Abstract
Background
Activated hepatic macrophages play a key role in inflammation and fibrosis progression in chronic liver disease.
Aim
To assess the prognostic value of soluble (s)CD163 and mannose receptor (sMR) in cirrhotic patients and explore associations with markers of intestinal permeability (lactulose-mannitol ratio, diamine oxidase), bacterial translocation (endotoxin, lipopolysaccharide-binding protein) and markers of systemic immune activation (interleukin-6, interleukin-8, sCD14).
Methods
We prospectively investigated 101 cirrhotic patients (Child-Pugh class A: n = 72, Child-Pugh classes B and C: n = 29) and 31 healthy controls. Patients were observed for a median follow-up of 37 months.
Results
Median plasma levels of sCD163 and soluble mannose receptor were significantly elevated in cirrhotic patients (P < .001) and increased with disease severity (sCD163 in healthy controls = 1.3, Child-Pugh class A = 4.2, Child-Pugh classes B and C = 8.4 mg/L; sMR in healthy controls = 15.8, Child-Pugh class A = 36.5, Child-Pugh classes B and C = 66.3 μg/dL). A total of 21 patients died during the observation period. Patients with sCD163 levels above 5.9 mg/L showed significantly reduced survival (survival rate after 36 months: 71% versus 98%, P < .001). Patients with soluble mannose receptor levels above 45.5 μg/dL developed significantly more complications of cirrhosis within 12 months (73% versus 9%, P < .001). Furthermore, both variables correlated with the lactulose-mannitol ratio, diamine oxidase, lipopolysaccharide and interleukin-8. Conclusion: Our data demonstrate the prognostic value of sCD163 in predicting long-term survival in patients with liver cirrhosis and identify soluble mannose receptor as a prognostic marker for occurrence of cirrhosis-associated complications. The correlation between gut barrier dysfunction and activation of macrophages points towards a link between them.
1. Introduction
Liver cirrhosis is a significant cause of global health burden, with more than one million deaths per year and increasing prevalence.1 Fibrosis development is considered the final response to hepatic injury and inflammation due to viral infection, alcohol consumption, toxins, autoimmune diseases and other factors.2 In advanced liver disease, hepatic inflammation is suspected to be additionally maintained by gut-derived pathogen-associated molecular patterns (PAMPs) that increasingly reach the liver due to a disrupted intestinal barrier and impaired immune responses.3 In the liver, recognition of PAMPs by toll-like receptors of immune cells leads to the secretion of pro-inflammatory cytokines,4 and subsequent hepatic as well as systemic inflammation.
Toll-like receptors are usually expressed by sentinel cells such as macrophages and dendritic cells. In the hepatic sinusoids, resident macrophages (Kupffer cells) constitute the first contact point for gutderived PAMPs like lipolpolysaccharide (LPS, endotoxin). Activation of Kupffer cells has recently been shown to play a crucial role in inflammation and fibrosis progression in different liver diseases.5–7 Markers of macrophage activation sCD163 and soluble mannose receptor (sMR) are detectable in peripheral blood and correlate well with liver disease severity and portal hypertension.8–13 CD163, the haemoglobin-haptoglobin scavenger receptor, is expressed on macrophages and monocytes,14 and is released into circulation as soluble (s)CD163 after shedding from immune cells. sCD163 levels are associated with prognosis in severe alcoholic hepatitis,15 cirrhosis of different aetiologies12,16,17 and acute or chronic liver failure.9 In alcoholic hepatitis, sCD163 levels correlate well with markers of the LPS-pathway15 supporting the hypothesis of gut-derived PAMPs as activators of liver macrophages. sMR, the shedding product of the mannose receptor located primarily on macrophages and dendritic cells, is not yet investigated as detailed as sCD163, but has been proven to be elevated in patients with liver disease and in patients with critical illness.8,18
The Child-Pugh and model for end-stage liver disease (MELD) scores represent the two most commonly used prognostic tools for patients suffering from liver cirrhosis. The Child-Pugh score was originally used to predict mortality in cirrhotic patients undergoing surgery19 and is based on standard laboratory and clinical characteristics. The MELD score was introduced as a prognostic tool in advanced cirrhotic patients scheduled for TIPSS (transjugulary intrahepatic porto-systemic shunt) placement with advanced liver cirrhosis20 and has shown its prognostic value especially in decompensated patients. However, these prognostic models are based on parameters that are predominantly manifestations of liver disease and do not take markers of disease pathogenesis or progression into account.
Therefore, our aim was to examine the predictive value of plasma levels of sCD163 and sMR (biomarkers of macrophage activation) and compare them to established models in a well-characterised cohort of predominantly compensated cirrhotics. Furthermore, we correlated sCD163 and sMR with markers of gut permeability (diamine oxidase, lactulose-mannitol ratio), bacterial translocation (endotoxin, lipopolysaccharide-binding protein) and markers of systemic immune activation (interleukin-6, interleukin-8, sCD14).
