Abstract
The actions and regulation of cardiomyocyte β1ARs differ in several respects from the properties described for the prototypical β2AR subtype; a mechanism to explain the unique properties of the β1AR subtype has never been obvious. This viewpoint summarizes recent studies that identify a novel signaling paradigm for the β1AR, implicating the N-terminus as a molecular determinant of β1AR responsiveness.
Keywords: Cell Signaling/Signal Transduction, heart failure, oxidant stress
SCIENTIFIC METHOD
It is a capital mistake to theorize before one has data. Insensibly one begins to twist the facts to suit theories, instead of theories to suit the facts.
SIR ARTHUR CONAN DOYLE
It is also a good rule not to put too much confidence in experimental results until they have been confirmed by theory.
SIR ARTHUR EDDINGTON
First get your facts; then you can distort them at your leisure.
MARK TWAIN
Beta-adrenergic receptors (βARs) are among the most intensively studied members of the G protein-coupled receptor (GPCR) superfamily. These receptors, which transduce signals derived from catecholamine binding to a cellular response, have garnered considerable interest as therapeutic targets because of their key roles in the physiologic control of cardiovascular performance as well as the pathogenesis of cardiac arrhythmias, ventricular remodeling, and the evolution of heart failure (HF). Breakthroughs in receptor biology, culminating most recently with the development of strategies to obtain high-resolution GPCR structures, have lead to a detailed understanding of the βAR’s two core functions - namely ligand binding to a transmembrane ligand-binding pocket and G protein- or β-arrestin-induced cellular activation 1. These studies also have exposed desensitization mechanisms that provide a critical homeostatic control to avoid excessive/unrelenting GPCR activation. However, it is important to note that much of our current concepts regarding the mechanisms that activate and/or regulate βARs come from studies of the β2AR subtype, the first hormone-activated GPCR to be cloned and structurally characterized. Based upon early studies that identified essentially equivalent signaling properties for β1ARs and β2ARs in certain model cell types as well as evidence that various aspects of the structural architecture, signaling mechanisms, and regulatory machinery are highly conserved across the GPCR superfamily, the literature has generally viewed the β2AR as a useful surrogate for the β1AR subtype. However, this assumption ignores literature that identifies discrepancies between the properties of β1 vs. β2ARs. My laboratory has struggled for some time with the nagging suspicion that some aspects of the actions and regulation of the cardiomyocyte β1AR may not adhere to conventional models described for the prototypical β2AR subtype.
Studies in transgenic models provided some of the earliest evidence for functional divergence between β1 and β2ARs showing that high levels of transgenic β2AR overexpression are well tolerated, whereas even relatively low levels of transgenic β1AR overexpression result in maladaptive cardiac remodeling and HF 2, 3. Studies in cell-based models also established that chronic catecholamine stimulation leads to cardiomyocyte apoptosis through the activation of β1ARs, whereas β2AR stimulation may in fact be cardioprotective 4.
Immunoblotting studies performed to validate the β1AR knockout mouse model identified another distinctive feature of β1ARs that defied obvious explanation. These Western blots identified molecular heterogeneity for the β1AR subtype that was not evident in immunoblotting studies of β2ARs 5. Recent studies from my laboratory establish that β1ARs are susceptible to N-terminal cleavage 6, similar to the processing mechanism originally described for the turkey βAR – the avian homologue of the mammalian β1AR subtype. The observation that β1ARs are expressed as both a full-length protein and a smaller N-terminally truncated species raises the first obvious question: What controls the generation of the distinct molecular forms of the β1AR? Do distinct β1AR species signal differently?
Other studies exposed differences in susceptibility to agonist-induced desensitization/internalization. The classical paradigm holds that agonist-activated βARs are phosphorylated by G protein-coupled receptor kinases (GRKs), leading to the recruitment of β-arrestin, and receptor desensitization/downregulation. Head-to-head comparisons of β1 versus β2ARs indicate that agonist-induced phosphorylation, internalization/sequestration, and/or down-regulation is blunted for the β1AR subtype, compared to the β2AR 7–10. Sequence comparisons provide a likely explanation; β1- and β2ARs share considerable sequence homology in their transmembrane ligand-binding pockets, but other regions of these receptors are more divergent. In particular, sites on the intracellular surface of the β2AR that serve as substrates for GRK phosphorylation and/or docking sites for β-arrestin are not conserved in β1ARs. Therefore, the observation that HF (a condition characterized by elevated catecholamine levels) leads to selective desensitization/downregulation of the β1AR subtype and is not accompanied by a commensurate loss of β2ARs seems paradoxical 11; the prediction based on cell-based studies is that chronic catecholamine-induced, GRK/β-arrestin-dependent desensitization would lead to a decrease in β2ARs, and that the β1AR subtype would be relatively spared. Hence, question #2: What mechanism underlies the HF-induced decrease in β1ARs?
