Abstract
Brain oscillatory activity from the midline prefrontal region has been shown to reflect brain dysfunction in subjects with Major Depressive Disorder (MDD). It is not known, however, whether electrodes from this area provide unique information about brain function in MDD. We examined a set of midline sites and two other prefrontal locations for detecting cerebral activity differences between subjects with MDD and healthy controls. Resting awake quantitative EEG (qEEG) data were recorded from 168 subjects: 47 never-depressed adults and 121 with a current major depressive episode. Individual midline electrodes (Fpz, Fz, Cz, Pz, and Oz) and prefrontal electrodes outside the hairline (Fp1, Fp2) were examined with absolute and relative power and cordance in the theta band. We found that MDD subjects exhibited higher values of cordance (p=0.0066) at Fpz than controls; no significant differences were found at other locations, and power measures showed trend-level differences. Depressed adults showed higher midline cordance than did never-depressed subjects at the most-anterior midline channel. Salient abnormalities in MDD may be detectable by focusing on the prefrontal midline region, and EEG metrics from focused electrode arrays may offer clinical practicality for clinical monitoring.
Keywords: depression, prefrontal cortex, EEG, neurophysiology, clinical monitoring
1. INTRODUCTION
Functional neuroimaging studies of Major Depressive Disorder (MDD) frequently report findings of abnormal activity in frontal mood regulating networks. These networks include the dorsolateral prefrontal cortex (DLPFC), dorsomedial prefrontal cortex (DMPFC), orbitofrontal cortex (OFC), and the anterior cingulate cortex (ACC), among other regions. Using positron emission tomography (PET), Buchsbaum and colleagues (1997), Mayberg and co-workers (1999), and other groups have differences in metabolism in between healthy control subjects and those with MDD in many of these areas. Sheline and colleagues (2010) have proposed that the frontopolar region constitutes a “dorsal nexus” where the function of many of these areas is linked and may differentiate between MDD and control subjects.
Quantitative electroencephalographic (qEEG) studies in MDD have employed arrays of scalp electrodes with varying densities to provide information on brain oscillatory activity that is characteristic of this disorder. Leuchter and colleagues (2012) have shown that widespread oscillatory synchrony between the frontopolar region and other brain areas distinguishes those with MDD from healthy controls. No study, however, has specifically focused solely on the oscillatory activity recorded from frontopolar electrodes to determine whether this may be uniquely useful for characterizing brain function in subjects with MDD. Differences have been reported in ACC (Holmes and Pizzagalli 2008; Korb et al., 2008; Mayberg et al., 1997; Mulert et al., 2002; Mulert et al., 2007a; Narushima et al., 2010; Pizzagalli et al., 2001; Pizzagalli et al., 2003; Poulsen et al., 2009; Saletu et al., 2010; Schrijvers et al., 2008; Schrijvers et al., 2009) as well as in broad frontal (Allen and Kline 2004; Bruder et al., 1997; Davidson 2004; Davidson and Irwin 1999) and prefrontal regions (Bares et al., 2007; Bares et al., 2008; Cook et al., 1998a; Cook et al., 2002; Cook et al., 2005), using surface measures. qEEG studies of MDD have generally employed full-head electrode montages, sampling activity from up to 256 (e.g., Plante et al., 2012) electrode locations.
While high-density electrode arrays contribute to our knowledge of brain function in MDD, some previous investigations of the relationships between prefrontal EEG signals and activity in deeper structures have reported that qEEG data recorded from midline anterior electrodes provides special information about frontal network function, particularly with regard to theta band activity (Asada et al., 1999; Ishii et al., 1999; Poulsen et al., 2009). Our group has reported (Korb et al., 2009; Korb et al., 2011) that consideration of midline prefrontal electrodes may be critical for characterizing ACC activity with the EEG technique “low resolution brain electromagnetic tomography” (LORETA) (Pascual-Marqui et al., 1994), suggesting that the midline prefrontal region may be a key location for observations related to the neurophysiology of depression.
The objective of our present study was to investigate whether various midline prefrontal electrodes provided unique information about differences in regional brain activity between depressed and non-depressed adults, regardless of the specific anatomic structure(s) responsible for a difference. We examined individual midline electrode locations, overlying the anterior, central, and posterior divisions of the cingulate gyrus, and those over adjacent prefrontal areas, and hypothesized that data from electrodes overlying some portions of the frontal lobe would be sensitive to brain dysfunction in MDD, while electrodes over other regions would not to detect these differences.
2. METHODS AND MATERIALS
2.1. Participants
2.1.1. Subjects with Depression
Depressed subjects were 121 adult outpatients diagnosed with unipolar MDD who had been recruited as subjects for antidepressant treatment trials in our laboratory. In accordance with principles of the Helsinki Declaration (as amended, 1975 – 2008), all protocols had been reviewed and approved by the UCLA Institutional Review Board (IRB), and written informed consent to participate in this research was obtained from all subjects. For the purposes of the present project, we examined each subject’s medication-free baseline EEG. Subjects were free of psychotropic medication for at least 2 weeks prior to enrollment (4 weeks for fluoxetine); the duration of this period reflected a balance between the scientific desirability of an extended washout period and the ethical considerations of imposing a treatment-free period on ill patients. Diagnoses were determined by trained, expert raters using a structured interview for DSM-IV, and inclusion criteria included intake scores ≥16 on the 17-item Hamilton Depression Rating Scale (HAM-D17) (Hamilton 1960). Exclusionary criteria included current dementia; delirium; bipolar disorder; substance-related or eating disorder; cluster A or B Axis II diagnoses; active suicidal plan or intent; the presence of any poorly controlled medical illness that could affect brain function (e.g., untreated hypothyroidism); concurrent use of medications that could interfere with EEG activity (e.g., benzodiazepines); ECT within the prior 6 months; or any history of head trauma, brain surgery, or skull defect. Recruitment mechanisms as well as inclusion and exclusion criteria were comparable for these protocols, and subjects showed no significant differences among trials with regard to age, gender balance, or depression severity. Reports of the treatment trial subjects and the healthy controls have previously appeared in the literature (e.g., Cook et al., 2002, 2009, Hunter et al 2006, 2010, 2013; Leuchter et al., 2002, 2008, 2012; Korb et al., 2008, 2009, 2011); none have focused on the questions addressed in this report.