2. Materials and Methods
2.1. Patients and study design
Outpatients aged 18-80 years with evidence of cirrhosis (clinical, biochemical and imaging techniques and/or biopsy-proven) of any aetiology were eligible for the study between July 2012 and September 2013 after we obtained written informed consent at outpatient clinics of the University Hospital of Graz, Austria (Department of Gastroenterology and Hepatology, Department of Transplantation Surgery). Patients with a Child-Pugh score of 12 or higher, alcohol abuse within 2 weeks prior to inclusion, active infection at screening, gastrointestinal haemorrhage within 2 weeks prior to inclusion, immunomodulation drugs, hepatic encephalopathy stage two or higher, renal failure (creatinine over 1.7 mg/dL), severe diseases unrelated to cirrhosis, malignancy or pregnancy were excluded.
This study was part of an intervention study (NCT01607528).21 Patient characteristics and blood samples from baseline visits were used to assess prognostic values of blood plasma levels of sCD163 and sMR. The study protocol was approved by the institutional ethics committee in Graz (23-096 ex 10/11) and performed according to the declaration of Helsinki. Data regarding occurrence of complications of cirrhosis were collected for 12 months during the active study period, data regarding survival were collected from out-patient clinic files or patients’ physicians. Complications of cirrhosis were defined as follows: development of new-onset ascites in a patient without ascites at baseline, development of new-onset hepatic encephalopathy, development of spontaneous bacterial peritonitis (SBP), new-onset jaundice (clinically detectable or bilirubin > 2 mg/dL), upper gastrointestinal bleeding due to portal hypertension (variceal bleeding or bleeding from gastric antral vascular ectasia), occurrence of portal vein thrombosis, or death from liver disease. Furthermore, infections requiring antibiotic treatment during the active study period were recorded. Severity of liver disease was assessed by Child-Pugh score, MELD score and by determination of D’Amico’s clinical stages of cirrhosis22 (stage 1 = no ascites and no varices; stage 2 = presence of varices; stage 3 = presence of ascites; stage 4 = variceal bleeding). Markers of macrophage activation in 101 cirrhotic patients were compared to 31 healthy controls.
2.2. Laboratory assays
Levels of sCD163 in plasma samples were measured by an in-house sandwich enzyme-linked immunosorbent assay (ELISA) using a BEP-2000 ELISA-analyser (Dade Behring) as previously described.9,23 Levels of sMR in plasma samples were measured by an in-house ELISA assay as previously described.18
A ready-to-use solid-phase sandwich ELISA (Immundiagnostik AG, Bensheim, Germany) was used to detect diamine oxidase (DAO) in serum samples. Lipopolysaccharide-binding protein and sCD14 levels were detected in EDTA plasma via a ready-to-use solid-phase sandwich ELISA (Hycult biotechnology, Uden, Netherlands). Cytokines (interleukin-6, interleukin-8) were measured by ProcartaPlex (eBioscience, Vienna, Austria). All tests were performed according to the manufacturer’s instructions. For endotoxin measurement, HEK-Blue LPS Detection Kit 2 (Invivogen, Toulouse, France) was used as previously described.21 The differential sugar absorption test was performed with lactulose (10 g) and mannitol (5 g) and analysed by nuclear magnetic resonance spectroscopy as previously described.21
2.3. Statistical analysis
Continuous variables are reported by medians and interquartile range, whereas categorical data are presented as frequencies and percentages. Between-group differences in continuous variables were assessed by Mann-Whitney U test for two-group comparisons and the Kruskal-Wallis test for several group comparisons with Bonferroni correction for multiple testing. Spearman’s rank test was used to determine correlations between biomarkers of macrophage activation and other variables of interest.
The predictive value of macrophage activation markers and established models on mortality were analysed by the Kaplan-Meier method and compared by log-rank test. Youden’s index was determined to identify the best cut-off within the cirrhosis group. A receiver operating characteristic curve (ROC) analysis and the area under the ROC curves (AUROC) were calculated to illustrate the predictive values of the separate variables. For comparison of AUROCs, methodology of Hanley &McNeil24,25 was used. For univariate Cox regression, coefficients for all variables with assumed prognostic relevance were calculated separately. Variables identified as potential covariates (P < .10) from univariate analyses were then tested in multivariable Cox proportional hazard regression (conditional backward selection) models. Hazard ratios (HRs) estimated from Cox models were reported as relative risks with corresponding 95% confidence intervals (CIs). The assumption of proportional hazards was checked by log-minus-log plots and residual analysis using Schoenfeld plots. Multicollinearity was assessed using the variation inflation factor (> 4) and tolerance value (< 0.25). Univariate binary logistic regression analysis was used to assess 1-year complication rate. Odds Ratios (OR) estimated from logistic regression were reported with corresponding 95% confidence intervals (95% CI). Nagelkerke’s Pseudo-R2 is given for each model as a measure of goodness-of-fit. A two-tailed P-value of less than .05 was considered as statistically significant. All statistical tests were performed using spss version 23.0 (spss Inc., Chicago, IL, USA) and MedCalc software (version 17.8, medcalc Software, Ostend, Belgium).
3. Results
3.1. Basic clinical and biochemical data
Between July 2012 and September 2013, in total 101 consecutive cirrhotic patients and 31 healthy controls were included in the study. 72 patients were Child-Pugh class A, 25 Child-Pugh class B and 4 Child-Pugh class C. Child-Pugh classes B and C were analysed together. During a median follow-up of 37 months (range 1-50), 21 patients (20.8%) died and nine patients (8.9%) received a liver transplantation. Patients were censored at the time of liver transplantation. For characteristics of patients and controls, see Table 1.
