels of propranolol in treated patients with coeliac disease and patients with Crohn’s disease. Brit Med J. 1976;2:794–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Atilano-Roque A, Roda G, Fogueri U, Kiser JJ, Joy MS. Effect of disease pathologies on transporter expression and function. J Clin Pharmacol. 2016;56 Suppl 7:S205–21. [DOI] [PubMed] [Google Scholar]
- [54].Thomson KA, Baeten JM, Mugo NR, Bekker LG, Celum CL, Heffron R. Tenofovir-based oral preexposure prophylaxis prevents HIV infection among women. Curr Opin HIV AIDS. 2016;11:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Radzio J, Henning T, Jenkins L, Ellis S, Farshy C, Phillips C, et al. Combination emtricitabine and tenofovir disoproxil fumarate prevents vaginal SHIV infection in macaques harboring C. trachomatis and T. vaginalis. J Infect Dis. 2016;213:1541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Radzio J, Aung W, Holder A, et al. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PloS One. 2012;7:e50632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Makarova N, Henning T, Taylor A, et al. Topical tenofovir protects against vaginal simian HIV infection in macaques coinfected with Chlamydia trachomatis and Trichomonas vaginalis. AIDS. 2017;31:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dobard C, Sharma S, Martin A, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol. 2012;86:718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Passmore JS, Jaspan HB, Masson L. Genital inflammation, immune activation and risk of sexual HIV acquisition. Curr Opin HIV AIDS. 2016;11(2):156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356:938–45. [DOI] [PubMed] [Google Scholar]
- [61].Hillier S, Meyn LA, Bunge K, et al. Impact of vaginal microbiota on genital tissue and plasma concentrations of tenofovir. Conference on Retroviruses and Opportunistic Infections Feb 13–16, 2017 Seattle, WA Abstract 86LB. [Google Scholar]
- [62].Hillier S, Meyn LA, Bunge K, et al. Impact of vaginal microbiota on genital tissue and plasma concentrations of dapivirine. International AIDS Conference 25 July 2017 Paris, France Abstract TUAC0104. [Google Scholar]
- [63].Heffron R, McClelland RS, Balkus JE, Celum C, Cohen CR, Mugo N, et al. Efficacy of oral pre-exposure prophylaxis (PrEP) for HIV among women with abnormal vaginal microbiota: a post-hoc analysis of the randomised, placebo-controlled Partners PrEP Study. Lancet HIV. 2017. 4(10):e449–e456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Donahue Carlson R, Sheth AN, Read TD, Frisch MB, Mehta CC, Martin A, et al. The female genital tract microbiome Is associated with vaginal antiretroviral drug concentrations in human immunodeficiency virus-infected women on antiretroviral therapy. J Infect Dis. 2017. 216(8):990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gianella S, Tsibris A, Barr L, Godfrey C. Barriers to a cure for HIV in women. J Int AIDS Soc. 2016;19:20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wira CR, Rodriguez-Garcia M, Shen Z, Patel M, Fahey JV. The role of sex hormones and the tissue environment in immune protection against HIV in the female reproductive tract. Am J Reprod Immunol. 2014;72:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Coles LD, Lee IJ, Voulalas PJ, Eddington ND. Estradiol and progesterone-mediated regulation of P-gp in P-gp overexpressing cells (NCI-ADR-RES) and placental cells (JAR). Mol Pharmaceut. 2009;6:1816–25. [DOI] [PubMed] [Google Scholar]
- [69].Shen Z, Fahey JV, Bodwell JE, et al. Estradiol regulation of nucleotidases in female reproductive tract epithelial cells and fibroblasts. PloS One. 2013;8:e69854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hunsucker SA, Mitchell BS, Spychala J. The 5’-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol Ther. 2005;107:1–30. [DOI] [PubMed] [Google Scholar]
- [71].Somjen D, Katzburg S, Shoaron O et al. Sex specific response of cultured human bone cells to ER-alpha and ER-beta specific agonists by modulation of cell proliferation and creatinine kinase activity. J Ster Biochem Mol Biol. 2011;125:226–30. [DOI] [PubMed] [Google Scholar]
- [72].Varga A, Graczer E, Chaloin L et al. Selectivity of kinases on the activation of tenofovir, an anti-HIV agent. Eur J Pharmaceut Sci. 2013;48:307–15. [DOI] [PubMed] [Google Scholar]
- [73].Shen Z, Fahey JV, Bodwell JE, Rodriguez-Garcia M, Kashuba AD, Wira CR. Sex hormones regulate tenofovir-diphosphate in female reproductive tract cells in culture. PloS One. 2014;9:e100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].White CW, Zieve D. “Estradiol blood test” Site: US National Library of Medicine MedlinePlus URL: https://medlineplus.gov/ency/article/003711.htm Last updated: 30Apr2018. Visited: 14May2018.
