Abstract
Mid-cervical spinal cord contusion disrupts both the pathways and motoneurons vital to the activity of inspiratory muscles. The present study was designed to determine if a rat contusion model could result in a measurable deficit to both ventilatory and respiratory motor function under “normal” breathing conditions at acute to chronic stages post trauma. Through whole body plethysmography and electromyography we assessed respiratory output from three days to twelve weeks after a cervical level 3 (C3) contusion. Contused animals showed significant deficits in both tidal and minute volumes which were sustained from acute to chronic time points. We also examined the degree to which the contusion injury impacted ventilatory pattern variability through assessment of Mutual Information and Sample Entropy. Mid-cervical contusion significantly and robustly decreased the variability of ventilatory patterns. The enduring deficit to the respiratory motor system caused by contusion was further confirmed through electromyography recordings in multiple respiratory muscles. When isolated via a lesion, these contused pathways were insufficient to maintain respiratory activity at all time points post injury. Collectively these data illustrate that, counter to the prevailing literature, a profound and lasting ventilatory and respiratory motor deficit may be modelled and measured through multiple physiological assessments at all time points after cervical contusion injury.
Keywords: Respiration, Cervical contusion, Ventilatory pattern variability, Minute volume, Tidal volume, Respiratory EMG
1. Introduction
Inspiration in mammals is largely caused by the contraction of the diaphragm. This powerful muscle is innervated by the phrenic motor pool, located between the third and fifth cervical spinal cord segments (C3–C5) in humans and rodents (Ellenberger and Feldman, 1988; Keswani and Hollinshead, 1955; Mantilla et al., 2009). Additional, accessory muscles are involved in facilitating inspiration. This includes the external intercostals (innervated at thoracic level T1–T12) and the pharyngeal dilator muscles (such as the genioglossus) (Drake et al., 2015). The latter muscles are important modulators of upper airway patency and innervated through the hypoglossal nerve, not via any spinally mediated pathway. Inspiratory activity is elicited in the rostral ventral respiratory group located within the medulla (Duffin, 2004; Feldman et al., 1985). Due to damage of the respiratory motoneurons and bulbospinal pathways, cervical spinal cord injury (SCI) causes respiratory compromise experimentally and clinically (DeVivo and Chen, 2011). Indeed, respiratory dysfunction is the major cause of morbidity and mortality following human cervical SCI (Brown et al., 2006; Hartkopp et al., 1997; Winslow and Rozovsky, 2003), with the mid-cervical contusion being the most prevalent form of trauma (Young, 2002).
Animal models of mid-cervical spinal cord contusion have explored the changes this injury evokes in respiratory function. Several studies have demonstrated a significant decrease in diaphragm electromyography (EMG), phrenic nerve activity, or ventilatory capacity under challenge (Awad et al., 2013; Baussart et al., 2006; el-Bohy et al., 1998; Golder et al., 2011; Lee et al., 2017; Lee and Kuo, 2017; Stamegna et al., 2011; Wen and Lee, 2017). For example, Wen and Lee (2017) have recently shown that tidal volume (VT) is reduced up to eight weeks following a cervical level 3/4 (C3/4) unilateral contusion. Interestingly, they show only transient effects upon inspiratory bursting in the bilateral diaphragm and external intercostal muscles, suggestive of some compensatory or endogenous plasticity occurring to facilitate function within this motor system. Indeed, a number of studies detail only a modest effect of mid-cervical contusion upon respiratory motor parameters (Alvarez-Argote et al., 2016; Choi, 2005; Gensel et al., 2006; Lane et al., 2012; Nicaise et al., 2012). While animals demonstrate an initial inability to respond to hypoxic or hypercapnic challenge (Alvarez-Argote et al., 2016; Choi, 2005; Lane et al., 2012), they typically show recovery in all respiratory parameters by four-to-eight weeks post trauma. However, this does not reflect the clinical situation. Following mid-cervical contusion, some patients may be able to be weaned off ventilation (Bluechardt et al., 1992; Brown et al., 2006), however, respiratory function does not typically return to “normal”, pre-injury values (Winslow and Rozovsky, 2003). An important question is how to achieve a model of respiratory contusion which accurately mimics clinical outcome measures overtime, and whether we can deepen this analysis to provide further insight into the effect of injury on respiratory function?
Respiratory control is known to be complex and exhibit deterministic behaviour (Askanazi et al., 1979; Brack et al., 2002; Ibrahim et al., 2008). A number of disorders lead to a decrease in ventilatory pattern variance including respiratory failure (Wysocki et al., 2006), sepsis (Askanazi et al., 1979), and restricted lung disease (Brack et al., 2002). As mid-cervical contusion damages respiratory motor pathways, and reduces the number of motoneurons, we hypothesised that this injury would cause a decrease in variability of the waveform pattern. Indeed, utilising a series of recently developed analytical tools (Chung et al., 2013; Dhingra et al., 2011), we quantified deterministic variance within the ventilatory pattern of unilateral mid-cervically contused rats from three days to twelve weeks post-trauma. Moreover, during this time frame we additionally assessed the classical ventilatory parameters of the animal during eupnea and the EMG of multiple inspiratory muscles including the diaphragm, external intercostals, and genio-glossus. These data enabled us to comprehensively assess the degree of respiratory motor deficit evident following mid-cervical contusion in a pre-clinical animal model, assess how any such deficit is altered from acute to chronic stages after injury, and quantify the degree of functional endogenous, reparative, or compensatory plasticity evident within the system.
2. Materials and methods
All experiments were approved by the Institutional Care and Use Committee at Case Western Reserve University, Cleveland. Animals were housed in groups of three, exposed to a normal dark-light cycle with free access to food, water, and environmental enrichment ad libitum. The health and welfare of the animals was monitored by the study investigators and veterinary staff at Case Western Reserve University.
2.1. Surgical procedures and diaphragm electromyography (diaEMG) recordings
2.1.1. Left lateral C3 contusion
Adult male Sprague Dawley rats (334.9 ± 3.48 g; n = 52; Harlan Laboratories Inc., Indianapolis, IN, USA) were anesthetized through intraperitonal (i.p.) injection with a ketamine/xylazine cocktail (70 mg kg–1/7 mg kg−1). Upon reaching a surgical plane of anaesthesia, animals were shaved and the dorsal neck cleansed with Betadine and 70% ethyl alcohol. Carprofen (5 mg/kg) was administered through subcutaneous (s.c.) injection while 0.002% bupivacaine hydrochloride injections were applied along the site of incision. Body temperature was maintained throughout surgery at 37 ± 1 °C. A dorsal midline incision, ~3 cm in length, was made over the cervical region and the skin and paravertebral muscles retracted. A laminectomy was performed over C2 and C3 exposing the spinal cord but keeping the dura intact. Animals were placed in the Infinite Horizon contusion impactor (Precision Systems and Instrumentation) with clamps placed around the C2 and C3 lamina to stabilise the cord during the injury. Animals received a 150 kD left lateral contusion with zero dwell time (n = 27). The anatomical completeness of the injury was confirmed through microscopy following cresyl violet staining. All procedures were performed on sham injured animals (n = 26), apart from the contusion. No animals died following the surgery, although contused animals typically required 10 mins of manual ventilation following the initial trauma. A subcutaneous injection of yohimbine (1.2mg/Kg, Tocris) was administered to reverse the effect of the respiratory depressant xylazine. The muscle layers were sutured together (3–0 vicryl) and the skin closed using wound clips. Following surgery, animals were given buprenorphine (30 μg/kg) and saline subcutaneously. Analgesics were maintained up to five-days post-surgery.