Table 1. Patients’ characteristics and routine laboratory measurements for the cirrhosis and control group.
| Parameter | Cirrhosis group (n = 101) | Control group (n = 31) |
|---|---|---|
| Age (y) | 57 (51; 63) | 58 (47; 64) |
| Sex (M/F) | 73/28 | 22/9 |
| Aetiology (Alc/HCV/others) | 54/21/26 | — |
| Child-Pugh class (A/B/C) | 72/25/4 | — |
| Child-Pugh score | 5 (5; 7) | — |
| MELD | 11 (8; 14) | — |
| ALT (U/L) | 36 (23; 51) a | 23 (19; 30) |
| AST (U/L) | 45 (35; 67)b | 25 (22; 29) |
| Crea (mg/dL) | 0.8 (0.7; 0.9) | 0.9 (0.8; 1.0) |
| Alb (g/dL) | 4.1 (3.5; 4.5)b | 4.5 (4.4; 4.8) |
| Bili (mg/dL) | 1.3 (0.7; 2.2) | 0.5 (0.4; 0.7) |
| INR | 1.25 (1.13; 1.40) b | 1.02 (0.99; 1.05) |
Data are given in median (Q1; Q3).
Alc, alcoholic cirrhosis; HCV, hepatitis C virus-associated cirrhosis; MELD, model for end-stage liver disease; ALT, alanine aminotransferase; AST, aspartate transaminase; Crea, creatinine; Alb, albumin; Bili, total bilirubin; INR, prothrombin time international normalised ratio.
Denotes a significant difference of P < .01.
Denotes a significant difference of P < .001.
3.2. sCD163 and sMR levels correlate with liver disease severity
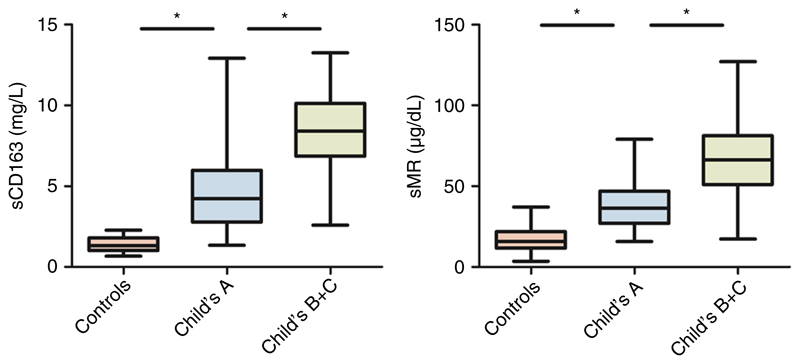
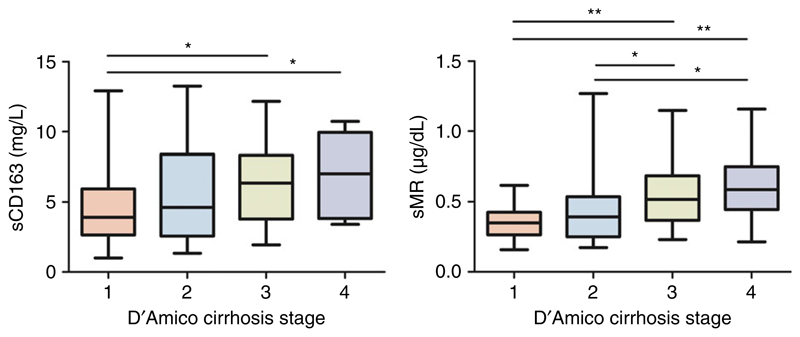
The median sCD163 and sMR levels were significantly higher in cirrhotic patients compared to healthy controls and gradually increased with disease severity (Figure 1, Table S1 and Supplementary Figure 1, published online). Furthermore, both markers sCD163 and sMR were significantly higher in patients with ascites at baseline (ascites/no ascites = 7.95/4.43 mg/L and 61/39 μg/dL, respectively) and showed a stepwise increase in relation to D’Amico’s clinical stages of cirrhosis (see Figure 2).
Figure 1.
sCD163 and sMR levels in cirrhotic patients classified according to disease severity and controls. (*) Denotes a significant difference of P < .001; Controls: n = 31; Child-Pugh class A: n = 72; Child-Pugh classes B+C: n = 29
Figure 2.
sCD163 and sMR levels in cirrhotic patients classified according to D’Amico’s clinical stages of cirrhosis. Kruskal-Wallis test showed significant differences in both groups (sCD163 P = .026; sMR P < .001). (*) Denotes a significant difference of P < .05; (**) Denotes a significant difference of P < .01; D’Amico 1 (no varices, no ascites): n = 28; D’Amico 2 (varices, no ascites): n = 26; D’Amico 3 (ascites, no variceal bleeding): n = 36; D’Amico 4 (variceal bleeding): n = 11
3.3. Markers of gut permeability and immune activation and correlations with sCD163 and sMR
Markers of gut permeability DAO and lactulose-mannitol ratio were higher in cirrhotic patients and gradually increased with disease severity. Endotoxin levels were higher in cirrhotic patients compared to controls; Lipopolysaccharide-binding protein was only slightly elevated in cirrhosis. Markers of systemic immune activation interleukin-6 and interleukin-8 gradually increased with disease severity, whereas levels of sCD14 were higher in cirrhotic patients compared to controls but did not change with disease severity (for further detail, see Supplementary Figure 2).