- [75].Mitchell C, Roemer E, Nkwopara E, et al. Correlation between plasma, intracellular, and cervical tissue levels of raltegravir at steady-state dosing in healthy women. Antimicrob Agents Chemother. 2014;58:3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sheth AN, Evans-Strickfaden T, Haaland R, et al. HIV-1 genital shedding is suppressed in the setting of high genital antiretroviral drug concentrations throughout the menstrual cycle. J Infect Dis. 2014;210:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cottrell ML, Prince HM, Allmon A, et al. Cervicovaginal and rectal fluid as a surrogate marker of Antiretroviral tissue concentration: implications for clinical trial design. 5th International Workshop on HIV and Women 21–22 Feb, 2015, Seattle, WA Abstract #21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Patterson K, Dumond JB, Prince HA, Kraft E, Eron J, Kashuba A. Pharmacokinetics of TDF in blood plasma and cervicovaginal fluid of HIV+ post-menopausal compared with pre-menopausal women. 18th Conference on Retroviruses and Opportunistic Infections Boston, MA 2011. [Google Scholar]
- [79].Gervasoni C, Meraviglia P, Landonio S, et al. Tenofovir plasma concentrations in post-menopausal versus pre-menopausal HIV-infected women. J Antimicrob Chemother. 2013;68:1206–7. [DOI] [PubMed] [Google Scholar]
- [80].Marsh EE, Shaw ND, Klingman KM, et al. Estrogen levels are higher across the menstrual cycle in African-American women compared with Caucasian women. J Clin Endocrinol Metab. 2011;96:3199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Nicol MR, Brewers LM, Kashuba ADM, Sykes C. The role of menopause in tenofovir diphosphate and emtricitabine triphosphate concentrations in cervical tissue. AIDS. 2018;32:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Scarsi KK, Darin KM, Chappell CA, Nitz SM, Lamorde M. Drug-Drug interactions, effectiveness, and safety of hormonal contraceptives in women living with HIV. Drug safety. 2016;39:1053–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Thurman AR, Anderson S, Doncel GF. Effects of hormonal contraception on antiretroviral drug metabolism, pharmacokinetics and pharmacodynamics. Am J Reprod Immunol. 2014;71:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Heffron R, Mugo N, Were E, et al. Preexposure prophylaxis is efficacious for HIV-1 prevention among women using depot medroxyprogesterone acetate for contraception. AIDS. 2014;28:2771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Radzio J, Hanley K, Mitchell J, et al. Depot-medroxyprogesterone acetate does not reduce the prophylactic efficacy of emtricitabine and tenofovir disoproxil fumarate in macaques. J Acquir Immune Defic Syndr. 2014;67:365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Shen Z, Rodriguez-Garcia M, Patel MV, Bodwell J, Kashuba ADM, Wira CR. Hormonal contraceptives differentially suppress TFV and TAF inhibition of HIV infection and TFV-DP in blood and genital tract CD4+ T cells. Sci Reports. 2017;7:17697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Coleman JS, Chaturvedula A, Hendrix C. Method of hormonal contraception is associated with lower tenofovir concentration in health women (MTN-001): implications for pre-exposure prophylaxis. XIX International AIDS Conference Washington DC, USA 2012. [Google Scholar]
- [88].Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72:714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Centers for Disease Control and Prevention. HIV among transgender people. https://www.cdc.gov/hiv/pdf/group/gender/transgender/cdc-hiv-transgender-factsheet.pdf. Assessed March 3, 2018.
