Abstract
Objectives
HIV infection has been associated with an impaired lung function in high-income countries, but the association between HIV infection and pulmonary function in Sub-Saharan Africa remains unclear. This study aims to investigate the relation between HIV infection and pulmonary function in a rural African population.
Methods
A cross-sectional study was conducted among HIV-positive and HIV-negative adults in a rural area in South Africa, as part of the Ndlovu Cohort Study. A respiratory questionnaire and post-bronchodilator spirometry were performed. Multivariable regression analysis was used to investigate whether HIV was independently associated with a decrease in post-bronchodilator FEV1/FVC ratio considering age, sex, body mass index, respiratory risk factors and a history of a pulmonary infection (tuberculosis (TB) or a pneumonia). Possible mediation by a history of pulmonary infection was tested by removing this variable from the final model.
Results
Two hundred and one consecutive participants were enrolled in the study in 2016, 84 (41.8%) were HIV-positive (82.1% on ART). The median age was 38 (IQR 29–51) years. Following multivariable analysis HIV was not significantly associated to a decline in post-bronchodilator FEV1/FVC ratio (β -0.017, p 0.18). However, upon removal of a history of a pulmonary infection from the final model HIV was significantly related to post-bronchodilator FEV1/FVC ratio, β -0.026, p 0.03.
Conclusions
Pulmonary function is affected by HIV infection which most likely results from co-infection with TB or other pneumonia. Further research should focus on the influence of a pulmonary infection, most notably TB, on pulmonary function, especially as the incidence of TB is high in HIV infection.
Introduction
Human Immunodeficiency virus (HIV) infection remains a major health problem in Sub-Saharan Africa (SSA). With nearly one in every 20 adults (4.9%) living with HIV in this part of the world, HIV/AIDS is the most important cause of morbidity and mortality in SSA [1,2].
Due to increased access to combination antiretroviral therapy (ART) the face of the epidemic is changing. Life expectancy of patients receiving ART is improving and long-term co-morbidities associated with HIV infection are becoming more relevant. Pulmonary emphysema and obstructive lung disease (OLD) are examples of frequently observed co-morbidities in HIV infection [3]. This intercedes with the existing burden of pulmonary diseases, with lower respiratory tract infections and tuberculosis ranking in the top five causes of years of life lost on the African continent [2].
OLD is more common in HIV-positive than in HIV-negative individuals, and HIV infection is independently associated with a decrease in lung function and diffusion capacity [4–10]. What role ART plays in the development of OLD is an unresolved issue. ART tapers inflammation, possibly reducing immunological damage on alveolar level, but a direct negative effect of ART on pulmonary function was also reported [4,11,12]. However, most data regarding OLD in HIV comes from high-income countries (HIC). Due to marked differences between the settings, such as sex distribution among HIV patients and occupational and environmental risk factors, these data cannot simply be applied to low- and middle-income countries (LMIC). [13,14]
Research into prevalence and pathogenesis of OLD in HIV in SSA is needed to guide medical management of HIV patients in low-resource settings and to reduce the burden of chronic pulmonary disease. This study sets out to investigate the relation between HIV infection and pulmonary function in a rural African population comparing HIV-positive and HIV-negative participants while taking pulmonary co-infection into account.
Methods
Study design and study population
Data was collected in a cross-sectional study, embedded in the Ndlovu Cohort Study (NCS). The NCS is a prospective study including approximately 2000 participants in rural South Africa (Limpopo province). The main aim is to investigate the epidemiology and pathogenesis of cardiovascular disease in the context of HIV infection. [15] Inclusion criteria for the NCS were age (≥18 years) and living in the proximity of the research site. All participants coming for a NCS baseline or follow-up visit in April and May 2016 were considered eligible for participation in this cross-sectional study. Exclusion criteria were myocardial infarction in the last month, surgery in the past six weeks, hypersensitivity to salbutamol or inability to undergo the procedure for any reason. The study was approved by the Human Ethics Research Committee of the University of Pretoria (Reference number 76/2016) and written informed consent was obtained from all subjects prior to study participation. The study was reported according to the STROBE guideline (S1 Appendix).
Baseline data collection
Socio-demographic, lifestyle and anthropometric data, HIV-status and most recent laboratory results (CD4 count and HIV viral load) were collected from the NCS database. Data collection followed standardised procedures as previously described [15]. Additional data on a history of tuberculosis (TB), a history of a pneumonia, smoking, occupational and environmental exposure was collected using an adapted respiratory questionnaire (S2 Appendix) based on The British Medical Research Council Respiratory Questionnaire [16], World Health Survey [17], ATS-DLD-78-A [18], MRC breathlessness scale [19], and questions used in other publications [20–22].
