Abstract
Context
Developing osteoarthritis is common after anterior cruciate ligament reconstruction (ACLR). Monitoring changes in femoral cartilage size after ACLR may be a way to detect the earliest structural alterations before the radiographic onset of osteoarthritis. Diagnostic ultrasonography (US) offers a clinically accessible and valid method for evaluating anterior femoral cartilage size.
Objective
To compare the US measurements of anterior femoral cross-sectional area and cartilage thickness between limbs in individuals with a unilateral ACLR and between the ACLR limbs of these individuals and the limbs of uninjured control participants.
Design
Case-control study.
Setting
Research laboratory.
Patients or Other Participants
A total of 20 volunteers with an ACLR (37.0 ± 26.6 months after surgery) and 28 uninjured volunteers.
Main Outcome Measure(s)
We used US to assess anterior femoral cartilage cross-sectional area and thickness (ie, medial, lateral, and intercondylar) in the ACLR and contralateral limbs of participants with ACLR and unilaterally in the reference limbs of uninjured participants.
Results
The ACLR limb presented with greater anterior femoral cartilage cross-sectional area (96.68 ± 22.68 mm2) than both the contralateral (85.69 ± 17.57 mm2, t19 = 4.47; P < .001) and uninjured (84.62 ± 15.89 mm2, t46 = 2.17; P = .04) limbs. The ACLR limb presented with greater medial condyle thickness (2.61 ± 0.61 mm) than both the contralateral (2.36 ± 0.47 mm, t19 = 2.78; P = .01) and uninjured limbs (2.22 ± 0.40 mm, t46 = 2.69; P = .01) and greater lateral condyle thickness (2.46 ± 0.65 mm) than the uninjured limb (2.12 ± 0.41 mm, t46 = 2.20; P = .03).
Conclusions
Anterior femoral cartilage cross-sectional area and thickness assessed via US were greater in the ACLR limb than in the contralateral and uninjured limbs. Greater thickness and cross-sectional area may have been due to cartilage swelling or hypertrophy after ACLR, which may affect the long-term health of the joint.
Keywords: diagnostic imaging, knee, cartilage thickness, cartilage cross-sectional area
Key Points
An ultrasonographic assessment of the anterior femoral cartilage indicated greater cartilage cross-sectional area and thickness in the anterior cruciate ligament reconstruction (ACLR) limb of patients approximately 3 years after surgery than both the contralateral limb and the limbs of uninjured participants.
When normalized to the contralateral limb, a longer time since ACLR was associated with a larger ACLR-limb cross-sectional area.
Studies are needed to examine the importance of early cartilage changes, as ultrasonography may be a clinically accessible tool capable of monitoring cartilage size after ACLR.
Approximately 50% of individuals who sustain an anterior cruciate ligament (ACL) injury and undergo ACL reconstruction (ACLR) develop radiographic knee osteoarthritis (OA) within 2 decades after injury.1,2 Alterations in joint metabolism coupled with chronic aberrant joint loading after injury and ACLR are theorized to disrupt knee-joint tissue homeostasis and lead to a decline in articular cartilage health.3–5 Monitoring cartilage size after ACLR may provide insight into the early mechanisms leading to the development of OA, which may allow for earlier and more effective administration of disease-modifying interventions.6 The radiographic diagnosis of OA relies on detecting bony osteophytes and tibiofemoral joint-space narrowing.7 However, radiographs provide an indirect assessment of cartilage structure and may not be sensitive enough to capture the earliest changes in cartilage size after ACLR.8 Therefore, more cartilage-specific imaging modalities are needed to detect the earliest changes in cartilage structure after ACLR to transition OA care from palliation to prevention.9
Ultrasonography (US) and magnetic resonance imaging (MRI) are valid and reliable imaging modalities for directly assessing in vivo femoral cartilage size.10–12 Authors5,13–17 of most femoral cartilage imaging studies post-ACLR have used MRI. Yet US provides a valid and much more accessible, inexpensive, and clinically oriented alternative that may allow for more routine cartilage health assessments.18 Using US, clinicians can specifically assess the size of the anterior femoral cartilage, which is traditionally determined by measuring thickness at 3 discrete locations on the anterior femur.11,19,20 Akkaya et al21 are the only researchers to report using US to assess femoral cartilage thickness after ACLR, and they observed no differences in cartilage thickness between the ACLR and contralateral limbs. However, no researchers to date have used US to compare femoral cartilage size between individuals with unilateral ACLR and uninjured control participants. In addition, no investigators have used a novel femoral cartilage US cross-sectional area technique that may provide a more representative indication of cartilage size when compared with the traditional US assessment of thickness. Therefore, the primary purpose of our study was to compare the US measurements of anterior femoral cross-sectional area and cartilage thickness between limbs in individuals with a unilateral ACLR and between the ACLR limbs in these individuals and the limbs of uninjured control participants. Based on previous MRI studies in which researchers demonstrated a progressive increase in medial femoral cartilage size over 1-year,14 2-year,13,22 and 5-year5 periods after ACLR, we hypothesized that anterior femoral cartilage thickness and cross-sectional area would be greater in the ACLR limbs than in the contralateral limbs of ACLR participants and the limbs of uninjured control participants. Our secondary purpose was to determine the association between cartilage structural outcomes and time from ACLR in a cross-sectional cohort of individuals with ACLR. We theorized that greater time from ACLR would be associated with greater cartilage size. If US is a clinically accessible imaging modality that can detect early alterations in anterior femoral cartilage among individuals after ACLR, it will be an important option for identifying those who may be at high risk for developing OA.