We observed an association between sCD163 and sMR (ρ = 0.864, P < .001). Furthermore, both markers correlated in various strengths with markers of gut permeability and immune activation (Table 2).
Table 2. Correlations between sCD163 and sMR and markers of gut permeability and immune activation.
| Correlations | Spearman’s rho | P value | |
|---|---|---|---|
| sCD163 | Endotoxinb | 0.476 | <.001 |
| Interleukin-8b | 0.761 | <.001 | |
| L/M ratiob | 0.444 | <.001 | |
| Diamine oxidaseb | 0.363 | <.001 | |
| LBP | 0.131 | .107 | |
| Interleukin-6a | 0.221 | .011 | |
| sCD14a | 0.200 | .022 | |
| sMR | Endotoxinb | 0.443 | <.001 |
| Interleukin-8b | 0.643 | <.001 | |
| L/M ratiob | 0.416 | <.001 | |
| Diamine oxidaseb | 0.359 | <.001 | |
| LBPa | 0.160 | .049 | |
| Interleukin-6a | 0.201 | .021 | |
| sCD14a | 0.282 | .001 | |
L/M ratio, lactulose-mannitol ratio; LBP, lipopolysaccharide-binding protein; sMR, soluble mannose receptor.
Denotes a significant difference of P < .05.
Denotes a significant difference of P < .001.
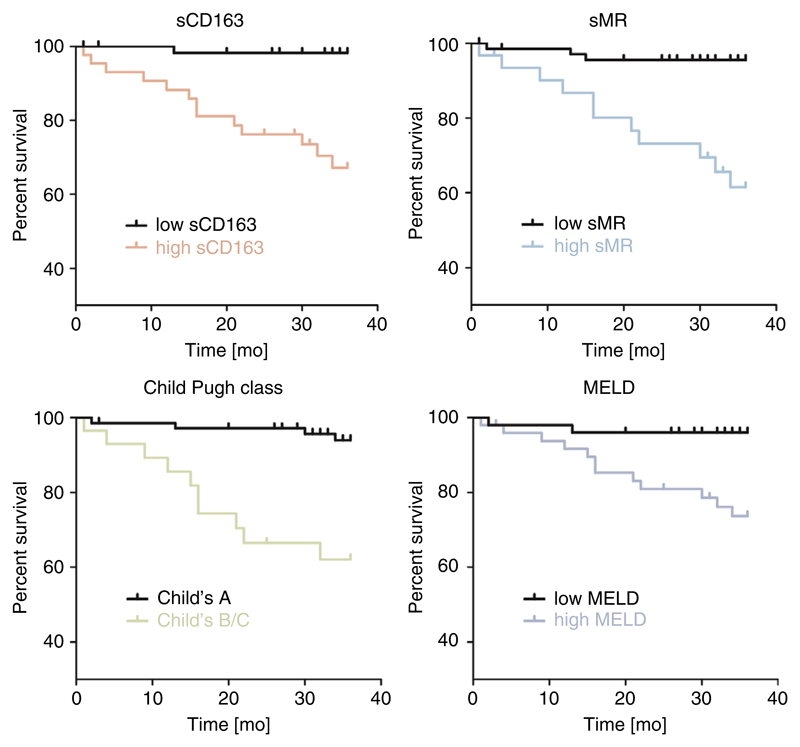
3.4. sCD163 and sMR levels predict mortality
Patients with high sCD163 or high sMR levels had significantly worse survival. Survival curves for macrophage activation biomarkers and standard prognostic models are depicted in Figure 3. According to Youden index, the optimal cut-off for survival prediction for sCD163 was 5.9 mg/L with a sensitivity of 76.2% and specificity of 65.0%, and for sMR 45.5 μg/dL with a sensitivity of 71.4% and a specificity of 65.0%. The cut-off value for MELD-score group classification was 13.6 (sensitivity 52.4%, specificity 80%).
Figure 3.
Kaplan-Meier curves of survival probability for low versus high for sCD163, sMR, Child-Pugh class and MELD. Log-rank test showed significant differences in survival curves in all parameters (P < .001); sCD163 low/high: n = 57/44; sMR low/high: n = 58/43; Child-Pugh class A/Child-Pugh classes B+C: n = 72/29; MELD low/high: n = 73/28
AUROCs for diagnostic accuracy of sCD163 and sMR were comparable to AUROCs of composite scores Child-Pugh and MELD (AUROC [95% CI] sCD163: 0.75 [0.64-0.86], sMR: 0.72 [0.61-0.83], Child-Pugh score 0.70 [0.57-0.84], MELD 0.67 [0.52-0.81]). There were no significant differences in the prognostic value of the different parameters when comparing AUROCs using methodology of Hanley & McNeil24,25 (Table S2, published online).