2.1.2. EMG recordings and right lateral C2 hemisection
At pre-determined end points, animals were anesthetised through i.p. injection of urethane (1.6 mg/kg). Upon reaching a surgical plane of anaesthesia, animals were shaved and the dorsal neck cleansed with Betadine and 70% ethyl alcohol. 0.002% bupivacaine hydrochloride injections were applied along the site of incision. Body temperature was maintained throughout surgery at 37 ± 1 °C. With the animal in prone position, a 5-cm laparotomy was performed to expose the abdominal surface of the diaphragm. Further, lateral 2 cm incisions were made over the left and right rib cage and the latissimus dorsi blunt dissected to expose the external intercostals at T1 and T2. Finally, a 1 cm midline incision was made down the animal’s throat. The digastric muscle was retracted to expose the genioglossus. Bipolar platinum electrodes (Grass Technology, Middleton, WI, USA) were placed in the crural region of the left and right hemidiaphrams (dorsal to the anterolateral branch of the inferior phrenic artery), into the left and right external intercostals at Tl, and into the left genioglossus muscle. Activity was amplified (gain 5000 ×; Quad-P511 Amplifier; Grass Technology), band pass filtered (30–3000 Hz; Grass Technology), digitized, and recorded using a data acquisition system (CED1401; Spike2; Cambridge Electronic Design). The integrated signal was rectified and smoothed at a time constant of 0.075 s. All recordings were obtained during eupnea prior to a 20-s nasal occlusion.
As described previously (Awad et al., 2013), during end-point EMG recordings, a lateral right C2 hemisection was performed in order to remove descending input to the contused phrenic motor pool from the contralateral uninjured side. A dorsal midline incision ~3 cm in length was made over the cervical region and the skin and paravertebral muscles were retracted. Using micro scissors, the C2/3 spinal cord was re-exposed and durotomy performed. Using a 21G needle (with the tip positioned towards the mid-line), a right lateral hemisection was performed caudal to the C2 dorsal roots. The incision was made till the needle had reached the ventral lamina surface. This process was repeated five times and extended from the midline to the most lateral extent of the spinal cord. The functional completeness of the injury was confirmed through absence of activity in the right diaEMG.
Time to cease left diaEMG activity was calculated from the last breath prior to completing the hemisection to the final peak on the integrated response. Statistical analysis was performed using a one-way analysis of variance (ANOVA) with post-hoc Bonferroni (SPSS). Divergences were considered significant if p < 0.05. Data are presented as mean ± SEM. At the time of recording and analysis, investigators were blind to the treatment group of each animal. A total of 43 animals underwent terminal EMG recordings (n = 22 contusion; n = 21 sham).
2.2. Whole body plethysmography (WBP) recordings
Individual animals were placed in Perspex plethysmography chambers (DSI). Airflow within the chamber was maintained at 2 L/min using a flow controllers (DSI). Prior to each recording, the chambers air-tight seal was assessed and air volumes calibrated (DSI) which was used to calculate tidal volume (VT) changes in respiratory activity. Animals were acclimated to the chambers by placing then in the closed environment for increasing lengths of time for five days prior to the first recording. The animals core body temperature was assessed at the conclusion of each experiment using a rectal thermometer (Physitemp). Measurements were continuously recorded through the proprietary Pomenah software (DSI). Animals were placed in the chambers for 100 mins, the first 60 of which acted as an acclimation period and was never analysed. Through the remaining 40 mins, rates of ventilation (VE) through normoxia (normal air) were assessed. Recordings were performed one day prior to the contusion (baseline), three days following injury, and then weekly up to twelve weeks post trauma. Baseline recordings were not statistically different from those conducted during the acclimation for each animal (p = ns) indicating the stability of the baseline. Statistical comparisons were made between treatment groups and within plethysmography measurements using one-way or two-way, repeated-measures ANOVA (SPSS). Divergences were considered significant if p < 0.05. Data are presented as mean ± SEM. At the time of recording and analysis, investigators were blind as to the treatment group of each animal. 20 animals underwent WBP recordings (n = 10 sham, n = 10 contusion). This includes the 5 sham and contusion animals that additionally had EMG recordings.
2.3. Data analysis and ventilatory pattern variability
The raw plethysmography data was transferred to a custom software program (Case Western Reserve University) and assessed in three 60 s epochs taken at ~70, 80, and 90 mins into the recording while the animal was at rest. Animals were monitored during the recording to verify their physiological state (i.e. relaxed, walking, sniffing, grooming). For each epoch, the software system identified discrete breaths which were manually verified and then data averaged for each animal.
As described previously (Chung et al., 2013; Dhingra et al., 2011), Mutual Information (MI) and Sample Entropy (SampEn) of raw and surrogate data were determined. Surrogate data for MI and SampEn were computed as previously described (Chung et al., 2013; Dhingra et al., 2011). Schematic diagrams describing the acquisition of data for both MI and SampEn are shown in (Dhingra et al., 2011). MI is an expression of the degree to which knowledge of a specific point in a data set x(t) reduces the uncertainty associated with a time advanced coordinate x(t + τ), where τ > 0 is the time interval between samples (determined by sampling frequency). It is assessed for each time lag (τ) from unity to one respiratory cycle length. In essentials, MI provides a measure of the statistical dependence between points in the data set and includes both linear and nonlinear correlations (Fraser, 1986; Shannon, 1997). A decrease in value represents reduced complexity and greater variance in a respiratory waveform.
SampEn is calculated through the creation of templates for each point (template = m points and m + 1 points separated by τ). A match is a point within a tolerance interval r. SampEn defines the negative natural logarithm of the conditional probability that a specific epoch will have the same number of matches for m and m + 1 number of points. We performed the assessment with m = 2 and r = 0.2 * SD. It is assessed for τ from unity to one cycle length with resulting values averaged. Values with high linear correlations were excluded (defined by the first minimum of the mutual information function). As such, SampEn measures temporal pattern variability, indicative of self-similarity within a given time frame. High values convey reduced self-similarity and predictability with greater complexity (Richman and Moorman, 2000). The nonlinear complexity index (NLCI) was calculated as the average of the statistically significant differences between the original and surrogate SampEn data sets at each τ. The higher the value of the NLCI, the greater the contribution of nonlinear and/or non-Gaussian sources of variability within the data set.
All experiments were assessed blind. A minimum of 3 repeats were conducted for each experiment. The parameters were compared between control and the test groups by repeated measures two-way analysis of variance (ANOVA; SPSS). Significance values represented as * = p < 0.05, ** = p < 0.01, *** = p < 0.001. Data are presented as mean ± SEM.
3. Results
3.1. Unilateral contusion of the spinal cord
Contused animals received an average 218.3 ± 16.2kDyne injury with 1546 ± 42.2 pm displacement. There were no deaths during or after any of the surgical procedures and no animal showed signs of blood in their urine or stool. While contused animals did show an initial decrease in weight 3 days following injury, this was recovered by 1 week after trauma (Fig. 2A). Moreover, regardless of group, all animals showed weight increase from pre-injury baseline (F(1,252) = 22.3, p < 0.0001). Sham and contused animals show no divergence in weight gain in the weeks following injury (Fig. 2A). This would suggest that alterations in weight are not a causal factor in any of the effects described.
Fig. 2.