Spirometric measurements
Spirometry testing with pre- and post-bronchodilator measurements was performed using a hand-held CareFusion 2009 spirometer and Spida 5 software. Procedures and review of acceptability and repeatability were performed according to American Thoracic Society and European Respiratory Society guidelines [23]. At least three acceptable and repeatable blows were required where the subject performed the manoeuvre with a maximum inspiration, a good start, a smooth continuous exhalation, and maximal effort. In addition, the largest and second-largest values for both forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) had to be within 150 mL of each other. In cases where acceptability and repeatability could not be achieved within three blows, the manoeuvre was repeated up to a maximum of eight times. After the initial measurements, a short-acting bronchodilator (salbutamol 100 μg, 4 doses) was administered using a spacer. Post-bronchodilator spirometry was repeated after a 15-minute waiting time using the same procedure as before. The respiratory parameters measured were: FEV1, FVC and FEV1/FVC ratio. The largest values for post-FEV1 and post-FVC were considered for analysis.
According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) OLD was defined as a FEV1/FVC-ratio below 0.70, post-bronchodilation [24]. As this definition can lead to underestimation of airflow limitation in young people and overestimation in older people, we chose to use a lower limit of normal (LLN) definition [25]. This was defined as a FEV1/FVC-ratio below the 5th percentile of the predicted value. The Global Lung Initiative equation category “Afro-American” was used to define normal values [26]. Reversibility was defined as a post-bronchodilator increase in FEV1 of more than 12% and over 200 ml [27].
Spirometry tests that did not meet acceptability and repeatability criteria, and thus could not be evaluated for the presence of obstruction, were excluded from further analysis (n = 6).
Statistical analysis
Continuous descriptive data was presented as means and standard deviations (SD) for normally distributed data and as medians and interquartile ranges (IQR) for non-normally distributed data. Categorical descriptive data was presented as frequencies and percentages. Selection bias was evaluated by comparing demographics of those who were enrolled in this sub-study to the demographics of the total NCS. The comparison included age, sex, HIV status, education level, marital status and body mass index (BMI). We used a t-test for continuous outcomes and a Chi square test for dichotomous outcomes.
Post-bronchodilator FEV1 and FVC outcomes, post-bronchodilation FEV1/FVC-ratio and the prevalence of OLD were compared between HIV-positive and HIV-negative individuals.
The association of HIV and FEV1/FVC ratio was assessed using the fixed post- bronchodilator FEV1/FVC ratio. We decided not to use the percentage of predicted FEV1/FVC generated by the GLI equation category “Afro-American”, as it is not certain if these values reflect the normal distribution in a Black African population. The relation between HIV and post-bronchodilator FEV1/FVC ratio was tested in a linear regression analysis. The following baseline variables were considered as confounders and tested in univariable analysis: age, gender, BMI, education, employment, current or ever smoking cigarettes or cigars, current smoking of marijuana, indoor smoking in childhood, a history of TB or pneumonia, use of wood fire outdoors and occupational exposure (mining work, having worked in a dusty job for more than a year or having working with chemicals and fumes for more than one year). All variables with p-value <0.2 in univariable analysis as well as age, sex and BMI (regardless of the p-value) were entered in a multivariable analysis. Second, we assessed mediation by a history of tuberculosis (TB) on the relation between HIV and post-bronchodilator FEV1/FVC ratio by removing TB from the final model. Analysis was performed using SPSS software (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.).
Results
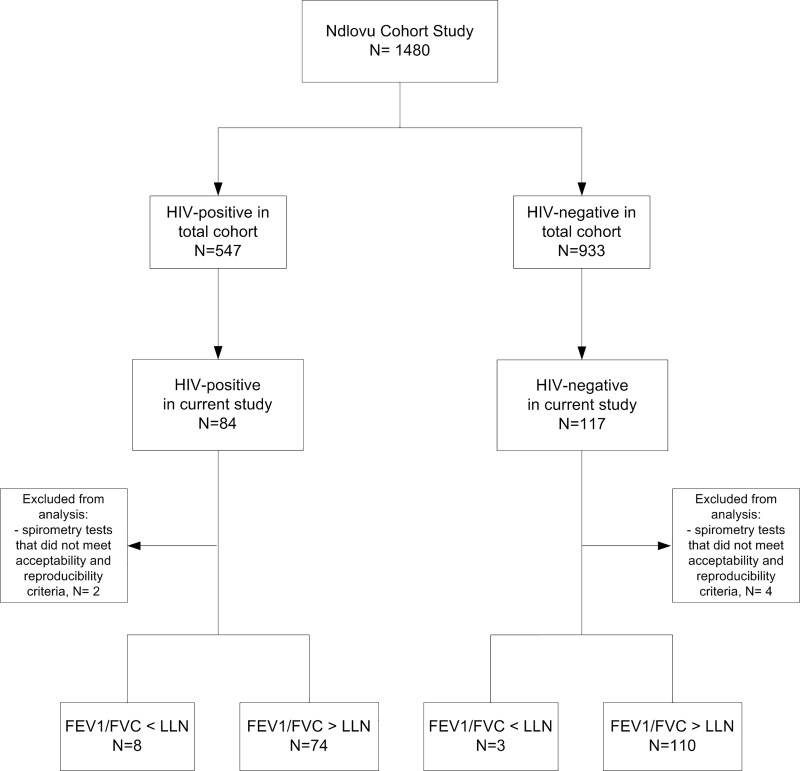
Two hundred and one participants (50% men) were enrolled in the study, 84 (42%) of whom were HIV-positive (Fig 1, Table 1). The age of participants in this sub-study was the only demographic factor that differed compared to the demographics of the participants in the NCS (the age in this sub-study was on average 2 years higher than the age in the NCS, p = 0.03) (S1 Table). The proportion of men was higher in the HIV-negative group (65%) as compared to the HIV-positive group (30%). HIV-positive participants were slightly older than HIV-negative participants.
