Abstract
Objectives:
Severe hemorrhage is a common cause of death despite the recent advances in critical care. Conventional resuscitation fluids are designed to reestablish tissue perfusion, but they fail to prevent lethal inflammatory responses. Our previous studies indicate that ethyl pyruvate (EP) inhibits tumor necrosis factor (TNF) production from macrophages. Here, we analyze whether EP can provide a therapeutic anti-inflammatory value to resuscitation fluids.
Design:
Laboratory animal experiments.
Setting:
Animal research laboratory at university medical school.
Subjects:
Adult male Sprague-Dawley rats.
Interventions:
Lethal hemorrhage over 15 minutes to reach a mean arterial blood pressure of 35–40 mm Hg and subsequent maintenance of this mean arterial blood pressure for another 15 minutes. Resuscitation was limited to 15 mL/kg Hextend with or without EP.
Results:
Resuscitation with Hextend supplemented with EP rescued all the animals from lethal hemorrhage. Unlike conventional fluids, EP inhibited the production of inflammatory and cardiodepressant factors such as TNF and high mobility group B protein-1. From a pharmacologic perspective, resuscitation with EP was particularly effective inhibiting TNF production in the spleen and the heart. Unlike other anti-inflammatory strategies, EP mitigated systemic inflammation through a mechanism independent of the spleen. At the molecular level, EP inhibited both poly(ADP-ribose) polymerase and p65RelA DNA binding without affecting IκBα activation.
Conclusions:
EP may be a promising anti-inflammatory supplement to improve survival during resuscitation in critical care. (Crit Care Med 2009; 37:860–868)
Keywords: inflammation, hemorrhage, ethyl pyruvate, tumor necrosis factor, resuscitation, high mobility group B protein-1
The innate immune system is an essential component of our defenses to trauma. But at the same time, it is one of the principle causes of morbidity and mortality in critical care (1, 2). This effect is particularly dramatic in severe hemorrhage. After losing over 50% of the total blood volume, the system is unable to reestablish tissue perfusion. Thus, resuscitation fluids are classically designed to restore circulatory volume and tissue perfusion. However, resuscitation can trigger systemic inflammatory responses that can be more dangerous than the original hemorrhage. Inflammatory cytokines such as tumor necrosis factor (TNF) cause lethal cardiovascular shock and multiple organ failure (1, 3). TNF is one of the most characteristic cardiodepressant factors, as it appears to be a sufficient and necessary mediator of “hemorrhagic shock” (4, 5): a) TNF is found in patients and experimental models of “hemorrhagic shock”; b) TNF itself is capable of triggering the spectrum of hemodynamic, metabolic, and pathologic sequelae of “hemorrhagic shock”; and c) TNF neutralization attenuates cardiovascular shock. For these reasons, there is a great interest in inhibiting the production of inflammatory cytokines during resuscitation.
Ringer lactated is the most common resuscitation fluid used to restore circulating volume in critical care (6, 7). The composition of Ringer’s lactate solution resembles the physiologic composition of plasma. Advanced resuscitation fluids include Hextend, the novel plasma volume expander containing 6% hydroxyethyl starch in Ringer’s lactate (8). Hetastarch creates oncotic pressure, which is normally provided by blood proteins and permits retention of intravascular fluid. Hextend is being established as standard care for the Army’s Special Forces because, as a plasma volume expander, it appears to be particularly indicated for scenarios of limited supplies including military operations and civilian mass casualties. Resuscitation with Hextend prevents multiple organ injury (9) and improves short-term survival as compared with saline (10). Still, advanced and common resuscitation fluids are based on lactated solutions. Lactic acid (2-hydroxypropanoic acid) is used as an inert compound to keep the osmolarity, but it fails to confer any therapeutic potential. Actually, recent studies suggest that lactate may exacerbate lactic acidosis during hypovolemia (11). Unlike lactate, pyruvic acid (2-ketopropanoic acid), a structurally similar natural molecule, has potential antioxidant and anti-inflammatory properties that protect mammalian cells against ischemic injury and cytotoxic damage (12). Solutions containing pyruvate are beneficial in experimental models of stroke and hemorrhage (13). Despite its therapeutic potential, the clinical use of pyruvate is hampered by its instability and toxicity (12). Aqueous solutions of pyruvate spontaneously degrade via condensation and cyclization reactions to form 2,4-dihydroxy-2-methylglutarate, a mitochondrial poison (14). These limitations appear to be overcome by ethyl pyruvate (EP), a stable lipophilic pyruvate derivative (15, 16). EP is compatible with blood products, but unlike lactate, it can provide a therapeutic benefit to the Ringer’s solution. EP is a stable, soluble, nontoxic compound that lacks the potential toxicity of pyruvate and is classified as general recognized as safe by the Food and Drug Administration.
Recently, we reported that EP was an effective anti-inflammatory compound that inhibited TNF production from human and murine macrophages (16, 17). In vivo, EP attenuates systemic inflammation and improves survival in experimental sepsis (16–21) and other models of systemic inflammation (22–25). However, a recent study indicates that a single dose of EP may worsen survival in endotoxemia (26) depending on the doses and time of administration (27). These results reveal the need to study the mechanism of action of EP and the factors affecting its therapeutic potential (27). These studies are of particular interest because a phase II trial using EP in cardiopulmonary bypass was recently terminated after the enrollment of 102 patients; yet the final results have not been reported (28). Here, we have analyzed whether EP could provide an anti-inflammatory value to advanced resuscitation fluids such as Hextend. Because our previous studies indicated that some anti-inflammatory compounds were not effective in splenectomized animals, we analyzed the anti-inflammatory potential of EP in both normal and splenectomized animals. If so, resuscitation with a small volume of Hextend supplemented with EP should provide a therapeutic advantage particularly relevant in scenarios of limited supplies. Our results indicate that all animals resuscitated with EP survived lethal hemorrhage. Resuscitation with EP mitigates systemic TNF and high mobility group B protein-1 (HMGB1) levels, and induces a complete and lasting protection against lethal hemorrhage in both normal and splenectomized animals. EP was particularly efficient at inhibiting TNF levels in the heart and the spleen. At the molecular level, EP inhibited poly(ADP-ribose) polymerase (PARP) activation and attenuated p65RelA DNA binding. EP might be a promising anti-inflammatory supplement to improve survival during resuscitation.
