Abstract
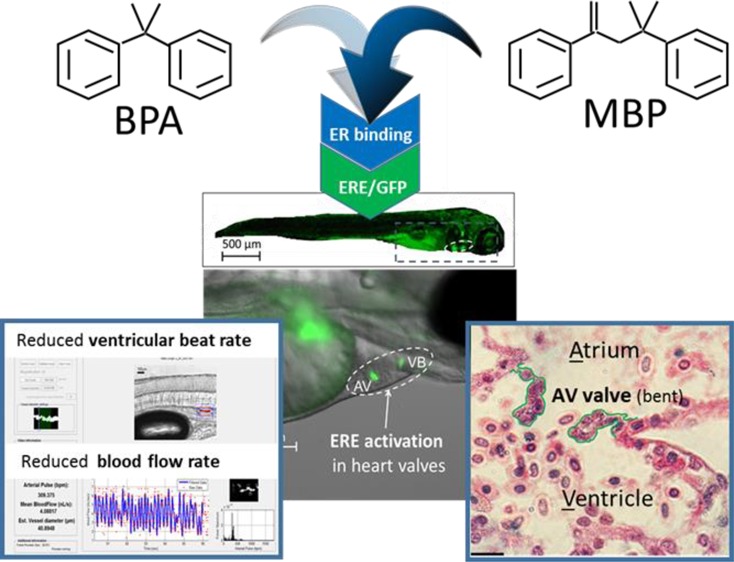
The plastic monomer bisphenol A (BPA) is one of the highest production volume chemicals in the world and is frequently detected in wildlife and humans, particularly children. BPA has been associated with numerous adverse health outcomes relating to its estrogenic and other hormonal properties, but direct causal links are unclear in humans and animal models. Here we simulated measured (1×) and predicted worst-case (10× ) maximum fetal exposures for BPA, or equivalent concentrations of its metabolite MBP, using fluorescent reporter embryo-larval zebrafish, capable of quantifying Estrogen Response Element (ERE) activation throughout the body. Heart valves were primary sites for ERE activation by BPA and MBP, and transcriptomic analysis of microdissected heart tissues showed that both chemicals targeted several molecular pathways constituting biomarkers for calcific aortic valve disease (CAVD), including extra-cellular matrix (ECM) alteration. ECM collagen deficiency and impact on heart valve structural integrity were confirmed by histopathology for high-level MBP exposure, and structural defects (abnormal curvature) of the atrio-ventricular valves corresponded with impaired cardiovascular function (reduced ventricular beat rate and blood flow). Our results are the first to demonstrate plausible mechanistic links between ERE activation in the heart valves by BPA’s reactive metabolite MBP and the development of valvular-cardiovascular disease states.
Introduction
Over 1400 chemicals have been identified as potential endocrine disrupting chemicals (EDCs)1 with potential to “alter function(s) of the endocrine system and consequently cause adverse health effects in an intact organism, or its progeny, or (sub)populations”.2−5 Over 100 of these chemicals are regarded internationally as priority EDCs and almost half (45%) are estrogenic, that is, estrogen receptor (ER) and/or estrogen-related receptor (ERR) agonists.6−8 Estrogens play a fundamental role in the formation and function of numerous organs and systems9 and imbalances are known to increase risks of cancers and disorders of reproductive, nervous, metabolic, immune, and cardiovascular systems in various animal models and humans.10−14 However, linking cause and effect remains a major challenge in chemical risk/safety assessment. Bisphenol A (BPA) is associated with the above disorders and is one of the world’s highest production volume chemicals,15 to which humans are continually exposed via plastic and other products.16−23 Reported BPA-effect mechanisms include agonism of nuclear ERs,24 ERRs,25,26 membrane ERs,27,28 epigenetic modulation of estrogen response elements (EREs)29,30 and weak agonism of androgen and thyroid (T3) receptors.31,32 Over 2700 peer-reviewed papers have been published on the endocrine effects of BPA (Scopus search, September 2018) illustrating the level of scientific interest in BPA. Despite this high number of studies, 10% of which are in vivo studies, regulatory authorities have concluded that there is insufficient evidence to establish causal links between BPA and adverse effects on human health.21,33,34 Nevertheless, public pressure has prompted the removal of BPA from baby products in Canada, Europe, and North America,35,36 due to higher exposures and lower competence for metabolizing BPA in infants.17,37 Some of the replacement products for BPA are also estrogenic in mammals38 and fish.39 Furthermore, the reactive BPA metabolite 4-methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (MBP) has been shown to be far more potent than the parent BPA in terms of: estrogen receptor binding and activation in vitro (×10–1000);40 stimulation of uterine growth in rats (×500);41 elevated estrogen receptor (esr1) and vitellogenin (vtg1, vtg2) gene and protein expression in medaka (Oryzias latipes) (×250–400);42,43 ERE activation in zebrafish (Danio rerio) (×1000) via esr1.44 These findings indicate an urgent need for more integrative test systems capable of evaluating multiple effect levels, linking key molecular events and adverse outcomes for chemicals like BPA and its analogues and metabolites.
Transgenic (TG) zebrafish models offer suitable integrative test systems, whereby key molecular events (e.g., (ant)agonism of hormone receptors, or hormone metabolism) can be identified and quantified by fluorescent protein reporters linked to specific enzymes, receptors or response elements.45 Spatial and temporal resolution of key molecular events in TG zebrafish can facilitate the detection of chemical effects throughout the body in vivo, in real time45−49 and can help establish causal links with subsequent adverse effects on biological development and/or function.39,44 Here we exploit TG(ERE:GFP)Casper zebrafish to study the effects of BPA and its highly estrogenic metabolite MBP on cardiovascular (CV) development and function, building on previous work using this model, which highlighted the heart, and heart valves in particular, as being key targets for these compounds.39,44,47,48
Materials and Methods
Test Substances
Bisphenol A or BPA: 2,2-bis(4-hydroxyphenyl)propane (99% pure, CAS No. 80–05–7) was obtained from Sigma-Aldrich Company Ltd., Dorset, UK.
The BPA derivative MBP: 4-methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (99% pure, CAS No. 13464–24–9) was synthesized at the University of Exeter (Supporting Information (SI) Figure S1).
Test Organisms
Test organisms were third generation homozygous TG(ERE:GFP)Casper zebrafish (Danio rerio),48 combining a green fluorescent protein (GFP) reporter system for estrogen response element (ERE) activation45 in a translucent Casper phenotype.50 This translucent model extends the use of fluorescent reporters to life-stages >5 dpf, which would otherwise gain skin pigmentation that interferes with GFP detection.48 This is an important feature of the model, as EDC effects may vary both within a tissue and between body tissues at different life-stages.4,51,52 The life-stages selected for this study represent two key landmarks in CV development: 5 days post fertilization (dpf) marking formation of the endocardial rings (precursors to the heart valve leaflets); 15 dpf marking elongation of the valve leaflets.53−55 The latter life-stage also corresponds with the depletion of the egg yolk and peaks in metabolism (oxygen consumption) and heart beat rate.56
The zebrafish is an established model for biomedical research on CV development, function, and disease,53,55,57 including heart valve (mal)formation.58−61 Despite some basic anatomical differences from the human heart,58,62 the cellular and molecular mechanisms of heart development are highly conserved between zebrafish and humans55,63 and zebrafish have several major advantages over other vertebrate models. Heart formation (including valvulogenesis and remodelling, outlined in detail in SI Table S1) is completed by 35 dpf,62,63 but a heartbeat is detectable as early as 1 dpf, with blood circulation beginning soon thereafter.64 Standard (resting) heart beat rate in 5–15 dpf embryo-larval zebrafish (160–260 beats per minute; bpm) is much closer to resting human fetal heart rate (130–170 bpm) than in rodents (300–600 bpm).56,65−67 Furthermore, respiration in embryo-larval stages relies mainly on cutaneous diffusion of O2 and CO2, rather than transport by convective blood circulation,68 enabling the in vivo study of late phenotypes of congenital CV malformations, which would be lethal in mammals.57
Ethical Statement
All experimental procedures with zebrafish were conducted in accordance with UK Home Office regulations for the use of animals in scientific procedures and followed local ethical review guidelines and approval processes. Water quality was assessed daily (SI Table S2).
Chemical Exposure
TG zebrafish embryos/larvae were exposed in the laboratory from 6 h post fertilization (hpf) to 5 days post fertilization (dpf) or from 6 hpf to 15 dpf, to aqueous concentrations of BPA (solvent (0.5% DMSO) control 0; low 100; high 1000 μg/L) or its metabolite MBP (solvent control 0; low 2.5; high 25 μg/L) in glass 1L aquaria (n = 6 aquaria per exposure treatment), positioned in random order and each containing ∼120 randomly assigned embryos. The lower BPA concentration was expected to represent maximum measured human maternal-fetal-placental unit concentrations of up to 105 ng/g,69,70 based on bioconcentration factors ranging from 0.25 to 5.7 in larval and adult zebrafish.39,71,72 Both lower and 10× higher (worst case) BPA concentrations were substantially below maximum tolerable concentrations.48 MBP exposure concentrations were based on a relative potency of 250× compared to BPA, measured in vivo in juvenile medaka.43 Stock solutions were prepared by dissolving pure test chemicals in analytical grade dimethyl sulfoxide (DMSO) and then diluting (200× ) in 400 mL of embryo culture water73 to give the desired nominal exposure concentrations in 0.5% DMSO. The pH of stock solutions was checked and adjusted to 7.5, as necessary. For the longer-term (0–15 dpf) exposure, 90% water changes were undertaken every 2 days, after commencing feeding twice a day at 6 dpf with excess <100 μm particulate fish food (ZM000) and with Artemia salinus nauplii from 10 dpf. Exposure solutions were maintained at 28 °C, under a 16h:8h light: dark photoperiod cycle with a 15 min dawn/dusk transition.