Fig 1. Subject recruitment.
Table 1. Baseline characteristics of study population.
| HIV-positive (n = 84) | HIV-negative (n = 117) | P value | |
|---|---|---|---|
| Demographic characteristics | |||
| Men | 25 (29.8%) | 76 (65.0%) | <0.01 |
| Age | 42.4 (10.4) | 39.0 (14.8) | 0.08 |
| Education level | 0.37 | ||
| None or Primary | 21 (25.0%) | 30 (25.6%) | |
| Secondary or higher | 63 (75.0%) | 87 (74.4%) | |
| Employment status | 0.02 | ||
| Unemployed | 41 (48.8%) | 55 (47.0%) | |
| Employed | 34 (40.5%) | 34 (29.1%) | |
| Retired | 9 (10.7%) | 17 (14.5%) | |
| Student | 0 (0.0%) | 11 (9.4%) | |
| Tobacco smoking | <0.01 | ||
| Never smoking | 57 (67.9%) | 50 (42.7%) | |
| Current smoking | 15 (17.9%) | 46 (39.3%) | |
| Former smoking | 12 (14.3%) | 21 (17.9%) | |
| No. cigarettes smoked daily* | 8.8 (9.3) | 6.3 (3.8) | 0.40 |
| Marijuana smoking | 10 (11.9%) | 27 (23.1%) | 0.04 |
| Cooking fuel | 0.83 | ||
| Gas | 6(7.1%) | 5 (4.3%) | |
| Electricity | 73 (86.9%) | 104 (88.9%) | |
| Paraffin | 2 (2.4%) | 3 (2.6%) | |
| Kerosene | 0 (0.0%) | 1 (0.9%) | |
| Solid fuels | 3 (3.6%) | 4 (3.4%) | |
| Use of fuel of heating (n = 77) | 0.28 | ||
| Gas | 0 (0.0%) | 3 (6.7%) | |
| Electricity | 31 (96.9%) | 39 (86.7%) | |
| Kerosene | 0 (0.0%) | 2 (4.4%) | |
| Solid fuels | 1 (3.1%) | 1 (2.2%) | |
| Occupational exposure | |||
| Mining work | 5 (6.0%) | 15 (12.8%) | 0.11 |
| Dusty job ≥1 year | 12 (14.3%) | 26 (22.2%) | 0.16 |
| Chemicals or fumes ≥ 1 year | 5 (6.0%) | 9 (7.7%) | 0.63 |
| Medical history | |||
| Pneumonia | 8 (9.5%) | 2 (1.7%) | 0.01 |
| Pulmonary TB | 29 (34.5%) | 6 (5.1%) | <0.01 |
| HIV-related characteristics | |||
| Years since diagnosis, mean (SD) | 5.3 (4.5) | - | |
| On ART | 69 (82.1%) | ||
| First line | 68 (98.6%) | - | |
| Years since start ART, median (IQR) | 5.0 (2.0–8.0) | - | |
| CD4 count cells/mm3 (n = 83) | |||
| <200 | 13 (15.7%) | - | |
| 200–350 | 11 (13.3%) | - | |
| >350 | 59 (71.1%) | - | |
| Viral load cp/ml (n = 78) | |||
| <50 | 54 (69.2%) | - | |
| 51–1000 | 7 (9.0%) | - | |
| >1000 | 17 (21.8%) | - | |
IQR, interquartile range, TB, tuberculosis.
Data are in n (%) or mean (SD) unless otherwise specified.
* Data for current smokers.