METHODS
Design
In this case-control study, a single examiner (M.S.H.) performed a bilateral US assessment of the anterior femoral articular cartilage in a group of individuals with a unilateral ACLR and a unilateral assessment of the reference limbs of uninjured participants with no history of lower extremity injury. In their large-scale US study, Ozcakar et al20 indicated no side-to-side difference in the cartilage thickness in uninjured participants; thus, unilateral US images were obtained of the dominant limb in the uninjured control participants. The dominant limb was defined as the limb that the participant preferred to use for kicking a ball.19 A subset of uninjured participants volunteered for an additional US assessment, and images from this session were used for an intersession reliability analysis of our novel US cross-sectional area measure. Participants arrived at the laboratory for each data-collection session at the same time of day (±2 hours of the previous session) to control for diurnal variation in femoral cartilage thickness.23
Participants
We recruited a convenience sample of 20 individuals with a history of primary unilateral ACLR (5 men, 15 women) and 28 uninjured individuals (10 men, 18 women) with no history of lower extremity joint injury (Table 1). All participants reported engaging in physical activity for at least 20 minutes, 3 days each week. Participants with ACLR had undergone surgery at least 6 months before enrolling in the study and were approved for unrestricted physical activity by their physicians. We excluded volunteers with ACLR who had been diagnosed with knee OA or reported symptoms of OA at the time of data collection (eg, pain, swelling, stiffness), any lower extremity surgery other than ACLR, bilateral ACLR, multiligament reconstruction, ACLR revision surgery, or any lower extremity injury within 6 months of participation. Uninjured participants reported no history or symptoms of OA, lower extremity surgery, ligamentous knee injury, or any major lower extremity injury. The uninjured participants were not individually matched to participants with ACLR; however, we recruited control participants from a similar population, and the key demographics were not different between groups (Table 1). An a priori power analysis (version 3.1.9.2; G*Power,24 Heinrich-Heine-Universität, Düsseldorf, Germany) indicated that we would need a sample size of at least 19 individuals with ACLR to determine a between-limbs difference (Cohen d = 0.6) in cartilage thickness to achieve 80% power with an α level of .05. All participants provided written informed consent, and the study was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Table 1.
Participant Demographics
| Characteristic |
Group |
t Value |
P Value |
|
| Anterior Cruciate Ligament Reconstruction (n = 20) |
Uninjured Control (n = 28) |
|||
| Males/females, No. (%) | 5/15 (25/75) | 10/18 (36/64) | NA | NA |
| Mean ± SD |
||||
| Age, y | 20.2 ± 2.1 | 20.5 ± 1.7 | 0.58 | .57 |
| Height, cm | 168.2 ± 7.3 | 170.8 ± 10.6 | 0.92 | .36 |
| Mass, kg | 69.7 ± 16.6 | 69.6 ± 18.5 | 0.02 | .98 |
| Time after surgery, mo | 37.0 ± 26.6 | NA | NA | NA |
Abbreviation: NA, not applicable.
Ultrasonographic Assessment of the Anterior Femoral Cartilage
Ultrasonographic Image Acquisition
Anterior femoral cartilage US assessment has been validated against cadaver specimens11 and MRI measurements.12 Our methods of US image acquisition were described in an earlier study.19 For this study, we recorded 3 images bilaterally in the participants with ACLR and unilaterally in the uninjured participants.
A subset of uninjured individuals (n = 17) returned for an identical data-collection session approximately 1 week (7.1 ± 1.6 days) after the initial session so that we could determine the intersession reliability of the novel US cross-sectional area measure. Using the recorded positioning of the heel and the locations of the femoral condyles on the transparency grid from the first session, we consistently positioned both the participant and the US probe.
Ultrasonographic Image Processing
A single, unblinded investigator (M.S.H.) manually segmented each US image using ImageJ software (version 1.50i; National Institutes of Health, Bethesda, MD). All 3 of the femoral cartilage US images for each knee were analyzed and averaged for the US outcomes of cross-sectional area and cartilage thickness.