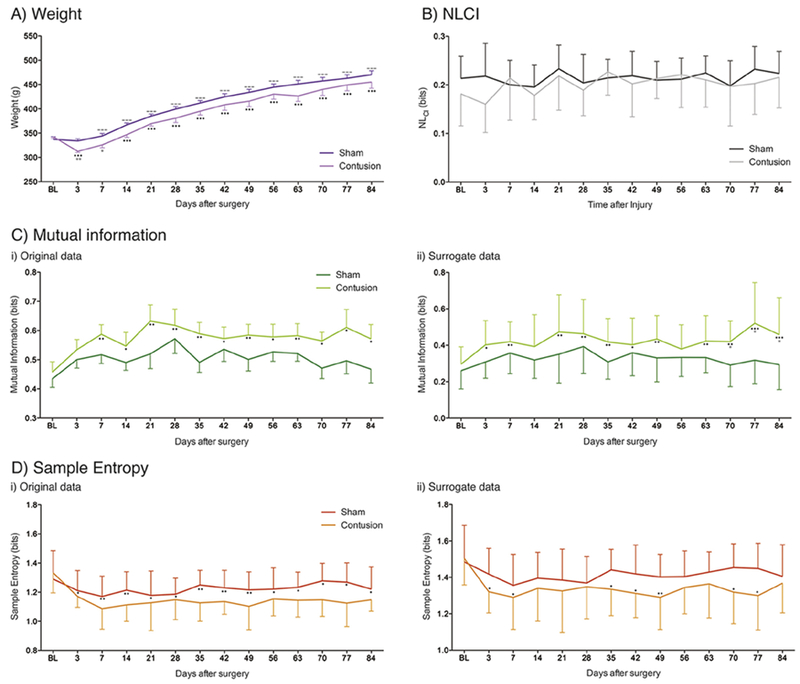
Contusion injury causes profound and robust decrease in linear ventilatory pattern variability. A) Contused animals (pink) show minimal deficit in weight compared to sham controls (purple) up to twelve weeks following trauma and weight gain compared to baseline (BL). B) Contusion (light grey) and sham (tungsten) injury do not cause an increase in nonlinear variability shown through the nonlinear complexity index (NLCI) However, C) Mutual Information, and D) Sample Entropy of i) the original and ii) surrogate data sets following pre-trauma baseline (BL), C3 contusion injury (light green/orange), and sham control (dark green/red). Graphs show means ± SEM, Comparisons between BL and contusion * = p < 0.05, ** = p < 0.01; *** = p < 0.001; contusion and sham * = p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Contusion injury causes a robust deficit in tidal and minute volume
In order to assess the effect that contusion injury had upon ventilatory parameters, the frequency (F), minute (VE) and tidal (VT) volume were assessed in eupnea weekly from acute to chronic stages following trauma (Fig. 1; Supplementary Table SI). Baseline values between groups for F, VE and VT were not statistically different (p = ns; Supplementary Table SI). Data show VE (F(1,252) = 49.82, p < 0.0001) and change in VE (F(1,252) = 128.78, p < 0.0001) statistically vary between our treatment groups and that this difference in response does not change with time (respectively: F(1,252) = 0.89, p = 0.562 and F(13,252) = 1.45, p = 0.952). Specifically, we demonstrate that contusion injury causes a robust decrease in change of VE from the acute stages after trauma as compared to both pre-injury baseline and sham controls (Fig. 1A). This would suggest that our contused animals exchange a smaller volume of air with time compared to baseline and sham controls, likely increasing blood carbon dioxide levels. This deficit is robust, lasting up to twelve weeks after the initial impact.
Fig. 1.
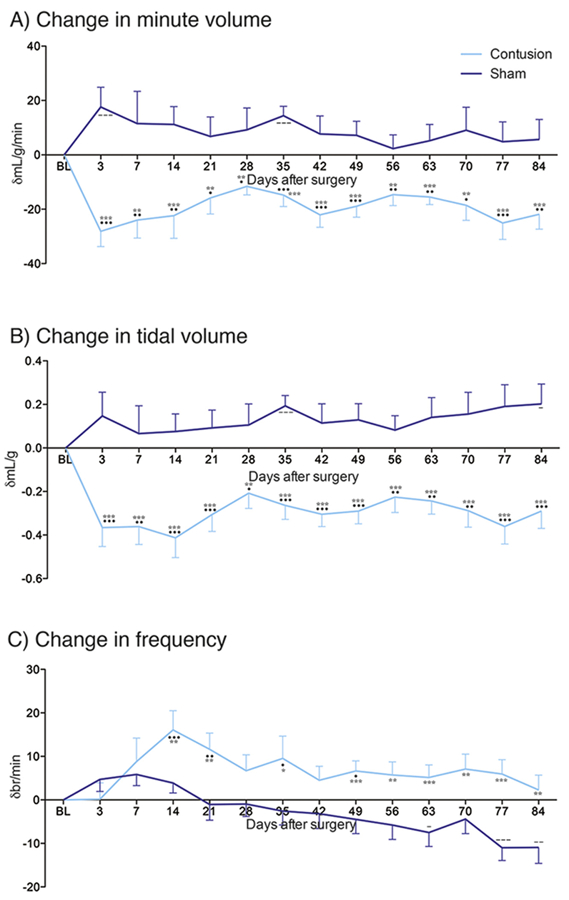
Contusion injury causes profound and robust deficit in respiratory parameters. Changes in A) minute volume (VE), B) tidal volume (VT), and C) frequency over time following pre-trauma baseline (BL), C3 contusion injury (light blue), and sham control (blue). Graphs show means ± SEM. Comparisons between BL and contusion * = p < 0.05, ** = p < 0.01, *** = p < 0.001; BL and sham - = p < 0.05, - = p < 0.01, — = P < 0.001; contusion and sham * = p < 0.05, ** = p < 0.01, *** = p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The change in VE exhibited through our data set is primarily caused by the significant decrease in VT (F(1,252) = 49.82, p < 0.0001) and change in VT (F(1,252) = 175.63, p < 0.0001) following contusion injury (Fig. 1B; Supplementary Table SI). These alterations are constant with time up to twelve weeks following injury (respectively: F (1,252) = 0.89, p =0.562 and F(13,252) = 0.62, p = 0.834). This would suggest that our contused animal’s breath a smaller volume of air with each breath compared to baseline and sham controls. While shams typically show an increase in VE and VT as compared to baseline, this is not typically statistically significant (Fig. 1A, B). Interestingly, we do not see such deficits in frequency of breaths following contusion injury (Fig. 1C; Supplementary Table SI). While there are significant differences between our treatment groups for both frequency of breaths (F (1,252) = 13.72, p = 0.0003) and change in breath frequency (F(1,252) = 52.4, p < 0.0001), these vary with time (respectively: F(1,252) = 3.41, p < 0.0001) and F(13,252) = 2.6, p = 0.0021). Contused animals show an increase in frequency change which peaks a month following the initial trauma, but then slowly decreases to baseline levels. Sham injured animals, demonstrate a gradual decrease in respiratory frequency nine weeks following the initial surgery (Fig. 1C; Supplementary Table SI). These alterations in breathing frequency suggest that endogenous changes are happening following trauma. However, collectively these data signify that any endogenous recovery which occurs within the respiratory motor system following mid-cervical contusion is not sufficient to mitigate the great effect the injury had upon ventilatory parameters. These data exhibit a profound deficit caused by mid-cervical contusion that lasts from acute to chronic time points.