Differences in socioeconomic, environmental, and occupational factors between the HIV-positive group and HIV-negative controls were minor. Current smoking was reported by 61 participants; 46 (75%) were HIV negative. Almost all smokers were males (n = 57, 93%). Occupational exposure was generally low and did not differ by HIV status. There were marked differences between men and women: 19% of all men reported having worked in a mine versus 1% of women, 32% of men had worked in a dusty job for more than one year versus 6% of women and 13% of men had worked in a job with exposure to chemical fumes or gases for more than one year compared to 1% of women. A history of pulmonary tuberculosis or pneumonia was reported significantly more often by HIV-positive than by HIV-negative participants (Table 1).
Acceptable spirometry test results could be obtained in 195 (97%) participants. Post-FEV1 and -FEV1/FVC values were significantly lower in the HIV-positive group than the HIV-negative group (Table 2). The prevalence of OLD, according to the LLN, was 10% (n = 8) in HIV-positive participants and 3% (n = 3) in HIV-negative participants (p = 0.06).
Table 2. Post-bronchodilator spirometry results.
| All (n = 195) |
HIV-positive (n = 82) |
HIV-negative (n = 113) | P value | |
|---|---|---|---|---|
| FEV1 (L) | 2.94 (0.79) | 2.62 (0.66) | 3.17 (0.80) | <0.01 |
| FEV1% predicted* | 101.0 (16.3) | 96.4 (17.7) | 104.0 (14.5) | <0.01 |
| FVC (L) | 3.51 (0.81) | 3.22 ((0.71) | 3.73 (0.82) | <0.01 |
| FVC% predicted* | 99.8 (14.5) | 97.4 (15.2) | 101.4(13.8) | 0.05 |
| FEV1/FVC-ratio | 0.84 (0.09) | 0.82 (0.10) | 0.85 (0.08) | 0.03 |
| FEV1/FVC-ratio predicted* | 101.1 (9.9) | 99.3 (12.2) | 102.3 (7.5) | 0.04 |
| FEV1 < 80% predicted* | 16 (8.2%) | 11 (13.4%) | 5 (4.4%) | 0.02 |
| FEV1/FVC < 0.70 | 9 (4.6%) | 6 (7.3%) | 3 (2.7%) | 0.17 |
| FEV1/FVC < LLN* | 11 (5.6%) | 8 (9.8%) | 3 (2.7%) | 0.06 |
FEV1 forced expiratory volume in 1 s; FVC, forced vital capacity; LLN, lower limit of normal
Data in mean (SE) or n(%).
* Based on GLI reference equations “Afro-American”
HIV was independently associated with a decrease in post- bronchodilator FEV1/FVC ratio following univariable regression analysis, β -0.028, p = 0.03.
Following multivariable regression analysis HIV was not related to post-bronchodilator FEV1/FVC ratio anymore (Table 3). However, when a history of TB or pneumonia was removed from the model the relation between HIV and post-bronchodilator FEV1/FVC ratio became significant again, indicating that the relation between HIV and post-bronchodilator FEV1/FVC ratio was mediated by TB (Table 3).The small number of HIV-positive participants not on ART (n = 15) did not allow us to evaluate the effect of ART on the post- bronchodilator FEV1/FVC ratio.
Table 3. Uni- and multivariable analysis and mediation analysis.
| Post FEV1/FVC | Univariable Unstandardized β coefficient (95% CI) |
p | Multivariable Unstandardized β coefficient (95% CI) |
p | Mediation Unstandardized β coefficient (95% CI) | p |
|---|---|---|---|---|---|---|
| HIV | -0.028 (-0.054–-0.003) |
0.030 | -0.017 (-0.043–0.008) |
0.177 | -0.026 (-0.050–-0.003) |
0.030 |
| Age | -0.004 (-0.004–-0.003) |
<0.001 | -0.003 (-0.004–-0.002) |
<0.001 | -0.003 (-0.004–-0.002) |
<0.001 |
| Female gender | 0.012 (-0.014–0.037) |
0.357 | 0.002 (-0.027–0.031) |
0.878 | 0.004 (-0.025–0.033) |
0.778 |
| Body mass index (kg/m2) | 0.000 (-0.002–0.003) |
0.675 | 0.001 (-0.001–0.003) |
0.315 | 0.001 (-0.001–0.003) |
0.281 |
| History of tuberculosis or a pneumonia | -0.047 (-0.078–-0.016) |
0.003 | -0.026 (-0.055–0.003) |
0.078 | ||
| Education, secondary or higher | 0.025 (0.013–0.037) |
<0.001 | 0.001 (-0.012–0.014) |
0.934 | 0.001 (-0.012–0.014) |
0.885 |
| Employment1 | 0.009 (-0.018–0.036) |
0.513 | ||||
| Indoor smoking in childhood | -0.027 (-0.056–0.003) |
0.074 | -0.020 (-0.046–0.005) |
0.116 | -0.019 (-0.045–0.006) |
0.137 |
| Current or ever smoking | -0.027 (-0.052–-0.001) |
0.039 | -0.007 (-0.034–0.020) |
0.620 | -0.005 (0.032–0.022) |
0.732 |
| Current smoking marijuana | 0.019 (-0.025–0.063) |
0.402 | ||||
| Exposure to wood fire outdoors | 0.012 (-0.020–0.044) |
0.467 | ||||
| Occupational exposure2 | -0.039 (-0.066–-0.012) |
0.005 | -0.026 (-0.053–0.001) |
0.062 | -0.027 (-0.054–0.000) |
0.048 |
CI; confidence interval.