MATERIALS AND METHODS
Animal Experiments.
Adult male Sprague-Dawley (350–450 g) rats from Harlen Sprague-Dawley (Indianapolis, IN) were allowed to acclimate for 5–7 days housed at 25°C on a 12-hour light/dark cycle. Animals were randomly grouped and assigned to a specific experiment. All animal experiments were performed in accordance with the National Institutes of Health Guidelines under protocols approved by the Institutional Animal Care and Use Committee of the North Shore University Hospital, and the University of Medicine and Dentistry of New Jersey. Investigators were blind to the experimental treatment.
Hemorrhage.
Animals were anesthetized by inhalation of isoflurane (5% induction, 2% maintenance; Minrad, Buffalo, NY) and subjected to surgical catheter placement into the femoral artery and vein using femoral artery catheter (Braintree Scientific, Braintree, MA). To avoid blood clot and maintain catheter patency, the catheters were flushed with 1% heparin solution immediately before placement. Heparin was not used in vivo during the experiment or observation period in agreement with previous publications (29). After the catheter implantation, the blood pressure and the heart rate were recorded for 15 minutes to establish a physiologic baseline. Then, the animals were subjected to hemorrhage for 15 minutes to reach a mean arterial blood pressure of 35–40 mm Hg and subsequent maintenance of this blood pressure by continued blood withdrawal for another 15 minutes. After the shock phase, resuscitated animals received specific resuscitation treatment over 40 minutes with a limited volume of 15 mL/kg (equivalent to 1000 mL of Hextend in a 70-kg man). Hextend was purchased from BioTime (Berkeley, CA). Shed blood was considered lost and it was not reinfused. Heart rate, mean arterial blood pressure, shed blood volume, and intravenous fluid volume were continuously monitored and recorded. After resuscitation treatment, the anesthesia was stopped and the animals were allowed to recover from anesthesia and housed individually in regular cages. Splenectomy was performed in a subgroup of Harlen Sprague-Dawley animals as described previously (30). Briefly, the spleen was identified following a midline laparotomy incision, and removed after appropriate blood vessel ligation. Sham animals underwent laparotomy without splenectomy.
Blood Chemistry and Cytokine Measurement.
Activation blood was collected at 2 hours after the hemorrhagic shock and analyzed using the i-Stat blood analyzer (Abbot Laboratories, Abbott Park, IL). Lung function was assessed by analyzing blood gases including total and partial carbon dioxide (TCO2, PCO2), bicarbonate (HCO3), pH, and the base excess of extracellular fluid. The organ function tests included anion sodium, potassium, chloride, anion gap, total plasma protein, and blood urea nitrogen. Blood chemistry also included glucose, hematocrit, and hemoglobin. The systemic inflammatory status was assessed by analyzing critical proinflammatory cytokines and cardiodepressant factors such as TNF and high mobility group box (HMGB)-1. TNF concentration in mouse serum and in conditioned media from mouse leukaemic monocyte macrophage cell line 264.7 cell cultures was measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). HMGB1 was analyzed by Western blot as previously described (31).
Nuclear factor-kappa B and PARP Analyses.
Homogenates were normalized by protein concentration, and the activation of the nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) was analyzed by Western blot by using anti-IκBα polyclonal antibody ab7217 and antiphosphorylated-IκBα(phospho S32 + S36) monoclonal antibody (clone39A1431) ab12135 (Abcam; Cambridge, MA). Mouse monoclonal [Ac-15] to beta actin anti-β-actin protein was used for Western blot as loading control. Transcriptional activity of splenic nuclear factor-kappa B (NF-κB) was analyzed by TransAM DNA-binding enzyme-linked immunosorbent assay (Active Motif; Cambridge, MA) following manufacturer’s instructions. The results were confirmed by electrophoretic mobility-shift assay from nuclear extracts performed as previously described (31). PARP activity in organ homogenates was analyzed using a commercially available kit (R&D Systems, Cat#4677–096K) following the manufacturer’s instructions. Protein levels were measured using the Bradford method (Bio-Rad, Hercules, CA), and PARP activity was normalized to protein content.
Statistical Analyses.
All data in the figures and text were expressed as mean ± SD. Analyses of normality and homogeneity of variance were performed to verify the assumptions of analysis of variance. Data were log transformed where applicable. Statistical analyses were performed using the one-way analysis of variance with the Bonferroni’s correction. Analysis of variance was used to compare all treatments and specific pairwise comparisons as stated in the experiments particularly to compare control vs. Hextend, Hextend vs. HEP (Hextend containing 50 mM EP), and control vs. HEP. The Student’s t test was used to compare mean values between two experimental groups. Statistical analyses of survival were determined using the log-rank test. Kaplan-Meier product-limit method was used for survival graphs. A p value <0.05 was considered statistically significant.
RESULTS
EP Provides Therapeutic Potential to Resuscitation Fluids.