2.1.2. Never-depressed Control Subjects
Healthy control subjects were 47 adults without current or past history of depression who had given informed consent to enroll in a study in our laboratory of the effects of antidepressant medication on healthy subjects (Leuchter et al., 2008) or in cognitive activation studies. Subjects underwent a structured clinical examination to confirm the absence of any history of mood, anxiety, psychotic, or cognitive illness or of substance abuse or dependence disorders. The control subjects did not differ significantly from the depressed group on age (CON: 37.9 (12.9) vs MDD: 40.6 (12.9); t166 = −1.24 p=0.22) or in gender balance (23M:24F vs 46M:75F; Chi square 1.47 df=1 p=0.23).
2.2. EEG Methods
2.2.1. Data Acquisition
Using procedures employed in our previous reports and summarized here, recordings were made with the QND System (Neurodata, Inc.; Pasadena, CA) or the NuAmps System (NeuroScan, Inc.; El Paso, Tx), calibrated to ensure equivalence across systems. Resting EEG was recorded in subjects while they lay with eyes closed in a quiet room. Subjects were instructed to remain still and inhibit blinks or eye movements during each recording period. Technicians monitored EEG throughout the recording and re-alerted subjects every 30–45 seconds as necessary to prevent drowsiness. Scalp electrodes were placed using an electrode cap (ElectroCap, Inc.; Eaton, OH, USA) using a 35-channel enhanced version the International 10–20 System of Electrode Placement, with additional electrodes located over prefrontal and parietooccipital regions. Electrode impedances were balanced and under 5kΩ for all channels. To control for ocular artifact, vertical and horizontal electro-oculograms (EOG) were recorded using bipolar electrodes placed at the supraorbital and infraorbital ridge of the right eye and the outer canthi of the left and right eye, respectively.
A minimum of 10 minutes of EEG data were recorded using a Pz referenced montage. Signals were digitized using a sampling rate of 256 Hz, a low-frequency filter of 0.3 Hz, a high-frequency filter of 70 Hz, as well as a notch filter at 60 Hz. Digital data were then imported into Brain Vision Analyzer (BVA) software (Brain Products GmbH; Gilching, Germany) in order to remove offsets, optimize scaling, re-reference the data, and segment the data into 2-second non-overlapping epochs. Using the BVA artifact rejection module, segments were removed according to standard thresholds likely to represent artifact based upon voltage step gradient (i.e., 100 μV), absolute values of difference within the epoch, or persistent low activity for greater than 100 msec. A semiautomated interactive process was then used to remove all epochs containing eye movement, muscle, or movement-related artifacts, or amplifier drift. Two technicians then independently inspected the data using multiple bipolar and referential montages, and isolated and removed any remaining data segments suspected of containing artifacts.
2.2.2. Calculation of Spectral Power and Cordance Measures
Absolute and relative power values were calculated with a linked-ears reference using BVA and theta band values (4.0–8.0 Hz) were exported for data analysis.
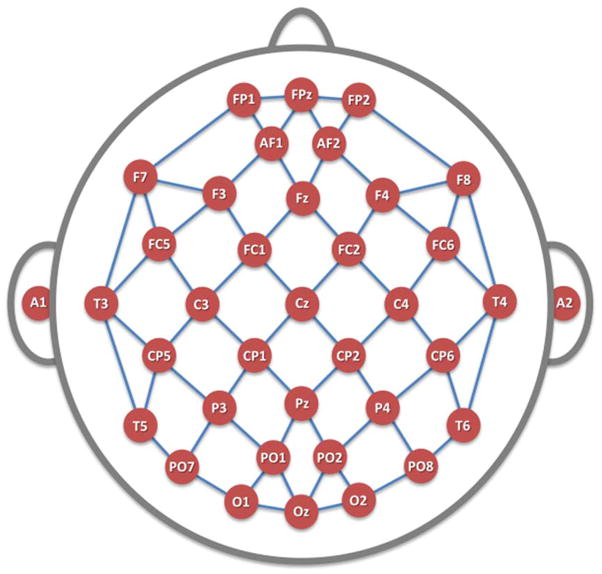
Cordance values were calculated using an algorithm that has been detailed elsewhere (Leuchter et al., 1999) and may be summarized as follows. Cordance is computed by a normalization and integration of absolute and relative power values from all electrode sites for a given EEG recording; cordance values are calculated in three steps. First, EEG power values are computed using a re-attributional electrode montage (figure 1) in which power values from pairs of electrodes that share a common electrode are averaged together to yield the re-attributed power (Cook et al., 1998b). This approach is similar to the single source method of Hjorth (1975) in which voltage signals are recombined, but the re-attributional montage approach has been shown to provide a higher association with regional cortical perfusion than the Hjorth method (Cook et al., 1998). These absolute power values are used to derive relative power (percentage of power in each frequency band) for each electrode.
FIGURE 1. Electrode Montage.
35 scalp channels and two ear leads, placed in accordance with the modified combinatorial nomenclature of the International 10–20 System. Line segments denote bipolar electrode pairs used in cordance calculations. Midline surface electrodes overlie midline cortical structures from the rostral-most anterior cingulate cortex (Fpz) to the caudal posterior cingulate region (Oz).
Second, these re-attributed absolute and relative power values for each individual EEG recording are normalized across electrode sites, using a z-transformation statistic for each electrode site s in each frequency band f (yielding Anorm(s,f) and Rnorm(s,f), respectively). This normalization process places absolute and relative power values into a common unit (standard deviation or z-score units) which allows them to be combined. It is worthwhile to note that these z-scores are based on the average power values in each band for all electrodes within a given QEEG recording (i.e., these are not z-scores referenced to some normative population).