1Employed versus no employment, retired or student.
2Defined as any of the following: mining work, exposure to chemicals or fumes at work or worked in a dusty environment, all for more than a year.
Discussion
In this African cohort the prevalence of OLD was 10% in HIV-positive participants compared to 3% in the HIV-negative group. HIV infection was not related to the post- bronchodilator FEV1/FVC ratio in multivariable analysis. However, when a history of a pulmonary infection was removed from the final model HIV became significantly associated with a decline in post-bronchodilator FEV1/FVC ratio. This indicates that a history of a pulmonary infection mediates the relation between HIV infection and a decline in pulmonary function.
Our results on the prevalence of OLD are roughly in line with the estimated worldwide prevalence of OLD of 11% [28] and 10.5% in people living with HIV [29]. Studies conducted amongst general populations in African countries reported a prevalence ranging from 4.1% to 24.8% [30], and 6.0% to 22.2% in HIV-positive populations[29,31–33]. Although not statistically significant in our cohort, the higher prevalence of OLD in HIV-positive participants is also in line with data from both HIC and LMIC. [5,6,9, 31]
Two recent studies conducted in HIV-positive cohorts found that immune defects as measured by a lower CD4 T cell count may be associated with a decline in FEV1 and FVC [34] and COPD [35]. In a large, international randomized controlled trial, the timing of ART initiation had no effect on rate of lung function decline in ART-naïve individuals with CD4 T cell counts of more than 500 per μL [36]. These findings support the newly updated WHO recommendations that all patients should be treated with ART regardless of their CD4 T cell counts [37]. Data from SSA countries regarding the role of immunomodulation and HIV and the effect of ART on OLD still remains limited.
In TB-endemic regions, such as SSA, the epidemiology of HIV infection and TB tend to be highly intertwined. Up to 65% of South Africans with TB also tested positive for HIV [38], and vice versa, globally approximately 30% of HIV-positive persons are estimated to have (usually latent) M. tuberculosis infection [39]. The risk of active TB doubles within the first year of HIV infection [40] and the life-time risk of active TB for HIV/TB co-infected persons in Africa has been estimated to be around 30–40% [41]. These figures highlight the relationship between HIV infection and pulmonary coinfection in SSA, yet few studies have taken the possible contribution of TB/pneumonia into account when looking at HIV and changes in lung function. Recent work investigating HIV infection, TB and COPD in a Ugandan cohort found a higher prevalence of COPD in HIV-positive individuals with a prior history of TB [33]. These findings are in agreement with our results and suggest decreased lung function in HIV infection in a SSA setting could be attributed to pulmonary co-infection rather than HIV infection.
Whereas associations between prior history of pneumonia and OLD have been established [42–44], the pathophysiological mechanisms behind airflow obstruction as a result of pulmonary infection are still incompletely understood. Postulated pathways include the development of bronchiectasis and bronchial stenosis due to aberrant lung tissue remodelling after inflammatory injury [44], as well as chronic inflammation caused by dysregulated macrophages [45]. The pathophysiological interactions of chronic inflammation in HIV infection and lung injury caused by pulmonary infections present interesting topics for future research.
Our analysis also showed that age was robustly associated with a decline in post-bronchodilator FEV1/FVC ratio. This was to be expected as pulmonary function decreases with age [46–49]. We also noted that the widely studied OLD-risk factor smoking was not associated with a decline in the post-FEV1/FVC ratio following multivariable analysis (either passive smoking in childhood, or former and current smoking versus never smoking). This may be due to the sample size, as well as different smoking habits between high income settings and low income settings [50, 51]. In our study smoking habits were often described as irregular and highly dependent on fluctuations in participants’ finances (average consumption of 7 cigarettes per day).