Hemorrhagic shock required withdrawing 21 ± 4.3 mL blood/kg body weight and the maintenance of that blood pressure for 15 minutes required another 7 ± 2.7 mL blood/kg body weight. All animals without resuscitation treatment (NR; n = 10) did not re-establish normal blood pressure (Fig. 1A), and died within the first 5 hours after shock (Fig. 1B). Resuscitation with Hextend (HXT) protected 11% of the animals in a statistically insignificant manner (n = 9; p > 0.05 vs. NR, log-rank test). The addition of 50 mM EP to the Hextend solution (HEP50) provided a statistically significant therapeutic potential and all the animals resuscitated with 50 mM EP survived (n = 7; p < 0.01 vs. NR; p < 0.05 vs. HXT, log-rank test). EP improved survival in hemorrhagic shock in a concentration-dependent manner (Fig. 1C). Addition of 25 mM EP to Hextend significantly protected 72% of the animals with hemorrhagic shock (n = 7; p < 0.05 Hextend + EP25 mM vs. Hextend, log-rank test), whereas 5 mM EP did not induce a statistically significant protection (p > 0.05 Hextend + EP5 mM vs. Hextend, log-rank test). Our analyses excluded the animals that died during the surgical procedure and counted only those animals that completed the resuscitation treatment. All nonsurviving animals died within the first 8 hours after hemorrhagic shock. All the animals that passed this critical period, survived the hemorrhagic shock, and when no late deaths were found the animals were followed up for up to 7 days.
Figure 1.
Ethyl pyruvate (EP) protects animals from lethal hemorrhage. Adult male Sprague-Dawley hemorrhagic rats received no resuscitation treatment (NR) or were resuscitated with 15 mL/kg (intravenous) Hextend (HXT) or HXT supplemented with 50 mM EP (HEP). A, Arterial blood pressure was recorded during the hemorrhage and the resuscitation treatment. B, Resuscitation with HXT allowed the animals to restore normal blood pressure and yet 89% of the animals died. Resuscitation with HXT supplemented with 50 mM of EP (HEP50) statistically protected all the animals (n = 7). C, EP improved survival in hemorrhage in a concentration-dependent manner in the animals treated with 50 mM (HEP50), 25 mM (HEP25), or 5 mM (HEP5) EP. *Represents p < 0.01 survival log-rank test vs. NR *Represents p < 0.05 survival log-rank test vs. HXT. BP, blood pressure.
EP Prevents Systemic Inflammation During Hemorrhage.
Characteristic pathologic markers of hemorrhage were assessed by arterial blood chemistry analyses. Blood was collected at 2 hours after hemorrhagic shock, which is approximately 30 minutes before the average time of death for the control animals without resuscitation treatment (161 ± 26 minutes). Animals without resuscitation were characterized by uremia, metabolic acidosis, and hyperglycemia (NR; n = 5; Fig. 2). Animals without resuscitation showed high levels of blood urea nitrogen (NR = 24 ± 3.5 mg/dL vs. control = 13 ± 0.5 mg/dL; n = 5/group, p < 0.05; Fig. 2A) representing a moderate renal failure. Although resuscitation with Hextend or Hextend supplemented with EP improved survival, they did not prevent uremia (n = 5/group, Fig. 2A). Hemorrhagic animals without resuscitation also showed metabolic acidosis that was associated with elevated anion gap (Fig. 2B and C), low levels of bicarbonate (Fig. 2E), and hypochloremia (Fig. 2D). Resuscitation with Hextend prevented metabolic acidosis and reestablished normal values of anion gap and bicarbonate but not hypochloremia. Hemorrhage caused a significant hyperglycemic response (NR = 561 ± 39 mg/dL vs. control = 173 ± 12 mg/dL; n = 5/group, p < 0.05; Fig. 2F) that was prevented by both resuscitation with Hextend and Hextend supplemented with 50 mM EP (Fig. 2F).
Figure 2.
Blood chemistry analyses after hemorrhage (Hem). Blood from control adult male Sprague-Dawley rats, or hemorrhagic animals without resuscitation treatment (NR, n = 5) or resuscitated with 15 mL/kg (intravenous) Hextend (HXT, n = 5) or HXT supplemented with 50 mM ethyl pyruvate (HEP, n = 5) was collected at 2 hours after the hemorrhagic load to analyze (A) blood urea nitrogen (BUN), (B) pH, (C) the anion gap (AnGap), (D) chloride (Cl), (E) bicarbonate (HCO3), and (F) glucose. *Represents p < 0.05 vs. control (n = 5/group; one-way analysis of variance with Bonferroni’s corrections).