Univariate Cox-regression analysis identified sCD163, sMR, Child-Pugh score, MELD, lactulose-mannitol ratio, interleukin-8 and DAO, but not age, gender, aetiology, interleukin-6 (showing a trend), lipopolysaccharide-binding protein, endotoxin or sCD14 as predictors of mortality (results are shown in Table S3, published online). Multiple multivariate Cox regression analyses including alternate variables of macrophage activation (sCD163, sMR), prognostic models (Child-Pugh score, MELD), intestinal permeability (lactulose-mannitol ratio, diamine oxidase) and systemic immune activation (interleukin-6, interleukin-8) were performed (Table S4, published online). sCD163 & DAO remained in the final model for survival prediction (Table 3).
Table 3. Prognostic factors regarding mortality in 101 cirrhotic patients.
| Variable | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| sCD163 | 1.26 | 1.05-1.50 | .013 |
| DAO | 1.03 | 1.01-1.06 | .023 |
Multivariate Cox proportional hazards analysis of sCD163 and DAO. 95% CI, 95% Confidence Interval.
A variance inflation factor of 1.04 and tolerance of 0.96 showed no multicollinearity between the two variables.
3.5. sCD163 and sMR predict occurrence of complications of cirrhosis within 12 months
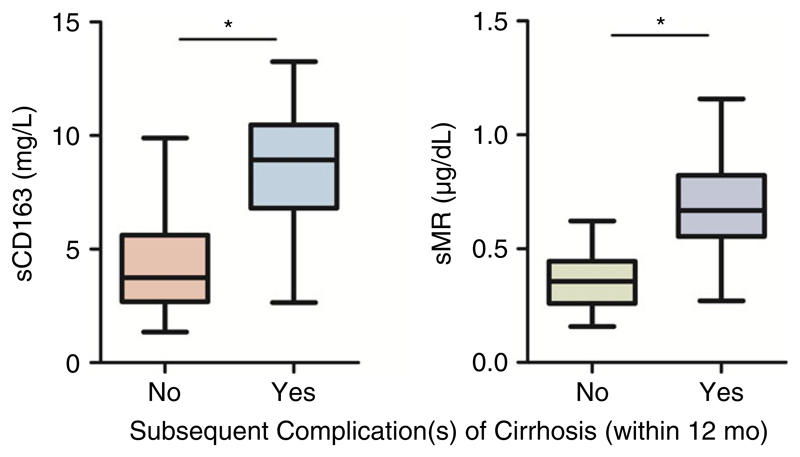
A total of 94 patients were included for analysis of predictive value of macrophage activation markers regarding occurrence of complications of cirrhosis within 12 months. Seven patients had to be excluded due to insufficient follow-up time (five patients withdrew consent, one patient died within 1 year (non liver-related) and one patient received a liver transplantation within 1 year). Overall, complications occurred in 28 of 94 patients (30%); usually more than one complication in one patient was recorded. Complications of cirrhosis were as follows: development of ascites (17 patients, 18%), development of hepatic encephalopathy (11 patients, 12%), development of jaundice (8 patients, 9%), development of spontaneous bacterial peritonitis (four patients, 4%), upper gastrointestinal bleeding due to portal hypertension (three patients, 3%), occurrence of portal vein thrombosis (2 patients, 2%), death from liver disease (two patients, 2%). Plasma levels of sCD163 and sMR were significantly higher in patients with subsequent complications than in those without (Figure 4). This was also observed when analysing subsequent development of ascites, hepatic encephalopathy or jaundice separately (Table S5). Markers of macrophage activation were not higher in patients developing infections requiring antibiotic therapy within 12 months (Table S5).
Figure 4.
Levels of sCD163 and sMR in cirrhotic patients with and without subsequent complications. (*) Denotes a significant difference of P < .001; Complication no/yes: n = 66/28
Univariate logistic regression analysis revealed sCD163, sMR, Child-Pugh score, MELD, diamine oxidase and aetiology (Table 4), but not age, gender, lactulose-mannitol ratio, interleukin-6, interleukin-8, lipopolysaccharide-binding protein, endotoxin or sCD14 as predictors of 1-year complication rate (Table S6, published online). Best Nagelkerke’s R square as goodness-of-fit measure was obtained using sMR.
Table 4. Prognostic factors regarding 1-year complication rate in 94 cirrhotic patients.
| Variable | Hazard ratio | 95% CI |
P value |
R2 | % correctly predicted |
|---|---|---|---|---|---|
| sCD163 | 1.67 | 1.36-2.06 | <.001 | 0.45 | 83.0 |
| sMR | 1.12 | 1.06-1.16 | <.001 | 0.56 | 84.0 |
| Child-pugh score | 3.47 | 2.07-5.83 | <.001 | 0.48 | 86.2 |
| MELD | 1.35 | 1.17-1.56 | <.001 | 0.30 | 78.7 |
| Diamine oxidase | 1.06 | 1.01-1-10 | .012 | 0.13 | 77.2 |
| Aetiology | 0.16 | 72.8 | |||
| alcohol | 3.51 | 1.02-13.39 | .047 | ||
| hepatitis C | 8.63 | 1.86-40.01 | .006 |
Univariate logistic regression analysis of clinical and biochemical factors associated with occurrence of complications; sMR, soluble mannose receptor.