3.3. Contusion injury causes dtficits in ventilatory pattern variability
To further assess the effect of contusion injury on respiratory parameters, the ventilatory pattern variability of the waveforms from each animal were assessed (Fig. 2). From one week post-surgery, MI was shown to increase in contused animals alone as compared to baseline (Fig. 2C). Sham injured animals showed no such increase in MI at any time-point following surgery. These alterations between treatment groups were significant (F(1,252) = 20.44, p < 0.0001) and robust over the 12week time frame (F(13, 252) = 1.44, p = 0.142), with no changes shown in the data-sets over this period. For surrogate data sets, MI was shown to have a similar pattern as that exhibited for the original data set (Fig. 2C). Namely, MI showed a significant increase compared to baseline from three days’ post trauma for the contused animals alone (F(1,252) = 33.8, p < 0.0001). No difference was shown in sham injured animals. These alterations in MI for the contused animals were robust over time, showing no statistical variance in the data set (F(l 3,252) = 1.38, p = 0.168). These data identify that contusion injury causes a robust increase in the statistical dependence between points in the plethysmography waveform. This suggests that the contusion injury altered the ventilatory pattern, with reduced variability that persisted up to twelve weeks.
SampEn was shown to significantly decrease in animals with contusion injury as compared to baseline (Fig. 2D; F(1,252) = 24.44, p < 0.0001). Sham injured animals showed no such decrease in SampEn at any time-point following surgery. Similar to MI, this response was shown to be robust from acute to chronic time points post injury, lasting up to twelve weeks following contusion. Interestingly, the effect of time is statistically significant on this data set (F (13,252) = 1.96, p = 0.0241). For surrogate data sets, SampEn was shown to have a similar pattern as that exhibited for the original data set (Fig. 2D). Differences between the treatment groups were determined to be statistically significant (F(1,252) = 15.53, p < 0.0001), and these alterations robust with time (F(13,252) = 1.18, p = 0.296). However, post-hoc tests do not show a robust decrease in the SampEn oi contused animals at every time point following injury. Collectively, these data suggest that contusion injury causes a reduction in temporal pattern variability within the respiratory waveform. As such, following the contusion injury, the waveform is more self-similar and predictable with greater complexity but less variance.
To determine the source of the differences in variance shown in our data sets in response to contusion injury the NLCI was determined. Differences between the SampEn of the original and surrogate data sets were used to assess and quantify the NLCI of the breathing patterns for each treatment group (Fig. 2B). NLCI was shown to not statistically alter between treatment group (F(1,252) = 3.13, p = 0.0782) or change with time (F(13,252) = 0.99, p = 0.466). This suggests that the changes in variance shown in our data in response to contusion injury are largely due to linear/Gaussian sources. Together, these data suggest that a mid-cervical contusion injury causes a decrease in linear sources of variability within breathing patterns which is permanent, showing no endogenous recovery with time.
3.4. Endogenous recovery is not sufficient to aid respiratory recovery through contused pathways
To further assess the effect of contusion injury upon respiratory motor function we evaluated the EMGs of a number of inspiratory muscles at acute (three days) and chronic (twelve weeks) time points post injury (Fig. 3). Sham injured animals show strong ipsilateral hemi-diaphragm and external intercostal activity at both three days and twelve weeks’ post-surgery (Fig. 3A). The activity of the genioglossus is indicative of moderate respiratory drive. Contused animals show significantly reduced diaphragm EMG activity which appears to diminish in amplitude between three days and twelve weeks’ post injury (Fig. 3B). The strong activity exhibited in the ipsilateral external intercostals during this time frame demonstrates that the multitude of decussating pathways which innovate the intercostal motor pool from the contralateral side of the spinal cord are still functioning appropriately, and they are partially compensating for the reduced activity of the ipsilateral hemi-diaphragm (Fig. 3B). Similar to sham animals there is also little activity in the genioglossus when breathing during epnea. Moreover, there is no difference in the EMG of these respiratory muscles from acute to chronic time points following injury (Fig. 3B). This would suggest that little endogenous recovery has occurred to facilitate a recovery of respiratory motor function over time.
Fig. 3.
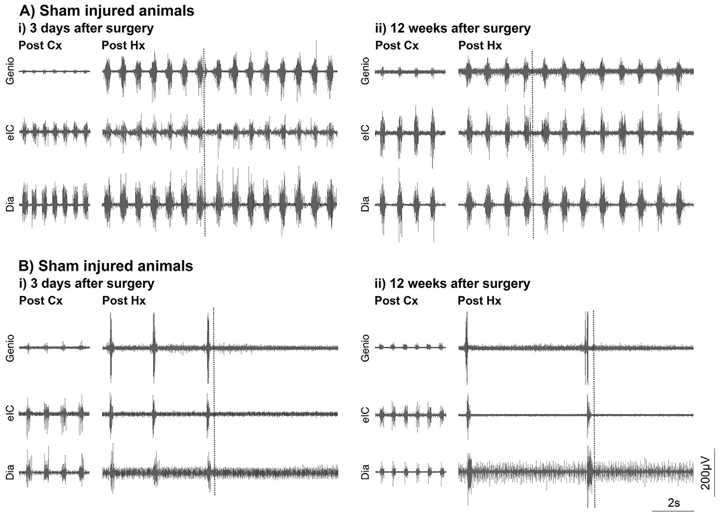
Endogenous recovery is not sufficient to mediate functional activity through the contused bulbospinal pathways at both acute and chronic time points post injury. EMG data is shown for the diaphragm (dia), external intercostals (elC) and genio-glossus (genio) ipsilateral to the A) sham or B) contusion injury i) 3 days and ii) 12 weeks after C3 contusion. While recovery in ipsilateral diaphragm activity following contusion (Cx) is evident at chronic stages it is not sufficient to maintain that activity when ipsilateral pathways are isolated through right contralateral hemisection (Hx; dashed line), regardless of time post injury. This effect is evident in all inspiratory muscles. All panels are recorded in the same animal.
In order to determine the extent to which any recovery occurred through the contused bulbospinal pathways at acute to chronic time points following injury a C2 hemisection was performed on the opposite side of the spinal cord to the original injury (Fig. 3). This removed any descending contralateral influence to the muscles under investigation (Awad et al., 2013; Baussart et al., 2006). Sham injured animals that underwent this procedure showed a compensatory increase in the amplitude of muscle EMG (particularly the genioglossus) at both acute and chronic time points post injury following completion of the hemisection (Fig. 3A). Indeed, animals continued to breath up to the maximal time under assessment following removal of the contralateral respiratory activity (Table 1). Spinal or neurogenic shock is likely to be the causal factor in any sham animal which stopped breathing during this time frame and all animals could be resuscitated if respiration failed during this time.
Table 1.
Time to cease EMG activity following completion of the contralateral C22 hemisection. Time given in seconds. Recordings were terminated at 5 min after completion of the hemisection. ANOVA shows a significant main effect (p < 0.0001; F(11, 50) = 16.63) with the sham data showing a significance difference to the contusion data at all time points with a minimum of p = 0.05.
| Days post contusion injury | Days post sham injury | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 21 | 42 | 84 | 3 | 7 | 21 | 42 | 84 | |
| n | 4 | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 5 |
| Mean (sec) | 0.0 | 6.757 | 8.981 | 12.13 | 21.14 | 262.2 | 281.3 | 300.0 | 300.0 | 196.8 |
| SEM | 0.0 | 6.757 | 8.981 | 12.13 | 12.43 | 37.77 | 18.7 | 0.0 | 0.0 | 63.93 |
| Min | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 148.9 | 225.2 | 300.0 | 300.0 | 11.38 |
| Max | 0.0 | 33.79 | 35.92 | 48.52 | 48.07 | 300.0 | 300.0 | 300.0 | 300.0 | 300.0 |
Following completion of the contralateral hemisection in animals with a prior contusion injury, a striking activity profile is seen compared to that of the sham group (Fig. 3B). Prior to completion of the hemisection there is typically strong activity in all of the ipsilateral respiratory muscles, perhaps indicative of an increase in drive. However, upon completion of the ipsilateral hemisection the animal typically stops all respiratory activity (Fig. 3B). This is even seen in the genioglossus, a muscle innervated above the level of injury. This would suggest considerable respiratory motor feedback is mediating pattern generation during this period. The near immediate cessation in all respiratory motor activity following hemisection was seen from acute to chronic time points in contused animal. While the average length of time animals continued to breath marginally increased with time post injury (Table 1), perhaps indicative of some endogenous recovery, this was not significant. Further, all times were significantly lower than that of the sham animals at all time points (F(l 1,50) = 16.63, p < 0.0001). None of these contused animals could be resuscitated once breathing had stopped. Collectively these data suggest that marginal endogenous recovery occurs to the respiratory motor system following mid-cervical contusion injury. However, this is not sufficient to mediate extensive breathing function either when offset by influence from the contralateral side of the cord or solely through the damaged ipsilateral bulbospinal pathways alone.