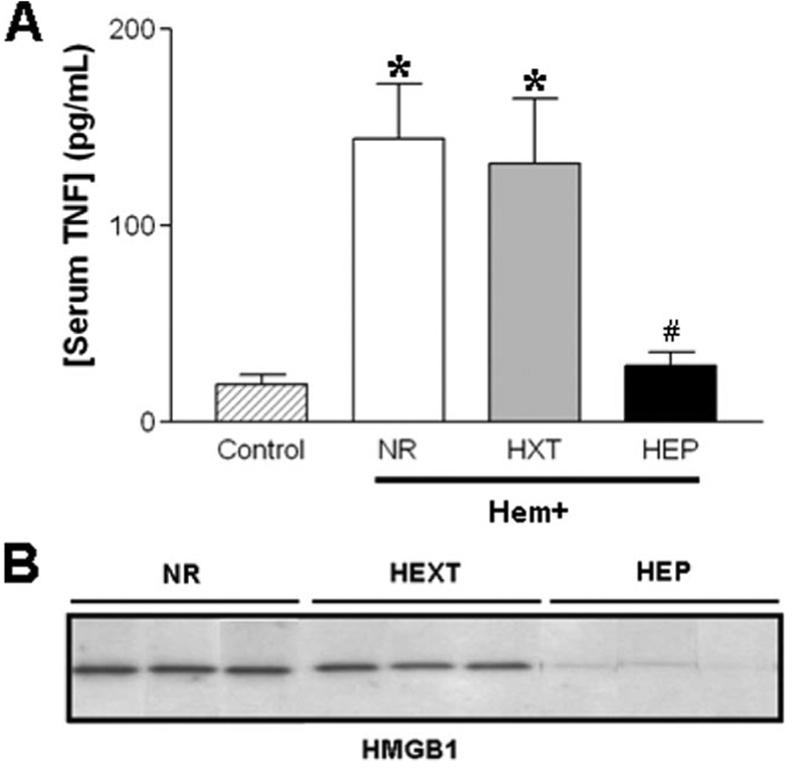
The most significant effect of EP was the inhibition of proinflammatory and cardiodepressant factors including TNF and HMGB1 (32, 33). Hemorrhage-induced lethal systemic TNF responses were not prevented by resuscitation with Hextend (NR = 144 ± 28 pg/mL; n = 12/group, Fig. 3A). However, resuscitation with Hextend containing EP prevented this lethal response (28 ± 7 pg/mL, n = 12/group, p < 0.05 vs. NR and HXT) inducing serum TNF levels statistically similar to those of control animals (p > 0.05 HEP vs. control animals without hemorrhage). Serum HMGB1 levels were analyzed by Western blots at 2 hours after the shock. Hemorrhage induced a lethal systemic HMGB1 response that was not prevented by resuscitation with Hextend (Fig. 3B). However, EP prevented this systemic HMGB1 response.
Figure 3.
Ethyl pyruvate (EP) diminishes circulating tumor necrosis factor (TNF) and high mobility group B protein-1 (HMGB1) levels. Blood from control and hemorrhagic animals was analyzed at 2 hours to determine circulating (A) TNF and (B) HMGB1 levels. *Means p < 0.01 vs. control. #Represents p < 0.01 for Hextend (HXT) supplemented with EP (HEP) vs. HXT. (n = 12/group; one-way analysis of variance with Bonferroni’s corrections). NR, without resuscitation treatment.
Next, we analyzed the effects of EP in specific organs (Fig. 4, n = 6/group). Resuscitation with Hextend attenuated TNF levels in most of the organs but not in the heart. Resuscitation with Hextend supplemented with EP induced a major effect inhibiting TNF levels in the heart and the spleen by 66% and 80%, respectively. Real-time polymerase chain reaction confirmed that EP inhibited TNF production in the spleen and the heart at the transcriptional level (data not shown) similar to that in macrophages (17). Splenic TNF levels of those animals resuscitated with EP were statistically similar to control animals without hemorrhage (Fig. 4D, p > 0.05 HEP vs. control animals without hemorrhage, n = 6/group).
Figure 4.
Ethyl pyruvate (EP) significantly attenuates cardiac and splenic tumor necrosis factor (TNF) levels. Blood and organs from control or hemorrhagic animals were analyzed at 2 hours to determine TNF concentration in the (A) lung, (B) liver, (C) heart, and (D) spleen. *Represents p < 0.01 as compared with control. #Represents p < 0.01 for Hextend (HXT) supplemented with EP (HEP) vs. HXT. n = 6/group; one-way analysis of variance with Bonferroni’s corrections.
Pharmacologic Implications of the Spleen in Resuscitation During Hemorrhage.
The pharmacologic implications of the spleen during resuscitation were studied in splenectomized animals with hemorrhage as described earlier (30). Blood chemistry analyses showed that hemorrhage induced similar effects in normal and splenectomized animals. Hemorrhage in splenectomized animals (n = 5/group) induced similar uremia, metabolic acidosis, and hyperglycemia (Fig. 5), but a potential milder hypochloremia and anion gap (Fig. 5C and D) than to that found in normal animals. Again, the most significant effect of EP was found on the inflammatory responses. Surprisingly, splenectomy exacerbated the systemic TNF levels during resuscitation (n = 4/group, Fig. 6A). These higher serum TNF levels correlated with higher TNF levels in the liver of splenectomized animals but not in the lung or the heart. Resuscitation with Hextend attenuated serum TNF levels during resuscitation (n = 4/group, p < 0.01 control vs. HXT, Fig. 6B). However, resuscitation with Hextend supplemented with EP was more effective, and there were no statistical differences between the TNF levels of these animals and control rats (n = 4/group, p > 0.05 control vs. HEP50, Fig. 6B). Resuscitation with Hextend attenuated TNF levels in the lung and the liver but not in the heart as compared with the animals without resuscitation. Similar to normal animals, resuscitation with Hextend supplemented with 50 mM EP attenuated TNF production in all the organs but particularly in the heart.
Figure 5.
Blood chemistry analyses in splenectomized animals. Blood from control splenectomized (SPX) or hemorrhagic (Hem) animals was collected at 2 hours after the hemorrhagic load to analyze (A) blood urea nitrogen (BUN), (B) pH, (C) the anion gap (AnGap), (D) chloride (Cl), (E) bicarbonate (HCO3), and (F) glucose. *Represents p < 0.05 vs. control (n = 5/group; one-way analysis of variance with Bonferroni’s corrections). HXT, Hextend supplemented with ethyl pyruvate (HEP).
Figure 6.