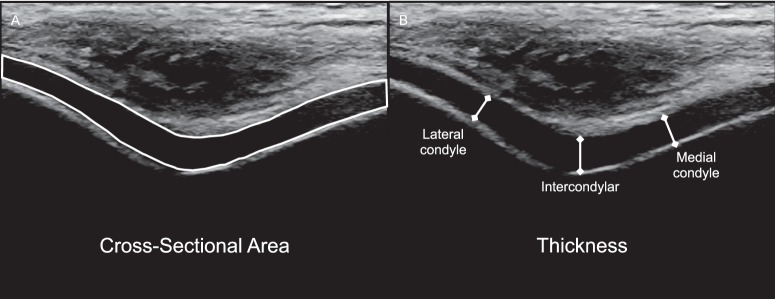
We assessed the anterior femoral cartilage cross-sectional area as a novel US outcome of cartilage size. Similar techniques for measuring cross-sectional area have often been used in US muscle studies, but we are the first to assess cartilage cross-sectional area using this technique. It involved segmenting the outline of the entire visualized cartilage-bone interface and soft tissue–cartilage interface of each US image. ImageJ software was used to calculate the cartilage cross-sectional area (mm2; Figure 1B) based on the segmented outline of the cartilage. Each cross-sectional area and thickness outcome was averaged across the 3 images for each participant for statistical analysis.
Figure 1.
Femoral cartilage ultrasonographic outcome measures. A, Femoral cartilage cross-sectional area was assessed by outlining the cartilage visualized on each ultrasonographic image. B, Femoral cartilage thickness was measured as the length of the straight line drawn from the cartilage-bone interface to the synovial space–cartilage interface.
We assessed anterior femoral cartilage thickness at the midpoint of the upslope of the medial and lateral femoral condyles and the intercondylar notch as the straight-line distance in millimeters from the cartilage-bone interface to the synovial space–cartilage interface (Figure 1A).11,19,20 Strong intrasession reliability and precision for the medial cartilage-thickness assessment have been established within our laboratory (intraclass correlation coefficient [ICC] [2,k] = 0.97, standard error of measurement [SEM] = 0.07 mm).19
Statistical Analysis
Independent t tests were used to compare demographics between the participants with ACLR and uninjured participants.
Intrasession and Intersession Reliability and Precision of US-Measured Femoral Cartilage Cross-Sectional Area
Intrasession reliability of the cartilage cross-sectional area was assessed by calculating separate ICCs (2,1) of the 3 individual images, and intrasession precision was determined by calculating the SEM in uninjured individuals. The ICCs were classified as weak (<0.5), moderate (0.5–0.69), or strong (≥0.7).25 Cartilage cross-sectional area SEM was calculated using Equation 1, where SD was the pooled standard deviation of the measurements and ICC was the calculated ICC.26
 |
We assessed the intersession reliability (ICC [2,k]) of the cartilage cross-sectional area for the 3-image average of each outcome measure at both times using the subset of uninjured individuals who returned for an additional data-collection session involving identical procedures. The precision of the intersession cartilage cross-sectional area was determined using the SEM.
Comparison of Femoral Cartilage US Outcome Measures
Dependent t tests were used to compare femoral cartilage cross-sectional area and thickness between the reconstructed and contralateral limbs in participants with ACLR. Independent t tests were used to compare femoral cartilage cross-sectional area and thickness between the dominant limb of the uninjured participants and both limbs of those with ACLR (ie, reconstructed and contralateral limbs).
Association Between Femoral Cartilage US Limb Symmetry Indices and Time Since ACLR
The average time since ACLR in our participants was approximately 3 years (37.0 ± 26.6 months); however, the range of time since ACLR was wide (7–103 months). Therefore, we wanted to determine if an association was present between cartilage size and time since ACLR. For this secondary analysis, a limb symmetry index (LSI) for each femoral cartilage US outcome (ie, cross-sectional area and cartilage thickness) was created for the participants with ACLR by dividing the value for the ACLR limb by the value for the contralateral limb and multiplying by 100. Thus, individuals with a greater cartilage cross-sectional area LSI had a larger cartilage cross-sectional area in their ACLR limb relative to their contralateral limb.
We used a Shapiro-Wilk test to determine if the outcomes were normally or non-normally distributed. When outcomes were normally distributed, separate Pearson product-moment correlations were used to determine the association between femoral cartilage US LSI (ie, cross-sectional area and thickness) and time since ACLR. When outcomes were non-normally distributed, a Spearman rank-order correlation was used to determine this association. The magnitude of association was defined as negligible (0.0–0.09), low (0.10–0.29), moderate (0.30–0.49), or large (0.50–1.00).27 All statistical analyses were performed using SPSS (version 21; IBM Corp, Armonk, NY). The α level was set a priori at .05.
RESULTS
Participant demographics are provided in Table 1. We found no differences between groups for any demographic variable.