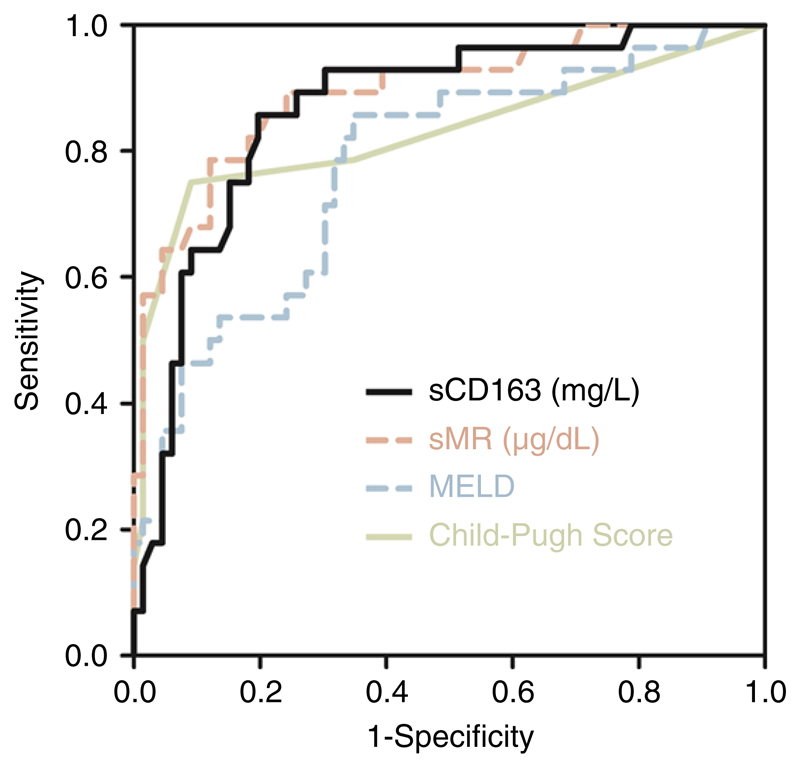
AUROC for diagnostic accuracy of sMR for 1-year complication rate was significantly higher compared to AUROC of MELD (P = .034). Predictive value of CPS was comparable to sCD163 and sMR (AUROC [95% CI] sCD163: 0.87 [0.78-0.93], sMR: 0.89 [0.81-0.95], Child-Pugh score 0.83 [0.74-0.90], MELD 0.78 [0.68-0.86]). AUROCs for 1-year complication rate are depicted in Figure 5.
Figure 5.
Receiver operating characteristic (ROC) curves of sCD163, sMR, model of end-stage liver disease (MELD) and Child-Pugh score as predictors of 1-year complication rate
4. Discussion
In this study, we investigated the prognostic value of macrophage activation markers sCD163 and sMR in a cohort of well-characterised, predominantly compensated cirrhotic patients over a median follow-up of 37 months. Patients with sCD163 levels above 5.9 mg/L or sMR levels above 45.5 μg/dL showed significantly reduced survival and developed significantly more complications of cirrhosis within 12 months. We identified sCD163 as an independent predictor of long-term survival and sMR as a predictor for occurrence of cirrhosis-associated complications. Furthermore, both variables correlated with markers of gut permeability and immune activation pointing towards important associations between disruption of gut barrier, macrophage activation and inflammation.
Liver macrophages represent >80% of the total macrophage population26 and play a key role in the development and progression of liver inflammation and fibrosis.5,6 Elevated plasma concentrations of sCD163 and sMR reflect activation and proliferation of hepatic macrophages11,15 and correlate well with disease severity and portal hypertension in patients with liver disorders9–12 In our study, a gradual increase in both values was observed in cirrhotic patients that correlated with increasing Child-Pugh score. sCD163 and sMR levels were significantly higher in patients with ascites at baseline and gradually increased in relation to D’Amico’s clinical stages of cirrhosis that mainly reflect severity of portal hypertension.22 This supports the hypothesis of macrophage involvement in portal hypertension, probably via secretion of vasoactive substances and activation of stellate cells.27 Progression of fibrosis and portal hypertension is inevitably associated with occurrence of complications of cirrhosis. Hence, recent studies have proven sCD163 to be a predictor of disease progression12 and variceal bleeding in cirrhosis.16 Both parameters have additionally been shown to predict survival in cirrhotic patients in different studies.9,12,16 The longest follow-up was reported in the study of Waidmann et al16 who observed patients for 7 months in median. In our study, patients were prospectively observed for a median follow-up of 37 months. Both sCD163 and sMR showed similar prognostic value regarding long-term survival compared to established composite scores (Child-Pugh-Score, MELD). In a multivariate Cox-regression analysis for survival prediction, only sCD163 and diamine oxidase, a marker of gut permeability, remained in the final model. Regarding occurrence of complications within 12 months, sMR was identified as an independent predictor. In a recent study, Laursen et al28 assessed levels of sMR in patients scheduled for TIPSS implantation and found that sMR levels were elevated in all patients, but especially in patients with acute variceal bleeding indicating sMR as a marker for cirrhosis complications. Actually, the mannose receptor is expressed not only on macrophages, but also on subsets of dendritic and especially endothelial cells29 that might be activated in patients with portal hypertension. Therefore, sMR might find its role as a slightly different biomarker compared to sCD163, possibly for prediction of complications and marker of portal hypertension.