Concentrations of exposure solutions were measured in three replicate aquaria per exposure treatment at the start and end of each exposure period. Chemical body burden was also measured in whole zebrafish embryo-larvae at 5 dpf, and in composite samples of ×30 hearts extracted from 5 dpf embryos (n = 3 composite samples per treatment). Heart extraction was performed en masse: × 50 larvae per aquarium were disrupted in ice-cold Leibovitz’s L-15 Medium (Invitrogen, UK) containing 10% fetal bovine serum, using a 5 mL syringe and 19 gauge needle,74 and ×30 hearts were isolated using a 30 μm mesh sieve followed by manual sorting in ice-cold Leibovitz’s L-15 Medium under a 5× objective on an Olympus SZX16 microscope (Olympus, UK). Fish/hearts were placed in embryo culture water with a terminal dose of anesthetic of 2 mg/mL tricaine methanesulfonate (MS222) at pH 7.5, then dried under vacuum, macerated and extracted in a solution of 80:20 water:acetonitrile containing an internal standard. Details of chromatographic separation and mass spectrometry analysis of BPA and MBP in water and fish tissues are provided in the SI Table S3. The limit of quantitation (LOQ) was 0.05 μg BPA/L and 0.05 μg MBP/L for water, and 0.5 ng BPA/g and 0.5 ng MBP/g for fish tissue. Bioconcentration factors (BCFwhole body and BCFheart) were calculated based on a mean whole body wet weight of 1200 μg for 5 dpf zebrafish larvae and a ventricle weight of 10% of the whole body weight at 5 dpf.75
Following chemical exposure, TG zebrafish larvae were selected randomly from each aquarium and were subject to the following effects analyses, which were conducted at 28 °C.
Quantifying ERE Activation (Estrogenicity)
ERE activation was quantified in the atrio-ventricular (AV) and ventricular-bulbus (VB) valves as follows. At 5 and 15 dpf ×6 larvae per replicate aquaria (n = 6) were washed and then anaesthetised in 0.1 mg/mL MS222 at pH 7.5 and mounted in vivo in 1% low melting agarose in embryo culture water with MS222, and then placed into a glass bottom 35 mm dish (MatTek, Ashland, MA). Larvae were orientated right side down and images were obtained using an inverted compound microscope (Zeiss Axio Observer, Cambridge, UK) with a 10× objective, under consistent GFP excitation for a scanning time of 180 ms, using filter set 38 HE: Excitation BP 470/40 nm, Beam splitter FT 495 nm, Emission BP 525/50 nm. GFP expression was quantified as the relative mean pixel intensity of green fluorescence (relative to the solvent control) in a defined region of interest (ROI) encompassing the heart, using ImageJ software.76
Histopathology of the Heart and Heart Valve Leaflets
Histopathology of the heart and heart valve leaflets was conducted on ×6 whole zebrafish larvae per replicate aquaria (n = 6) at 5 and 15 dpf, following terminal anesthesia in 2 mg/mL MS222 at pH 7.5, and destruction of the brain. For visible light microscopy, zebrafish were fixed in Bouin’s solution (Sigma-Aldrich, Dorset, UK), progressively dehydrated in 70–100% industrial methylated spirits and embedded in paraffin wax. For transmission electron microscopy (TEM), zebrafish were fixed in 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M PIPES buffer (pH 7.2) for 24 h, washed with buffer (3 × 5 min) and postfixed for 1 h in 1% osmium tetroxide (reduced with 1.5% w/v potassium ferrocyanide) in 0.1 M sodium cacodylate buffer (pH 7.2). After a series of washes in deionized water (3 × 5 min) the larvae were subject to in-block staining with 1% uranyl acetate for 30 min, then 3 × 5 min washes with deionized water and then the larvae were dehydrated through an ethanol gradient and embedded in Spurr resin (TAAB Laboratories, Aldermaston, UK). Sagittal sections were obtained through the midline of the atrium and ventricle (to examine the AV valves). For visible light microscopy, serial sections (5 μm) were obtained and placed on glass slides, stained using Masson’s trichrome and examined using a Leitz Diaplan light microscope (×10–100) magnification) to assess for structural pathologies (focusing on valvular cells and interstitial extracellular matrix). For TEM, 70 nm ultrathin sections were collected on pioloform-coated EM copper slot grids (Agar Scientific, Stansted, UK) and were analyzed using a JEOL JEM 1400 operated at 120 kV, and images taken (×3000–20 000) magnification with a digital camera (ES 100W CCD, Gatan, Abingdon, UK) to assess for any ultrastructural effects on the heart valves.
Quantifying CV function
Noninvasive video analysis of the heart and dorsal aorta was used to measure multiple cardiovascular (CV) end points simultaneously.67 At 15 dpf ×6 larvae per replicate aquaria (n = 6), were anaesthetized in 0.1 mg/mL MS222 and embedded right side down in 1% agarose in a single well of a press-to-seal silicon isolator (Sigma-Aldrich, Poole, UK) on a clear microscope slide. The slide was then viewed using an inverted light microscope (Leica DM IRB, Leica Microsystems UK Ltd., Milton Keynes, UK, 5× objective) fitted with two high speed video cameras. The first camera (Grasshopper GRAS-50S5C–C, Richmond, Canada) recorded ventricular heart beat at 30 frames per second (the atrium was obscured at 15 dpf). The second camera recorded blood flow in the dorsal aorta, caudal to the swim bladder, at 120 frames per second (Grasshopper GRAS-03K2M-C, Richmond, Canada). Both cameras were focused independently on their respective regions of interest, to ensure optimal image quality, and set to record simultaneously for 5 min; recordings from the last 3 min were subject to image analysis as follows. Resting (standard) ventricular beat rate (bpm, beats per minute) was measured using MicroZebraLab image analysis software (v3.5, ViewPoint, Lyon, France). Resting (standard) aortic blood flow rate (nL/s) was measured using ZebraBlood (v1.3.2, ViewPoint, Lyon, France).
Quantifying Metabolic Scope
Metabolic scope, combining scope for growth and scope for movement, were measured in terms of specific growth rate (SGR)77 and critical swimming speed (Ucrit),78 respectively. SGR was calculated by measuring individual standard body length (±0.01 mm from snout to caudal peduncle) of TG zebrafish larvae (×10 per aquarium) under anesthetic (0.1 mg/mL MS222) at 5 and 15 dpf, using an Olympus SZX16 microscope and cellSens image analysis software. Since individual fish were not tagged, mean specific growth rate (mean SGR as % standard body length per day) was calculated for each aquarium (eq 1). Critical swimming speed (Ucritb as standard body lengths/sec) was then assessed at 15 dpf, by placing ×5 larvae at a time in a cylindrical swim flume of length 25 cm, internal diameter 2 cm and volume 78.55 mL, and increasing laminar water flow incrementally by 1.33 cm/sec every 300 s (5 min) until the time to exhaustion for all individual larvae. Ucritb was calculated based on aquarium-mean standard body length at 15 dpf (eq 2).
Equations
| 1 |
Where SL is mean standard length per aquarium (mm); T is time interval (days)
| 2 |
Where U is penultimate swimming speed (mean standard body lengths/sec); Ui is velocity increment (1.33 cm/sec); t is time swum in final velocity increment (secs); ti is time interval for each increment (300 s).
Transcriptomic Profiling of Heart Tissues
Hearts were removed from TG zebrafish larvae following terminal anesthesia in 2 mg/mL MS222 at pH 7.5, followed by destruction of the brain. At 5 dpf ×50 larvae per replicate aquaria (n = 4) were disrupted in ice-cold Leibovitz’s L-15 Medium (Invitrogen, UK) containing 10% fetal bovine serum, and hearts were extracted en masse, as described above. Hearts from larvae of 15 dpf were microdissected individually in ice-cold Leibovitz’s L-15 Medium using fine tip forceps (Dumont Inox #5SF) and an Olympus SZX16 microscope. Hearts for the 15 dpf larvae were pooled (×30 per aquarium) and then snap frozen in liquid nitrogen. Total mRNA was extracted from each pool of 30 hearts using RNeasy microkits with on-column DNase treatment (Qiagen, UK) and RNA integrity (RIN) scores were confirmed to be in the range 7.4–8.7 using an Agilent 2200 TapeStation (Agilent Technologies Ltd. Berkshire, UK). cDNA libraries were prepared with polyA isolation using Illumina TruSeqTM 2 Stranded mRNA Library Preparation kits and subsequent cluster generation was conducted using TruSeqTM Paired-End Cluster Generation kits (Illumina, San Diego CA). Up to 24 cDNA libraries were prepared for sequencing for each test substance (BPA and MBP): nominally n = 4 replicate pooled samples from each of three treatment groups (solvent control, low, high exposure), for two time points (5 dpf and 15 dpf). Libraries were sequenced with 12 per lane using an Illumina HiSeq 2500 in standard mode, generating 100 base pair reads (paired-end).
Statistical Analysis
Prior to statistical analysis, phenotypic data were tested for normality (Anderson–Darling test) and homogeneity of variance (Bartlett’s or Levene’s tests) using Minitab 16 (Minitab, Coventry, UK). Statistical analysis of phenotypic/functional end points was performed using linear mixed effects (lme) models (R-statistics version 3.3.2, R Foundation for Statistical Computing) and all lme models included aquarium as a random effect. A multivariate MANOVA was used to assess the fixed effect of exposure treatment on cardiovascular function, combining both ventricular heart beat rate and blood flow (using the function “cbind”). Statistical significance (p < 0.05) of treatment effects on these and other individual phenotypic end points was also determined by “one-way” comparisons of untransformed heart beat or blood flow data, and lme model fit was measured using the Akaike Information Criterion (AIC). Phenotypic/functional effects data are presented in the text or shown graphically as the mean ±95% confidence interval.