Ethyl pyruvate (EP) significantly attenuates systemic tumor necrosis factor (TNF) levels in splenectomized (Spx) animals after resuscitation. A, Blood collected from control (c) or Spx (X) animals with hemorrhage (Hem) and no resuscitation treatment were analyzed for TNF levels. Higher serum TNF levels in Spx animals correlated with higher TNF levels in the liver but not in the lung or the heart. *Represents p < 0.05 (Student’s t test). Hemorrhagic Spx animals were subjected to the different treatments and TNF concentrations were determined in the (B) serum, (C) lung, (D) heart, and (E) liver. *Represents p < 0.01 as compared with control. #Represents p < 0.05 for Hextend (HXT) supplemented with 50 mM EP (HEP50) vs. HXT (one-way analysis of variance with Bonferroni’s correction, n = 4/group). NR, without resuscitation treatment.
EP Inhibits the NF-κB Pathway and PARP During Resuscitation.
NF-κB binding to DNA was analyzed by electrophoretic mobility-shift assay in different organs at 2 hours after hemorrhage (Fig. 7A). Resuscitation with Hextend enhanced NF-κB activation, whereas resuscitation with Hextend supplemented with EP inhibited p65RelA binding to DNA. We also analyzed its effect on IκBα phosphorylation, the first step in the activation of the NF-κB transcriptional complex. IκBα was analyzed by Western blot using anti-IκBα polyclonal antibody and antiphosphorylated-IκBα (phospho S32 + S36) monoclonal antibody in homogenates normalized by protein concentration (Fig. 7B). Actin protein was used as loading control. Hemorrhage was characterized by a phosphorylation of splenic IκBα at the serines 32 and 36. Resuscitation with either Hextend or Hextend supplemented with 50 mM of EP did not prevent IκBα phosphorylation. We also analyzed the effects of EP on PARP, a regulator of NF-κB and HMGB1 (34–37). Hemorrhage caused PARP activation, and resuscitation with Hextend did not prevent it. However, resuscitation with Hextend supplemented with EP inhibited PARP activity in the liver during resuscitation (Fig. 7C). Similar results were observed in the spleen (data not shown).
Figure 7.
Ethyl pyruvate (EP) inhibits in vivo nuclear factor-kappa B (NF-κB) DNA binding and poly(ADP) polymerase (PARP) activity during resuscitation. The spleen from control and hemorrhagic animals was collected at 2 hours after the hemorrhage. The effects of the EP on the NF-κB pathway were analyzed at two levels: (A) NF-κB DNA binding analyzed by electrophoretic mobility-shift assay and (B) phosphorylation of IκBα analyzed by Western blot. EP inhibited NF-κB DNA binding without affecting the activation of the IκBα. (C) EP inhibits PARP activity in the liver, a major source of high mobility group B protein-1 during hemorrhage. #Represents p < 0.05 for Hextend (HXT) vs. HXT supplemented with EP (HEP) (n = 5/group, one-way analysis of variance with Bonferroni’s corrections). NR, without resuscitation treatment. C, control.
DISCUSSION
Conventional resuscitation fluids are designed to re-establish tissue perfusion, but they fail to prevent deleterious inflammatory responses. For instance, resuscitation with Hextend re-established arterial blood pressure and tissue perfusion, but still 89% of the animals died within the first 6 hours after hemorrhage. Indeed, resuscitation can exacerbate systemic inflammatory responses that can be more dangerous than the original hemorrhage. Here, we report that all the animals resuscitated with Hextend supplemented with 50 mM EP survived hemorrhagic shock. These results are consistent with previous studies showing the potential of EP to prevent the permeability of the ileal mucosa during resuscitation (22–24). As compared with these previous studies, our results are particularly significant in four considerations. First, this is an experimental model of severe hemorrhage with more than 75% of estimated blood volume lost. Second, shed blood was considered lost and it was not reinfused. Furthermore, animals were treated with a small volume of 15 mL/kg resuscitation (equivalent to 1000 mL of Hextend in a 70-kg patient) that represents approximately 50% of the total shed blood volume. Third, our studies use Hextend as a control solution. This is a critical consideration as recent studies indicate that resuscitation with Hextend prevents multiple organ injury (9) and improves short-time survival as compared with saline (10). Indeed, unlike previous studies, all our animals resuscitated with control solution survived the initial response (<4 hours), but 90% died in the secondary inflammatory phase 4–10 hours postresuscitation. Fourth, the animals were followed up for up to 1 week to analyze the total survival including late deaths. Together, these considerations are of particular interest in scenarios of limited supplies including critical care of both military operations and civil mass casualties. These considerations are particularly pronounced on the battle-field characterized by low supplies of resuscitation fluid, long transport times to a medical facility, and hemorrhage associated with exacerbated inflammatory responses produced by collateral trauma.
The most significant results correlating with the therapeutic potential of EP during resuscitation were its anti-inflammatory potential to inhibit systemic inflammation similar to that described in other studies including experimental sepsis (16–20). However, a recent study indicates that a single dose of EP administered at the same time as lipopolysaccharide may worsen survival in endotoxemia (26) depending on the dose and time of administration (27). These studies are of particular interest because a phase II trial using EP in cardiopulmonary bypass has recently been terminated after the enrollment of 102 patients; yet the final results have not been reported (28). Because our studies indicate that some anti-inflammatory compounds are not effective in splenectomized animals (30), we have analyzed the effects of EP in specific organs and splenectomized animals. Our previous studies indicate that the spleen is a major source of systemic TNF, and inhibition of TNF production in the spleen can prevent cardiovascular shock in experimental sepsis. The vagus nerve and cholinergic agonists prevent systemic inflammation during endotoxemia by inhibiting TNF production in the spleen (30). Splenectomy moderated systemic TNF levels and abolished the anti-inflammatory potential of these strategies (30). Likewise, here we report that EP is particularly efficient in attenuating TNF levels in the spleen during resuscitation. In contrast to septic shock, splenectomy failed to prevent systemic inflammation in hemorrhagic shock. Actually, serum TNF levels during hemorrhagic shock were significantly higher in splenectomized than in nonsplenectomized animals. These higher serum TNF levels in splenectomized animals correlated only with higher TNF levels in the liver. One potential explanation is that splenectomy may prevent the regulation of hepatic TNF production induced by the splenic release of anti-inflammatory cytokines such as transforming growth factor-β into the splenic and portal vein. These results reveal a fundamental difference between the systemic inflammatory response in experimental sepsis and hemorrhage. From a pharmacologic perspective, EP significantly attenuates systemic and cardiac TNF levels in normal animals. This anti-inflammatory potential of EP was significantly diminished in splenectomized animals as compared with the resuscitation with Hextend. These results indicate that the spleen and the heart can contribute directly to the anti-inflammatory potential of the EP. They also imply that the anti-inflammatory and therapeutic potential of EP could be compromised in patients with traumatized or dysfunctional spleen (30) or during cardiopulmonary bypass. Future studies are needed to determine the anti-inflammatory potential of EP in different clinical populations.