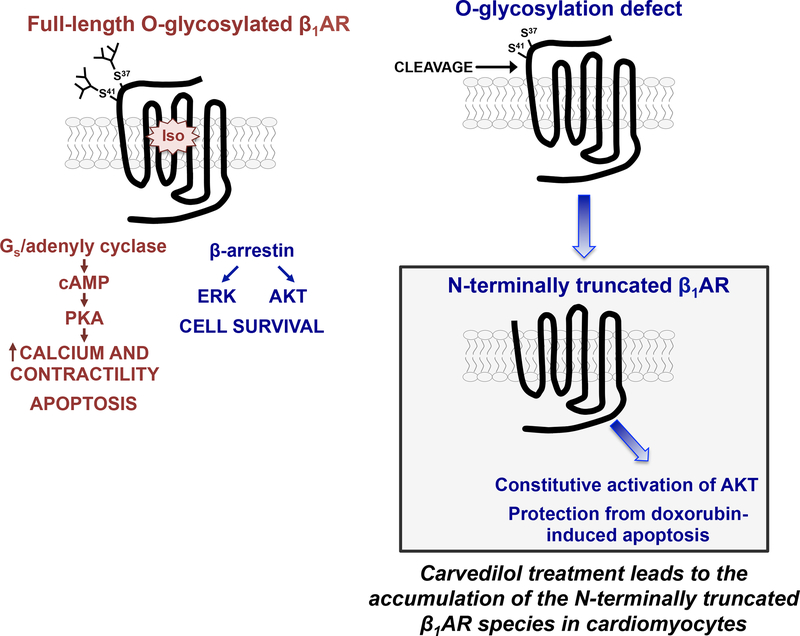
We recently made progress toward answering both of these questions through studies of the β1AR N-terminus, the relatively short/unstructured extracellular portion of the receptor that differs in length, sequence, and post-translational processing from the β2AR N-terminus. We showed that the β1AR N-terminus contains two O-linked glycosylation sites at Ser37 and Ser41 and that O-glycosylation is required for full-length β1AR expression; β1ARs accumulate as N-terminally truncated species under conditions that prevent β1AR O-glycosylation (6 as if the mucin-like O-glycans attached to the N-terminus act as a barrier to prevent protease access and cleavage at an adjacent site, Figure 1). An O-glycosylation-regulated N-terminal cleavage mechanism that gives rise to distinct molecular forms of the β1AR provides the first credible explanation for the molecular heterogeneity displayed by native β1ARs in various cardiac preparations. It also emphasizes that previous literature using immunblotting or immunohistochemical methods to track β1AR expression and/or localization using antibodies to a transgene’s N-terminal tag should be interpreted with caution, since these techniques do not capture N-terminally truncated β1AR species that lack the N-terminal epitope tag.
Figure 1:
β1AR N-terminal processing (see text).
Does β1AR molecular heterogeneity matter?
We recently reported that N-terminally truncated β1ARs remain signaling competent, but N-terminal truncation alters signaling bias to the cAMP/PKA vs. ERK pathways 6. The notion that an O-glycosylation-regulated N-terminal cleavage mechanism can regulate signaling by a GPCR is quite novel and is predicted to have pathophysiological significance since O-linked glycan structures are highly regulated during developmental and in response to inflammatory stimuli, metabolic disorders, and abnormalities in cell growth; alterations in glycan structures are detected in cardiac hypertrophy 12.
How does the N-terminus function as a molecular determinant of β1AR responsiveness?
In theory, the N-terminus might function as an allosteric regulator of the β1AR structure itself, a regulator of β1AR trafficking to subcellular compartments, and/or a regulator of β1AR coupling to signaling partners and downstream effector responses. These mechanisms are not mutually exclusive and are the focus of ongoing studies.
What is the mechanism underlying the HF-induced decrease in β1AR density?
In an effort to identify stimuli that might regulate β1AR cleavage, we stumbled upon evidence that oxidative stress (a stimulus that contributes to the pathogenesis of HF) leads to a selective decrease in cardiomyocyte β1AR expression that is not associated with a change in the abundance of the β2AR subtype 13.
Carvedilol to the rescue!
Attempts to identify a mechanism for (and strategies to prevent) the ROS-dependent decrease in β1AR expression lead to two intriguing observations regarding the pharmacologic properties of carvedilol. First, our studies showed that the redox-dependent decrease in β1AR expression is prevented by carvedilol (but not a panel of other βAR ligands) and that this cannot be attributed to carvedilol’s ancillary anti-oxidant properties 13. While the molecular mechanism underlying carvedilol’s ability to protect β1ARs from redox-inactivation remains uncertain, it is worth noting that carvedilol contains a bulky aromatic amine substitution (not present in most other adrenergic ligands) that makes unique contacts with an extended β1AR ligand binding pocket that includes the redox-sensitive cysteines in extracellular loop 2 14. In theory, carvedilol might protect β1ARs from redox-inactivation either by shielding these cysteines from redox-inactivation or producing a conformational rearrangement of the extracellular surface so as to bury the redox-sensitive disulfide bonds.