Some limitations of our study need to be mentioned. First, as a result of the small sample size we could not investigate the clinically relevant outcome OLD. However, we do not expect that HIV would be associated with OLD, as we found no relation with post-bronchodilator FEV1/FVC when a history of a pulmonary infection was considered. Due to the relatively low number of study participants we were also unable to explore the role of other HIV-related factors, such as ART, CD4 T cell counts and viral loads. Second, as our data collection was constrained to one time-point, studying changes in lung function in HIV infection was beyond the scope of this study. Third, the cross-sectional design limits causal inference. Fourth, participants in this analysis were slightly older than participants in the NCS. However, the other demographic factors did not differ, so it is unlikely that this sub-study is subjected to selection bias. The older age is also unlikely to influence the generalizability of our findings to all cohort participants as there is no reason to assume that the influence of HIV on pulmonary function would change with a small increase in age. Finally, for some of the data we relied on self-reporting, which may have subjected some of our measured parameters to recall or reporting bias.
Strengths to be mentioned are the comparison between HIV-positive and HIV-negative individuals, the rural setting and the extensive evaluation of pulmonary risk factors like smoking in childhood and occupational exposure. As a result, our study contributes to the current understanding on the influence of HIV on post-bronchodilator FEV1/FVC ratio and it highlights the influence of a history of a pulmonary infection on pulmonary function in a rural SSA setting
In conclusion, HIV infection is associated with a decline in pulmonary function measured with post-bronchodilator FEV1/FVC ratio, but this effect seems to be mediated by a history of a pulmonary infection, notably TB. TB is still highly prevalent, especially in the HIV-positive population and may pose a significant burden of pulmonary disease in these patients. We would therefore recommend to investigate the influence of TB on pulmonary function, considering HIV infection, in a larger cohort using the clinically more relevant outcome ‘OLD’. In the meantime, the regular contact of HIV-positive participants with the health care system should be used to direct clinical care towards prevention and early treatment of TB infections.
Supporting information
(DOC)
(PDF)
(DOCX)
Acknowledgments
We would like to acknowledge P. Zanen, lung physiologist in the UMC Utrecht, The Netherlands, for a quality check of the spirometries and the Ndlovu Research Consortium (South Africa and The Netherlands) for hosting this project.
Data Availability
Data underlying the study cannot be made publicly available due to ethical considerations, as the data set contains potentially identifying information of participants. The Faculty of Health Sciences Research Ethics Committee has therefore advised against the publication of the dataset. Researchers interested in the data should contact Dr. K. Scheuermaier, scientific coordinator of the Ndlovu Research Consortium, Email address: Karine.Scheuermaier@wits.ac.za. Request will be reviewed on an individual basis and the advice of the Faculty of Health Sciences Research Ethics Committee will be sought to determine if the dataset can be shared without risks of confidentiality breaches.
Funding Statement
A.G. Vos received a once-off grand of 1,700 euros from Boehringer Ingelheim, The Netherlands, to support the acquisition of disposables for the spirometry measurement. Boehringer Ingelheim was not involved in the design of the study, data collection, data analysis, data interpretation, writing of the paper or the decision to submit the paper.
References
- 1.Joint United Nations Program on HIV/AIDS (UNAIDS) (2012). Global Report: UNAIDS report on the global AIDS epidemic, 2012 Geneva: UNAIDS; Available from: http://www.unaids.org/sites/default/files/media_asset/20121120_UNAIDS_Global_Report_2012_with_annexes_en_1.pdf. [Google Scholar]
- 2.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017; 390(10100):1151–1210. 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeks SG, Lewin SR, Havlir DV. The End of AIDS: HIV infection as a chronic disease. Lancet. 2013; 382(9903):1525–33. 10.1016/S0140-6736(13)61809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz PT, Wewers MD, Pacht E, Drake J, Nagaraja HN, Clanton TL. Respiratory symptoms among HIV-seropositive individuals. Chest. 2003; 123(6):1977–82. [DOI] [PubMed] [Google Scholar]
- 5.Crothers K, Butt AA, Gibert CL, Rodriguez- Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006; 130(5):1326–33. 10.1378/chest.130.5.1326 [DOI] [PubMed] [Google Scholar]
- 6.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011; 183(3):388–95. 10.1164/rccm.201006-0836OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrache I, Diab K, Knox KS, Twigg HL 3rd, Stephens RS, Flores S, et al. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax. 2008; 63(5):463–9. 10.1136/thx.2007.079111 [DOI] [PubMed] [Google Scholar]
- 8.Kristoffersen US, Lebech AM, Mortensen J, Gerstoft J, Gutte H, Kjaer A. Changes in lung function of HIV-infected patients: a 4.5-year follow-up study. Clin Physiol Funct Imaging. 2012; 32(4):288–95. 10.1111/j.1475-097X.2012.01124.x [DOI] [PubMed] [Google Scholar]
- 9.Drummond MB, Kirk GD, Astemborski J, Marshall MM, Mehta SH, McDyer JF, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012; 67(4):309–14. 10.1136/thoraxjnl-2011-200702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MB, Huang L, Diaz PT, Kirk GD, Kleerup EC, Morris A, et al. Factors associated with abnormal spirometry among HIV-infected individuals. AIDS. 2015; 29(13):1691–700. 10.1097/QAD.0000000000000750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Twigg HL, Knoz KS. HIV-related lung disorders. Drug Discov Today Dis Mech. 2007; 4(2): 95–101. 10.1016/j.ddmec.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009; 4(7):e6328 10.1371/journal.pone.0006328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNAIDS: The Gap Report. Geneva 2014. Available at: http://www.unaids.org/en/resources/documents/2014/20140716_UNAIDS_gap_report.