In patients with liver cirrhosis, macrophages seem to be constitutively activated, since in our patients, levels of sCD163 and sMR were significantly higher in patients compared to healthy controls. Activation is suspected to be maintained by gut-derived PAMPs like endotoxins15,30–32 that are especially detectable in portal blood of cirrhotic patients33 and therefore, are suspected to enter the body via a disrupted gut barrier.34 Our data emphasises this hypothesis, since markers of macrophage activation correlated well with several markers of gut permeability, bacterial translocation and systemic inflammation. Both macrophage activation parameters correlated well with the lactulose-mannitol ratio and diamine oxidase (both markers of gut permeability), endotoxin (a marker of bacterial translocation) and interleukin-8 (a marker of systemic inflammation and an obligatory part in the LPS signaling cascade). Associations between interleukin-6 and macrophage activation parameters were not as strong as with interleukin-8. Although interleukin-6 can induce the expression of the membrane bound proteins, CD163 and MR, it is not involved in their shedding in response to inflammatory stimuli.35–37 sCD14 can bind endotoxin and present it to cells which do not express the membrane bound form.38 The correlations between sCD163/sMR and sCD14 were rather weak in our cohort although correlations have been found before. This might be due to the low serum concentrations of sCD14 that were comparable to healthy controls within this cohort, probably due to the inclusion of patients with stable disease. Since sCD14 can bind to endotoxin and then to a cell, increased endotoxin levels might lead to a decreased bioavailability of sCD14 in serum, making simple numeric correlation hard to interpret. However, experimental studies are needed to better define the causal relationship between gut barrier dysfunction and macrophage activation.
Our study has some limitations: Our study was performed as a single-centre study; however, we managed to recruit a relatively large number of patients with different aetiologies and observed them for a median follow-up of 37 months. Since overall mortality was low with only 21 deaths in our cohort, further analysis in terms of model development was not possible.
Taken together, the overexpression of macrophage activation markers, sCD163 and sMR, and its relation to gut barrier defects in cirrhotic patients reflect the detrimental impact of bacterial translocation on liver function. Both markers have shown predictive value regarding long-term survival. In addition, sMR was identified as a promising predictor of cirrhosis-associated complications. Our data also suggest macrophages as a possible therapeutic target to interrupt inflammation and fibrosis progression in patients with chronic liver disease.
Supplementary Material
Additional Supporting Information will be found online in the supporting information tab for this article.
Acknowlegdements
Declaration of personal interests: None.
Declaration of funding interests: This study was mainly funded by the Austrian Science Fund (FWF): P 24362. The nuclear magnetic resonance analysis for the sugar absorption test was partly funded by Institut Allergosan, Graz, Austria via the FFG basic program (Project 854419/6497141). AH was supported by the Austrian Science Fund (FWF): P24362. AH was supported by the Medical University Graz through the PhD Program Molecular Fundamentals of Inflammation (DK-MOLIN). HG and HJM received funding from The Danish Strategic Research Council (TRAIN 10-092797), HG received additional funding from the NOVO Nordisk Foundation and “Savværksejer Jeppe Juhl og hustru Ovita Juhls mindelegat.”
Funding information
Austrian Science Fund, Grant/Award Number: P 24362; Österreichische Forschungsförderungsgesellschaft, Grant/Award Number: 854419/6497141; Danish Strategic Research Council, Grant/Award Number: TRAIN 10-092797; Institut Allergosan; Savværksejer Jeppe Juhl og hustru Ovita Juhls mindelegat; PhD Program Molecular Fundamentals of Inflammation (DK-MOLIN); Novo Nordisk
Footnotes
Authorship
Guarantor of the article: Dr. Florian Rainer.
Author contributions: FR, acquisition of data, analysis & interpretation of data, drafting of the manuscript, guarantor of article; AH, acquisition of data, analysis and interpretation of data, drafting of the manuscript; TDS, acquisition of data; BL, acquisition of data, administrative and technical support; BS, acquisition of data, administrative and technical support; AB, acquisition of data; AGS, critical revision of the manuscript for important intellectual content; RES, acquisition of data; PF, critical revision of the manuscript for important intellectual content; PS, acquisition of data, obtained funding; HJM, acquisition of data; HG, acquisition of data; VS, study concept and design, interpretation of data, drafting of the manuscript, obtained funding. All authors approved the final version of the article, including the authorship list.