Transcriptomic data were quality-trimmed and filtered (to remove sequencing adapters) and then processed using the TopHat/Cufflinks pipeline,79 followed by differential gene expression analysis comparing chemical exposure treatments with control treatments using DESeq2 v3.6 and an adjusted p-value of <0.05 set as the false discovery rate.80
Gene Set Enrichment Analysis (GSEA) using DAVID v6.8,81 Enrichr82 and Reactome v6683 was conducted on sets of differentially expressed genes (for each chemical exposure treatment) to identify over-represented Gene Ontology (GO) terms for biological processes and functional pathways, including KEGG v8884 and Reactome v66 pathways83 referenced to the zebrafish (GRCz10) and the human genome (GRCh38.p12).
Transcription Factor Binding Site (TFBS) motif enrichment analysis was conducted on 5 and 50 kilobase (kB) long DNA flanking sequences both up and downstream of the differentially expressed genes to quantify the potential for estrogen receptor interactions; flanking sequences were retrieved using BioMart from Ensembl v94.85 Over-represented motifs in each gene set were identified using AME86 in MEME Suite v5.0.2 (http://meme.nbcr.net). Each gene set was “shuffled” to generate a control gene set (with matched GC content), and enriched TFBS motifs were subsequently identified using the JASPAR 2018 (CORE:vertebrate) database.87
Gene groups were considered to be enriched when enrichment scores (EASE scores) were >1.3, and when p-values adjusted for multiple-testing (Benjamini-Hochberg) were <0.1.
Results and Discussion
Chemical Exposure
Mean measured concentrations of aqueous exposure solutions were 117% and 103% of nominal values for 100 and 1000 μg/L BPA (117 ± 4, 1028 ± 23 μg/L) and 84% and 113% of nominals for 2.5 and 25 μg/L MBP (2.1 ± 0.1, 28.2 ± 0.35), and both sets of controls contained no measurable test chemical (SI Table S4). Mean measured bioconcentration factors for BPA were 5 day BCFwhole body = 2.5 and 3.8 for 100 and 1000 μg/L BPA exposures, respectively, corresponding with whole body concentrations of ∼250 and ∼3700 ng/g, which are equivalent to ×2.5 and ×37 maximum human maternal-fetal-placental unit concentrations of 105 ng/g.69,70 Mean measured 5 day BCFheart = 0.09 for the 1000 μg/L BPA exposure was substantially lower than the corresponding BCFwhole body. The mean measured bioconcentration factor for MBP was: 5 day BCFwhole body = 27 for the 25 μg/L MBP exposure, corresponding with a whole body concentration of 675 ng/g. MBP was not detectable above the LOQ (0.5 ng/g) in heart tissue, so a 5 day BCFheart could not be determined.
ERE Activation
Exposure to BPA or MBP induced fluorescence from the ERE:GFP reporter in the liver and in the region of interest (ROI) encompassing the heart, specifically in the atrio-ventricular (AV) and the ventricular-bulbus (VB) valves (Figure 1), which is consistent with previous studies.44,48 Higher fluorescence intensity in the heart/valves was not due to chemical partitioning, since heart tissue concentrations for BPA were more than an order of magnitude lower than in whole body tissue (and the water concentration). Instead, greater ERE activation and fluorescence in the heart valves may be due to tissue-specific expression of different ERs and receptor subtypes. Relative mean fluorescence intensity in the ROI (relative to the solvent control) increased with BPA exposure concentration (100 to 1000 μg/L) from 2.2 ± 0.3 to 31 ± 2 at 5 dpf, and showed a similar concentration-related response, increasing from 15.3 ± 1.1 to 26.9 ± 0.2, at 15 dpf. There was a similar response pattern in relative mean fluorescence intensity in the ROI for MBP, increasing with MBP exposure (2.5 to 25 μg/L) from 34 ± 3 to 54 ± 5 at 5 dpf, and from 3.0 ± 0.4 to 14.6 ± 1.5 at 15 dpf (SI Figure S2). Comparing relative mean fluorescence of MBP versus BPA for the lower-level exposure treatments, which can be assumed to lie on the linear sections of the dose response curves,44,48 we calculated the relative potency of MBP compared to BPA to be 710× at 5 dpf and 23× at 15 dpf (SI Table S5), which is consistent with results from previous in vivo studies on zebrafish,39,44 medaka42,43 and rats.41
Figure 1.
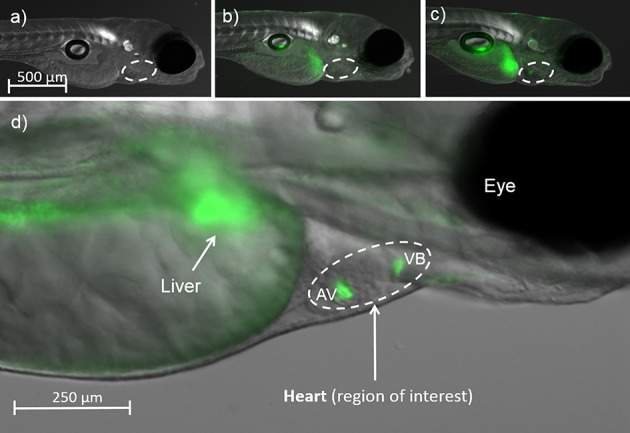
GFP fluorescence locating and quantifying ERE activation by BPA and MBP in the heart in in embryo-larval zebrafish at 5 dpf: (a) Solvent control; (b) 1000 μg/L BPA; (c) 25 μg/L MBP; (d) Close up of the region of interest showing the heart (encircled). Fluorescence indicating Estrogen Response Element (ERE) activation was concentrated in the Atrio-Ventricular (AV) and Ventricular-Bulbous (VB) valves.
Greater potency of MBP compared with BPA may be explained, at least in part, by the bioconcentration of MBP from water (5 day BCFwhole body = 27), which was 1 order of magnitude greater than for BPA. Chemical exposure level- and time- related changes in ERE/GFP expression in zebrafish heart valves are likely to be influenced by range of interacting factors. The lower relative potency of MBP in larvae at 15 dpf, compared with that in 5dpf larvae, followed a net reduction in ERE:GFP fluorescence intensity, possibly corresponding (to some extent) with tissue thickening in older fish. Furthermore, the reduction in fluorescence intensity over time was greater in MBP compared to BPA treatments. These results are consistent with metabolic activation of BPA in fish42,43,88 and greater metabolic activity in 15 dpf compared to 5 dpf zebrafish larvae.56 The observed variation in levels of ERE activation at these different life stages may also be due in part to age-related variation in the expression of different ERs and receptor subtypes.52
Effects on the Structure of the Heart Valve Leaflets
There was qualitative evidence of ultrastructural changes in the AV valve leaflets in both BPA and MBP high-level exposure treatments at 15 dpf. TEM images (×3000–20 000 magnification) showed the dislocation of valvular cells and the loss of collagen filaments from the interstitial extra-cellular spaces (Figure 2). In the high-level MBP exposure there were also gross-structural changes in the AV valve leaflets. Light microscopy images (×100 magnification), taken following Masson’s trichromatic staining, showed that valve leaflets were misshapen (bent) and that the extra-cellular matrix between the bilayer of valvular cells was narrower and lacked collagen (indicated by the lack of blue stain) compared to solvent controls (Figure 2). Although these ultra- and gross- structural effects were clearly evident, they could not be quantified morphometrically, due to the limited number (n = 2 or 3 per treatment) of sagittal sections in which the AV valve leaflets were discernible. No other heart valve pathologies were detected in any other treatment or time point, and there was no evidence of edema to indicate general cardiotoxicity. Histological assessment of ventricular-bulbus (VB) valves was not possible from the sagittal sections taken to assess the AV valves, due to their alternative orientation/alignment.
Figure 2.
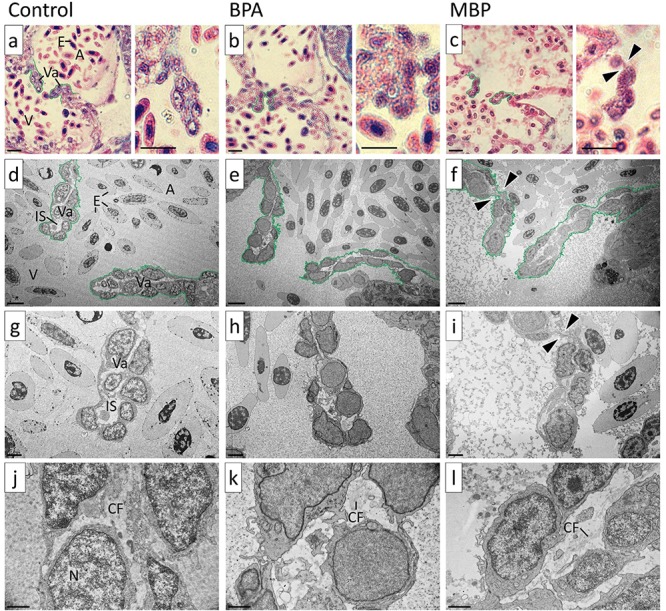
Images of atrio-ventricular (AV) valve leaflets from high-level BPA and MBP exposure treatments versus solvent controls at 15 days post fertilization (dpf) Bright field images a-c are shown at ×100 magnification: AV valve leaflets were bent in the high-level MBP exposure. TEM images d-l are shown at ×3000, ×6000, and ×20 000 magnification: The extra-cellular matrix between the bilayer of valvular cells was narrower and lacked collagen in the high-level BPA and MBP exposure treatments compared to solvent controls (qualitative assessment). Annotations: A = atrium, V = ventricle, E = erythrocytes, Va = valve leaflet, IS = interstitial space, CF = collagen fibers, N = nucleus, arrow heads indicate bent AV valve leaflet. Scale bar (bottom left in each image): a–c = 10 μm; d–f = 5 μm; g–i = 2 μm; j–l = 1 μm.