Our previous studies indicate that EP prevents NF-κB activation in RAW264.7 macrophage cells (16, 17). Han et al (39) further analyzed the mechanism showing that in a cell-free system, binding of p50 homodimers to an NF-κB consensus oligonucleotide sequence was unaffected by EP over a wide range of concentrations, indicating that EP probably does not modify or interact with the p50 subunit of NF-κB. In contrast, EP inhibited DNA binding by ectopically overexpressed wild-type p65 homodimers in human embroynic kidney 293 cells. However, EP failed to inhibit the DNA-binding activity of homodimers of an overexpressed mutant form of a p65 with substitution of serine for cysteine 38 (39). Taken together, these results suggest that EP inhibits DNA binding by covalently modifying p65 at the cysteine position (38, 39). Here, we report that EP inhibits p65RelA DNA binding without affecting IκBα phosphorylation during resuscitation. These results constitute the first evidence of the anti-inflammatory mechanism of EP in vivo. In addition to NF-κB, EP also inhibited the activation of PARP. This result has significant implications as PARP inhibitors or PARP deficiency provides survival benefit and prevents organ injury in hemorrhagic shock (35–37, 40) and it provides an additional mechanism for the anti-inflammatory potential of EP. PARP activation is known to promote cell death via necrosis (41, 42) and PARP inhibitors abrogate HMGB1 release in necrotic cells (34). Thus, the inhibition of PARP may explain the regulation of systemic HMGB1 by EP during resuscitation. In addition, as PARP regulates NF-kB and PARP inhibitors suppress the production of proinflammatory cytokines (37), the inhibition of PARP may contribute to the regulation of NF-kB by EP. As EP has been shown to act as an antioxidant (43), this could be a potential mechanism to modulate PARP. These results warrant future studies to determine the effect of EP in other critical pathways contributing to hemorrhagic shock.
EP inhibited systemic HMGB1 levels during resuscitation. Originally described as an intracellular protein, HMGB1 can be released into the extracellular milieu where it functions as an inflammatory cytokine (1, 44–46). Extracellular HMGB1 acts as a proinflammatory cytokine that causes abrupt cardiac standstill (32, 33), intestinal dearrangement (47), acute lung injury (48), and sustains the inflammatory response by activating immune cells (46). HMGB1 appears to be a pharmacologic target for hemorrhagic shock as: a) serum HMGB1 levels are increased in patients with hemorrhagic shock (49) and b) inhibition of HMGB1 activity with neutralizing antibody significantly decreased liver damage after ischemia and reperfusion (50). Our previous studies supported HMGB1 as a late pharmacologic target for systemic inflammation because it appeared in the serum at 18–24 hours after the induction of sepsis. However, this study shows an “early” serum HMGB1 level at only 2 hours after hemorrhage (16, 17). Recent studies also reported similar “early” extracellular HMGB1 release after hepatic ischemia/reperfusion (50). These differences between “late” secretion in sepsis and “early” release in hemorrhage may be explained by two different mechanisms of HMGB1 production (46). The first mechanism may represent a time-consuming “active secretion” from immune cells to act as a proinflammatory cytokine during an immunologic challenge, such as sepsis (51). The second mechanism may represent a “passive release” of HMGB1 from damaged or necrotic cells during hemorrhage. In this hemorrhagic scenario, HMGB1, an intracellular protein, can represent an optimal signal selected by the innate immune system to recognize tissue damage and initiate reparative responses (52). From an immunologic perspective, HMGB1 depicts a characteristic “necrotic marker” or damage-associated molecular pattern molecule (53, 54). This emerging family of specific intracellular proteins symbolizes optimal chemotactic markers selected by the innate immune system to recognize tissue damage and initiate reparative responses (55, 56). From a pathologic perspective, HMGB1 can be used as a marker for cellular necrosis and tissue injury. In this sense, resuscitation with EP, but not Hextend, prevented serum HMGB1 levels confirming its therapeutic potential. From the study, we suggest that EP seems to provide therapeutic anti-inflammatory potential to advanced resuscitation fluids, which limit critical inflammatory and cardiodepressant factors during resuscitation.
ACKNOWLEDGMENTS
We thank Dr. Nina Kohn and Martin Lesser from the Biostatistics unit of the Feinstein Institute for Medical Research for their statistical analyses. We also thank Drs. Vadim Pisarenko and Danielle Doucet for their thoughtful discussions and revision of the manuscript. Drs. Fink, Tracey, and Ulloa are patent inventors related to EP.