ORCID
F. Rainer: http://orcid.org/0000-0002-8339-8821
References
- 1.Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiest R, Lawson M, Geuking M, et al. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 4.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boltjes A, Movita D, Boonstra A, Woltman AM. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J Hepatol. 2014;61:660–671. doi: 10.1016/j.jhep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dultz G, Gerber L, Farnik H, et al. Soluble CD163 is an indicator of liver inflammation and fibrosis in patients chronically infected with the hepatitis B virus. J Viral Hepat. 2015;22:427–432. doi: 10.1111/jvh.12309. [DOI] [PubMed] [Google Scholar]
- 8.Andersen ES, Rødgaard-Hansen S, Moessner B, Christensen PB, Møller HJ, Weis N. Macrophage-related serum biomarkers soluble CD163 (sCD163) and soluble mannose receptor (sMR) to differentiate mild liver fibrosis from cirrhosis in patients with chronic hepatitis C: a pilot study. Eur J Clin Microbiol Infect Dis. 2014;33:117–122. doi: 10.1007/s10096-013-1936-3. [DOI] [PubMed] [Google Scholar]
- 9.Grønbæk H, Rødgaard-Hansen S, Aagaard NK, et al. Macrophage activation markers predict mortality in patients with liver cirrhosis without or with acute-on-chronic liver failure (ACLF) J Hepatol. 2016;64:813–822. doi: 10.1016/j.jhep.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Grønbaek H, Sandahl TD, Mortensen C, Vilstrup H, Møller HJ, Møller S. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Aliment Pharmacol Ther. 2012;36:173–180. doi: 10.1111/j.1365-2036.2012.05134.x. [DOI] [PubMed] [Google Scholar]
- 11.Holland-Fischer P, Gronbaek H, Sandahl TD, et al. Kupffer cells are activated in cirrhotic portal hypertension and not normalised by TIPS. Gut. 2011;60:1389–1393. doi: 10.1136/gut.2010.234542. [DOI] [PubMed] [Google Scholar]
- 12.Rode A, Nicoll A, Møller HJ, et al. Hepatic macrophage activation predicts clinical decompensation in chronic liver disease. Gut. 2013;62:1231–1232. doi: 10.1136/gutjnl-2012-304135. [DOI] [PubMed] [Google Scholar]
- 13.Kazankov K, Barrera F, Møller HJ, et al. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology. 2014;60:521–530. doi: 10.1002/hep.27129. [DOI] [PubMed] [Google Scholar]
- 14.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 15.Sandahl TD, Grønbæk H, Møller HJ, et al. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: a prospective cohort study. Am J Gastroenterol. 2014;109:1749–1756. doi: 10.1038/ajg.2014.262. [DOI] [PubMed] [Google Scholar]
- 16.Waidmann O, Brunner F, Herrmann E, Zeuzem S, Piiper A, Kronenberger B. Macrophage activation is a prognostic parameter for variceal bleeding and overall survival in patients with liver cirrhosis. J Hepatol. 2013;58:956–961. doi: 10.1016/j.jhep.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Waidmann O, Köberle V, Bettinger D, et al. Diagnostic and prognostic significance of cell death and macrophage activation markers in patients with hepatocellular carcinoma. J Hepatol. 2013;59:769–779. doi: 10.1016/j.jhep.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Rødgaard-Hansen S, Rafique A, Christensen PA, et al. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clin Chem Lab Med. 2014;52:453–461. doi: 10.1515/cclm-2013-0451. [DOI] [PubMed] [Google Scholar]
- 19.Child CG, Turcotte JG. The Liver and Portal Hypertension. Philadelphia: Saunders; 1964. Surgery and portal hypertension; pp. 50–64. [Google Scholar]
- 20.Kamath P, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 21.Horvath A, Leber B, Schmerboeck B, et al. Randomised clinical trial: the effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment Pharmacol Ther. 2016;44:926–935. doi: 10.1111/apt.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Møller HJ, Hald K, Moestrup SK. Characterization of an enzyme-linked immunosorbent assay for soluble CD163. Scand J Clin Lab Invest. 2002;62:293–299. doi: 10.1080/003655102760145852. [DOI] [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 25.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 26.Ishibashi H, Nakamura M, Komori A, Migita K, Shimoda S. Liver architecture, cell function, and disease. Semin Immunopathol. 2009;31:399–409. doi: 10.1007/s00281-009-0155-6. [DOI] [PubMed] [Google Scholar]
- 27.Cavaillon JM. Cytokines and macrophages. Biomed Pharmacother. 1994;48:445–453. doi: 10.1016/0753-3322(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 28.Laursen TL, Rødgaard-Hansen S, Møller HJ, et al. The soluble mannose receptor is released from the liver in cirrhotic patients, but is not associated with bacterial translocation. Liver Int. 2016 doi: 10.1111/liv.13262. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 30.Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22:S53–S56. doi: 10.1111/j.1440-1746.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- 31.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 32.Hume DA, Underhill DM, Sweet MJ, Ozinsky AO, Liew FY, Aderem A. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001;2:11. doi: 10.1186/1471-2172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Llorente C, Hartmann P, Yang A-M, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods. 2015;421:44–53. doi: 10.1016/j.jim.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cirera I, Bauer TM, Navasa M, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–37. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 35.Ritter M, Buechler C, Langmann T, Orso E, Klucken J, Schmitz G. The scavenger receptor CD163: regulation, promoter structure and genomic organization. Pathobiology. 1999;67:257–261. doi: 10.1159/000028105. [DOI] [PubMed] [Google Scholar]
- 36.Funding M, Vorum H, Nexø E, Moestrup SK, Ehlers N, Møller HJ. Soluble CD163 and interleukin-6 are increased in aqueous humour from patients with endothelial rejection of corneal grafts. Acta Ophthalmol Scand. 2005;83:234–9. doi: 10.1111/j.1600-0420.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 37.Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS ONE. 2014;9:e94188. doi: 10.1371/journal.pone.0094188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frey EA, Miller DS, Jahr TG, et al. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information will be found online in the supporting information tab for this article.