Effects on CV Function
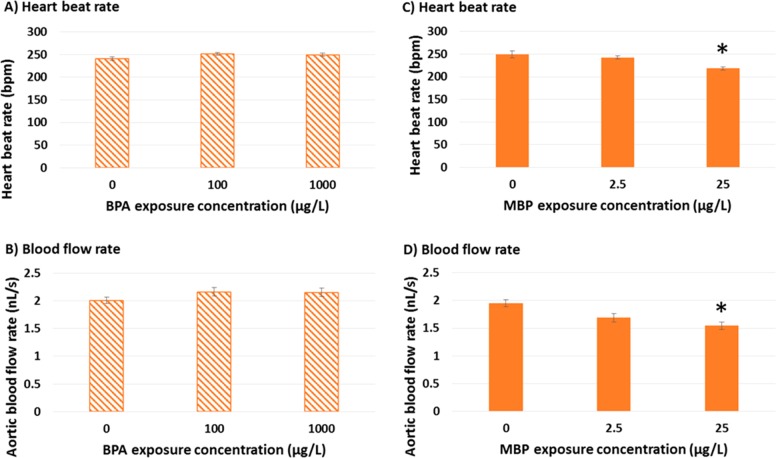
There were no discernible effects of BPA exposure on CV function in agreement with a previous study,44 but high-level MBP exposure resulted in a statistically significant reduction (versus solvent controls) in ventricular heart beat rate (218 ± 4 versus 249 ± 7 bpm; p = 0.023) and aortic blood flow rate (1.55 ± 0.07 versus 1.95 ± 0.06 nL/s; p = 0.03) in 15 dpf zebrafish, according to the linear mixed effect model lme(Rate ∼ Treatment, random = ∼1|Tank1) (Figure 3; SI Table S6). These results fall within ranges reported at 28 °C for zebrafish aged 3.5–21 dpf for resting heart beat rate (160–260 bpm)56,65−67 and resting blood flow rate (500 to 1860 μm/s; equivalent to 0.25–2.1 nL/s).67,89,90 Ontogeny of embryo-larval development in zebrafish is such that heart beat and blood flow rate vary substantially with development time and peak at 10–20 dpf, a period which corresponds with a peak in aerobic metabolism.56 Therefore, CV performance is likely to be most critical for our selected life-stage (15 dpf) and reduced CV performance (20% reduction in blood flow rate) could be biologically significant. The proportional reduction in blood flow (20 ± 3.6%) was greater than for heart beat rate (12 ± 2.3%), potentially indicating valve prolapse (although note the variation in these CV parameters). We were unable to observe valve function directly because of tissue thickening, nor were we able to demonstrate AV decoupling as being symptomatic of valve prolapse,91 since organ growth prevented imaging of both atrial and ventricular beating at 15 dpf. Nevertheless BPA exposures up to 2500 μg/L have been shown to induce erratic AV beat ratios in 5 dpf zebrafish.44
Figure 3.
Effects of BPA and MBP exposure on cardiovascular function in zebrafish larvae at 15 days post fertilization (dpf) Hatched bar charts (A-B) represent BPA, solid bar charts (C–D) represent MBP. Data represent six individual fish taken randomly from each of six separate aquaria (n = 6 experimental replicates) per exposure treatment. Bar heights represent means, error bars represent 95% confidence intervals. Significant differences from 0 μg/L solvent control (p > 0.05) are highlighted with an asterisk.
Effects on Metabolic Scope
No effects of BPA or MBP were seen on metabolic scope (i.e., SGR and Ucritb) (SI Figure S3 and Table S7). These energetic measures provide a general indication of individual metabolic scope, that is, energetic reserves beyond basal metabolism.92Ucritb integrates anaerobic as well as aerobic scope for movement93 and also cardio-respiratory performance.65 The lack of significant effects (despite downward trends for MBP) may have been due to substantial interindividual variation across the exposure treatments. Interindividual differences in locomotory performance traits have been observed in larval zebrafish between 3 and 21 dpf, and shown to be due to heritable genetic factors not necessarily related to body size.65 Our test organisms were third generation transgenic zebrafish maintained in up to six spawning groups with 30 fish in each group, therefore genetic variation may have been a confounding factor, but this was not quantified. High interindividual variation may also be related to larval swimming behavior, which is characterized by intermittent bursts of swimming,94 such that prolonged swim challenge tests >10 min may not reliably indicate swimming stamina.65 Our swim challenge tests typically ran for 5–10 min and mean Ucritb ranged from 10.7 to 12.6 body lengths/sec, which is comparable to values reported elsewhere, ranging from 13 to 18 body lengths/sec in juvenile and adult zebrafish.95,96
Effects on the Heart Transcriptome
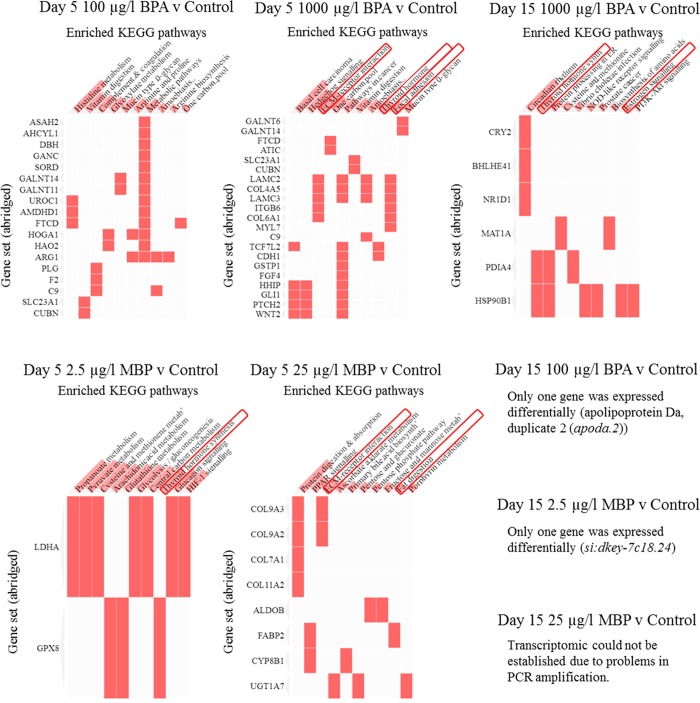
BPA (100, 1000 μg/L) and MBP (2.5, 25 μg/L) exposures at 5 and 15 dpf resulted in significant (adjusted p-value < 0.1) differential expression (predominantly down-regulated expression, compared to solvent controls) in a range of genes governing heart valve development and function (SI Figures S4–S5; Tables S8–S10). The down-regulation of a range of genes by ER signaling (via esr1, esr2 and heterodimers of these) has been demonstrated elsewhere in humans and mammalian models.97,98 The number of genes affected and the level of effect were greatest at 5 dpf for high-level BPA exposure (371 genes: 329 down-regulated by log2 fold changes of −0.5 to −9), followed by low-level BPA exposure (131 genes: 115 down-regulated by log2 fold changes of −0.9 to −9) and high-level MBP exposure (127 genes: 101 down-regulated by log2 fold changes of −2.5 to −9). There was some overlap between high and low-level BPA exposures at 5 dpf, with 62 genes being common to both treatments, whereas only one gene (elastin microfibril interfacer 3 - emilin3) was common to both MBP treatments at 5 dpf, due in part to the low number of genes (8 genes) that were differentially expressed in the low-level MBP exposure (SI Figure S6–S7). Collectively, however, the genes affected by both BPA and MBP at 5 dpf shared significant enrichment for molecular pathways concerning (i) nuclear receptor and calcium signaling (estrogen and (para)thyroid), (ii) lipid metabolism, and (iii) extra-cellular matrix (ECM) interactions99 (Figure 4; SI Figure S8; Tables S8–S10). ECM-related pathways comprising collagen and cartilage formation and organization were particularly prominent for the high-level MBP exposure, and can be related directly to phenotypic effects on the heart valves observed therein. ECM interactions were found to involve Notch signaling (dre04330) and calcium ion binding (GO:0005509) (SI Table S9) which, along with the aforementioned pathways (i-iii), have previously been linked to the progression of calcific aortic valve disease99,100 (CAVD - fibrosis, and subsequent calcification and thickening of the aortic valves), which affects up to 13% of human populations in the western world99 (see later discussion). At 15 dpf fewer genes were differentially expressed compared to 5 dpf. Although only five genes overlapped between time points for the high-level BPA exposure treatment (SI Figure S7), there was gene set enrichment for thyroid hormone synthesis (also seen at 5 dpf for MBP) and for estrogen signaling for the 15 dpf high-level BPA exposure (Figure 4). Both hormone signaling pathways have been highlighted in mammalian studies on BPA.31,101 Overall, gene sets from 15 dpf represented pathways associated with heart function (more so than development), particularly cardiac muscle contraction (SI Tables S8–S9). Four of the 32 genes down-regulated by the high-level BPA exposure at 15 dpf: actinin alpha 3b (actn3b); myosin light chain, phosphorylatable, fast skeletal muscle a (mylpfa); myosin, heavy polypeptide 2, fast muscle specific (myhz2); and troponin I type 2a (skeletal, fast), tandem duplicate 4 (tnni2a.4) have previously been identified as potential biomarkers for cardiac disease in animal models, including zebrafish.102 Low level BPA exposure at 15 dpf led to the differential expression of only gene: apolipoprotein Da, duplicate 2 (apoda.2 transport protein). The transcriptomic effects of high-level MBP exposure at 15 dpf could not be established due to problems encountered in sample processing (PCR amplification during library preparation), while low-level MBP exposure at 15 dpf led to the differential expression of only one (unannotated) gene (si:dkey-7c18.24) (SI Figure S7, Table S8).
Figure 4.