Intrasession and Intersession Reliability and Precision of US-Measured Femoral Cartilage Cross-Sectional Area
Strong intrasession reliability (n = 28) was demonstrated for femoral cartilage cross-sectional area (ICC [2,1] = 0.98; 95% confidence interval = 0.96, 0.99). The cartilage cross-sectional area SEM was small, indicating acceptable precision between measurements within the same session (SEM = 2.23 mm2).
Strong intersession reliability (n = 17) was demonstrated for the femoral cartilage cross-sectional area (ICC [2,k] = 0.95; 95% confidence interval = 0.87, 0.98). The cartilage cross-sectional area SEM was small, indicating acceptable precision between measurements within different sessions (SEM = 3.11 mm2).
Comparison of Femoral Cartilage US Outcome Measures
The ACLR limb presented with greater anterior femoral cartilage cross-sectional area than the contralateral limb (t19 = 4.47, P < .001, Cohen d = 0.50; Table 2). Specifically, the ACLR limb displayed greater medial condyle thickness than the contralateral limb (t19 = 2.78, P = .01, Cohen d = 0.46), but we observed no difference between limbs for the lateral condyle (t19 = 2.02, P = .06, Cohen d = 0.42) or intercondylar thickness (t19 = 1.99, P = .06, Cohen d = 0.30).
Table 2.
Comparisons of Limb Ultrasound Measures
| Outcome Measure |
Group |
No. |
Mean ± SD |
Comparison With Uninjured Control Limb |
Comparison With Contralateral Limb |
||||
|
t46 Value |
P Value |
Cohen d Value |
t19 Value |
P Value |
Cohen d Value |
||||
| Medial condyle thickness, mm | ACLR limb | 20 | 2.61 ± 0.61 | 2.69 | .01a | 0.79 | 2.78 | .01a | 0.46 |
| Contralateral limb | 20 | 2.36 ± 0.47 | 1.12 | .27 | 0.33 | ||||
| Uninjured control limb | 28 | 2.22 ± 0.40 | |||||||
| Lateral condyle thickness, mm | ACLR limb | 20 | 2.46 ± 0.65 | 2.20 | .03a | 0.65 | 2.02 | .06 | 0.42 |
| Contralateral limb | 20 | 2.24 ± 0.37 | 1.03 | .31 | 0.30 | ||||
| Uninjured control limb | 28 | 2.12 ± 0.41 | |||||||
| Intercondylar thickness, mm | ACLR limb | 20 | 2.46 ± 0.66 | 1.79 | .08 | 0.53 | 1.99 | .06 | 0.30 |
| Contralateral limb | 20 | 2.29 ± 0.47 | 0.88 | .39 | 0.27 | ||||
| Uninjured control limb | 28 | 2.16 ± 0.49 | |||||||
| Cross-sectional area, mm2 | ACLR limb | 20 | 96.68 ± 22.68 | 2.17 | .04a | 0.64 | 4.47 | <.001a | 0.50 |
| Contralateral limb | 20 | 85.69 ± 17.57 | 0.22 | .83 | 0.06 | ||||
| Uninjured control limb | 28 | 84.62 ± 15.89 | |||||||
Abbreviation: ACLR, anterior cruciate ligament reconstruction.
Difference between limbs (P < .05).
The ACLR limb had greater anterior femoral cartilage cross-sectional area than the dominant limb of the uninjured individuals (t46 = 2.17, P = .04, Cohen d = 0.64). Specifically, the ACLR limb demonstrated greater medial (t46 = 2.69, P = .01, Cohen d = 0.79) and lateral (t46 = 2.20, P = .03, Cohen d = 0.65) condyle thickness than the dominant limb of the uninjured individuals. Intercondylar thickness was not different between the ACLR limb and the dominant limb of the uninjured individuals (t46 = 1.79, P = .08, Cohen d = 0.53). The contralateral limb of the ACLR participants was similar to the dominant limb of the uninjured participants for cross-sectional area (t46 = 0.22, P = .83, Cohen d = 0.06; Table 2) and each thickness outcome (medial condyle: t46 = 1.12, P = .27, Cohen d = 0.33; lateral condyle: t46 = 1.03, P = .31, Cohen d = 0.30; intercondylar: t46 = 0.88, P = .39, Cohen d = 0.27).
Association Between Femoral Cartilage US Limb Symmetry Indices and Time Since ACLR
All variables were normally distributed; therefore, only Pearson product moment correlations were used in the analysis. Greater time since ACLR was moderately associated with greater femoral cartilage area LSI (r = 0.47, P = .04; Figure 2). Time since ACLR was not associated with the medial condylar (r = 0.02, P = .93), intercondylar (r = 0.27, P = .25), or lateral condylar (r = 0.20, P = .39) femoral cartilage thickness LSI.
