Abstract
Background:
AAV2-neurturin (CERE-120) is designed to deliver the neurotrophic-factor, neurturin, to the striatum to restore and protect degenerating nigrostriatal neurons in Parkinson’s disease (PD). A common hypothesis is that following expression in the striatum, neurotrophic-factors like neurturin (NRTN) will be transported from degenerating terminals to their cell bodies in the substantia nigra pars compacta (SNc).
Methods:
We tested this concept, comparing the bioactivity of AAV2-neurturin in brains of PD patients versus those of nonhuman primates similarly treated using immunohistochemistry. NRTN-immunostaining in the targeted striatum was seen in all PD cases (mean putaminal coverage: ~15% by volume); comparable expression was observed in young, aged, and parkinsonian monkeys. In the SNc cell bodies, however, only rare evidence of neurturin was seen in PD, while ample evidence of intense nigral-NRTN was observed in all monkeys. NRTN-expression was associated with occasional, sparse TH-induction in the striatum of PD, but nothing apparent in the SNc. In primates, NRTN produced robust TH-induction throughout the nigrostriatal neurons.
Discussion:
These data provide the first evidence that gene therapy can increase expression of a neurotrophic-factor deep in the PD brain and that clear but modest enhancement of degenerating neurons can be induced. They also provide important insight regarding deficiencies in the status of nigrostriatal neurons in advanced PD, suggesting that serious axon-transport deficits reduced the bioactivity of AAV2-NRTN by limiting the protein exposed to the cell body. Thus, future efforts using neurotrophic-factors to treat neurodegenerative diseases will need to target both the terminal fields and the cell bodies of degenerating neurons to assure maximal benefit is achieved.
Keywords: neurotrophic factors, translational research, axonal transport, neurodegeneration, neural repair
Parkinson’s disease (PD) is a chronic motor disorder whose symptoms are associated with degeneration of dopamine-producing neurons originating in the substantia nigra pars compacta (SNc) and projecting to the striatum (including the putamen). No treatments protect against the continued degeneration of these neurons and, over time, all therapies fail. CERE-120 is an AAV2-based gene transfer vector designed to deliver the neurotrophic factor human neurturin (NRTN) to these degenerating neurons as a treatment for PD. Neurotrophic factors are naturally-occurring proteins that restore function and prevent death of damaged neurons. NRTN and glial cell line-derived neurotrophic factor (GDNF) are structural and functional homologs shown to restore and protect dying dopaminergic neurons in animal models of PD.1–4 However, the use of neurotrophic factors as a treatment for neurodegenerative diseases has proven extremely difficult, in large part due to obstacles that preclude targeted delivery of adequate and sustained levels of protein to the degenerating neurons.5–9
Gene therapy may provide a means to overcome these obstacles by distributing protein throughout the targeted region for the life of the transduced cells. For these reasons, AAV2-NRTN (CERE-120) was developed, and following extensive preclinical testing,4,10–15 was tested in PD patients using stereotactic delivery to the putamen. A Phase 1 trial in 12 moderately advanced PD patients identified no safety issues, while suggesting possible improvement on several measures of motor function.16 A subsequent double-blind-controlled Phase 2 trial in 58 subjects further supported the safety of CERE-120, but failed to discern any benefit compared to sham surgery on the primary endpoint (UPDRS-motor-”off” at 12-months). However, several secondary endpoints suggested modest clinical benefit, while no measurement favored sham-control. Moreover, a protocol-prescribed analysis of all data from patients whose treatment remained blinded at 15 to 18 months (n 5 30) suggested significant benefit with CERE-120 on the primary and secondary endpoints with no measurement favoring sham (Marks et al., in press).
Two PD patients in the Phase 2 trial died from non AAV2-NRTN-related events, and their brains were examined histologically. Here, we report the effects of delivering AAV2-NRTN to the putamen in these patients, comparing the expression of NRTN in putamen and nigra, and TH-induction in nigrostriatal neurons, to that following the same treatment in young, aged, and parkinsonian monkeys.
