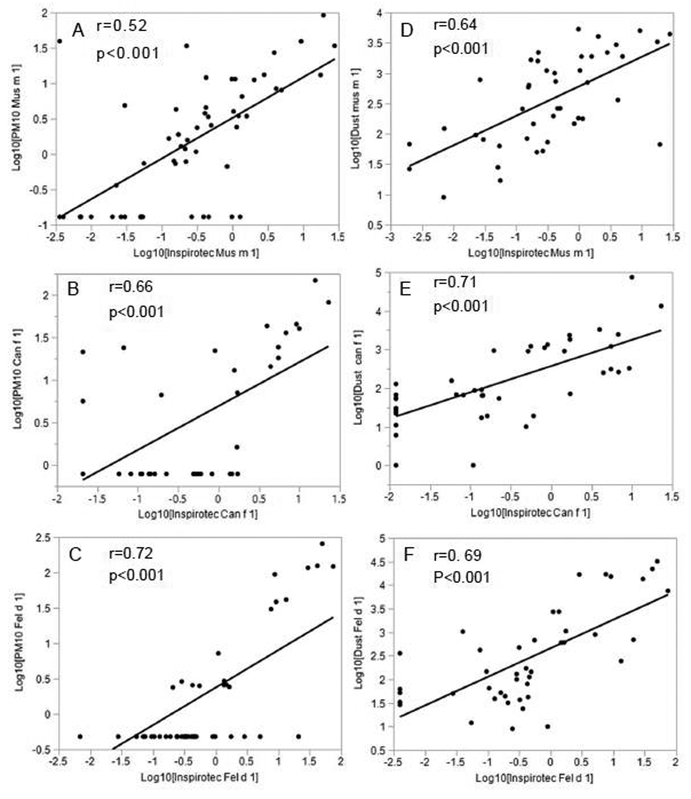
Allergens are distributed between the settled dust and airborne compartments. The airborne compartment represents inhalable allergen that penetrates deeply in the respiratory tract and may trigger asthma symptoms (1). Larger airborne particles may also trigger allergic rhinitis. Because methods of air sampling have been technically demanding and limited by assay sensitivity, dust collection has been a standard surrogate. Methods for measurement of airborne allergens were reviewed (1). More recent publications described potentially improved methods. Barnes et al (2, 3) used capture of allergens by air filters in homes that run over a period of months. We (4) introduced a simple plug-in device (Inspirotec Inc, Chicago, IL) for air sampling. It is silent, unobtrusive and requires no technical skill to operate.
Due to the relatively low concentration of allergens in the airborne compartment, their detection, especially of dust mite, has been problematic. The Inspirotec sampler has been optimized for sample capture by ionic propulsion (US patent numbers 8,038,944, 9,216,421, 9,360,402, 9,481,904 and 9,618,431). The combination of Inspirotec’s high air flow rate and highly sensitive MARIA® (Indoor Biotechnology, Charlottesville, VA) multiplex immunoassays facilitates the detection of a full spectrum of airborne allergens, including those previously non detectable. Hitherto, others have been able to find some significant correlations between allergen content of dust and air for mouse (5, 6), cat (7–10) and dog (7–9). Here, we wish to evaluate the performance of the Inspirotec sampler by establishing correlations with reference methods of air sampling and dust collection for multiple allergens. This was done as part of a larger on-going randomized clinical trial of home environmental intervention where multiple air and dust samples were collected in low income Baltimore homes. We showed significant correlations for Inspirotec sampling compared both with standard PM10 (particle sizes ≤10 μm) air filtration, and dust collection, for mouse, cat as well as dog allergens (Fig 1).
Fig 1. Bivariate analysis of Inspirotec airborne allergen capture compared with two reference methods.
Reference methods were collection by air sampling and dust sampling. Samples were from twenty-five urban, low income Baltimore homes at baseline, 17 at 3 months, and 12 at 6 months. Whether a home is in the active intervention group or the control group is still blinded. Air sampling by PM10 (particles > 10 μm selectively removed with an impinger) and dust collection were as described elsewhere (5). In parallel, Inspirotec samplers were plugged into wall sockets and run for the same period of 5– 7 days. Inspirotec samplers had pre-calibrated flow rates of 100±20 liters/min and were stable within ±10% over multiple usages. PM10 samplers were adjusted to 4 liters/min. Samples from Inspirotec electrodes and PM10 filters were extracted in 1 mL and assayed by Indoor Biotechnologies MARIA™ assays for the 12 common household allergens: Der p 1, Der f 1, mite group 2, Fel d 1, Can f 1, Mus m 1, Rat n1, Bla g 1, Alt a 1, Bet v 1, Asp f 1, Phl p 5. Dust samples were weighed, extracted by standard methods and assay by Indoor Biotech ELISA for Der f 1, Fel d 1, Can f 1, Mus m 1 and Bla g 2. Air sample results were expressed as pg/m3 and dust as μg/g. JMP® Pro 13.0.0 (SAS Institute Inc.Cary, NC) and StataSE 13.0 (StataCorp LLC, College Station, Texas) statistics packages were used. Allergen variables were log10-transformed and r values shown are Pearson’s rho (r) from StataSE. Because there was repeated sampling within the same home, r and p-values were generated using Generalized Estimating equations, which account for the within-home correlation. The best straight line fit to the log10 of the values are shown. Values below the LLOD were assigned a value of LLOD/2.