Third, the cordance values are formed by summing the z-scores for normalized absolute and relative power ( Z(s,f) = Anorm(s,f) + Rnorm(s,f) ), for each electrode site and in each frequency band. Cordance values have been shown to have higher correlations with regional cerebral blood flow than absolute or relative power alone (Leuchter et al., 1999), and thus this combination measure can be placed in context with prior work in depression that have employed functional measures of brain activity such as PET scan data. As with absolute and relative power measures, we focused on theta band cordance, as activity in that band has been associated with the presence of MDD and with treatment response (Bares et al., 2007; Bares et al., 2008; Broadway et al., 2012; Cook et al., 2002; Cook et al., 2005; Cook et al., 2009; Cook and Leuchter 2001; Hunter et al., 2012; Knott et al., 1996; Leuchter 1997; Leuchter et al., 2009a; Leuchter et al., 2009b; Mulert et al., 2007b; Pizzagalli et al., 2001; Ulrich et al., 1988a; Ulrich et. al 1988b; Ulrich et. al 1994).
In this study, we first directed our attention to the five individual midline electrodes placed in accordance with the extended International 10–20 montage locations, in prefrontal (Fpz), frontal (Fz), central (Cz), parietal (Pz), and occipital (Oz) regions (Sharbrough et al., 1991). These electrodes overlie the cingulate cortex, moving from anterior regions (e.g., Brodmann Areas (BA) 32, 24 for Fpz) to more posterior locations (e.g., BA 23, 31 for Oz). To examine two other locations of interest for practical clinical monitoring, we evaluated the prefrontal electrodes Fp1 and Fp2 that flank Fpz laterally, as these are comparison channels located outside the hairline, that overlie prefrontal but not midline structures.
2.3. Data Analysis
Analyses were performed using the SPSS version 21 software package (IBM Inc.; Armonk, NY). Data were analyzed with 2-tail t-tests, the chi-square statistic, and ANOVA/ANCOVA models. Within each EEG measure, we adjusted the significance threshold using the Bonferroni approach to correct for tests of seven electrode sites (i.e., .05/7 = .007). Effect sizes were computed with GPower (Erdfelder et al., 1996).
3. RESULTS
3.1. Overall Physiologic Differences
As an omnibus test for the presence of differences between groups in physiologic measures, we performance a multiple analysis of variance (MANOVA), examining all EEG measures (absolute power at 7 locations, relative power and 7 locations, and cordance at 7 locations) as dependent variables, and diagnostic group as a fixed factor. The overall test was significant (Wilk’s Lambda 0.791, F 1.84, p=0.02 η2=0.21).
3.2. Absolute and Relative Power Group Differences
In comparing depressed and control subject groups, no significant differences were found in absolute or relative power for any of the five midline electrodes or the two lateral prefrontal locations, after correcting for multiple comparisons (Table 1). Some trend-level associations were observed for absolute power but not for relative power.
TABLE 1. Group Differences in Power and Cordance.
Mean (s.d.) absolute and relative power and cordance values at all locations are shown comparing CON and MDD subjects (with t-statistics and uncorrected 2-tail p-values). Only at the Fpz location did cordance differ between controls and all depressed subjects after a Bonferroni correction was applied; no significant differences were found at other electrode sites in any of the three measures.
| ABSOLUTE POWER | |||||||
|---|---|---|---|---|---|---|---|
| Fpz | Fz | Cz | Pz | Oz | Fp1 | Fp2 | |
| CON | 19.88 (13.82) | 29.51 (19.41) | 35.26 (24.32) | 36.35 (27.16) | 27.74 (21.07) | 20.28 (13.90) | 20.47 (14.17) |
| MDD | 24.95 (22.51) | 38.66 (32.66) | 46.90 (42.90) | 49.68 (52.50) | 40.07 (42.20) | 27.54 (24.96) | 27.77 (25.01) |
| t | −1.76; df 135a | −2.23; df 139a | −2.21; df 144a | −2.15; df 153a | −2.51; df 156a | −2.38; df 146a | −2.38; df 144a |
| p | 0.080 | 0.027 | 0.029 | 0.033 | 0.013 | 0.018 | 0.019 |
| RELATIVE POWER | |||||||
|---|---|---|---|---|---|---|---|
| Fpz | Fz | Cz | Pz | Oz | Fp1 | Fp2 | |
| CON | 17.29 (6.54) | 19.35 (6.66) | 18.10 (6.74) | 15.88 (6.65) | 14.70 (6.22) | 17.20 (6.28) | 17.14 (6.49) |
| MDD | 18.23 (8.74) | 20.58 (8.60) | 19.60 (8.50) | 17.22 (8.28) | 15.86 (7.92) | 18.37 (8.51) | 18.37 (8.52) |
| t | −0.66; df 166 | −0.88; df 166 | −1.09; df 166 | −0.99; df 166 | −0.90; df 166 | −0.86; df 166 | −0.89; df 166 |
| p | 0.508 | 0.382 | 0.277 | 0.322 | 0.371 | 0.394 | 0.374 |
| CORDANCE | |||||||
|---|---|---|---|---|---|---|---|
| Fpz | Fz | Cz | Pz | Oz | Fp1 | Fp2 | |
| CON | −1.46 (1.44) | 0.14 (1.43) | 0.61 (1.18) | −1.19 (0.85) | −0.96 (1.55) | −0.88 (1.27) | −1.07 (1.35) |
| MDD | −0.78 (1.45) | 0.11 (1.30) | 0.21 (1.35) | −1.32 (1.06) | −1.02 (1.41) | −0.95 (1.29) | −1.02 (1.37) |
| t | −2.75; df 166 | 0.14; df 166 | 1.78; df 166 | 0.75; df 166 | 0.25; df 166 | 0.33; df 166 | −0.25; df 166 |
| p | 0.0066 * | 0.888 | 0.077 | 0.456 | 0.806 | 0.739 | 0.804 |
t-test for equality of means performed for unequal variances based on significant Levene’s test finding