- 14.Institute for Health Metrics and Evaluation, Human Development Network, The World Bank. The Global Burden of Disease: Generating evidence, guiding policy—Sub-Saharan Africa Regional Edition Seattle, WA: IHME, 2013. [Google Scholar]
- 15.Vos AG, Tempelman H, Devillé W, Barth R, Wensing A, Kretzschmar M, et al. HIV and risk of cardiovascular disease in Sub-Saharan Africa: Rationale and design of the Ndlovu Cohort Study. Eur J Prev Cardiol. 2017; 24(10):1043–1050. 10.1177/2047487317702039 [DOI] [PubMed] [Google Scholar]
- 16.Cotes JE. Medical Research Council Questionnaire on respiratory symptoms. Lancet. 1987; 2:1028. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization, Evidence and Information for Policy. World Health Survey 2002. B–Individual Questionnaire, Rotation A. Available from: http://www.who.int/healthinfo/survey/whslongindividuala.pdf
- 18.Ferris B. Epidemiology standardization project (American Thoracic Society). Am Rev Respir Dis. 1978. December; 118:1–120. [PubMed] [Google Scholar]
- 19.Stenton C. The MRC breathlessness scale. Occup Med (Lond). 2008; 58(3):226–7. [DOI] [PubMed] [Google Scholar]
- 20.Mengersen K, Morawska L, Wang H, Murphy N, Tayphasavanh F, Darasavong K, et al. The effect of housing characteristics and occupant activities on the respiratory health of women and children in Lao PDR. Sci Total Environ. 2011; 409(8):1378–84. 10.1016/j.scitotenv.2011.01.016 [DOI] [PubMed] [Google Scholar]
- 21.Isara AR, Aigbokhaode AQ. Household cooking fuel use among residents of a sub-urban community in Nigeria: Implications for indoor air pollution. Eurasian J Med. 2014; 46(3):203–8 10.5152/eajm.2014.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Gemert F, Kirenga B, Chavannes N, Kamya M, Luzige S, Musinguzi P, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health. 2015; 3(1):e44–51 10.1016/S2214-109X(14)70337-7 [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005; 26(2):319–38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 24.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. Available from: http://goldcopd.org/. [DOI] [PubMed]
- 25.Bakke PS, Ronmark E, Eagan T, Pistelli F, Annesi-Maesano I, Maly M, et al. Recommendations for epidemiological studies on COPD. Eur Respir J. 2011; 38(6):1261–77 10.1183/09031936.00193809 [DOI] [PubMed] [Google Scholar]
- 26.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. , and the ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95 year age range: the global lung function 2012 equations. Eur Respir J. 2012; 40(6):1324–43. 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005; 26(5):948–68. 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 28.Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global Health Epidemiology Reference Group (GHERG). Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. 2015; 5(2):020415 10.7189/jogh.05-020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bigna JJ, Kenne AM, Asangbeh SL, Sibetcheu AT. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(2):e193–e202. 10.1016/S2214-109X(17)30451-5 [DOI] [PubMed] [Google Scholar]
- 30.Finney LJ, Feary JR, Leonardi-Bee J, Gordon SB, Mortimer K. Chronic obstructive pulmonary disease in sub-Saharan Africa: a systematic review. Int J Tuberc Lung Dis. 2013; 17(5):583–9. 10.5588/ijtld.12.0619 [DOI] [PubMed] [Google Scholar]
- 31.Akanbi MO, Taiwo BO, Achenbach CJ, Ozoh OB, Obaseki DO, Sule H, et al. HIV-associated chronic obstructive pulmonary disease in Nigeria. J AIDS Clin Res. 2015; 6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pefura-Yone EW, Fodjeu G, Kengne AP, Roche N, Kuaban C. Prevalence and determinants of chronic obstructive pulmonary disease in HIV infected patients in an African country with low level of tobacco smoking. Respir Med. 2015; 109(2):247–54. 10.1016/j.rmed.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 33.North CM, Allen JG, Okello S, Sentongo R, Kakuhikire B, Ryan ET, et al. HIV Infection, Pulmonary Tuberculosis, and COPD in Rural Uganda: A Cross-Sectional Study. Lung. 2018;196(1):49–57. 10.1007/s00408-017-0080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Risso K, Guillouet-de-Salvador F, Valerio L, Puglièse P, Naqvi A, Durant J, et al. COPD in HIV-Infected Patients: CD4 Cell Count Highly Correlated. PLoS One. 2017;12(1):e0169359 10.1371/journal.pone.0169359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronit A, Lundgren J, Afzal S, Benfield T, Roen A, Mocroft A, et al. Airflow limitation in people living with HIV and matched uninfected controls.Thorax. 2018;73(5):431–438. 10.1136/thoraxjnl-2017-211079 [DOI] [PubMed] [Google Scholar]