- [90].Sevelius JM, Deutsch MB, Grant R. The future of PrEP among transgender women: the critical role of gender affirmation in research and clinical practices. J int AIDS Soc. 2016;19:21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Deutsch MB, Bhakri V, Kubicek K. Effects of cross-sex hormone treatment on transgender women and men. Obstetrics Gynecol. 2015;125:605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Grant RM, Anderson PL, McMahan V et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microb. 2012;10:852–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Garcia-Lerma JG, Heneine W. Animal models of antiretroviral prophylaxis for HIV prevention. Curr Opin HIV AIDS. 2012;7:505–13. [DOI] [PubMed] [Google Scholar]
- [95].Tsai CC, Follis KE, Sabo A, Beck TW, Grant RF, Bischofberger N, et al. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–9. [DOI] [PubMed] [Google Scholar]
- [96].Tsai CC, Emau P, Follis KE, Beck TW, Benveniste RE, Bischofberger N, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Cong ME, Youngpairoj AS, Zheng Q, et al. Protection against rectal transmission of an emtricitabine-resistant simian/human immunodeficiency virus SHIV162p3M184V mutant by intermittent prophylaxis with Truvada. J Virol. 2011;85:7933–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cong ME, Mitchell J, Sweeney E, et al. Prophylactic efficacy of oral emtricitabine and tenofovir disoproxil fumarate combination therapy against a tenofovir-resistant simian/human immunodeficiency virus containing the K65R mutation in macaques. J Infect Dis. 2013;208:463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Radzio J, Spreen W, Yueh YL, et al. The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Transl Med. 2015;7:270ra5. [DOI] [PubMed] [Google Scholar]
- [101].Andrews CD, Yueh YL, Spreen WR, et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med. 2015;7:270ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Neff CP, Ndolo T, Tandon A, Habu Y, Akkina R. Oral pre-exposure prophylaxis by anti-retrovirals raltegravir and maraviroc protects against HIV-1 vaginal transmission in a humanized mouse model. PloS One. 2010;5:e15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Veselinovic M, Yang KH, LeCureux J, Sykes C, Remling-Mulder L, Kashuba AD, et al. HIV pre-exposure prophylaxis: mucosal tissue drug distribution of RT inhibitor Tenofovir and entry inhibitor Maraviroc in a humanized mouse model. Virology. 2014;464–465: 253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kovarova M, Swanson MD, Sanchez RI, et al. A long-acting formulation of the integrase inhibitor raltegravir protects humanized BLT mice from repeated high-dose vaginal HIV challenges. J Antimicrob Chemother. 2016. 71(6):1586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nicol MR, Emerson CW, Prince HM, et al. Models for predicting effective HIV chemoprevention in women. J Acquir Immune Defic Syndr. 2015;68:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cost M, Dezzutti CS, Clark MR et al. Characterization of UC781-tenofovir combination gel products for HIV-1 infection prevention in an ex vivo ectocervical model. Antimicrob Agents Chemother. 2012;56:3058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PloS one. 2010;5(2):e9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Fletcher P, Harman S, Azijn H, et al. Inhibition of human immunodeficiency virus type 1 infection by the candidate microbicide dapivirine, a nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2009;53:487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Fletcher P, Herrera C, Armanasco N, Nuttall J, Shattock RJ. Limited anti-HIV-1 activity of maraviroc in mucosal tissues. AIDS Res Hum Retrovirus. 2016. 32(4):334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Dezzutti CS, Else LJ, Yandura SE, et al. Distinct pharmacodynamic activity of rilpivirine in ectocervical and colonic explant tissue. Antimicrob Agents Chemother. 2016;60(5):2765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dezzutti CS, Yandura S, Wang L, et al. Pharmacodynamic activity of dapivirine and maraviroc single entity and combination topical gels for HIV-1 prevention. Pharmaceut Res. 2015. 32(11):3768–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Seifer SM, Chen X, Meditz AL et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. Aids Res Hum Retrovirus. 2016;32:981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kashuba AD, Gengiah TN, Werner L, et al. Genital tenofovir concentrations correlate with protection against HIV infection in fhte CAPRISA 004 trial: importance of adherence for microbicide effectiveness. J Acquir Immun Def Syndr. 2015;69:264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Dezzutti CS, Richardson-Harman N, Rohan LC, et al. Pharmacodynamic correlations using fresh and cryopreserved tissue following use of vaginal rings containing dapivirine and/or maraviroc in a randomized, placebo controlled trial. Medicine. 2016;95:e4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chen BA, Panther L, Marzinke MA, et al. Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Def Syndr. 2015;70(3):242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Def Syndr. 2014;66:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hendrix CW, Chen BA, Guddera V, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir gel and oral tablets in vaginal tissue and other compartments. PLoS One.2013;8:e55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rees H, Delany-Moretlwe S, Baron D, et al. FACTS 001 phase III trial of pericoital tenofovir 1% gel for HIV prevention in women. Conference on Retroviruses and Opportunistic Infections, Feb 23–26, 2015 Seattle, WA Abstract 26LB. [Google Scholar]
- [121].Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. [DOI] [PubMed] [Google Scholar]
- [122].Gulick R, Wilkin T, Chen Y, et al. HPTN 069/ACTG A5305:phase II study of maraviroc-containing regiments for HIV PrEP in United States (U.S.) women. 21st International AIDS Conference (AIDS 2016) July 18–22, 2016 Durban, South Africa Abstract TUAC 0102. [Google Scholar]
- [123].Chapman EH, Kurec AS, Davey FR, et al. Cell volumes of normal and malignant mononuclear cells. J Clin Pathol. 1981;43:1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]