Dr. Ulloa is supported by the faculty program of the Department of Surgery, UMDNJ; and grants from the US Army Medical Research Command (USAMRMC#05308004), and the American Heart Association (AHA06352230N), and the NIH (RO1-GM084125).
Footnotes
The authors have not disclosed any potential conflicts of interest.
For information regarding this article, Mail@LuisUlloa.com
REFERENCES
- 1.Ulloa L, Tracey KJ: The “cytokine profile”: A code for sepsis. Trends Mol Med 2005; 11: 56–63 [DOI] [PubMed] [Google Scholar]
- 2.Ulloa L: The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov 2005; 4:673–684 [DOI] [PubMed] [Google Scholar]
- 3.Ulloa L, Doody J, Massague J: Inhibition of transforming growth factor-beta/SMAD signaling by the interferon-gamma/STAT pathway. Nature 1999; 397:710–713 [DOI] [PubMed] [Google Scholar]
- 4.Tracey KJ, Cerami A: Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol 1993; 9:317–343 [DOI] [PubMed] [Google Scholar]
- 5.Tracey KJ, Cerami A: Tumor necrosis factor: A pleiotropic cytokine and therapeutic target. Annu Rev Med 1994; 45:491–503 [DOI] [PubMed] [Google Scholar]
- 6.Hartmann AF, Senn MJ: Studies in the metabolism of sodium r-lactate. III Response of human subjects with liver damage, disturbed water and mineral balance, and renal insufficiency to the intravenous injection of sodium r-lactate. J Clin Invest 1932; 11: 345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordell AR: Milestones in the development of cardioplegia. Ann Thorac Surg 1995; 60: 793–796 [DOI] [PubMed] [Google Scholar]
- 8.Gan TJ, Bennett-Guerrero E, Phillips-Bute B, et al. : Hextend, a physiologically balanced plasma expander for large volume use in major surgery: A randomized phase III clinical trial Hextend Study Group. Anesth Analg 1999; 88:992–998 [DOI] [PubMed] [Google Scholar]
- 9.Nielsen VG, Tan S, Brix AE, et al. : Hextend (hetastarch solution) decreases multiple organ injury and xanthine oxidase release after hepatoenteric ischemia-reperfusion in rabbits. Crit Care Med 1997; 25:1565–1574 [DOI] [PubMed] [Google Scholar]
- 10.Kellum JA: Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: Improved short-term survival and acid-base balance with hextend compared with saline. Crit Care Med 2002; 30:300–305 [DOI] [PubMed] [Google Scholar]
- 11.Baskett TF: The resuscitation greats: Sydney Ringer and lactated Ringer’s solution. Resuscitation 2003; 58:5–7 [DOI] [PubMed] [Google Scholar]
- 12.Montgomery CM, Fairhurst AS, Webb JL: Metabolic studies on heart mitochondria. III. The action of parapyruvate on alpha-ketoglutaric oxidase. J Biol Chem 1956; 221: 369–376 [PubMed] [Google Scholar]
- 13.Slovin PN, Huang CJ, Cade JR, et al. : Sodium pyruvate is better than sodium chloride as a resuscitation solution in a rodent model of profound hemorrhagic shock. Resuscitation 2001; 50:109–115 [DOI] [PubMed] [Google Scholar]
- 14.Vonkorff RW: Pyruvate-C14, purity and stability. Anal Biochem 1964; 8:171–178 [DOI] [PubMed] [Google Scholar]
- 15.Fink MP: Ringer’s ethyl pyruvate solution: A novel resuscitation fluid. Minerva Anestesiol 2001; 67:190–192 [PubMed] [Google Scholar]
- 16.Ulloa L, Ochani M, Yang H, et al. : Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A 2002; 99: 12351–12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulloa L, Fink MP, Tracey KJ: Ethyl pyruvate protects against lethal systemic inflammation by preventing HMGB1 release. Ann N Y Acad Sci 2003; 987:319–321 [Google Scholar]
- 18.Yang R, Uchiyama T, Watkins SK, et al. : Ethyl pyruvate reduces liver injury in a murine model of extrahepatic cholestasis. Shock 2004; 22:369–375 [DOI] [PubMed] [Google Scholar]
- 19.Sappington PL, Cruz RJ Jr, Harada T, et al. : The ethyl pyruvate analogues, diethyl oxaloproprionate, 2-acetamidoacrylate, and methyl-2-acetamidoacrylate, exhibit anti-inflammatory properties in vivo and/or in vitro. Biochem Pharmacol 2005; 70:1579–1592 [DOI] [PubMed] [Google Scholar]
- 20.Su F, Wang Z, Cai Y, et al. : Beneficial effects of ethyl pyruvate in septic shock from peritonitis. Arch Surg 2007; 142:166–171 [DOI] [PubMed] [Google Scholar]
- 21.Riedemann NC, Guo RF, Ward PA: Novel strategies for the treatment of sepsis. Nat Med 2003; 9:517–524 [DOI] [PubMed] [Google Scholar]
- 22.Tawadrous ZS, Delude RL, Fink MP: Resuscitation from hemorrhagic shock with Ringer’s ethyl pyruvate solution improves survival and ameliorates intestinal mucosal hyperpermeability in rats. Shock 2002; 17: 473–477 [DOI] [PubMed] [Google Scholar]
- 23.Yang R, Gallo DJ, Baust JJ, et al. : Ethyl pyruvate modulates inflammatory gene expression in mice subjected to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol 2002; 283:G212–G221 [DOI] [PubMed] [Google Scholar]
- 24.Mulier KE, Beilman GJ, Conroy MJ, et al. : Ringer’s ethyl pyruvate in hemorrhagic shock and resuscitation does not improve early hemodynamics or tissue energetics. Shock 2005; 23:248–252 [PubMed] [Google Scholar]
- 25.Fink MP: Ethyl pyruvate: A novel treatment for sepsis and shock. Minerva Anestesiol 2004; 70:365–371 [PubMed] [Google Scholar]
- 26.Su J, Li X, Cui X, et al. : Ethyl pyruvate decreased early nuclear factor-kappaB levels but worsened survival in lipopolysaccharide-challenged mice. Crit Care Med 2008; 36: 1059–1067 [DOI] [PubMed] [Google Scholar]
- 27.Tenhunen JJ: Bull’s eye missed by the magic bullet: Preclinical investigations, publication bias, and promising new interventions. Crit Care Med 2008; 36:1361–1363 [DOI] [PubMed] [Google Scholar]
- 28.Critical Therapeutics Announces Decision to Discontinue Trial of CTI-01, Daily updates. Available at: http://www.thefreelibrary.com/Critical+Therapeutics+Announces+Results+of+CTI-01+Phase+II+Trial.-a0151425989. Accessed May 30, 2008.