Figure 2.
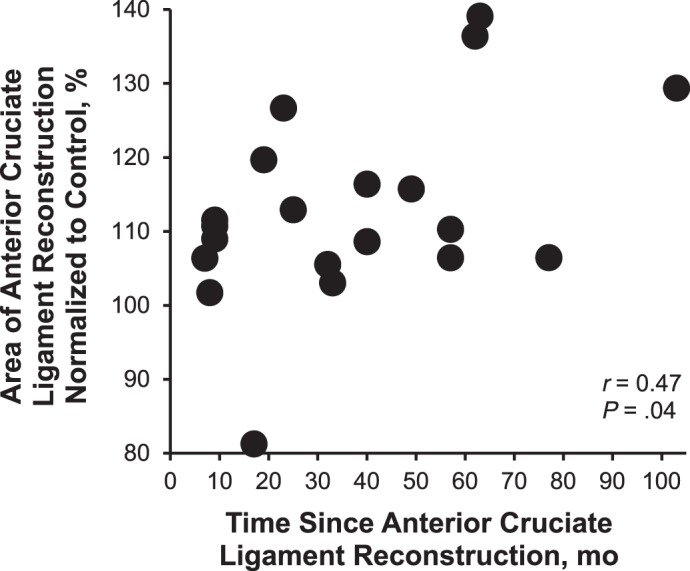
A greater time since anterior cruciate ligament reconstruction was associated with a larger femoral cartilage cross-sectional area limb symmetry index.
DISCUSSION
Our main finding was that the ACLR limb demonstrated greater anterior femoral cartilage cross-sectional area than the contralateral limbs of participants with ACLR and the dominant limbs of uninjured control participants. Specifically, the ACLR limb demonstrated greater medial condyle thickness than the contralateral and uninjured control limbs and greater lateral condyle thickness than the uninjured control limb. The cartilage thickness that we observed was consistent with the results of a previous large-scale US study of 1544 individuals.20 Consistent with our hypothesis, greater femoral cartilage area LSI was moderately associated with greater time since ACLR. The importance of these results is 2-fold: (1) alterations in cartilage morphology are evident within 3 years after ACLR and (2) US is a clinically accessible technique that may be used as a simple tool for detecting early cartilage changes after ACLR.
Greater cross-sectional area and cartilage thickness after ACLR appear contradictory to traditional OA theories, as end-stage OA is characterized by femoral cartilage thinning.28 However, researchers29,30 studying idiopathic OA have theorized that the earliest stages may be defined by cartilage thickening before the eventual thinning of the cartilage. Our findings of greater femoral cartilage cross-sectional area and medial thickness on US are similar to those of previous MRI studies in which investigators observed longitudinal increases in medial femoral cartilage thickness at 1 year,14 2 years,13,22 and 5 years5 after ACL injury. Radiographic changes to the anterior medial femoral condyle may occur early in the development of knee OA because of impingement of the medial femoral condyle on the medial tibial spine.31 In addition, investigators using compositional MRI techniques have indicated that the anterior medial femur demonstrates worse cartilage composition (ie, depletions in proteoglycans32 and glycosaminoglycans33) as early as 1 year after ACLR.32,33 Using an MRI definition of OA, 6%, 11%, and 17% of individuals presented with OA in the medial tibiofemoral, lateral tibiofemoral, and patellofemoral compartment, respectively, within 1 year after ACLR.31,34 The positive association between time since surgery and cross-sectional area LSI that we observed provides evidence that complements the previously noted longitudinal increases in medial femoral cartilage.5,13,14,22 Together, these data suggest that the thickening of femoral cartilage early after ACLR, especially in the anteromedial femur,5,13,14,22 is progressive and detectable using a clinically accessible US technique. Whereas the precise mechanism for increased cartilage size after ACLR and in early OA is not completely understood, researchers35,36 have hypothesized that an increase in cartilage thickness may be due to either swelling of the cartilage or hypertrophy of the extracellular matrix.
Investigators37–39 have also hypothesized that, as the cartilage extracellular matrix begins to break down (ie, proteoglycan depletion or collagen network disorganization), an influx of water creates swelling of the cartilage and presents as an increase in cartilage thickness. Conversely, the initial increase in cartilage size can be explained by increased synthesis of extracellular matrix components, which results in decreased relative water content.35 Given that the interaction between water and the extracellular matrix provides most of the compressive stiffness attributed to the cartilage, alterations in the healthy relative water content may disrupt the ability of the cartilage to properly respond to loading that occurs during activities of daily living.40 Increased cartilage size, whether due to swelling or hypertrophy, may result in aberrant joint mechanics and altered viscoelastic properties of the cartilage,41 which may then contribute to chronic negative effects on the joint tissue and increase the likelihood of OA development.