Gene set enrichment for KEGG pathways in heart tissues from 5 and 15 day old larval zebrafish in BPA and MBP exposure treatments (versus solvent controls). Sequence data were generated from hearts pooled from ∼30 individuals from each of four separate aquaria (nominally n = 4 experimental replicates) per exposure treatment. Enriched pathways were identified using Enrichr and referenced to the KEGG database (2016). Pathways highlighted in red boxes are calcific aortic valve disease (CAVD) biomarkers. Also see enriched Reactome pathways (in SI Figure S8).
Analysis of TFBS motif enrichment showed that differentially expressed genes in both BPA and MBP exposures were similarly enriched and were flanked (within 5 kB) by binding sites for transcription factors associated with estrogen receptor signaling, including: estrogen receptor 2 (esr2); specificity proteins constituting ERE tethering factors (sp1, sp3, sp4); pioneer factors facilitating ERE binding (foxa1, nfkb2, pbx1, runx1); CAMP responsive element binding proteins (creb1, creb5) (SI Figure S9, Tables S11–S13). There was also enrichment for estrogen receptors (esr1, esr2) beyond proximal promoter regions, up to 50 kB from differentially expressed genes (SI Table S13). Examination of these findings versus the established roles of estrogen in normal (and abnormal) heart valve development indicate plausible links between substantial ERE activation by BPA and MBP and the observed transcriptomic and phenotypic effects on the heart valves in our zebrafish. Endogenous estrogen (17β-estradiol) is known to be protective of the CV system in later life, which is one of the reasons for hormone replacement therapy in postmenopausal women. The results of studies on the pathogenesis of CAVD share some similarities with the findings in our study (in terms of effect pathways) and also show that major risk factors include polymorphisms of the estrogen receptor.103 Inactivation of estrogen receptors is generally associated with increased calcification of the valve leaflets in diseased patients.104,105 Empirical studies in mammalian models have shown that estrogen inhibits collagen synthesis in rat cardiac fibroblasts106 and, more specifically, estrogen decreases collagen I and III gene expression in fibroblasts from female rats.107 BPA has also been shown to cause sex-specific alterations in gene expression profiles, changes in the composition of the cardiac collagen extracellular matrix and altered CV function in CD-1 mice.101 Furthermore, disruption of collagen in the extracellular matrix has been shown to directly promote valve leaflet calcification in vitro.108 Based on these studies it is entirely plausible that the estrogenic action of high-level MBP exposure led to collagen deficiency and valve weakening in our larval zebrafish. This preliminary (precalcification) condition resembles “myxomatous degeneration” characterized by collagen degradation and elastic fiber fragmentation, resulting in a “floppy” valve that is prone to prolapse and regurgitation.109 As with CAVD, it may be speculated that more progressive effects of MBP (and BPA) on heart valve development and function may occur in the longer-term in zebrafish, but this remains to be proven.
Linking Cause and Effect
Although estrogen is known to be cardio-protective in later life, inappropriate estrogenic exposures, including from estrogenic chemicals, in early life, can lead to cardiac malformation.110 We demonstrate that aqueous exposure to the weak estrogen BPA at 1× and 10× maximum human maternal-fetal-placental unit concentrations, or its more potent metabolite MBP, at equivalent potency concentrations, activate estrogen responsive elements (EREs via estrogen receptor signaling) in the heart valves and alter the expression of a range of genes, including several governing CV development and function in embryo-larval zebrafish. BPA and MBP perturbed similar downstream effect pathways, but only the high-level MBP exposure resulted in gross phenotypic effects including, malformation of the AV valves, and reduced heart beat and blood flow, at 15 dpf. Our findings provide a substantial chain of evidence, but further work is required to fully define adverse outcome pathways for the effects of BPA and its metabolites, including MBP, on heart development and function. Longer-term studies, including lower level chemical exposures, are needed, since heart valve formation and remodelling (e.g., AV valve transitioning from two to four leaflets) is not complete in zebrafish until 35 dpf62,63 and effects may not become functionally manifest until in later life. Vertebrate models with short life spans, such as zebrafish, are highly amenable for this work.
Acknowledgments
We are grateful to the anonymous referees whose comments helped to improve the quality of our paper. We are grateful to the Exeter Sequencing Service for undertaking mRNA sequencing, data processing and providing expert guidance on differential gene expression analysis. We are also grateful to Ronny van Aerle for his advice on gene ontology, pathway and motif analysis. This project (and ARB) was funded directly by a BBSRC Flexible Interchange Programme (BB/L01548X/1). Other authors of this work were supported by the following institutes, organizations and grants: S.M. by NERC (NE/L007371/1), J.M.G. on a BBSRC Industrial CASE research studentship with AstraZeneca Global SHE (ref:620033640), J.M. on a NERC research studentship (ref: 610040829) and LG by AstraZeneca Global SHE Research Programme grant to C.R.T. The sequencing was conducted at the Exeter Sequencing Service, funded by Medical Research Council Clinical Infrastructure award (MR/M008924/1), Wellcome Trust Institutional Strategic Support Fund (WT097835MF), Wellcome Trust Multi User Equipment Award (WT101650MA) and BBSRC LOLA award (BB/K003240/1).
Glossary
Abbreviations
- AIC
Akaike information criterion
- AV
atrio-ventricular (valves)
- BCF
bioconcentration factor
- BPA
bisphenol A
- CAVD
calcific aortic valve disease
- CV
cardiovascular
- DMSO
dimethyl sulfoxide
- ECM
extra-cellular matrix
- EDC
endocrine disrupting chemical
- ER
estrogen receptor
- ERE
estrogen response element
- GFP
green fluorescent protein
- LOQ
limit of quantitation
- MBP
4-methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene
- PCR
polymerase chain reaction
- ROI
region of interest
- SGR
specific growth rate
- TEM
transmission electron microscopy
- TFBS
transcription factor binding site
- TG
transgenic
- VB
ventricular-bulbus (valves)
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.8b04281.
Tables describing: zebrafish heart and heart valve morphogenesis (valvulogenesis); water quality; test substance analysis; ERE response - relative mean fluorescence intensity induced by BPA and MBP in the heart valves; effects on CV function; specific growth rate (SGR) and critical swimming speed (Ucritb); differentially expressed genes and enrichment for GO terms, KEGG and Reactome pathways and TFBS motifs in BPA and MBP exposure treatments. Figures showing: Nuclear Magnetic Resonance Spectrum for MBP; relative fluorescence in ERE-GFP transgenic zebrafish larvae following exposure to BPA and MBP; effects of BPA and MBP exposure on specific growth rate (SGR) and critical swimming speed (Ucritb); heat maps showing differentially expressed genes; Venn diagrams showing overlap in differentially expressed genes for BPA and for MBP exposure treatments and for time points 5 and 15 dpf; cluster plots showing enrichment of Reactome pathways and TFBS motifs in BPA and MBP exposure treatments (PDF) (XLXS)
Author Present Address
§ (A.R.B.) Biosciences, College of Life and Environmental Sciences University of Exeter, Lab 201, Geoffrey Pope Building, Stocker Road, Exeter, EX4 4QD, United Kingdom.
Author Contributions
The overall study was designed and implemented by A.R.B., R.C., M.J.H., and C.R.T. A.R.B. ran the study, and analyzed all the data presented in the manuscript; J.M.G. helped to evaluate ERE activation via image analysis of GFP reporters and to measure chemical effects on specific growth rate and critical swimming speed; J.M., L.G., and S.M. helped undertake microdissection of zebrafish hearts, DNA extraction and library preparation for sequencing; J.B. and M.J.W. helped to design and undertake assays to evaluate CV function; M.T. undertook chemical analysis of water and zebrafish embryo-larvae; A.C. and C.H. undertook microscope and TEM work to assess for pathologies of the heart valves, A.P. and M.E.W. synthesized the MBP used in the study; M.J.H., R.A.C., and C.R.T. helped to manage the overall study. The manuscript was written through the contributions of each author, all of whom have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- TEDX . TEDX list of potential endocrine disruptors. 2018; (accessed 26 June 2018): https://endocrinedisruption.org/interactive-tools/tedx-list-of-potential-endocrine-disruptors/.
- EC European Commission . European workshop on the impact of endocrine disrupters on human health and wildlife. Weybridge, UK. 1996; (accessed 1 February 2018): http://www.iehconsulting.co.uk/IEH_Consulting/IEHCPubs/EndocrineDisrupters/WEYBRIDGE.pdf.
- WHO . IPCS Global assessment of the state-of-the-science of endocrine disruptors WHO/PCS/EDC/02.2. 2002; (accessed 15 January 2017): http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/.
- WHO/UNEP . State of the science of endocrine disrupting chemicals – 2012. An assessment of the state of the science of endocrine disruptors prepared by a group of experts for the United Nations Environment Programme (UNEP) and World Health Organisation (WHO). 2012; (accessed 15 January 2017): http://www.who.int/ceh/publications/endocrine/en/.
- EEA European Environment Agency . Impacts of endocrine disrupters on wildlife, people and their environments. The Weybridge+15 (1996–2011) report. EEA Technical report No 2/2012, ISSN 1725-2237. 2012; (accessed March 2018): https://www.eea.europa.eu/publications/the-impacts-of-endocrine-disrupters.
- EC European Commission . Endocrine disruptors, strategy, what is being done, priority list. 2017; (accessed Jan 2018): http://ec.europa.eu/environment/chemicals/endocrine/strategy/being_en.htm.
- EPA Environmental Protection Agency . Endocrine Disruption Screening Program for the 21st Century Dashboard. 2017; (accessed 10 January 2018): https://actor.epa.gov/edsp21/.
- MOE, Ministry of the Environment - Government of Japan . Endocrine Disrupting Effects of Substances. 2017; (accessed 2 November 2017): http://www.env.go.jp/en/chemi/ed.html.