4. Discussion
The present study demonstrates long-term deficits in both VE and VT during eupnea caused by unilateral mid-cervical contusion injury as assessed through WBP. Further, ventilatory patterns of contused animals show a significant increase in Mutual Information and decrease in SampEn. This reduction in variability is primarily linear in nature and suggests that these animals are likely less able to adapt to alterations in activity or changes in their surrounding environment. These effects are evident from acute to chronic stages following the initial trauma, demonstrating the great effect mid-cervical contusion has upon ventilatory activity. Finally, we establish a pronounced deficit in ipsilateral diaphragm EMG activity due to the contusion injury, becoming more pronounced at chronic stages. Conversely, the activity of the ipsilateral external intercostal muscle did regain function within three days post-injury, demonstrating substantial compensatory plasticity which lasted up to twelve weeks following trauma. However, without contralateral descending input, activity in all respiratory muscles ceased, indicating little endogenous recovery occurs through the contused respiratory pathways regardless of time post-injury and the negative effect of mid-cervical contusion on afferent feedback. These data show that mid-cervical contusion can result in extensive deficits throughout the respiratory motor system. That, while plasticity is evident, there is little evidence of endogenous recovery. Functional shortfalls impact clinical outcome measures from acute to chronic time points.
4.1. Mid-cervical contusion injury
There is a division within the field regarding the nature of respiratory function following mid-cervical contusion injury. While, a number of studies show some degree of deficit within motor function, such as phrenic nerve output or diaphragm EMG amplitude following trauma, this is often transient (Baussart et al., 2006; el-Bohy et al., 1998; Stamegna et al., 2011; Wen and Lee, 2017). Moreover, compromise to ventilatory parameters is often limited to the first few days following injury (Alvarez-Argote et al., 2016; Awad et al., 2013; Golder et al., 2011; Lane et al., 2012; Nicaise et al., 2013). There are notable exceptions to these generalisations. However, we have uniquely shown that mid-cervical contusion causes a decrease from baseline in both VT and VE, a reduction in ventilatory pattern variability, and diminished ipsilateral diaphragm function, none of which recover with time. These data suggest our cervical contusion model causes a deficit in ventilation and respiratory motor control which persists from acute to chronic stages of injury.
We suggest that three factors influence the longevity of the respiratory deficit produced. First, the configuration of our animal at the point of injury ensures considerable stability of the spinal cord and column (Lee et al., 2017) and, with the pia removed (Baussart et al., 2006), guarantees the production of a severe cervical SCI with little variance between animals (Awad et al., 2013). Despite the severity of the injury and effects produced, the unilateral nature of the contusion ensured the animals survival without respiratory assistance or obvious bladder or bowel problems meaning we can produce clinically relevant effects on the respiratory motor system without adversely affecting the animals quality of life. Second, recovery could be subject to sex and strain differences. For example, following cervical hemisection, female rats demonstrate superior recovery of VT and phrenic nerve activity under challenge then males, conceivably due to alterations in hormone levels aiding respiratory responses (Doperalski et al., 2008). The use of male Sprague Dawley rats in this study could prolong the respiratory deficit produced. Third, it is possible that models of mid-cervical contusion performed by other investigators are activating spared or latent tissue, regaining ipsilateral functionality. Due to the bilateral innervation of the phrenic motor neurons from the premotor neurons of the rostral ventral respiratory group (Lipski et al., 1994) it is possible that either the contralateral descending pathway or crossed spinal pathway are activating residual ipsilateral phrenic motoneurons, supporting ventilation and respiratory motor function. We see little evidence of this in the present study except, perhaps, in conditions of extreme respiratory drive through ipsilateral diaphragm EMG recordings. Nonetheless, the significant and robust deficit in ventilation and respiratory motor function we obtain following our model of mid-cervical contusion is an advantage in both accurately mimicking the respiratory effects of clinical cervical contusion and providing a baseline from which to address recovery following trauma.
4.2. Reductions in ventilatory parameters and variance of contused animals
We demonstrate a transient increase in respiratory frequency in response to mid-cervical contusion injury. This short-term alteration in response to the injury has been demonstrated in similar contusion models (Golder et al., 2011; Lee and Kuo, 2017; Nicaise et al., 2013), although we illustrate that this effect occurs for longer than shown previously (seven rather than two weeks). The effect is likely caused by alterations in pathways affecting afferent feedback and chemoreflexes (Golder et al., 2011; Lee and Kuo, 2017) or due to an increase in compensatory drive from the supraspinal pathways (Choi, 2005). Interestingly, we do see profound decreases in VT and VE in eupnea which are constant from three days to twelve weeks following mid-cervical contusion. Ventilatory parameters have either been shown not to change following contusion (Alvarez-Argote et al., 2016; Lane et al., 2012) or to recover in the first few weeks following mid-cervical trauma (Choi, 2005; Nicaise et al., 2013). In support of our study, Stamegna et al. (2011) demonstrated a reduction in epnea VE in animals following mid-cervical contusion 3.5 months following the initial trauma. Moreover, Lee et al. (2017) established that both VE and VT were decreased one month subsequent to the injury. Further, significant reductions in VT following cervical contusion have been show to remain constant for eight weeks following the surgery, although VE was only similarly affected at acute stages (Lee and Kuo, 2017; Wen and Lee, 2017). Similar to transection models (Fuller et al., 2006, 2009; Golder et al., 2001; Goshgarian et al., 1986), the reduction in ventilatory parameters exhibited within this study is likely caused by significant damage to unilateral cervical white matter at, and rostral to, the injury site (Lane et al., 2012) damaging the bulbospinal pathways responsible for inspiratory activity. Moreover, this would further the effect of phrenic motor neuron (Alvarez-Argote et al., 2016; Golder et al., 2011; Lee et al., 2017; Lee and Kuo, 2017) and diaphragm neuromuscular junction (Alvarez-Argote et al., 2016; Nicaise et al., 2013) loss typified by this injury. This lack of innervation causes muscle atrophy and decreases in muscle fibre conduction velocity, diameter, and isometric force (Gill et al., 2014; Smuder et al., 2016; Wu et al., 2014) which are not recovered upon potential reinnervation (Nicaise et al., 2013; van der Meulen et al., 2003). While there is potential for compensatory plasticity originating from the contralateral respiratory motor pathways to augment ventilatory parameters (Lee et al., 2017), this does not occur within our model.