Aberrant joint mechanics during walking and running are common for years after ACL injury and ACLR.42,43 Similar to the time-dependent changes we observed in femoral cartilage after ACLR, researchers44–46 have also reported evidence of temporal changes in knee kinematics and kinetics depending on the time since ACL injury and ACLR. The consequences of these subtle alterations in knee mechanics after ACLR are seen as a shift in the location of cartilage loading coupled with decreased contact area and increased peak cartilage deformation.47 In their simulation study of ACL-injured knees, Andriacchi et al48 indicated that 5° of tibial internal rotation may offset knee-cartilage contact patterns that can lead to a rapid rate of cartilage loss compared with uninjured knees. Alternatively, declines in cartilage health have been evident in compositional MRI studies in which authors have demonstrated proteoglycan disruption32,33 and type II collagen disorganization41 in the tibiofemoral cartilage of individuals within the first year after ACLR. Declines in cartilage composition have been theorized to disrupt the healthy viscoelastic properties of the tissue and alter the cartilage response to acute loading.49 Evidence of a decline in these viscoelastic cartilage properties has been observed after ACLR with type II collagen disorganization, as these same individuals also presented with decreased cartilage resiliency after an acute bout of running.41 Therefore, alterations in both joint mechanics and cartilage viscoelastic properties occur after ACLR and may be risk factors for developing OA.
Our study highlights the ability of US to provide an accessible and inexpensive method of imaging cartilage that can potentially detect differences in anterior femoral cartilage size in individuals at an average of 3 years after ACLR compared with both the contralateral knees and the knees of uninjured participants. However, future researchers will need to determine if early cartilage alterations assessed with US affect patient-reported outcomes and objective measures of patient function. This knee-cartilage US assessment was limited to the anterior femoral cartilage and cannot determine the morphology of central or posterior femoral cartilage or tibial cartilage. However, the US protocol provides an affordable, easily attained assessment that may allow for future longitudinal studies in which anterior femoral cartilage is routinely monitored. We observed a cross-sectional association between femoral cartilage area LSI and time since ACL injury, but a larger-scale longitudinal study is needed to definitively establish the longitudinal changes in cartilage morphology after ACLR. Given that OA has been described as a disease of multiple tissues within the entire joint organ,50 researchers should use US to assess alterations in other joint tissues (ie, tendons, menisci, synovium, Hoffa fat pad).51 In our study, a single experienced reader conducted all US image analyses, yet this reader was not blinded to the status of the participants' knee joints. In future studies, investigators should consider involving blinded readers who are unaware of the knee status of participants to minimize potential bias in the analysis. Our US protocol consistently used 140° of knee flexion to ensure imaging of the same location of femoral cartilage among individuals, but this position may not be feasible in a population with range-of-motion restrictions (ie, acutely injured, knee OA). If a participant cannot achieve 140° of knee flexion, maximum knee flexion can be used to sufficiently image the femoral cartilage.20,52,53 We did not have access to the surgical notes for the individuals with ACLR, which limited our ability to further understand how concomitant surgical procedures or injuries may have influenced changes in cartilage size in the ACLR limb. In future studies, researchers should determine if specific meniscal or cartilage injuries or surgeries at the time of ACLR affect the results. Our secondary analysis indicated a progressive thickening of anterior femoral cartilage as time since ACLR increased, but we need to assess individuals at longer times since ACLR to determine when they begin to experience the cartilage thinning that may lead to OA.
CONCLUSIONS
A US assessment of the anterior femoral cartilage of patients approximately 3 years after ACLR indicated greater cartilage cross-sectional area and thickness in the ACLR limbs than in both the contralateral limbs and the limbs of uninjured participants. We observed a positive association between time since ACLR and cross-sectional area LSI, indicating that a longer time since ACLR was associated with a larger ACLR limb cross-sectional area when normalized to the contralateral limb. Future studies are needed to evaluate the importance of early cartilage changes, as US may be a clinically accessible tool capable of monitoring cartilage size after ACLR.