3.3. Cordance Group Differences
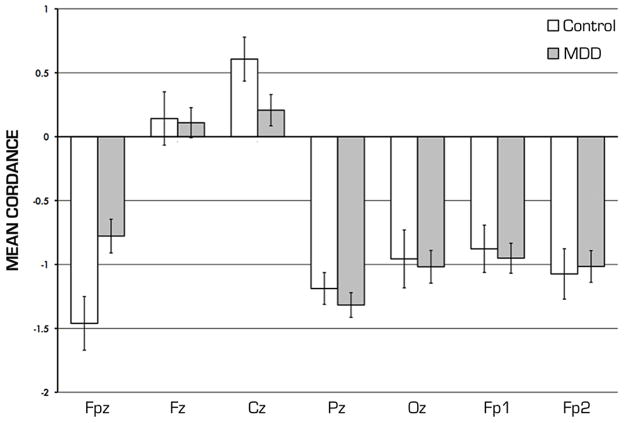
Cordance values at the Fpz electrode were significantly higher in the depressed subjects (mean −0.78 (1.45 sd)) than in the control group (mean −1.46 (1.44 sd), t166 = 2.748, 2-tail p=0.0067, effect size d 0.47). No significant differences were found at the other midline or prefrontal locations after correction for multiple tests (Table 1, Figure 2). While not statistically significant, there were numerical trends for imbalances in age and gender between MDD and CON group, so we also performed ANCOVA to examine differences between MDD and CON groups while controlling for these two factors. In this analysis, we found that the main effect on Fpz cordance of diagnostic group was significant in this model (F=7.21, p=0.008), while neither age nor gender were significant covariates.
FIGURE 2. Midline Cordance Values.
Group average cordance values for midline electrodes (Fpz through Oz) and forehead prefrontal electrodes (Fp1, Fp2) differ significantly between MDD and CON subjects only at Fpz location after Bonferroni correction.
3.4. Depression Severity
The relationships between symptom severity (HAM-D17 score) and physiologic measures were evaluated within the 121 subjects with MDD with regression analyses. Depression severity was not related to the value of cordance, absolute power, or relative power at any of the electrode sites (e.g., the strongest correlation was in cordance at the Pz location, with r=0.14 p=0.74).
4. DISCUSSION
The central finding in this study is that depressed individuals differed from healthy control subjects in qEEG values measured at the prefrontal midline location (Fpz) overlying the far-anterior midline structures, and did not differ at remaining midline sites or at more lateral prefrontal sites. Importantly, this suggests that clinically salient features may be detectable using observations focusing on a very limited number of electrode sites. These findings indicate that brain oscillatory activity, measured at the frontal pole in the midline, may uniquely reflect features that are highly characteristic of individuals experiencing an episode of MDD.
These findings are consistent with recent work on brain connectivity, which suggest that the far prefrontal region constitutes a “nexus” (Sheline et al., 2010) or a “hub node” of functional connectivity (Leuchter et al., 2012) that differentiates individuals with depression from healthy controls. The mood regulating network in the frontopolar region consists of a number of structures that demonstrate abnormal activity in subjects with MDD, including the DLPFC, DMPFC, ACC, and OFC. While rhythmic oscillatory activity from the frontal midline has been correlated with activity in the ACC (Asada et al., 1999; Gevins et al., 1997; Iramina et al., 1996; Ishii et al., 1999; Sauseng et al., 2007; Tsujimoto et al., 2006), it is possible if not likely that many of the structures in this network contribute to the rhythmic oscillations recorded from the Fpz electrode. Other studies have also reported differences between depressed subjects and healthy controls, in frontal activity in all these structures (Bench (1992), Drevets (1992), Ito (1996), Brody (2001), Kennedy (2001), Kimbrell (2002), Fitzgerald (2008)).
It is noteworthy that in our resting state EEGs, the control subjects had significantly lower cordance values than the depressed subjects. This would be consistent with low levels of activity in this region when healthy individuals are observed in the resting state. The ACC has been tied to attentional and emotional processing, and low activity levels have been observed in the resting state in the healthy, well-regulated brain (Posner et al., 2007), so the cognitive dysfunction in depression could reflect impairment of that regulation in MDD (Porter et al., 2007; Zakzanis et al., 1998). Resting state activity has been reported as lower in controls than in MDD subjects in portions of the PFC broadly (Drevets 1992; Brody 2001), consistent with our finding, though some others have reported the reverse pattern (cf Rigucci et al., 2010).
These differences in prefrontal midline function also may be particularly relevant to interpreting and designing studies of depression using EEG, and to reconciling past discrepancies in the literature. While our data suggest that recording from the Fpz location appears to be particularly important for detecting differences between MDD and control subjects, the classic 19- or 21-channel versions of the International 10–20 system montage do not incorporate this location (Bocker et al., 1994; Jasper 1958; Nuwer 1987; Sharbrough et al., 1991). In contrast, later extensions of the 10–20 system do routinely include a midline recording location at the frontal pole (Chatrian et al., 1998; Jurcak et al., 2007). Discrepant prior reports using EEG to study depression could reflect inconsistent inclusion or omission of this recording site, as recent work has examined the impact of the presence or absence of Fpz data (Korb et al., 2009).