Further to the robust decrease in VT and VE demonstrated, we have uniquely shown that the variance of ventilatory patterns is also compromised from acute to chronic stages following mid-cervical contusion. This is illustrated through an increase in the statistical dependence between points on the waveform and a decrease in self-similarity of the ventilatory pattern waveform (Dhingra et al., 2011). These data suggest that animals display a more regular breathing pattern following mid-cervical contusion injury. Evaluation of surrogate data sets reveals this decrease in ventilatory variance to be caused by the contribution of linear Gaussian stochastic sources of variability. These differences are unlikely to be caused by alterations in respiratory rate, as this changed over time for each treatment group following the injury. One could hypothesise that reductions in ventilatory variance would be caused by similar means to that which affects ventilator parameters (namely denervation of nerves or muscle which cannot be compensated for through induction of plasticity) although this physiological explanation has not been proved within the current study. Nonetheless, the robust decrease in ventilatory pattern variability shown may further explain why previous investigators have demonstrated the inability of contused animals to respond to hypoxic or hypercapnic conditions (Alvarez-Argote et al., 2016; Golder et al., 2011; Lane et al., 2012; Stamegna et al., 2011). Similarly, the hypercapnic response is blunted in humans with cervical SCI (Lin et al., 1998; Manning et al., 1992). The reduction in pattern variability is likely a manifestation of an injury respiratory control system that is unable to adequately respond to changes in environment or activity. As such it is a highly significant and clinically relevant measure of respiratory function following cervical SCI.
4.3. Compromised diaphragm activity from acute to chronic time points
Similar to the robust decrease in ventilatory parameters, we find that the mid-cervical contusion causes a substantial impairment of the ipsilateral hemidiaphragm which lasts from three days to twelve weeks following the injury. Sham injured animals displayed strong activity in both the ipsilateral diaphragm and external intercostal EMG during eupnea with little variation. These data are regularly confirmed in similar mid-cervical contusion models (Alvarez-Argote et al., 2016; Baussart et al., 2006; Mantilla et al., 2011; Stamegna et al., 2011; Wen and Lee, 2017). Indeed, even after the advent of the contralateral C2 hemisection, only small increases in amplitude of the diaphragm are noted (Baussart et al., 2006). The compensatory plasticity within the intact system is sufficient to mediate respiratory activity.
A distinctly different effect on muscle EMG function is caused following mid-cervical contusion. We show that ipsilateral diaphragm activity remains minimal from acute to chronic time points after trauma during eupnea. This activity may be mediated through either, or both, ipsi- or contralateral innervation and has been demonstrated in a selection of similar contusion models. For example, Baussart et al. (2006) showed minimal ipsilateral diaphragm EMG activity up to one month following contusion injury. Negligible recovery, or compensatory function, was demonstrated at three months following trauma in the same model (Stamegna et al., 2011). Wen and Lee (2017) showed an initial decrease in diaphragm EMG activity which partially recovered at eight weeks following injury. Further functional impairment following mid-cervical contusion has been shown through assessment of phrenic nerve activity (Baussart et al., 2006; el-Bohy et al., 1998; Golder et al., 2011; Lane et al., 2012; Nicaise et al., 2012, 2013). For example, Nicaise et al. (2012, 2013) showed that immediately after contusion phrenic nerve compound muscle action potential was decreased and remained so for two weeks. However, some studies have shown a minimal effect of contusion on ipsilateral diaphragm EMG output when the data was normalised to maximal output (Alvarez-Argote et al., 2016; Rana et al., 2017). Although this may be limited by electrode placement within the diaphragm (Baussart et al., 2006; Li et al., 2015), their use of indwelling electrodes may suggest that the data in our study could be confounded through the use of anaesthetic at the time of recording (Chung et al., 2013; Lee and Kuo, 2017). The reduction in diaphragm EMG activity following spinal contusion is likely mediated through injury-dependent loss of ventral horn grey matter, and thus the phrenic motor neurons and propriospinal interneurons (Lane et al., 2012).
In our model, compensatory activity allows for an increase in diaphragm EMG function when the respiratory motor system is further compromised (e.g. performing a contralateral C2 hemisection) which is mediated through the contralateral, decussating pathways (Huang and Goshgarian, 2009; Lipski et al., 1994). The contused, ipsilateral pathways do not recover sufficient function at either acute or chronic time points following injury to alone cause respiratory activity. The lack of restorative plasticity within our model is likely caused by the position of our uni-lateral mid-cervical contusion with the impact affecting both the neurons in the phrenic motor pool and the bulbospinal pathways (Baussart et al., 2006). Conversely, we demonstrate substantial compensatory activity in the external intercostals ipsilateral to the contusion, which regain and retain function from three days to twelve weeks following trauma. These data confirm the findings of previous studies (Wen and Lee, 2017). The recovery in activity of the ipsilateral external intercostals is more rapid then the seven-fourteen day time-lag shown following C2 hemisection (Beth Zimmer et al., 2015; Dougherty et al., 2012; Navarrete-Opazo et al., 2015) and is likely mediated through propriospinal interneurons which upregulate following trauma (Lane et al., 2008).
As expected, the effect of mid-cervical contusion upon the EMG activity of the genioglosus is fairly limited as the muscle is innervated above the level of trauma. Interestingly, the muscle activity was attenuated at the point of contralateral C2 hemisection completion in previously contused animals. This occurred at all time points following trauma and would suggest that sensory feedback may be robustly altered following contusion injury due to chronic changes in respiratory circuitry (Felix et al., 2014; Lee and Kuo, 2017). However, further evidence would be required to substantiate this hypothesis. Nonetheless, this is something that needs to be assessed in greater detail due to potential clinical implications. There is substantial information to illustrate that spinal injuries cause dysfunction to the major inspiratory and expiratory muscles through both paralysis and atrophy. However, there is a deficit of information relating to how cervical SCI affects the muscles function and morphology of the upper airway. Our data suggest that this effect is substantial through modifications to sensory feedback, and may be a causal factor in the development of spinal injury associated dysfunctions such as dysphagia. Dysphagia, or swallowing difficulty, affects between 17 and 41% of SCI individuals (Lee et al., 2016) and can lead to pneumonia, airway obstruction, and chemical pneumonitis (Palmer et al., 2000). These data require substantial further assessment. Further, with a reduction in ventilatory pattern variance, dysfunction to respiratory sensory feedback could be a causal factor in the development of secondary pulmonary complications associated with SCI, such as sleep disordered breathing (SDB) and obstructive sleep apnea (Biering-Sorensen et al., 2009). This is a condition which affects ~25% of SCI patients (Fuller et al., 2013). The reduction in ventilatory pattern variability after contusion injury means that the respiratory motor system could be less likely to cope with the normal reductions in excitability and output associated with sleep. Indeed, combined with other factors demonstrated here such as the reduction in lung volume, loss of muscle function, and alterations to sensory feedback there is an increased likelihood of developing SDB following mid-cervical contusion injury. The prevalence and causal mechanisms of these secondary effects following SCI contusion models requires greater investigation to ensure that they can be better predicted and treated.
4.4. Physiological and clinical significance
Ethically and practically, it is not feasible to produce a pre-clinical model of a ventilator dependent animal (Powers et al., 2009; Sieck and Mantilla, 2008). Our model of mid-cervical spinal cord contusion (Awad et al., 2013) yields an injury with measurable and prolonged deficits in clinically relevant ventilatory parameters, variability, and motor function. As such, this model can enable the transparent and accurate analysis and evaluation of potential treatment methods for respiratory motor function following contusion injury. Moreover, clinical outcomes to cervical contusion are variable. Patients may show evidence of endogenous plasticity and respiratory compensation, eventually being weaned from the ventilator and achieving eupnic breathing (Bluechardt et al., 1992). However, this does not occur through the restoration of pre-injury motor control, but through the introduction of accessory muscle activation (Ledsome and Sharp, 1981; Winslow and Rozovsky, 2003), which is accurately reflected within our model. In combination, these data suggest that mid-cervical contusion can produce severe deficits throughout the respiratory motor system affecting multiple ventilatory and functional outcome measures which will not recover with time unless some form of treatment is imposed.