REFERENCES
- 1.Harris KP, Driban JB, Sitler MR, Cattano NM, Balasubramanian E, Hootman JM. Tibiofemoral osteoarthritis after surgical or nonsurgical treatment of anterior cruciate ligament rupture: a systematic review. J Athl Train. 2017;52(6):507–517. doi: 10.4085/1062-6050-49.3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49(6):806–819. doi: 10.4085/1062-6050-49.3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harkey MS, Luc BA, Golightly YM, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthritis Cartilage. 2015;23(1):1–12. doi: 10.1016/j.joca.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Favre J. The nature of in vivo mechanical signals that influence cartilage health and progression to knee osteoarthritis. Curr Rheumatol Rep. 2014;16(11):463. doi: 10.1007/s11926-014-0463-2. [DOI] [PubMed] [Google Scholar]
- 5.Eckstein F, Wirth W, Lohmander LS, Hudelmaier MI, Frobell RB. Five-year followup of knee joint cartilage thickness changes after acute rupture of the anterior cruciate ligament. Arthritis Rheumatol. 2015;67(1):152–161. doi: 10.1002/art.38881. [DOI] [PubMed] [Google Scholar]
- 6.Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther. 2012;14(3):212. doi: 10.1186/ar3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guermazi A, Hayashi D, Roemer FW, Felson DT. Osteoarthritis: a review of strengths and weaknesses of different imaging options. Rheum Dis Clin North Am. 2013;39(3):567–591. doi: 10.1016/j.rdc.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Chu CR, Millis MB, Olson SA. Osteoarthritis: from palliation to prevention. AOA critical issues. J Bone Joint Surg Am. 2014;96(15):e130. doi: 10.2106/JBJS.M.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14(suppl A):A46–A75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Naredo E, Acebes C, Moller I, et al. Ultrasound validity in the measurement of knee cartilage thickness. Ann Rheum Dis. 2009;68(8):1322–1327. doi: 10.1136/ard.2008.090738. [DOI] [PubMed] [Google Scholar]
- 12.Ostergaard M, Court-Payen M, Gideon P, et al. Ultrasonography in arthritis of the knee: a comparison with MR imaging. Acta Radiol. 1995;36(1):19–26. [PubMed] [Google Scholar]
- 13.Frobell RB. Change in cartilage thickness, posttraumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption: a two-year prospective MRI study of sixty-one subjects. J Bone Joint Surg Am. 2011;93(12):1096–1103. doi: 10.2106/JBJS.J.00929. [DOI] [PubMed] [Google Scholar]
- 14.Frobell RB, Le Graverand MP, Buck R, et al. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage. 2009;17(2):161–167. doi: 10.1016/j.joca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Andreisek G, White LM, Sussman MS, et al. Quantitative MR imaging evaluation of the cartilage thickness and subchondral bone area in patients with ACL-reconstructions 7 years after surgery. Osteoarthritis Cartilage. 2009;17(7):871–878. doi: 10.1016/j.joca.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Hosseini A, Li JS, Gill TJ IV, Li G. Quantitative magnetic resonance imaging (MRI) morphological analysis of knee cartilage in healthy and anterior cruciate ligament-injured knees. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1496–1502. doi: 10.1007/s00167-011-1723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okafor EC, Utturkar GM, Widmyer MR, et al. The effects of femoral graft placement on cartilage thickness after anterior cruciate ligament reconstruction. J Biomech. 2014;47(1):96–101. doi: 10.1016/j.jbiomech.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iagnocco A. Imaging the joint in osteoarthritis: a place for ultrasound? Best Pract Res Clin Rheumatol. 2010;24(1):27–38. doi: 10.1016/j.berh.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Harkey MS, Blackburn JT, Davis H, Sierra-Arevalo L, Nissman D, Pietrosimone B. Ultrasonographic assessment of medial femoral cartilage deformation acutely following walking and running. Osteoarthritis Cartilage. 2017;25(6):907–913. doi: 10.1016/j.joca.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 20.Ozcakar L, Tunc H, Oken O, et al. Femoral cartilage thickness measurements in healthy individuals: learning, practicing and publishing with TURK-MUSCULUS. J Back Musculoskelet Rehabil. 2014;27(2):117–124. doi: 10.3233/BMR-130441. [DOI] [PubMed] [Google Scholar]
- 21.Akkaya S, Akkaya N, Gungor HR, Agladioglu K, Ok N, Ozcakar L. Sonoelastographic evaluation of the distal femoral cartilage in patients with anterior cruciate ligament reconstruction. Eklem Hastalik Cerrahisi. 2016;27(1):2–8. doi: 10.5606/ehc.2016.02. [DOI] [PubMed] [Google Scholar]
- 22.Williams A, Winalski CS, Chu CR. Early articular cartilage MRI T2 changes after anterior cruciate ligament reconstruction correlate with later changes in T2 and cartilage thickness. J Orthop Res. 2017;35(3):699–706. doi: 10.1002/jor.23358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilic G, Kilic E, Akgul O, Ozgocmen S. Ultrasonographic assessment of diurnal variation in the femoral condylar cartilage thickness in healthy young adults. Am J Phys Med Rehabil. 2015;94(4):297–303. doi: 10.1097/PHM.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 24.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 25.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 26.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19(1):231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences Rev ed. New York, NY: Academic Press; 1977. pp. 145–178. [Google Scholar]