Only the three allergens in Fig 1 were in a sufficient number of positive samples to permit the bivariate analysis. Of the 12 common household allergens tested, all except birch pollen, Bet v 1, showed relatively more positive samples (higher sensitivity) with the Inspirotec sampler than the PM10 (e-Table 1). This is likely accounted for by the larger air volume sampled by the Inspirotec device during the time of running in the homes (100 liters/min compared with 5 liters/min). Results in e-Table 1 also show a higher proportion of samples positive for dust and negative for airborne samples for dustmite, dog and cockroach. This is consistent with preponderance of larger particles containing those allergens.
e-Table 1.
Percent detectable and undetectable with air sampling methods.
| Inspirotec | Positive | Negative | |
|---|---|---|---|
| PM10 | |||
| Total mite | Positive | 1.8 | 12.7 |
| Fel d 1 | Positive | 30.9 | 52.7 |
| Can f 1 | Positive | 29.8 | 35.1 |
| Mus m 1 | Positive | 67.3 | 27.3 |
| Rat n 1 | Positive | 1.8 | 9.1 |
| Bla g 2 | Positive | 1.8 | 23.6 |
| Alt a 1 | Positive | 0 | 5.5 |
| Bet v 1 | Positive | 7.3 | 9.1 |
| Asp f 1 | Positive | 1.8 | 18.2 |
| Phl p 5 | Positive | 0 | 16.4 |
| Dust | |||
| Total mite v. Der f 1 | Positive | 9.1 | 6.8 |
| Feld 1 | Positive | 95.3 | 0 |
| Can f 1 | Positive | 68.2 | 2.3 |
| Mus m 1 | Positive | 95.5 | 0 |
| Bla g 2 v. Bla g 1 | Positive | 18.2 | 9.1 |
Numbers are percent of samples that were above (positive) or below (negative) field LLODs. Assays were as described in legend to Fig 1. Total mite was the sum of Der f 1, Der f 2 and Mite Group 2 values.
The higher sensitivity of the Inspirotec sampler also appears when the airborne allergen values are characterized by percentile ranges, as shown in e-Table 2. Although allergens were more likely to be detectable with Inspirotec sampling, the concentrations of the allergens tended to be lower than those measured from PM10 sampling (e-Table 2). These differences in allergen concentrations likely reflects the lower capture efficiency of the Inspirotec sampler. A model system of fluorescent latex particles estimated capture efficiency to be 23%. Although theoretically, a correction factor could be been applied to account for capture efficiency, the capture efficiency of allergens would likely differ from that for latex particles, which have different particle characteristics.
e-Table 2.
Airborne Allergen Concentration by Percentile
| Percentile (pg/m3) | |||||
|---|---|---|---|---|---|
| Allergen | Sampler | 25 | 50 | 75 | 95 |
| Total mite | PM10 | 4.00 | |||
| Inspirotec | 0.29 | ||||
| Fel d 1 | PM10 | 2.51 | 118.96 | ||
| Inspirotec | 0.33 | 1.36 | 34.37 | ||
| Can f 1 | PM10 | 7.06 | 42.39 | ||
| Inspirotec | 0.14 | 1.57 | 9.54 | ||
| Mus m 1 | PM10 | 0.13 | 1.78 | 7.99 | 36.00 |
| Inspirotec | 0.05 | 0.31 | 1.22 | 12.67 | |
| Rat n 1 | PM10 | 1.74 | |||
| Inspirotec | 0.04 | ||||
| Bla g 2 | PM10 | 4.65 | |||
| Inspirotec | 0.06 | 0.55 | |||
| Alt a 1 | PM10 | ||||
| Inspirotec | 0.01 | ||||
| Bet v 1 | PM10 | 3.43 | 13.47 | ||
| Inspirotec | 0.42 | ||||
| Asp f 1 | PM10 | ||||
| Inspirotec | 0.34 | ||||
| Phl p 5 | PM10 | 10.78 | |||
| Inspirotec | 0.32 | ||||
Values are in pg/m3 of air. Values below LLOD were assigned a value of LLOD/2 for the purposes of this calculation of the percentiles. For clarity, the blanks are where the values were below the LLOD. Total mite was the sum of Der f 1, Der f 2 and Mite Group 2 values. Note that when the majority of samples are negative, there are only significant values in the highest percentile range. There were no positives for Bla g 2 and Bet v 1 by PM10 sampling.