While MDD and control groups exhibited a clear difference in activity at Fpz, within the MDD group there was no relationship between Fpz activity and depression severity (HAM-D17 score), suggesting that these deviations from healthy patterns of brain activity reflect something other than simple symptom burden. It is possible that Fpz activity reflects the presence of MDD or of trait vulnerability to development of MDD, rather than marking a point along a continuum between states of illness and health. This distinction might be clarified by a cross-sectional examination of other individuals with MDD of lesser severity, or with “minor” or “subsyndromal” depression diagnoses, or longitudinal comparisons of MDD subjects when ill vs in remission, or of healthy subjects transiently experiencing depressed mood (e.g., from immersion in an affective task); this might also aid in interpreting the numerical value of Fpz cordance in this context. Alternatively, the brain dysfunction detected at Fpz could reflect an aspect of being ill with MDD that is not reflected in that rating scale score. Although the HAM-D17 scale is a widely-employed measure, its limitations in comprehensively capturing aspects of MDD have been acknowledged (Bagby et al., 2004; Gibbons et al., 1993; Trivedi et al., 2009). Other work from our lab (Hunter et al., 2013) found that measures of rACC activity varied over the course of a depressive episode, and that the level measured closest in time to the start of antidepressant treatment was the better predictor of clinical response, independent of HAM-D17 score. Future work might examine relationships between Fpz activity and other metrics that characterize MDD, such as neurocognitive performance or functional abilities (e.g., in the home and workplace, or in relationships).
Because our clinical subjects all met diagnostic criteria for unipolar major depression, our study also cannot evaluate the specificity of this finding to depression in that illness (e.g., in contrast with the depressed phase of bipolar disorder). Structural and functional abnormalities have been reported in the midline structure in bipolar disorder (Fountoulakis et al., 2008). Importantly, however, research evidence also points to ACC involvement in psychotic depression (McCormick et al., 2009), dementia (Assal and Cummings 2002), schizophrenia (Baiano et al., 2007), social phobia (Ahs et al., 2009; Rauch et al., 1995), pain syndromes (Peyron et al., 2000), borderline personality disorder (Minzenberg et al., 2008), autism (Delmonte et al., 2013; Hall et al., in press) and other disorders, possibly reflecting the role of the this region in mental functions that are disrupted across a variety of illnesses (van Veen and Carter 2002; van Veen and Carter 2006). As such, we believe it would not be appropriate to consider qEEG measurements at Fpz as a substitute for conventional clinical diagnosis. Future projects may determine whether Fpz-detected differences are disorder-specific or reflect a common substrate for multiple neuropsychiatric illnesses, perhaps by including subjects with a CNS disorder where the midline frontal structures are not believed to be as central to the manifestations of the illness (e.g., Parkinson’s disease). Another concern is that we were able to include data from more MDD subjects than from healthy control individuals. Future work should strive to include a larger sample of healthy adults. A technical consideration is the possibility of misattributing cerebral activity to residual artifact, either from subtle eye movements or from muscle activity in the face. We have employed a well-established set of procedures to eliminate these and other types of artifact (Cook et al., 1998a, 1998b, 2001, 2002, 2005, 2009; Hunter et al, 2010, 2013, 2006; Korb et al., 2008, 2009, 2011; Leuchter et al., 1999, 2002, 2008, 2012). Indeed, facial muscle and/or eye movement artifact would be expected to be found at Fpz and also at the adjacent electrodes, so the localization of the significant differences to the Fpz location alone argues against contamination and in favor of this finding reflecting a true neurophysiologic aspect of depression.
Some limitations of our findings relate to our subject pool’s characteristics. Because our subjects were all adults, it is unknown whether these differences would be found in depressed children and adolescents. Individuals with co-morbid substance use disorders were excluded from this project, yet represent a large subset of those seeking care for depression, and the applicability of these findings in that population are unknown. An additional limitation of the present report is that only midline and prefrontal sites were considered in our hypothesis, but the standard cordance calculation algorithm incorporates a spatial normalization step requiring data from electrodes across the head. While the midline, far prefrontal Fpz location may offer a key answer to the question of “where to look” for salient physiologic differences, it appears that some different metric, computed using signals from a limited set of locations, will be needed to offer a more practical way to address the matter of “how to look.” Future extensions of this work could examine new approaches to spatial normalization from a more limited set of locations, particularly those outside the hairline and easily accessible for electrode placement, or altogether different measures derived from these channels.
Similarly, this examination focused on theta band activity, because prior work had led us to hypothesize that theta activity may reveal physiologic differences between groups. Other bands could also exhibit differences, or slowing of the posterior dominant rhythm from the alpha range to the theta range could also have occurred. These questions can be addressed in future research. Nonetheless, our findings suggest a useful approach to studying clinically-relevant issues in depression using qEEG, and future extensions may seek to examine a wider frequency range.
Previous qEEG studies have indicated that early and pre-treatment physiologic biomarkers may predict response and/or remission from antidepressant treatment (Bares et al., 2007; Bares et al., 2008; Cook et al., 2002; Cook et al., 2005; Cook and Leuchter 2001; Juckel et al., 2007; Mulert et al., 2007b; Pizzagalli et al., 2001). Most of these studies have utilized a “full head” recording montage. To the extent that both MDD and treatment response affect aspects of frontal activity, a greatly reduced montage focusing on the midline prefrontal regions may be able to provide much of the information necessary to monitor salient aspects of brain function related to treatment outcome in MDD. Indeed, the recent BRITE-MD trial (“Biomarkers for Rapid Identification of Treatment Effectiveness in Major Depression,” NCT00289523) employed a focused array (Fpz, FT9, FT10, A1, A2 electrodes) and found that EEG features at baseline and emerging in the first week of treatment were significantly predictive of later clinical outcome (Leuchter et al., 2009a; Leuchter et. al 2009b). The qEEG biomarker used in the BRITE-MD trial also may predict the likelihood and speed of achieving a sustained remission (Cook et al., 2013), so the use of practical neurophysiologic monitoring in MDD may soon be justifiable as an evidence-based aspect of practice. For physiologic monitoring to be integrated into routine clinical practice, however, a streamlined electrode array would be central to a practical recording and monitoring system. Future studies should focus on the unique information on brain function that may be recorded from the Fpz site and other electrode locations that may reflect activity in salient brain regions.