- Deroo B. J.; Korach K. S.. Estrogen receptors and human disease. J. Clin. Invest. 2006, 116( (3), ), 561–570. PMID: 16511588 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi A.; Ciana P.; Belcredito S.; Vegeto E.. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu. Rev. Physiol. 2004, 66, 291–313. PMID: 14977405 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- Ma L.Endocrine disruptors in female reproductive tract development and carcinogenesis. Trends Endocrinol. Metab. 2009, 20( (7), ), 357–363. PMID: 19709900 10.1016/j.tem.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EC European Commission . 4th Report on the implementation of the ″Community Strategy for Endocrine Disrupters″ a range of substances suspected of interfering with the hormone systems of humans and wildlife (COM (1999) 706). Commission staff working paper SEC (2011) 1001 final, Brussels. http://ec.europa.eu/environment/chemicals/endocrine/documents/index_en.htm#SubThemes2 (accessed 1 February 2018).
- Kortenkamp A.; Martin O.; Faust M.; Evans R.; McKinlay R.; Orton F.; Rosivatz E.. State of the art assessment of endocrine disrupters. Final Report to the European Commission Project Contract Number 070307/2009/550687/SER/D3. 2011; URL (accessed March 2018): http://ec.europa.eu/environment/chemicals/endocrine/pdf/sota_edc_final_report.pdf.
- Murphy E.; Kelly D. P.. Estrogen signalling and cardiovascular disease. Circ. Res. 2011, 109( (6), ), 687–696. PMID: 21885836 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC, Merchant Research & Consulting . Bisphenol A (BPA): 2014 World Market Outlook and Forecast up to 2018. Market Publishers Ltd. 2014; (accessed 7 December 2017): http://www.prweb.com/releases/2014/04/prweb11761146.htm.
- Calafat A. M.; Ye X.; Wong L. Y.; Reidy J. A.; Needham L. L.. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116( (1), ), 39–44. PMID: 18197297 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US NTP-CERHR - National Toxicology Program Center for the Evaluation of Risks to Human Reproduction. NTP-CERHR Monograph on the potential human reproductive and developmental effects of bisphenol A; 2008. NIH Publication no: 80–5994. URL (accessed 20 April 2018): www.cerhr.niehs.nih.gov/chemicals/bisphenol/bisphenol.pdf.
- Melzer D.; Rice N. E.; Lewis C.; Henley W. E.; Galloway T. S.. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One 2010, 5( (1), ), e8673. PMID: 20084273 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO Food and Agriculture Organisation/World Health Organisation . Joint FAO/WHO expert meeting to review toxicological and health aspects of bisphenol A: final report, including report of stakeholder meeting on bisphenol A, 1–5 November 2010, Ottawa, Canada. Publ. WHO, Geneva, Switzerland, 2011. (accessed 1 September 2016): http://apps.who.int/iris/bitstream/10665/44624/1/97892141564274_eng.pdf?ua=1.
- Careghini A.; Mastorgio A. F.; Saponaro S.; Sezenna E.; Bisphenol A.. nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ. Sci. Pollut. Res. 201522( (8), ), 5711–41. PMID: 25548011 10.1007/s11356-014-3974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, CEF Panel (EFSA Panel on Food Contact Materials Enzymes, Flavourings and Processing Aids). Draft Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. European Food Safety Authority (EFSA), Parma, Italy. 2014; (accessed 1 September 2016): https://www.efsa.europa.eu/sites/default/files/consultation/140117.pdf.
- EFSA, CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: executive summary. EFSA J. 2015, 13( (1), ), 3978. 10.2903/j.efsa.2015.3978. [DOI] [Google Scholar]
- Corrales J.; Kristofco L. A.; Steele W. B.; Yates B. S.; Breed C. S.; Williams E. S.; Brooks B. W.. Global assessment of bisphenol A in the environment: review and analysis of its occurrence and bioaccumulation. Dose-Response 2015, 13( (3), ) PMID: 26674671, 155932581559830. 10.1177/1559325815598308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons W. V.; Thayer K. A.; Judy B. M.; Taylor J. A.; Curran E. M.; vom Saal F. S.. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 2003, 111, 994–1006. PMID: 12826473. 10.1289/ehp.5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H.; Tokunaga T.; Liu X.; Takayanagi S.; Matsushima A.; Shimohigashi Y.. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ. Health Perspect. 2008, 116( (1), ), 32–38. PMID: 18197296 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohmé M.; Prud’homme S. M.; Boulahtouf A.; Samarut E.; Brunet F.; Bernard L.; Bourguet W.; Gibert Y.; Balaguer P.; Laudet V.. Estrogen-related receptor γ is an in vivo receptor of bisphenol A. FASEB J.. 2014;28( (7), ):3124–3133. PMID: 24744145 10.1096/fj.13-240465. [DOI] [PubMed] [Google Scholar]
- Wozniak A. L.; Bulayeva N. N.; Watson C. S.. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ. Health Perspect. 2005, 113( (4), ), 431–439. PMID: 15811834 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z. G.; Huang W.; Liu Y. X.; Zhu B. Z.. Bisphenol A at a low concentration boosts mouse spermatogonial cell proliferation by inducing the G protein-coupled receptor expression. Toxicol. Appl. Pharmacol.. 2012, 267( (1), ), 88–94. PMID: 23274518. 10.1016/j.taap.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Bromer J. G.; Zhou Y.; Taylor M. B.; Doherty L.; Taylor H. S.. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J.. 2010, 24( (7), ), 2273–2280. PMID: 20181937. [DOI] [PMC free article] [PubMed]
- Mileva G.; Baker S. L.; Konkle A. T.; Bielajew C.. Bisphenol-A: epigenetic reprogramming and effects on reproduction and behavior. Int. J. Environ. Res. Public Health. 2014, 11( (7), ), 7537–7561. PMID: 25054232 10.3390/ijerph110707537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K.; Tagami T.; Akamizu T.; Usui T.; Saijo M.; Kanamoto N.; Hataya Y.; Shimatsu A.; Kuzuya H.; Nakao K.. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. PMID: 12414890 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- Lee H. J.; Chattopadhyay S.; Gong E. Y.; Ahn R. S.; Lee K.. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol. Sci. 2003, 75( (1), ), 40–46. PMID: 12805653 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- US EPA (Environmental Protection Agency) . Bisphenol A (CASRN 80–05–7) Action Plan, 3/29/2010, 2010; (accessed 20 April 2018): https://www.epa.gov/sites/production/files/2015-09/documents/bpa_action_plan.pdf.
- EC JRC, European Commission Joint Research Centre . Summary dossier review: Bisphenol A-DRAFT-JRC-2015, 2015; (accessed 30 November 2017): https://circabc.europa.eu/···/Summary%20dossier%20review_Bisphenol%20A-DRAFT.
- Health Canada . Bisphenol A: Update on the Food Directorate’s risk management commitments for infant formula. December 15, 2014; (accessed 1 December 2017): http://www.hc-sc.gc.ca/fn-an/alt_formats/pdf/securit/packag-emball/bpa/bpa-formula-nourrissons-eng.pdf.
- FDA Food and Drug Administration . Update on Bisphenol A (BPA) for Use in Food Contact Applications, Updated November 2014; (accessed 1 September 2016): http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm064437.htm.
- Health Canada . Significant New Activity Notice No. 15290 (Section 85 of the Canadian Environmental Protection Act, 1999) Publication of final decision on the screening assessment of a substance – Phenol, 4,4’-(1-methylethylidene)bis-(bisphenol A),CASNo. 80–05–7 – specified on the Domestic Substances List [subsection 77(6) of the Canadian Environmental Protection Act, 1999]; Canada Gazette 2008;142( (42), ); (accessed 7 November 2017): www.canadagazette.gc.ca/partI/2008/20081018/html/notice-e.html#d101.
- Usman A.; Ahmad M.. From BPA to its analogues: Is it a safe journey? Chemosphere 2016, 158, 131–142. PMID: 27262103 10.1016/j.chemosphere.2016.05.070. [DOI] [PubMed] [Google Scholar]
- Moreman J.; Lee O.; Trznadel M.; David A.; Kudoh T.; Tyler C. R.. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ. Sci. Technol. 2017, 51( (21), ), 12796–12805. PMID: 29016128 10.1021/acs.est.7b03283. [DOI] [PubMed] [Google Scholar]
- Yoshihara S.; Mizutare T.; Makishima M.; Suzuki N.; Fujimoto N.; Igarashi K.; Ohta S.. Potent estrogenic metabolites of bisphenol a and bisphenol b formed by rat liver s9 fraction: their structures and estrogenic potency. Toxicol. Sci. 2004, 78, 50–59. PMID: 14691209 10.1093/toxsci/kfh047. [DOI] [PubMed] [Google Scholar]
- Okuda K.; Takiguchi M.; Yoshihara S.. In vivo estrogenic potential of 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, an active metabolite of bisphenol A, in uterus of ovariectomized rat. Toxicol. Lett. 2010, 197, 7–11. PMID: 20435109 10.1016/j.toxlet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A.; Ishibashi H.; Kohra S.; Arizono K.; Tominaga N.. Short-term effects of endocrine-disrupting chemicals on the expression of estrogen-responsive genes in male medaka (Oryzias latipes). Aquat. Toxicol. 2005, 72, 239–249. PMID: 15820104 10.1016/j.aquatox.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Ishibashi H.; Watanabe N.; Matsumura N.; Hirano M.; Nagao Y.; Shiratsuchi H.; Kohra S.; Yoshihara S.; Arizono K.. Toxicity to early life stages and an estrogenic effect of a bisphenol A metabolite, 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene on the medaka (Oryzias latipes). Life Sci.. 2005, 77, 2643–2655. PMID: 15961118 10.1016/j.lfs.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Moreman J.; Takesono A.; Trznadel M.; Winter M. J.; Perry A.; Wood M. E.; Rogers N. J.; Kudoh T.; Tyler C. R.. Estrogenic mechanisms and cardiac responses following early life exposure to Bisphenol A (BPA) and its metabolite 4-methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (MBP) in zebrafish. Environ. Sci. Technol. 2018, 52( (11), ), 6656–6665. PMID: 29738667 10.1021/acs.est.8b01095. [DOI] [PubMed] [Google Scholar]
- Lee O.; Takesono A.; Tada M.; Tyler C. R.; Kudoh T.. Biosensor zebrafish provide new insights into potential health effects of environmental estrogens. Environ. Health Perspect. 2012, 120( (7), ), 990–996. PMID: 22510978 10.1289/ehp.1104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion F.; Le Page Y.; Piccini B.; Cardoso O.; Tong S.-K.; Chung B. C.; Kah O.. Screening estrogenic activities of chemicals or mixtures in vivo using transgenic (cyp19a1b-GFP) zebrafish embryos. PLoS One 2012, 7( (5), ), e36069. PMID: 22586461 10.1371/journal.pone.0036069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick D. A.; Iwanowicz L. R.; Hung A. L.; Blazer V. S.; Halpern M. E.. Transgenic zebrafish reveal tissue-specific differences in estrogen signalling in response to environmental water samples. Environ. Health Perspect. 2014, 122( (4), ), 356–362. PMID: 24425189 10.1289/ehp.1307329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. M.; Metz J.; Lee O.; Trznadel M.; Takesono A.; Brown A. R.; Owen S. F.; Kudoh T.; Tyler C. R.. High-content and semi-automated quantification of responses to estrogenic chemicals using a novel translucent transgenic zebrafish. Environ. Sci. Technol. 2016, 50( (12), ), 6536–6545. PMID: 27227508 10.1021/acs.est.6b01243. [DOI] [PubMed] [Google Scholar]
- Green J. M.; Lange A.; Scott A.; Trznadel M.; Wai H. A.; Takesono A.; Brown A. R.; Owen S. F.; Kudoh T.; Tyler C. R. Early life exposure to ethinylestradiol enhances subsequent responses to environmental estrogens measured in a novel transgenic zebrafish. Sci. Rep. 2018, 8 (1), 2699. 10.1038/s41598-018-20922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; PMID: 29426849.