Important to our study is the use of ventilatory pattern variability to evaluate the effect of mid-cervical contusion injury upon the respiratory system. As injury size does not predict functional respiratory outcomes in human SCI (Bluechardt et al., 1992) it is important to find other clinically relevant measures that may achieve this effect. Ventilatory variance has been shown to be an advantageous predictor of success following ventilator weaning (Seely et al., 2014; Wysocki et al., 2006). Data collection for this technique can be achieved simply and in eupnea, achieving clear indications of ventilatory deficit. We believe that this measure could be used as an ‘additional vital sign’ in the continued clinical assessment of patients with spinal cord injury, facilitating risk stratification or facilitating the development of individualised treatment strategies.
Supplementary Material
Acknowledgements
We thank the veterinary staff at Case Western Reserve University for their assistance with the animals. Financial support was provided by Wings for Life (WFL-US-027/14 to PMW), The International Spinal Research Trust (STR117 to WJA and PMW), Craig H. Neilsen Foundation (221988 to WJA), ISSF Welcome Trust Fellowship (105615/Z/14/Z to PMW), Department of Defense/Congressionally Directed Medical Research Program (DoD/CDMRP; SC140243 W81XWH-15–1-0378 to WJA), NIH (R21OD018297 to WJA), and VA Research Service Merit Review Award (I01BX000873 to FJJ).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.expneurol.2018.04.005.
References
- Alvarez-Argote S, Gransee HM, Mora JC, Stowe JM, Jorgenson AJ, Sieck GC, Mantilla CB, 2016. The impact of Midcervical contusion injury on diaphragm muscle function. J. Neurotrauma 33, 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askanazi J, Silverberg PA, Hyman AI, Rosenbaum SH, Foster R, Kinney JM, 1979. Patterns of ventilation in postoperative and acutely ill patients. Crit. Care Med 7, 41–46. [DOI] [PubMed] [Google Scholar]
- Awad BI, Warren PM, Steinmetz MP, Alilain WJ, 2013. The role of the crossed phrenic pathway after cervical contusion injury and a new model to evaluate therapeutic interventions. Exp. Neurol 248, 398–405. 10.1016/j.expneurol.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Baussart B, Stamegna JC, Polentes J, Tadie M, Gauthier P, 2006. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol. Dis 22, 562–574. [DOI] [PubMed] [Google Scholar]
- Beth Zimmer M, Grant JS, Ayar AE, Goshgarian HG, 2015. Ipsilateral inspiratory intercostal muscle activity after C2 spinal cord hemisection in rats. J. Spinal Cord. Med 38, 224–230. 10.1179/2045772314Y.0000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biering-Sorensen F, Jennum P, Laub M, 2009. Sleep disordered breathing following spinal cord injury. Respir. Physiol. Neurobiol 169, 165–170. http://dx.doi.org/10. 1016/j.resp.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Bluechardt MH, Wiens M, Thomas SG, Plyley MJ, 1992. Repeated measurements of pulmonary function following spinal cord injury. Paraplegia 30, 768–774. [DOI] [PubMed] [Google Scholar]
- Brack T, Jubran A, Tobin MJ, 2002. Dyspnea and decreased variability of breathing in patients with restrictive lung disease. Am. J. Respir. Crit. Care Med 165, 1260–1264. 10.1164/rccm.2201018. [DOI] [PubMed] [Google Scholar]
- Brown R, DiMarco AF, Hoit JD, Garshick E, 2006. Respiratory dysfunction and management in spinal cord injury. Respir. Care 51 (853–68–discussion 869–70). [PMC free article] [PubMed] [Google Scholar]
- Choi H, 2005. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J. Neurosci 25, 4550–4559. 10.1523/JNEUROSCI.5135-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A, Fishman M, Dasenbrook EC, Loparo KA, Dick TE, Jacono FJ, 2013. Isoflurane and ketamine anesthesia have different effects on ventilatory pattern variability in rats. Respir. Physiol. Neurobiol 185, 659–664. http://dx.doi.org/10. 1016/j.resp.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVivo MJ, Chen Y, 2011. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch. Phys. Med. Rehabil 92, 332–338. http://dx.doi.org/10. 1016/j.apmr.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Dhingra RR, Jacono FJ, Fishman M, Loparo KA, Rybak IA, Dick TE, 2011. Vagal-dependent nonlinear variability in the respiratory pattern of anesthetized, spontaneously breathing rats. J. Appl. Physiol 111, 272–284. 10.1152/japplphysiol.91196.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doperalski NJ, Sandhu MS, Bavis RW, Reier PJ, Fuller DD, 2008. Ventilation and phrenic output following high cervical spinal hemisection in male vs. female rats. Respir. Physiol. Neurobiol 162, 160–167. 10.1016/j.resp.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Lee KZ, Gonzalez-Rothi EJ, Lane MA, Reier PJ, Fuller DD, 2012. Recovery of inspiratory intercostal muscle activity following high cervical hemisection. Respir. Physiol. Neurobiol 183, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RL, Vogle AW, Mitchell AWM, 2015. Grey’s Anatomy for Students. Churchill Livingstone, Philadelphia. [Google Scholar]
- Duffin J, 2004. Functional organization of respiratory neurones: a brief review of current questions and speculations. Exp. Physiol 89, 517–529. 10.1113/expphysiol.2004.028027. [DOI] [PubMed] [Google Scholar]
- el-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG, 1998. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp. Neurol 150, 143–152. 10.1006/exnr.1997.6757. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, 1988. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J. Comp. Neurol 269, 47–57. 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Loewy AD, Speck DF, 1985. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J. Neurosci 5, 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix MS, Bauer S, Darlot F, Muscatelli F, Kastner A, Gauthier P, Matarazzo V, 2014. Activation of Akt/FKHR in the medulla oblongata contributes to spontaneous respiratory recovery after incomplete spinal cord injury in adult rats. Neurobiol. Dis 69, 93–107. 10.1016/j.nbd.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Fraser Swinney, 1986. Independent coordinates for strange attractors from mutual information. Phys. Rev. A Gen. Phys 33, 1134–1140. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Mitchell GS, 2006. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J. Appl. Physiol 100, 800–806. 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ, 2009. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir. Physiol. Neurobiol 165, 245–253. 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Lee K-Z, Tester NJ, 2013. The impact of spinal cord injury on breathing during sleep. Respir. Physiol. Neurobiol 188, 344–354. 10.1016/j.resp.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Tovar CA, Hamers FPT, Deibert RJ, Beattie MS, Bresnahan JC, 2006. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J. Neurotrauma 23, 36–54. 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- Gill LC, Ross HH, Lee KZ, Gonzalez-Rothi EJ, Dougherty BJ, Judge AR, Fuller DD, 2014. Rapid diaphragm atrophy following cervical spinal cord hemisection. Respir. Physiol. Neurobiol 192, 66–73. 10.1016/j.resp.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Davenport PW, Bolser DC, 2001. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J. Appl. Physiol 91, 2451–2458. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Lovett-Barr MR, Vinit S, Resnick DK, Mitchell GS, 2011. Breathing patterns after mid-cervical spinal contusion in rats. Exp. Neurol 231, 97–103. 10.1016/j.expneurol.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Moran MF, Prcevski P, 1986. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH, and respiratory rate in the adult rat. Exp. Neurol 93, 440–445. [DOI] [PubMed] [Google Scholar]