- 36.Kunisaki KM, Niewoehner DE, Collins G, Aagaard B, Atako NB, Bakowska E et al. Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial. Lancet Respir Med. 2016;4(12):980–989. 10.1016/S2213-2600(16)30319-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV Geneva, Switzerland: World Health Organization, 2015. [PubMed] [Google Scholar]
- 38.Mayosi BM, Lawn JE, van Niekerk A, Bradshaw D, Abdool Karim SS, Coovadia HM; Lancet South Africa team. Health in South Africa: changes and challenges since 2009. Lancet. 2012;380(9858):2029–43. 10.1016/S0140-6736(12)61814-5 [DOI] [PubMed] [Google Scholar]
- 39.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50 Suppl 3:S201–7. [DOI] [PubMed] [Google Scholar]
- 40.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191(2):150–8. 10.1086/426827 [DOI] [PubMed] [Google Scholar]
- 41.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009–21. 10.1001/archinte.163.9.1009 [DOI] [PubMed] [Google Scholar]
- 42.Amaral AF, Coton S, Kato B, Tan WC, Studnicka M, Janson C et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J. 2015;46(4):1104–12. 10.1183/13993003.02325-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayden LP, Hobbs BD, Cohen RT, Wise RA, Checkley W, Crapo JD, et al. Childhood pneumonia increases risk for chronic obstructive pulmonary disease: the COPDGene study. Respir Res. 2015; 16(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gautam SS, O’Toole RF. Convergence in the Epidemiology and Pathogenesis of COPD and Pneumonia. COPD. 2016;13(6):790–798. 10.1080/15412555.2016.1191456 [DOI] [PubMed] [Google Scholar]
- 45.Chakrabarti B, Calverley PM, Davies PD. Tuberculosis and its incidence, special nature, and relationship with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2(3):263–72. [PMC free article] [PubMed] [Google Scholar]
- 46.Lalley PM. The aging respiratory system—pulmonary structure, function and neural control. Respir Physiol Neurobiol. 2013;187(3):199–210. 10.1016/j.resp.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 47.Janssens JP. Aging of the respiratory system: impact on pulmonary function tests and adaptation to exertion. Clin Chest Med. 2005;26(3):469–84, vi-vii. 10.1016/j.ccm.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 48.Brändli O, Schindler C, Künzli N, Keller R, Perruchoud AP. Lung function in healthy never smoking adults: reference values and lower limits of normal of a Swiss population. Thorax. 1996;51(3):277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dockery DW, Ware JH, Ferris BG Jr, Glicksberg DS, Fay ME, Spiro A 3rd, Speizer FE. Distribution of forced expiratory volume in one second and forced vital capacity in healthy, white, adult never-smokers in six U.S. cities. Am Rev Respir Dis. 1985;131(4):511–20. 10.1164/arrd.1985.131.4.511 [DOI] [PubMed] [Google Scholar]
- 50.Pampel F. Tobacco use in sub-Sahara Africa: estimates from the demographic health surveys. Soc Sci Med. 2008;66(8):1772–83. 10.1016/j.socscimed.2007.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sreeramareddy CT, Harper S, Ernstsen L. Educational and wealth inequalities in tobacco use among men and women in 54 low-income and middle-income countries. Tob Control. 2018; 27(1):26–34 10.1136/tobaccocontrol-2016-053266 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(DOCX)
Data Availability Statement
Data underlying the study cannot be made publicly available due to ethical considerations, as the data set contains potentially identifying information of participants. The Faculty of Health Sciences Research Ethics Committee has therefore advised against the publication of the dataset. Researchers interested in the data should contact Dr. K. Scheuermaier, scientific coordinator of the Ndlovu Research Consortium, Email address: Karine.Scheuermaier@wits.ac.za. Request will be reviewed on an individual basis and the advice of the Faculty of Health Sciences Research Ethics Committee will be sought to determine if the dataset can be shared without risks of confidentiality breaches.