- White R. M.; Sessa A.; Burke C.; Bowman T.; LeBlanc J.; Ceol C.; Bourque C.; Dovey M.; Goessling W.; Burns C. E.; Zon L. I.. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008, 2( (2), ), 183–189. PMID: 18371439 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal F. S.; Akingbemi B. T.; Belcher S. M.; Birnbaum L. S.; Crain D. A.; Eriksen M.; Farabollini F.; Guillette L. J. Jr; Hauser R.; Heindel J. J.; Ho S. M.; Hunt P. A.; Iguchi T.; Jobling S.; Kanno J.; Keri R. A.; Knudsen K. E.; Laufer H.; LeBlanc G. A.; Marcus M.; McLachlan J. A.; Myers J. P.; Nadal A.; Newbold R. R.; Olea N.; Prins G. S.; Richter C. A.; Rubin B. S.; Sonnenschein C.; Soto A. M.; Talsness C. E.; Vandenbergh J. G.; Vandenberg L. N.; Walser-Kuntz D. R.; Watson C. S.; Welshons W. V.; Wetherill Y.; Zoeller R. T.. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007, 24( (2), ), 131–138. PMID: 17768031 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondesson M.; Hao R.; Lin C. Y.; Williams C.; Gustafsson J.-Å.. Estrogen receptor signalling during vertebrate development. Biochim. Biophys. Acta, Gene Regul. Mech. 2015, 1849( (2), ), 142–151. PMID: 24954179 10.1016/j.bbagrm.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartman T.; Walsh E. C.; Wen K. K.; McKane M.; Ren J.; Alexander J.; Rubenstein P. A.; Stainier D. Y.. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol.. 2004, 2( (5), ), E129. PMID: 15138499 10.1371/journal.pbio.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. T.; Bartman T.. Analysis of heart valve development in larval zebrafish. Dev. Dyn. 2009, 238( (7), ), 1796–1802. PMID: 19449301 10.1002/dvdy.21976. [DOI] [PubMed] [Google Scholar]
- Staudt D.; Stainier D.. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu. Rev. Genet. 2012, 46, 397–418. PMID: 22974299 10.1146/annurev-genet-110711-155646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo W. R.; Burggren W. W.. O2 consumption and heart rate in developing zebrafish (Danio rerio): influence of temperature and ambient O2. Am. J. Physiol. 1999, 276( (2 Pt 2), ), R505–13. PMID: 9950931. 10.1152/ajpregu.1999.276.2.R505 [DOI] [PubMed] [Google Scholar]
- Chávez M. N.; Aedo G.; Fierro F. A.; Allende M. L.; Egaña J. T.. Zebrafish as an emerging model organism to study angiogenesis in development and regeneration. Front. Physiol. 2016, 8, (7), , 56. PMID: 27014075 10.3389/fphys.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes A. C.; Stadt H. A.; Shepherd I. T.; Kirby M. L.. Solving an enigma: Arterial pole development in the zebrafish heart. Dev. Biol. 2006, 290( (2), ), 265–276. PMID: 16405941 10.1016/j.ydbio.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Hove J. R., Koster R. W.; Forouhar A. S.; Acevedo-Bolton G.; Fraser S. E.; Gharib M.. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 2003, 421, 172 -177. PMID: 12520305 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Chen I. H.; Wang H. H.; Hsieh Y. S.; Huang W. C.; Yeh H. I.; Chuang Y. J.. PRSS23 is essential for the Snail-dependent endothelial-to-mesenchymal transition during valvulogenesis in zebrafish. Cardiovasc. Res. 2013, 97( (3), ):443–453. PMID: 23213106 10.1093/cvr/cvs355. [DOI] [PubMed] [Google Scholar]
- Vermot J.; Forouhar A. S.; LieblingM Wu D.; Plummer D.; Gharib M.; Fraser S. E.. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol.. 2009, 7( (11), ), e1000246. PMID: 19924233 10.1371/journal.pbio.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S.; Marrs J. A.. Zebrafish as a vertebrate model system to evaluate effects of environmental toxicants on cardiac development and function. Int. J. Mol. Sci. 2016, 17( (12), ), 2123. PMID: 27999267 10.3390/ijms17122123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beis D.; Bartman T.; Jin S. W.; Scott I. C.; D’Amico L. A.; Ober E. A.; Verkade H.; Frantsve J.; Field H. A.; Wehman A.; Baier H.; Tallafuss A.; Bally-Cuif L.; Chen J. N.; Stainier D. Y.; Jungblut B.. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 2005, 132, 4193–4204. PMID: 16107477 10.1242/dev.01970. [DOI] [PubMed] [Google Scholar]
- Stainier D. Y.; Lee R. K.; Fishman M. C.. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development 1993, 119, 31–40. PMID: 8275863. [DOI] [PubMed] [Google Scholar]
- Gore M.; Burggren W. W.. Cardiac and metabolic physiology of early larval zebrafish (Danio rerio) reflects parental swimming stamina. Front. Physiol. 2012, 3, 35. PMID: 22375123 10.3389/fphys.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca E.; Zaccaria G. M.; Hadhoud M.; Rizzo G.; Ponzini R.; Morbiducci U.; Santoro M. M.. ZebraBeat: A flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci. Rep. 2014 5, 4, 4898. PMID 25790189. [Google Scholar]
- Parker T.; Libourel P.-A.; Hetheridge M. J.; Cumming R. I.; Sutcliffe T. P.; Goonesinghe A. C.; Ball J. S.; Owen S. F.; Chomis Y.; Winter M. J.. A multi-endpoint in vivo larval zebrafish (Danio rerio) model for the assessment of integrated cardiovascular function. J. Pharmacol. Toxicol. Methods 2014, 69( (1), ), 30–38. PMID: 24140389 10.1016/j.vascn.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Pelster B.; Burggren W. W.. Disruption of hemoglobin oxygen transport does not impact oxygen-dependent physiological processes in developing embryos of zebrafish (Danio rerio). Circ. Res. 1996, 79, 358–362. PMID: 8756015 10.1161/01.RES.79.2.358. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y.; Tsutsumi O.; Takai Y.; Kamei Y.; Taketani Y.. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod.. 2002;17:2839–2841. PMID: 12407035 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Schonfelder G.; Wittfoht W.; Hopp H.; Talsness C. E.; Paul M.; Chahoud I.. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002, 110, A703–A707. PMID: 12417499. 10.1289/ehp.021100703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholst C.; Wynne P.; Marriott P.; Pedersen S.; Bjerregaard P.. Metabolism of bisphenol A in zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss) in relation to estrogenic response. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2003,135, 169–177. PMID: 12860056 10.1016/S1532-0456(03)00088-7. [DOI] [PubMed] [Google Scholar]
- Fang Q.; Shi Q.; Guo Y.; Hua J.; Wang X.; Zhou B.. Enhanced bioconcentration of bisphenol A in the presence of nano-TiO2 can lead to adverse reproductive outcomes in zebrafish. Environ. Sci. Technol. 2016, 50( (2), ), 1005–1013. PMID: 26694738 10.1021/acs.est.5b05024. [DOI] [PubMed] [Google Scholar]
- ISO . Water Quality Sampling, ISO 5667, Part 16. Guidance on Biotesting of Samples; Wiley-VCH: Weinheim-New York. 1997; 30, http://www.iso.org (accessed 3 April 2018). [Google Scholar]
- Burns C. G.; MacRae C. A.. Purification of hearts from zebrafish embryos. BioTechniques 2006, 40( (3), ), 278–281. PMID: 16568816. 10.2144/000112135 [DOI] [PubMed] [Google Scholar]
- Hu N.; Sedmera D.; Yost H. J.; Clark E. B.. Structure and function of the developing zebrafish heart. Anat. Rec. 2000, 60( (2), ), 148–57. PMID: 10993952. [DOI] [PubMed] [Google Scholar]
- Schneider C. A.; Rasband W. S.; Eliceiri K. W.. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9( (7), ), 671–675. PMID: 22930834 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker W. E.Growth rates and models. In Fish Physiology, Volume VIII. Bioenergetics and Growth; Hoar W. S., Randall D. J., Brett J. R., Eds.; Academic Press: New York, NY, 1979; p 677–743. [Google Scholar]
- Brett J. R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish. Res. Board Can. 1964, 21, 1183–1226. 10.1139/f64-103. [DOI] [Google Scholar]