- Hartkopp A, Bronnum-Hansen H, Seidenschnur AM, Biering-Sorensen F, 1997. Survival and cause of death after traumatic spinal cord injury. A long-term epidemiological survey from Denmark. Spinal Cord 35, 76–85. [DOI] [PubMed] [Google Scholar]
- Huang Y, Goshgarian HG, 2009. Identification of the neural pathway underlying spontaneous crossed phrenic activity in neonatal rats. Neuroscience 163, 1109–1118. 10.1016/j.neuroscience.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim LH, Patel SR, Modarres M, Johnson NL, Mehra R, Kirchner HL, Redline S, 2008. A measure of ventilatory variability at wake-sleep transition predicts sleep apnea severity. Chest 134, 73–78. 10.1378/chest.07-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani NH, Hollinshead WH, 1955. The phrenic nucleus. III. Organization of the phrenic nucleus in the spinal cord of the cat and man. Proc. Staff Meet. Mayo Clin 30, 566–577. [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ, 2008. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J. Comp. Neurol 511, 692–709. 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee K-Z, Salazar K, O’Steen BE, Bloom DC, Fuller DD, Reier PJ, 2012. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp. Neurol 235, 197–210. 10.1016/j.expneurol.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledsome JR, Sharp JM, 1981. Pulmonary function in acute cervical cord injury. Am. Rev. Respir. Dis 124, 41–44. 10.1164/arrd.1981.124.l.41. [DOI] [PubMed] [Google Scholar]
- Lee K-Z, Kuo H-C, 2017. Vagal control of breathing pattern after midcervical contusion in rats. J. Neurotrauma 34, 734–745. 10.1089/neu.2016.4645. [DOI] [PubMed] [Google Scholar]
- Lee JC, Gross BW, Rittenhouse KJ, Vogel AR, Vellucci A, Alzate J, Gillio M, Rogers FB, 2016. A bitter pill to swallow: dysphagia in cervical spine injury. J. Surg. Res 201, 388–393. 10.1016/j.jss.2015.ll.031. [DOI] [PubMed] [Google Scholar]
- Lee K-Z, Chiang S-C, Li Y-J, 2017. Mild acute intermittent hypoxia improves respiratory function in unanesthetized rats with midcervical contusion. Neurorehabil. Neural Repair 31, 364–375. 10.1177/1545968316680494. [DOI] [PubMed] [Google Scholar]
- Li K, Javed E, Hala TJ, Sannie D, Regan KA, Maragakis NJ, Wright MC, Poulsen DJ, Lepore AC, 2015. Transplantation of glial progenitors that overexpress glutamate transporter GLT1 preserves diaphragm function following cervical SCI. Mol. Ther 23, 533–548. 10.1038/mt.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KH, Wu HD, Chang CW, Wang TG, Wang YH, 1998. Ventilatory and mouth occlusion pressure responses to hypercapnia in chronic tetraplegia. Arch. Phys. Med. Rehabil 79, 795–799. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R, 1994. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 640, 171–184. [DOI] [PubMed] [Google Scholar]
- Manning HL, Brown R, Scharf SM, Leith DE, Weiss JW, Weinberger SE, Schwartzstein RM, 1992. Ventilatory and P0.1 response to hypercapnia in quadriplegia. Respir. Physiol 89, 97–112. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Zhan W-Z, Sieck GC, 2009. Retrograde labeling of phrenic motoneurons by intrapleural injection. J. Neurosci. Methods 182, 244–249. http://dx.doi. org/10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan W-Z, Sieck GC, 2011. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir. Physiol. Neurobiol 177, 176–182. 10.1016/j.resp.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS, 2015. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp. Neurol 266, 1–10. 10.1016/j.expneurol.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, Hala TJ, Frank DM, Parker JL, Authelet M, Leroy K, Brion J-P, Wright MC, Lepore AC, 2012. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp. Neurol 235, 539–552. 10.1016/j-expneurol.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Nicaise C, Frank DM, Hala TJ, Authelet M, Pochet R, Adriaens D, Brion J-P, Wright MC, Lepore AC, 2013. Early phrenic motor neuron loss and transient respiratory abnormalities after unilateral cervical spinal cord contusion. J. Neurotrauma 30, 1092–1099. 10.1089/neu.2012.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JB, Drennan JC, Baba M, 2000. Evaluation and treatment of swallowing impairments. Am. Fam. Physician 61, 2453–2462. [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, Levine S, 2009. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit. Care Med 37, S347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Sieck GC, Mantilla CB, 2017. Diaphragm electromyographic activity following unilateral midcervical contusion injury in rats. J. Neurophysiol 117, 545–555. 10.1152/jn.00727.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JS, Moorman JR, 2000. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol 278, H2039–49. [DOI] [PubMed] [Google Scholar]
- Seely AJE, Bravi A, Herry C, Green G, Longtin A, Ramsay T, Fergusson D, McIntyre L, Kubelik D, Maziak DE, Ferguson N, Brown SM, Mehta S, Martin C, Rubenfeld G, Jacono FJ, Clifford G, Fazekas A, Marshall J, Canadian Critical Care Trials Group (CCCTG), 2014. Do heart and respiratory rate variability improve prediction of extubation outcomes in critically ill patients? Crit. Care 18, R65. 10.1186/ccl3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon CE, 1997. The mathematical theory of communication. 1963. MD Comput 14, 306–317. [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB, 2008. Effect of mechanical ventilation on the diaphragm. N. Engl. J. Med 358, 1392–1394. 10.1056/NEJMe0801226. [DOI] [PubMed] [Google Scholar]
- Smuder AJ, Gonzalez-Rothi EJ, Kwon OS, Morton AB, Sollanek KJ, Powers SK, Fuller DD, 2016. Cervical spinal cord injury exacerbates ventilator-induced diaphragm dysfunction. J. Appl. Physiol 120, 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamegna JC, Felix MS, Roux-Peyronnet J, Rossi V, Feron F, Gauthier P, Matarazzo V, 2011. Nasal OEC transplantation promotes respiratory recovery in a subchronic rat model of cervical spinal cord contusion. Exp. Neurol 229, 120–131. 10.1016/j.expneurol.2010.07.002. [DOI] [PubMed] [Google Scholar]
- van der Meulen JH, Urbanchek MG, Cederna PS, Eguchi T, Kuzon WM, 2003. Denervated muscle fibers explain the deficit in specific force following reinnervation of the rat extensor digitorum longus muscle. Plast. Reconstr. Surg 112, 1336–1346. 10.1097/01.PRS.0000081464.98718.E3. [DOI] [PubMed] [Google Scholar]
- Wen M-H, Lee K-Z, 2017. Diaphragm and intercostal muscle activity following midcervical spinal cord contusion in the rat. J. Neurotrauma 2017, 5128–5142. 10.1089/neu.2017.5128. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J, 2003. Effect of spinal cord injury on the respiratory system. Am. J. Phys. Med. Rehabil 82, 803–814. [DOI] [PubMed] [Google Scholar]
- Wu P, Chawla A, Spinner RJ, Yu C, Yaszemski MJ, Windebank AJ, Wang H, 2014. Key changes in denervated muscles and their impact on regeneration and reinnervation. Neural Regen. Res 9, 1796–1809. 10.4103/1673-5374.143424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki M, Cracco C, Teixeira A, Mercat A, Diehl J-L, Lefort Y, Derenne J-P, Similowski T, 2006. Reduced breathing variability as a predictor of unsuccessful patient separation from mechanical ventilation. Crit. Care Med 34, 2076–2083. 10.1097/01.CCM.0000227175.83575.E9. [DOI] [PubMed] [Google Scholar]
- Young W, 2002. Spinal cord contusion models. Prog. Brain Res 137, 231–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