- 29.Handrigan MT, Bentley TB, Oliver JD, et al. : Choice of fluid influences outcome in prolonged hypotensive resuscitation after hemorrhage in awake rats. Shock 2005; 23:337–343 [DOI] [PubMed] [Google Scholar]
- 30.Huston JM, Ochani M, Rosas-Ballina M, et al. : Splenectomy inactivates the cholinergic anti-inflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 2006; 203:1623–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Liao H, Ochani M, et al. : Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 2004; 10:1216–1221 [DOI] [PubMed] [Google Scholar]
- 32.Mantell LL, Parrish WR, Ulloa L: HMGB1 as a therapeutic target for infectious and inflammatory disorders. Shock 2006; 25:4–11 [DOI] [PubMed] [Google Scholar]
- 33.Li W, Sama AE, Wang H: Role of HMGB1 in cardiovascular diseases. Curr Opin Pharmacol 2006; 6:130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ditsworth D, Zong WX, Thompson CB: Activation of poly(ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol Chem 2007; 282:17845–17854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabo C: Potential role of the peroxynitratepoly(ADP-ribose) synthetase pathway in a rat model of severe hemorrhagic shock. Shock 1998; 9:341–344 [DOI] [PubMed] [Google Scholar]
- 36.Virag L, Szabo C: The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 2002; 54:375–429 [DOI] [PubMed] [Google Scholar]
- 37.Jagtap P, Szabo C: Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov 2005; 4:421–440 [DOI] [PubMed] [Google Scholar]
- 38.Messmer D, Yang H, Telusma G, et al. : High mobility group box protein 1: An endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol 2004; 173:307–313 [DOI] [PubMed] [Google Scholar]
- 39.Han Y, Englert JA, Yang R, et al. : Ethyl pyruvate inhibits nuclear factor-kappaB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther 2005; 312:1097–1105 [DOI] [PubMed] [Google Scholar]
- 40.Liaudet L, Soriano FG, Szabó E, et al. : Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A 2000; 97:10203–10208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virag L, Scott GS, Cuzzocrea S, et al. : Peroxynitrite-induced thymocyte apoptosis: the role of caspases and poly (ADP-ribose) synthetase (PARS) activation. Immunology 1998; 94:345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ha HC, Snyder SH: Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A 1999; 96:13978–13982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo YJ, Taylor MD, Cohen JE, et al. : Ethyl pyruvate preserves cardiac function and attenuates oxidative injury after prolonged myocardial ischemia. J Thorac Cardiovasc Surg 2004; 127:1262–1269 [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Bloom O, Zhang M, et al. : HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999; 285:248–251 [DOI] [PubMed] [Google Scholar]
- 45.Lotze MT, Tracey KJ: High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat Rev Immunol 2005; 5:331–342 [DOI] [PubMed] [Google Scholar]
- 46.Ulloa L, Messmer D: High-mobility group box 1 (HMGB1) protein: Friend and foe. Cytokine Growth Factor Rev 2006; 17:189–201 [DOI] [PubMed] [Google Scholar]
- 47.Sappington PL, Yang R, Yang H, et al. : HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology 2002; 123:790–802 [DOI] [PubMed] [Google Scholar]
- 48.Abraham E, Arcaroli J, Carmody A, et al. : HMG-1 as a mediator of acute lung inflammation. J Immunol 2000; 165:2950–2954 [DOI] [PubMed] [Google Scholar]
- 49.Ombrellino M, Wang H, Ajemian MS, et al. : Increased serum concentrations of high-mobility-group protein 1 in haemorrhagic shock. Lancet 1999; 354:1446–1447 [DOI] [PubMed] [Google Scholar]
- 50.Tsung A, Sahai R, Tanaka H, et al. : The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 2005; 201:1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardella S, Andrei C, Ferrera D, et al. : The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 2002; 3:995–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scaffidi P, Misteli T, Bianchi ME: Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418: 191–195 [DOI] [PubMed] [Google Scholar]
- 53.Matzinger P: The danger model: A renewed sense of self. Science 2002; 296:301–305 [DOI] [PubMed] [Google Scholar]
- 54.Rubartelli A, Lotze MT: Inside, outside, upside down: Damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol 2007; 28:429–436 [DOI] [PubMed] [Google Scholar]
- 55.Dumitriu IE, Baruah P, Manfredi AA, et al. : HMGB1: Guiding immunity from within. Trends Immunol 2005; 26:381–387 [DOI] [PubMed] [Google Scholar]
- 56.Akira S, Takeda K: Toll-like receptor signaling. Nat Rev Immunol 2004; 4:499–511 [DOI] [PubMed] [Google Scholar]