Although threshold concentrations considered clinically meaningful have limitations, physicians and other health care providers and patients need such information to guide decision-making. As a result, thresholds of allergens that are expected to be of clinical significance have been proposed. Salo et al (11) proposed 10 μ/g of dust for Can f 1 and 8 μg/g for Fel d 1 as cut-off points for “elevated levels”. Although there are limitations to extrapolating from one study, corresponding airborne levels can be calculated from the Generalized Estimating regression equations used to estimate relationships between Inspirotec and settled dust allergen concentrations. This calculation would lead to equivalent levels of 0.04 pg/m3 for both Can f 1 and for Fel d 1
In summary, the results obtained with the Inspirotec sampler show that it is more sensitive, but yields lower allergen concentrations than airborne allergen sampling with PM10. The Inspirotec sampler is compact, easily deployed and unobtrusive, so larger scale studies can be done without logistical problems that would be associated with more technically demanding and noisier alternative equipment. It is also a reasonable alternative to dust collection because the use of a vacuum cleaner in a controlled manner and vacuuming of defined areas is technically demanding, and airborne samples are closer approximation to the air inhaled. The results here, combined with the ease of deployment and unobtrusiveness of the Inspirotec samplers, show that direct measurement of allergens in the airborne fraction, which is most relevant for assessing exposure in allergic asthma or rhinitis, can be used in large scale studies involving multiple sites.
Acknowledgements.
We gratefully acknowledge the technical support of MS Andrea Wachter and coordination of samples and data by MS Michelle Newman. We also gratefully acknowledge the provision of the original pollen counts for the Baltimore area by Dr Jon Matz. This work was supported by grants U01 Al083238 and K24 AI114769https://projectreporter.nih.gov/project_info_details.cfm?aid=8803514&icde=27336897&ddparam=&ddvalue=&ddsub=&cr=2&csb=default&cs=ASCfrom the National Institute of Allergy and Infectious Diseases and R01 ES023447 and R01 ES026170 from the National Institute of Environmental Health Sciences.to Elizabeth Matsui, and by Inspirotech Inc.
Funding sources: grants U01Al083238 and K24 AI114769https://projectreporter.nih.gov/project_info_details.cfm?aid=8803514&icde=27336897&ddparam=&ddvalue=&ddsub=&cr=2&csb=default&cs=ASCfrom the National Institute of Allergy and Infectious Diseases and R01 ES023447 and R01 ES026170 from the National Institute of Environmental Health Sciences. to EM, and from Inspirotec Inc
Abbreviations:
- ELISA
Enzyme Linked Immunoassay
- MARIA®
Multiplex Array for Indoor Allergens
- LLOD
Lower Limit of Detection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: JG, RR and PG are employees of Inspirotec Inc and JG and PG are co-founders and stockholders.
References
- 1.Raulf M, Buters J, Chapman M, Cecchi L, et al. Monitoring of occupational and environmental aeroallergens - EAACI Position Paper Concerted action of the EAACI IG Occupational Allergy and Aerobiology & Air Pollution. Allergy. 2014. October;69(10):1280–99. [DOI] [PubMed] [Google Scholar]
- 2.Barnes CS, Allenbrand R, Mohammed M, et al. Measurement of aeroallergens from furnace filters. Annals of Allergy Asthma & Immunology. 2015. March;114(3):221–5. [DOI] [PubMed] [Google Scholar]
- 3.Allenbrand R, Barnes CS, Mohammed M, et al. Comparison of allergens collected from furnace filters and vacuum floor dust. Ann Allergy Asthma Immunol. 2017. January;118(1):108–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon J, Detjen P, Kelso D, Gandhi P. A new patient-operated sampling device for measurement of aeroallergens. Ann Allergy Asthma Immunol. 2016. May;116(5):475–6. [DOI] [PubMed] [Google Scholar]
- 5.Matsui EC, Simons E, Rand C, et al. Airborne mouse allergen in the homes of inner-city children with asthma. J Allergy Clin Immunol. 2005. February;115(2):358–63. [DOI] [PubMed] [Google Scholar]
- 6.Permaul P, Hoffman E, Fu CX, et al. Allergens in urban schools and homes of children with asthma. Pediatr Allergy Immunol. 2012. September;23(6):543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Custovic A, Green R, Fletcher A, et al. Aerodynamic properties of the major dog allergen Can f 1: Distribution in homes, concentration, and particle size of allergen in the air. American Journal of Respiratory and Critical Care Medicine. 1997. January;155(1):94–8. [DOI] [PubMed] [Google Scholar]
- 8.Custovic A, Simpson B, Simpson A, Hallam C, Craven M, Woodcock A. Relationship between mite, cat, and dog allergens in reservoir dust and ambient air. Allergy. 1999. June;54(6):612–6. [DOI] [PubMed] [Google Scholar]
- 9.Custovic A, Simpson A, Pahdi H, Green RM, Chapman MD, Woodcock A. Distribution, aerodynamic characteristics, and removal of the major cat allergen Fel d 1 in British homes. Thorax. 1998. January;53(1):33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raja S, Xu Y, Ferro AR, Jaques PA, Hopke PK. Resuspension of indoor aeroallergens and relationship to lung inflammation in asthmatic children. Environment International. 2010. January;36(1):8–14. [DOI] [PubMed] [Google Scholar]
- 11.Salo PM, Arbes SJ, Crockett PW, Thorne PS, Cohn RD, Zeldin DC. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol. 2008. March;121(3):678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]