- 28.Hunter DJ. Osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25(6):801–814. doi: 10.1016/j.berh.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Buck RJ, Wirth W, Dreher D, Nevitt M, Eckstein F. Frequency and spatial distribution of cartilage thickness change in knee osteoarthritis and its relation to clinical and radiographic covariates: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2013;21(1):102–109. doi: 10.1016/j.joca.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Cotofana S, Buck R, Wirth W, et al. Cartilage thickening in early radiographic knee osteoarthritis: a within-person, between-knee comparison. Arthritis Care Res (Hoboken) 2012;64(11):1681–1690. doi: 10.1002/acr.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairclough JA, Graham GP, Dent CM. Radiological sign of chronic anterior cruciate ligament deficiency. Injury. 1990;21(6):401–402. doi: 10.1016/0020-1383(90)90130-m. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2: initial experience with 1-year follow-up. Radiology. 2011;258(2):505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming BC, Oksendahl HL, Mehan WA, et al. Delayed gadolinium-enhanced MR imaging of cartilage (dGEMRIC) following ACL injury. Osteoarthritis Cartilage. 2010;18(5):662–667. doi: 10.1016/j.joca.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Culvenor AG, Collins NJ, Guermazi A, et al. Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheumatol. 2015;67(4):946–955. doi: 10.1002/art.39005. [DOI] [PubMed] [Google Scholar]
- 35.Vignon E, Arlot M, Hartmann D, Moyen B, Ville G. Hypertrophic repair of articular cartilage in experimental osteoarthrosis. Ann Rheum Dis. 1983;42(1):82–88. doi: 10.1136/ard.42.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvo E, Palacios I, Delgado E, et al. Histopathological correlation of cartilage swelling detected by magnetic resonance imaging in early experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12(11):878–886. doi: 10.1016/j.joca.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Buck RJ, Wyman BT, Le Graverand MP, et al. Osteoarthritis may not be a one-way-road of cartilage loss: comparison of spatial patterns of cartilage change between osteoarthritic and healthy knees. Osteoarthritis Cartilage. 2010;18(3):329–335. doi: 10.1016/j.joca.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Lusse S, Claassen H, Gehrke T, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging. 2000;18(4):423–430. doi: 10.1016/s0730-725x(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 39.Chou MC, Tsai PH, Huang GS, et al. Correlation between the MR T2 value at 4.7 T and relative water content in articular cartilage in experimental osteoarthritis induced by ACL transection. Osteoarthritis Cartilage. 2009;17(4):441–447. doi: 10.1016/j.joca.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Woo SLY, Buckwalter JA. Injury and Repair of the Musculoskeletal Soft Tissues. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1988. [Google Scholar]
- 41.Van Ginckel A, Verdonk P, Victor J, Witvrouw E. Cartilage status in relation to return to sports after anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(3):550–559. doi: 10.1177/0363546512473568. [DOI] [PubMed] [Google Scholar]
- 42.Noehren B, Wilson H, Miller C, Lattermann C. Long-term gait deviations in anterior cruciate ligament-reconstructed females. Med Sci Sports Exerc. 2013;45(7):1340–1347. doi: 10.1249/MSS.0b013e318285c6b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 44.Webster KE, Feller JA, Wittwer JE. Longitudinal changes in knee joint biomechanics during level walking following anterior cruciate ligament reconstruction surgery. Gait Posture. 2012;36(2):167–171. doi: 10.1016/j.gaitpost.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Zabala ME, Favre J, Andriacchi TP. Relationship between knee mechanics and time since injury in ACL-deficient knees without signs of osteoarthritis. Am J Sports Med. 2015;43(5):1189–1196. doi: 10.1177/0363546514567296. [DOI] [PubMed] [Google Scholar]
- 46.Slater LV, Hart JM, Kelly AR, Kuenze CM. Progressive changes in walking kinematics and kinetics after anterior cruciate ligament injury and reconstruction: a review and meta-analysis. J Athl Train. 2017;52(9):847–860. doi: 10.4085/1062-6050-52.6.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van de Velde SK, Bingham JT, Hosseini A, et al. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60(12):3693–3702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 49.Cohen NP, Foster RJ, Mow VC. Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J Orthop Sports Phys Ther. 1998;28(4):203–215. doi: 10.2519/jospt.1998.28.4.203. [DOI] [PubMed] [Google Scholar]
- 50.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oo WM, Bo MT. Role of ultrasonography in knee osteoarthritis. J Clin Rheumatol. 2016;22(6):324–329. doi: 10.1097/RHU.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 52.Tuna S, Balci N, Ozcakar L. The relationship between femoral cartilage thickness and muscle strength in knee osteoarthritis. Clin Rheumatol. 2016;35(8):2073–2077. doi: 10.1007/s10067-016-3271-4. [DOI] [PubMed] [Google Scholar]
- 53.Akkaya S, Akkaya N, Ozcakar L, et al. Ultrasonographic evaluation of the femoral cartilage thickness after unilateral arthroscopic partial meniscectomy. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1104–1110. doi: 10.1007/s00167-012-2081-8. [DOI] [PubMed] [Google Scholar]