Carvedilol treatment also leads to the accumulation of N-terminally truncated β1ARs that constitutively activate the AKT pathway and protect against doxorubicin-induced apoptosis (Figure 1 13). This second cardioprotective property for carvedilol (not shared by other βAR ligands) is predicted to have important clinical and therapeutic implications. First, it identifies a mechanism that presumably underlies the protective effects of prophylactic carvedilol against anthracycline-induced cardiotoxicity in the clinic. Second, these findings may be pertinent to the lingering controversy regarding the interpretation of the COMET trial results, which ascribed significant survival advantage to carvedilol over metoprolol in the treatment of HF. The unique pharmacologic action of carvedilol not shared with metoprolol or other βAR blockers identified in our studies is predicted to offer to meaningful cardioprotection.
This viewpoint presents a revised model of β1AR action and regulation that challenges conventional dogma in that it identifies a novel role for the β1AR N-terminus as a molecular determinant of catecholamine responsiveness. We recognize (and are open to the fact that) some aspects of our model might not withstand the scrutiny of future research. Nevertheless, these newer studies set the stage for future research that examines factors (and proteases) that control β1AR N-terminal cleavage and the role of glycosylation-defective/N-terminally truncated β1ARs in the evolution of catecholamine-induced pathologic cardiac remodeling in vivo. Our hope is that this line of research will reveal novel strategies targeted to processing events localized to the β1AR N-terminus that might ultimately be used for therapeutic advantage.
Acknowledgments
Funding: NHLBI grant HL138468.
Footnotes
Disclosures: none
REFERENCES
- 1.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human β2-adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387 [DOI] [PubMed] [Google Scholar]
- 2.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice Proc Natl Acad Sci USA. 1999;96:7059–7064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science. 1994;264:582–586 [DOI] [PubMed] [Google Scholar]
- 4.Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol. 2001;189:257–265 [DOI] [PubMed] [Google Scholar]
- 5.Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP, Barsh GS, Bernstein D, Kobilka BK. Targeted disruption of the mouse β1-adrenergic receptor gene: Developmental and cardiovascular effects. Proc Natl Acad Sci USA. 1996;93:7375–7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park M, Reddy GR, Wallukat G, Xiang YK, Steinberg SF. β1-adrenergic receptor O-glycosylation regulates N-terminal cleavage and signaling responses in cardiomyocytes. Sci Rep. 2017;7:7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiina T, Kawasaki A, Nagao T, Kurose H. Interaction with β-arrestin determines the difference in internalization behavor between β1- and β2-adrenergic receptors. J Biol Chem. 2000;275:29082–29090 [DOI] [PubMed] [Google Scholar]
- 8.Green SA, Liggett SB. A proline-rich region of the third intracellular loop imparts phenotypic β1-versus β2-adrenergic receptor coupling and sequestration. J Biol Chem. 1994;269:26215–26219 [PubMed] [Google Scholar]
- 9.Suzuki T, Nguyen CT, Nantel F, Bonin H, Valiquette M, Frielle T, Bouvier M. Distinct regulation of β1- and β2-adrenergic receptors in chinese hamster fibroblasts. Mol Pharmacol. 1992;41:542–548 [PubMed] [Google Scholar]
- 10.Eichel K, Jullie D, von Zastrow M. Β-arrestin drives map kinase signalling from clathrin-coated structures after GPCR dissociation. Nature Cell Biol. 2016;18:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Port JD, Bristow MR. Altered β-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol. 2001;33:887–905 [DOI] [PubMed] [Google Scholar]
- 12.Rong J, Han J, Dong L, Tan Y, Yang H, Feng L, Wang QW, Meng R, Zhao J, Wang SQ, Chen X. Glycan imaging in intact rat hearts and glycoproteomic analysis reveal the upregulation of sialylation during cardiac hypertrophy. J Am Chem Soc. 2014;136:17468–17476 [DOI] [PubMed] [Google Scholar]
- 13.Park M, Steinberg SF. Carvedilol prevents redox inactivation of cardiomyocyte β1-adrenergic receptors. JACC; Basic Trans Sci. 2018;3:521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warne T, Edwards PC, Leslie AG, Tate CG. Crystal structures of a stabilized β1-adrenoceptor bound to the biased agonists bucindolol and carvedilol. Structure. 2012;20:841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]