Acknowledgments
ROLE OF THE FUNDING SOURCE
This work was supported by grants R01 MH069217 and R01 AT003479 from the National Institute of Mental Health, and by grants from Eli Lilly and Company, Wyeth-Ayerst Laboratories, and Aspect Medical Systems. These entities had no influence over the study design; in the collection, analysis, and interpretation of data; in the writing of this report; and in the decision to submit this paper for publication.
Footnotes
CONTRIBUTORS
Dr. Cook developed the hypothesis tested in this work and the approach, and wrote the initial draft of the manuscript. Drs. Cook and Leuchter designed and executed the underlying clinical studies, assessed the research subjects clinically, and reviewed EEG recordings for reliability, and contributed to the revisions of the paper. Drs. Cook, Hunter, and Korb performed the statistical analyses, and contributed to the conceptualization of the findings presented in this report. Drs. Cook, Hunter, Korb, and Leuchter and all contributed substantively to the final manuscript and approved it.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahs F, Sollers JJ, 3rd, Furmark T, Fredrikson M, Thayer JF. High-frequency heart rate variability and cortico-striatal activity in men and women with social phobia. NeuroImage. 2009;47:815–820. doi: 10.1016/j.neuroimage.2009.05.091. [DOI] [PubMed] [Google Scholar]
- Allen JJ, Kline JP. Frontal EEG asymmetry, emotion, and psychopathology: the first, and the next 25 years. Biological Psychology. 2004;67:1–5. doi: 10.1016/j.biopsycho.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Asada H, Fukuda Y, Tsunoda S, Yamaguchi M, Tonoike M. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neuroscience Letters. 1999;274:29–32. doi: 10.1016/s0304-3940(99)00679-5. [DOI] [PubMed] [Google Scholar]
- Assal F, Cummings JL. Neuropsychiatric symptoms in the dementias. Current Opinion in Neurology. 2002;15:445–450. doi: 10.1097/00019052-200208000-00007. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? American Journal of Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophrenia Research. 2007;93:1–12. doi: 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bares M, Brunovsky M, Kopecek M, Novak T, Stopkova P, Kozeny J, Sos P, Krajca V, Höschl C. Early reduction in prefrontal theta QEEG cordance value predicts response to venlafaxine treatment in patients with resistant depressive disorder. European Psychiatry. 2008;23:350–355. doi: 10.1016/j.eurpsy.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Bares M, Brunovsky M, Kopecek M, Stopkova P, Novak T, Kozeny J, Höschl C. Changes in QEEG prefrontal cordance as a predictor of response to antidepressants in patients with treatment resistant depressive disorder: a pilot study. Journal of Psychiatric Research. 2007;41:319–325. doi: 10.1016/j.jpsychires.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia--focal abnormalities of cerebral blood flow in major depression. Psychological Medicine. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Bocker KB, van Avermaete JA, van den Berg-Lenssen MM. The international 10–20 system revisited: cartesian and spherical co-ordinates. Brain Topography. 1994;6:231–235. doi: 10.1007/BF01187714. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Phelps ME, Huang SC, Wu HM, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR., Jr Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Archives of General Psychiatry. 2001;58:631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, McGrath PJ, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biological Psychiatry. 1997;41:939–48. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, Reynolds C. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biological Psychiatry. 1997;41:15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Modified nomenclature for the “10%” electrode system. Journal of Clinical Neurophysiology. 1998;5:183–186. [PubMed] [Google Scholar]
- Cook IA, Hunter AM, Abrams M, Siegman B, Leuchter AF. Midline and right frontal brain function as a physiologic biomarker of remission in major depression. Psychiatry Research. 2009;174:152–7. doi: 10.1016/j.pscychresns.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook IA, Hunter AM, Gilmer WS, Iosifescu DV, Zisook S, Burgoyne KS, Howland RH, Trivedi MH, Jain R, Greenwald S, Leuchter AF. Quantitative electroencephalogram biomarkers for predicting likelihood and speed of achieving sustained remission in major depression: a report from the biomarkers for rapid identification of treatment effectiveness in major depression (BRITE-MD) trial. Journal of Clinical Psychiatry. 2013;74:51–56. doi: 10.4088/JCP.10m06813. [DOI] [PubMed] [Google Scholar]
- Cook IA, Leuchter AF. Prefrontal changes and treatment response prediction in depression. Seminars in Clinical Neuropsychiatry. 2001;6:113–120. doi: 10.1053/scnp.2001.21844. [DOI] [PubMed] [Google Scholar]
- Cook IA, Leuchter AF, Morgan ML, Stubbeman W, Siegman B, Abrams M. Changes in prefrontal activity characterize clinical response in SSRI nonresponders: a pilot study. Journal of Psychiatric Research. 2005;39:461–466. doi: 10.1016/j.jpsychires.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Cook IA, Leuchter AF, Morgan M, Witte E, Stubbeman WF, Abrams M, Rosenberg S, Uijtdehaage SH. Early changes in prefrontal activity characterize clinical responders to antidepressants. Neuropsychopharmacology. 2002;27:120–131. doi: 10.1016/S0893-133X(02)00294-4. [DOI] [PubMed] [Google Scholar]
- Cook IA, Leuchter AF, Uijtdehaage SH, Osato S, Holschneider DH, Abrams M, Rosenberg-Thompson S. Altered cerebral energy utilization in late life depression. Journal of Affective Disorders. 1998a;49:89–99. doi: 10.1016/s0165-0327(97)00192-4. [DOI] [PubMed] [Google Scholar]