- Trapnell C.; Roberts A.; Goff L.; Pertea G.; Kim D.; Kelley D. R.; Pimentel H.; Salzberg S. L.; Rinn J. L.; Pachter L.. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. PMID: 22383036 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M. I.; Huber W.; Anders S.. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.. 2014, 15, 550. PMID: 25516281 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. W.; Sherman B. T.; Lempicki R. A.. Systematic and integrative analysis of large gene 569 lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4( (1), ), 44–57. PMID: 19131956 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Kuleshov M. V.; Jones M. R.; Rouillard A. D.; Fernandez N. F.; Duan Q.; Wang Z.; Koplev S.; Jenkins S. L.; Jagodnik K. M.; Lachmann A.; McDermott M. G.; Monteiro C. D.; Gundersen G. W.; Ma’ayan A.. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res.. 2016, 44, Web Server issue gkw377: W90–W97. PMID: 27141961, W90. 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabregat A.; Jupe S.; Matthews L.; Sidiropoulos K.; Gillespie M.; Garapati P.; Haw R.; Jassal B.; Korninger F.; May B.; Milacic M.; Roca C. D.; Rothfels K.; Sevilla C.; Shamovsky V.; Shorser S.; Varusai T.; Viteri G.; Weiser J.; Wu G.; Stein L.; Hermjakob H.; D’Eustachio P.. The Reactome Pathway Knowledgebase. Nucleic Acids Res.. 2018, 4, 46( (D1), ), D649-D655. PMID: 29145629 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M.; Furumichi M.; Tanabe M.; Sato Y.; Morishima K.. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res.. 2017, 45, D353–D361. PMID: 27899662 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffier M.; Kähäri A.; Komorowska M.; Keenan S.; Laird M.; Longden I.; Proctor G.; Searle S.; Staines D.; Taylor K.; Vullo A.; Yates A.; Zerbino D.; Flicek P.. Ensembl core software resources: storage and programmatic access for DNA sequence and genome annotation. Database 2017, bax020 PMID: 201728365736, 10.1093/database/bax020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeay R. C.; Bailey T. L.. Motif Enrichment Analysis: a unified framework and an evaluation on ChIP data. BMC Bioinf.. 2010, 11, 165. PMID: 20356413 10.1186/1471-2105-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.; Fornes O.; Stigliani A.; Gheorghe M.; Castro-Mondragon J. A.; van der Lee R.; Bessy A.; Chèneby J.; Kulkarni S. R.; Tan G.; Baranasic D.; Arenillas D. J.; Sandelin A., Vandepoele K.; Lenhard B.; Ballester B.; Wasserman W. W.; Parcy F.; Mathelier A.. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res.. 2018, 46( (D1), ), D260–D266. PMID: 29140473 10.1093/nar/gkx1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A.; Ishibashi H.; Arizono K.; Tominaga N.. In vivo and in silico analyses of estrogenic potential of bisphenol analogs in medaka (Oryzias latipes) and common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2015, 120, 198–205. PMID: 26086576 10.1016/j.ecoenv.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Kwon H.-B.; Wang S.; Helker C. S. M.; Rasouli S. J.; Maischein H.-M.; Offermanns S.; Herzog W.; Stainier D. Y. R.. In vivo modulation of endothelial polarization by Apelin receptor signalling. Nat. Commun. 2016, 7, 11805. PMID: 27248505 10.1038/ncomms11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieramonti L.; Foglia E. A.; Malavasi S.; D’Andrea C.; Valentini G.; Cotelli F.; Bassi A.. Quantitative measurement of blood velocity in zebrafish with optical vector field tomography. J. Biophotonics 2015, 8( (1–2), ), 52–59. PMID: 24339189 10.1002/jbio.201300162. [DOI] [PubMed] [Google Scholar]
- Noseworthy P. A.; Asirvatham S. J.. The knot that binds mitral valve prolapse and sudden cardiac death. Circulation 2015, 132( (7), ), 551–552. PMID: 26160860 10.1161/CIRCULATIONAHA.115.017979. [DOI] [PubMed] [Google Scholar]
- Elliott J. M.The energetics of feeding, metabolism and growth of brown trout (Salmo trutta L.) in relation to body weight, water temperature and ration size. J. Anim. Ecol. 1976, 45( (3), ), 923–948. 10.2307/3590. [DOI] [Google Scholar]
- Ejbye-Ernst R.; Michaelsen T. Y.; Tirsgaard B.; Wilson J. M.; Jensen L. F.; Steffensen J. F.; Pertoldi C.; Aarestrup K.; Svendsen J. C.. Partitioning the metabolic scope: the importance of anaerobic metabolism and implications for the oxygen- and capacity-limited thermal tolerance (OCLTT) hypothesis. Conserv. Physiol. 2016, 4( (1), ). PMID: 27293766 cow019. 10.1093/conphys/cow019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatto B.; Pelster B.; Burggren W. W.. Growth and metabolism of larval zebrafish: effects of swim training. J. Exp. Biol. 2001, 204, 4335–4343. PMID: 11815657. [DOI] [PubMed] [Google Scholar]
- Plaut II; Gordon M.. Swimming metabolism of wild-type and cloned zebrafish Brachydanio rerio. J. Exp. Biol. 1994, 194, 209–223. PMID: 9317659. [DOI] [PubMed] [Google Scholar]
- Palstra A. P.; Tudorache C.; Rovira M.; Brittijn S. A.; Burgerhout E.; van den Thillart G.; Spaink H. P.; Planas J. V.. Establishing zebrafish as a novel exercise model: swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS One 2010, 5( (12), ), e14483. PMID: 21217817 10.1371/journal.pone.0014483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welboren W. J.; Stunnenberg H. G.; Sweep F. C.; Span P. N.. Identifying estrogen receptor target genes. Mol. Oncol.. 2007, 1( (2), ), 138–43. PMID: 19383291 10.1016/j.molonc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal J. F.; Lenfant F.; Metivier R.; Flouriot G.; Henrion D.; Adlanmerini M.; Fontaine C.; Gourdy P.; Chambon P.; Katzenellenbogen B.; Katzenellenbogen J.. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol. Rev.. 2017, 97( (3), ), 1045–1087. PMID: 28539435 10.1152/physrev.00024.2016. [DOI] [PubMed] [Google Scholar]
- Small A.; Kiss D.; Giri J.; Anwaruddin S.; Siddiqi H.; Guerraty M.; Chirinos J. A.; Ferrari G.; Rader D. J.. Biomarkers of calcific aortic valve disease. Arterioscler., Thromb., Vasc. Biol. 2017, 37( (4), ), 623–632. PMID: 28153876 10.1161/ATVBAHA.116.308615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusanescu G.; Weissleder R.; Aikawa E.. Notch signaling in cardiovascular disease and calcification. Curr. Cardiol. Rev. 2008, 4( (3), ): 148–156. PMID: 19936191 10.2174/157340308785160552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher S. M.; Gear R. B.; Kendig E. L.. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology, 2015, 156( (3), ), 882–895. PMID: 25594700 10.1210/en.2014-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forough R.; Scarcello C.; Perkins M.. Cardiac biomarkers: a focus on cardiac regeneration. J. Tehran Heart Cent. 2011, 6( (4), ), 179–86. PMID: 23074366. [PMC free article] [PubMed] [Google Scholar]
- Nordström P.; Glader C. A.; Dahlen G.; Birgander L. S.; Lorentzon R.; Waldenstrom A.; Lorentzon M.. Oestrogen receptor alpha gene polymorphism is related to aortic valve sclerosis in postmenopausal women. J. Intern. Med. 2003, 254, 140–146. PMID: 12859695 10.1046/j.1365-2796.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- Bosse Y.; Mathieu P.; Pibarot P.. Genomics: the next step to elucidate the etiology of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 2008, 51, 1327–1336. PMID: 18387432 10.1016/j.jacc.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Elmariah S.; Mohler E. R.. The pathogenesis and treatment of the valvulopathy of aortic stenosis: Beyond the SEAS. Curr. Cardiol. Rep. 2010, 12( (2), ), 125–132. PMID: 20425167 10.1007/s11886-010-0089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L.; Shao Y.; Huang Y.; Yao T.; Lu L. M.. 17β-Estradiol inhibits angiotensin II-induced collagen synthesis of cultured rat cardiac fibroblasts via modulating angiotensin II receptors. Eur. J. Pharmacol. 2007, 567, 186–192. PMID:17511985 10.1016/j.ejphar.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Petrov G.; Regitz-Zagrosek V.; Lehmkuhl E.; Krabatsch T.; Dunkel A.; Dandel M.; Dworatzek E.; Mahmoodzadeh S.; Schubert C.; Becher E.; Hampl H.; Hetzer R.. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation 2010, 14, 122( (11 Suppl), ), S23–8. PMID: 20837918 10.1161/CIRCULATIONAHA.109.927764. [DOI] [PubMed] [Google Scholar]
- Rodriguez K. J.; Piechura L. M.; Porras A. M.; Masters K. S.. Manipulation of valve composition to elucidate the role of collagen in aortic valve calcification. BMC Cardiovasc. Disord. 2014, 14, 29. PMID: 24581344 10.1186/1471-2261-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton R. B.; Yutzey K. E.. Heart valve structure and function in development and disease. Annu. Rev. Physiol. 2011, 3, 29–46. PMID: 20809794 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E. B.Cardiovascular birth defects and prenatal exposure to female sex hormones: A reevaluation of data reanalysis from a large prospective study. Teratology 1994, 49, 162–166. PMID: 8059421 10.1002/tera.1420490303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.