- Cook IA, O’Hara R, Uijtdehaage SH, Mandelkern M, Leuchter AF. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalography and Clinical Neurophysiology. 1998b;107:408–414. doi: 10.1016/s0013-4694(98)00092-3. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology. 2006;115:174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biological Psychology. 2004;67:219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Science. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Delmonte S, Gallagher L, O’Hanlon E, McGrath J, Balsters JH. Functional and structural connectivity of frontostriatal circuitry in Autism Spectrum Disorder. Front Hum Neurosci. 2013;7:430. doi: 10.3389/fnhum.2013.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. Journal of Neuroscience. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPower: A general power analysis program. Behavior Research Methods, Instruments, & Computers. 1996;28:1–11. [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Human Brain Mapping. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Giannakopoulos P, Kovari E, Bouras C. Assessing the role of cingulate cortex in bipolar disorder: Neuropathological, structural and functional imaging data. Brain Research Reviews. 2008;59:9–21. doi: 10.1016/j.brainresrev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cerebral Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Clark DC, Kupfer DJ. Exactly what does the Hamilton Depression Rating Scale measure? Journal of Psychiatric Research. 1993;27:259–273. doi: 10.1016/0022-3956(93)90037-3. [DOI] [PubMed] [Google Scholar]
- Hall SS, Jiang H, Reiss AL, Greicius MD. Identifying Large-Scale Brain Networks in Fragile X Syndrome. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.247. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth B. An on-line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalography and Clinical Neurophysiology. 1975;39:526–530. doi: 10.1016/0013-4694(75)90056-5. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AM, Cook IA, Leuchter AF. Impact of antidepressant treatment history on clinical outcomes in placebo and medication treatment of major depression. Journal of Clinical Psychopharmacology. 2010;30:748–51. doi: 10.1097/JCP.0b013e3181faa474. [DOI] [PubMed] [Google Scholar]
- Hunter AM, Korb AS, Cook IA, Leuchter AF. Rostral anterior cingulate activity in major depressive disorder: state or trait marker of responsiveness to medication? Journal of Neuropsychiatry and Clinical Neuroscience. 2013;25:126–33. doi: 10.1176/appi.neuropsych.11110330. [DOI] [PubMed] [Google Scholar]
- Hunter AM, Leuchter AF, Morgan ML, Cook IA. Changes in brain function (quantitative EEG cordance) during placebo lead-in and treatment outcomes in clinical trials for major depression. American Journal of Psychiatry. 2006;163:1426–32. doi: 10.1176/ajp.2006.163.8.1426. [DOI] [PubMed] [Google Scholar]
- Iramina K, Ueno S, Matsuoka S. MEG and EEG topography of frontal midline theta rhythm and source localization. Brain Topography. 1996;8:329–331. doi: 10.1007/BF01184793. [DOI] [PubMed] [Google Scholar]
- Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, Hirabuki N, Asada H, Kihara T, Robinson SE, Takeda M. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999;10:675–679. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- Ito H, Kawashima R, Awata S, Ono S, Sato K, Goto R, Koyama M, Sato M, Fukuda H. Hypoperfusion in the limbic system and prefrontal cortex in depression: SPECT with anatomic standardization technique. Journal of Nuclear Medicine. 1996;37:410–414. [PubMed] [Google Scholar]
- Jasper H. The 10–20 electrode system of the international federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Juckel G, Pogarell O, Augustin H, Mulert C, Müller-Siecheneder F, Frodl T, Mavrogiorgou P, Hegerl U. Differential prediction of first clinical response to serotonergic and noradrenergic antidepressants using the loudness dependence of auditory evoked potentials in patients with major depressive disorder. Journal of Clinical Psychiatry. 2007;68:1206–1212. doi: 10.4088/jcp.v68n0806. [DOI] [PubMed] [Google Scholar]
- Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. NeuroImage. 2007;34:1600–1611. doi: 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Krüger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, Willis MW, Herscovitch P, Post RM. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biological Psychiatry. 2002;51:237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Telner JI, Lapierre YD, Browne M, Horn ER. Quantitative EEG in the prediction of antidepressant response to imipramine. Journal of Affective Disorders. 1996;39:175–84. doi: 10.1016/0165-0327(96)00003-1. [DOI] [PubMed] [Google Scholar]
- Korb AS, Cook IA, Hunter AM, Leuchter AF. Brain electrical source differences between depressed subjects and healthy controls. Brain Topography. 2008;21:138–146. doi: 10.1007/s10548-008-0070-5. [DOI] [PubMed] [Google Scholar]
- Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. Clinical Neurophysiology. 2009;120:1313–1319. doi: 10.1016/j.clinph.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex activity and early symptom improvement during treatment for major depressive disorder. Psychiatry Res. 2011;192:188–94. doi: 10.1016/j.pscychresns.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, DeBrota DJ, Hunter AM, Potter WZ, McGrouther CC, Morgan ML, Abrams M, Siegman B. Changes in brain function during administration of venlafaxine or placebo to normal subjects. Clinical EEG and Neuroscience. 2008;39:175–181. doi: 10.1177/155005940803900405. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Gilmer WS, Burgoyne KS, Howland RH, Trivedi MH, Zisook S, Jain R, Fava M, Iosifescu D, Greenwald S. Effectiveness of a Quantitative Electroencephalographic Biomarker for Predicting Differential Response or Remission with Escitalopram and Bupropion in Major Depressive Disorder. Psychiatry Research. 2009a;169:132–138. doi: 10.1016/j.psychres.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Hunter AM, Cai C, Horvath S. Resting-state quantitative electroencephalography reveals increased neurophysiologic connectivity in depression. PLoS One. 2012;7:e32508. doi: 10.1371/journal.pone.0032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Marangell LB, Gilmer WS, Burgoyne KS, Howland RH, Trivedi MH, Zisook S, Jain R, McCracken JT, Fava M, Iosifescu D, Greenwald S. Comparative effectiveness of biomarkers and clinical indicators for predicting outcomes of SSRI treatment in Major Depressive Disorder: results of the BRITE-MD study. Psychiatry Research. 2009b;169(2):124–131. doi: 10.1016/j.psychres.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Witte EA, Morgan M, Abrams M. Changes in brain function of depressed subjects during treatment with placebo. American Journal of Psychiatry. 2002;159:122–9. doi: 10.1176/appi.ajp.159.1.122. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Uijtdehaage SH, Cook IA, O’Hara R, Mandelkern M. Relationship between brain electrical activity and cortical perfusion in normal subjects. Psychiatry Research. 1999;90:125–140. doi: 10.1016/s0925-4927(99)00006-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McCormick LM, Yamada T, Yeh M, Brumm MC, Thatcher RW. Antipsychotic effect of electroconvulsive therapy is related to normalization of subgenual cingulate theta activity in psychotic depression. Journal of Psychiatric Research. 2009;43:553–560. doi: 10.1016/j.jpsychires.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Frontolimbic structural changes in borderline personality disorder. Journal of Psychiatric Research. 2008;42:727–733. doi: 10.1016/j.jpsychires.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Juckel G, Augustin H, Hegerl U. Comparison between the analysis of the loudness dependency of the auditory N1/P2 component with LORETA and dipole source analysis in the prediction of treatment response to the selective serotonin reuptake inhibitor citalopram in major depression. Clinical Neurophysiology. 2002;113:1566–1572. doi: 10.1016/s1388-2457(02)00252-3. [DOI] [PubMed] [Google Scholar]
- Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, Möller HJ, Hegerl U, Pogarell O. Rostral anterior cingulate cortex activity in the theta band predicts response to antidepressive medication. Clinical EEG and Neuroscience. 2007a;38:78–81. doi: 10.1177/155005940703800209. [DOI] [PubMed] [Google Scholar]
- Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, Möller HJ, Hegerl U, Pogarell O. Prediction of treatment response in major depression: integration of concepts. Journal of Affective Disorders. 2007b;98:215–225. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Narushima K, McCormick LM, Yamada T, Thatcher RW, Robinson RG. Subgenual cingulate theta activity predicts treatment response of repetitive transcranial magnetic stimulation in participants with vascular depression. Journal of Neuropsychiatry and Clinical Neuroscience. 2010;22:75–84. doi: 10.1176/appi.neuropsych.22.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwer MR. Recording electrode site nomenclature. Journal of Clinical Neurophysiology. 1987;4:121–133. doi: 10.1097/00004691-198704000-00002. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. International Journal of Psychophysiology. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis Neurophysiologie clinique. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: an EEG/PET study of normal and depressed subjects. Psychophysiology. 2003;40:939–949. doi: 10.1111/1469-8986.00112. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. American Journal of Psychiatry. 2001;158:405–15. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Plante DT, Landsness EC, Peterson MJ, Goldstein MR, Wanger T, Guokas JJ, Tononi G, Benca RM. Altered slow wave activity in major depressive disorder with hypersomnia: a high density EEG pilot study. Psychiatry Research. 2012;201:240–244. doi: 10.1016/j.pscychresns.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RJ, Bourke C, Gallagher P. Neuropsychological impairment in major depression: its nature, origin and clinical significance. Australian and New Zealand Journal of Psychiatry. 2007;41:115–128. doi: 10.1080/00048670601109881. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cognitive, Affective & Behavioral Neuroscience. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Poulsen C, Luu P, Crane SM, Quiring J, Tucker DM. Frontolimbic activity and cognitive bias in major depression. Journal of Abnormal Psychology. 2009;118:494–506. doi: 10.1037/a0015920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Miguel EC, Baer L, Breiter HC, Fischman AJ, Manzo PA, Moretti C, Jenike MA. A positron emission tomographic study of simple phobic symptom provocation. Archives of General Psychiatry. 1995;52:20–28. doi: 10.1001/archpsyc.1995.03950130020003. [DOI] [PubMed] [Google Scholar]
- Rigucci S, Serafini G, Pompili M, Kotzalidis GD, Tatarelli R. Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. World Journal of Biological Psychiatry. 2010;11(2 Pt 2):165–80. doi: 10.1080/15622970903131571. [DOI] [PubMed] [Google Scholar]
- Saletu B, Anderer P, Saletu-Zyhlarz GM. EEG topography and tomography (LORETA) in diagnosis and pharmacotherapy of depression. Clinical EEG and Neuroscience. 2010;41:203–210. doi: 10.1177/155005941004100407. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Hoppe J, Klimesch W, Gerloff C, Hummel FC. Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. European Journal of Neuroscience. 2007;25:587–593. doi: 10.1111/j.1460-9568.2006.05286.x. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, De Bruijn ER, Maas Y, De Grave C, Sabbe BG, Hulstijn W. Action monitoring in major depressive disorder with psychomotor retardation. Cortex. 2008;44:569–579. doi: 10.1016/j.cortex.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, De Bruijn ER, Maas YJ, Vancoillie P, Hulstijn W, Sabbe BG. Action monitoring and depressive symptom reduction in major depressive disorder. International Journal of Psychophysiology. 2009;71(3):218–224. doi: 10.1016/j.ijpsycho.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Sharbrough FW, Chatrian GE, Lesser R, Luders H, Nuwer MR, Picton TW. American Electroencephalographic Society guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1991;8:200–202. [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11020–5. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Corey-Lisle PK, Guo Z, Lennox RD, Pikalov A, Kim E. Remission, response without remission, and nonresponse in major depressive disorder: impact on functioning. International Clinical Psychopharmacology. 2009;24:133–138. doi: 10.1097/YIC.0b013e3283277614. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. Journal of Neurophysiology. 2006;95:2987–3000. doi: 10.1152/jn.00730.2005. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology and Behavior. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Error detection, correction, and prevention in the brain: a brief review of data and theories. Clinical EEG and Neuroscience. 2006;37:330–335. doi: 10.1177/155005940603700411. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry, NMeuropsychology, and Behavioral Neurology. 1998;11:111–119. [PubMed] [Google Scholar]