PATIENTS AND METHODS
Vector Design and Construction
CERE-120 is an AAV2 vector genetically engineered to express only human NRTN.11
Parkinson’s Autopsy Cases
Patients who previously met entry criteria and signed an IRB-approved informed consent received CERE-120 (5.4 × 1011 vg), distributed via four separate needle tracts per hemisphere and two deposits per tract. One subject (#1802) was a 59-year-old man who had been diagnosed 10.2 years earlier and died from a pulmonary embolism on Day 90, post-CERE-120 treatment. The second subject (#1904) was a 73-year-old man who had been diagnosed 9.5 years earlier and suffered a fatal myocardial infarction on Day 47 post-CERE-120. Subject #1904 had baseline UPDRS motor off/on scores of 34/21 and a self-reported diary off time of 1.33 hr/day. Subject #1802 had baseline UPDRS motor off/on scores of 51/34 and a self-reported diary off time of 4.7 hr/day. Brains were fixed following postmortem intervals of 6 and 13 hours, respectively, with a modified Zamboni’s solution and sectioned at 40 μm. Only one hemisphere was available from the second subject. Brains were stained for NRTN and tyrosine hydroxylase (TH), using non-PD aged humans as positive controls. Both brains demonstrated typical pathological features of PD, with marked loss of cells in SNc, coupled with multiple α-synuclein-stained Lewy bodies.
Nonhuman Primate Cases
Ten Rhesus monkeys (Macaca mulatta) were administered intraputaminal doses of AAV2-NRTN within the range administered to the PD patients, based on relative striatal volume, except for two young monkeys who intentionally received a substantially lower dose (4% of human dose by striatal volume) and their brains evaluated only 28 days later. All housing and experimentation was IACUC approved.4,12–14 Following CERE-120 administration, monkeys were anesthetized, perfused transcardially with 0.9% saline, followed by a modified Zamboni’s fixative and the brains sectioned. Some of these monkeys were part of previous publications,4,12,13 though the specific data presented here were not previously published.
Two young monkeys (~5 years old) were administered 0.5 × 1011 vg of CERE-120 per hemisphere in two deposits in a total volume of 25 μL within the striatum and euthanized 1 month later, and an additional young monkey received 1.0 × 1011 vg per hemisphere of CERE-120 and was euthanized three months later.
Three aged monkeys (22–25 years old) were administered 3 × 1011 vg of CERE-120, unilaterally, via five deposits (30 μL each) distributed throughout the striatum and euthanized 8 months later.
Five monkeys (age range: 6–10 years) received unilateral, intracarotid infusions of MPTP resulting in motor dysfunction. They were then administered 1.5 × 1011 vg of CERE-120 4 days later, via five deposits (15 μL each) distributed throughout the striatum, as well as a single, 10 μL injection into the SNc (0.2 × 1011 vg dose) and euthanized 10.5 months later.
Immunohistochemical Analysis of Neurturin and Tyrosine Hydroxylase
Immunoperoxidase labeling was used to visualize NRTN and TH within the human and nonhuman primate striatum and SN, as previously reported.4 A stereologic sampling method, combined computer-assisted imaging software, and the Cavalieri method was used to quantify volume of NRTN expression in the primate striatum. For the human cases, two different methods were independently employed to compute volume of NRTN expression in putamen. One method (at Rush University) employed volumetric analyses based on stereological sampling of 6 to 11 sections throughout each putamen. The second method (Ceregene) sampled all sections found to contain putamen (19–29 sections per each case).The percent of NRTN expressed within each targeted structure was then calculated based on the volumes of the entire target, using values from in house human and primate histological sections and MRI scans, as well as published values to provide an estimate of nonhuman primate caudate/putamen of ~1200 mm3/hemisphere and human putamen of ~4000 mm3/hemisphere).
RESULTS
NRTN Expression
For the two PD cases, neurturin expression was quantified in the three available hemispheres 7 weeks or 3+ months post-AAV2-NRTN treatment following death from unrelated causes. NRTN-immunoreactivity was seen in all hemispheres, restricted to the targeted putamen (see Fig. 1). Two independent, blinded analyses conservatively estimated NRTN protein covered ~15% of the entire putamen by volume. The dosing paradigm employed for CERE-120 was intended to distribute the AAV2-NRTN as widely as possible throughout the putamen, while limiting spread to surrounding sites to reduce potential side effects.5,6,17 Detectable NRTN protein was seen in 93%, 58%, and 80% of all putaminal sections analyzed, in each of the three hemispheres studied.
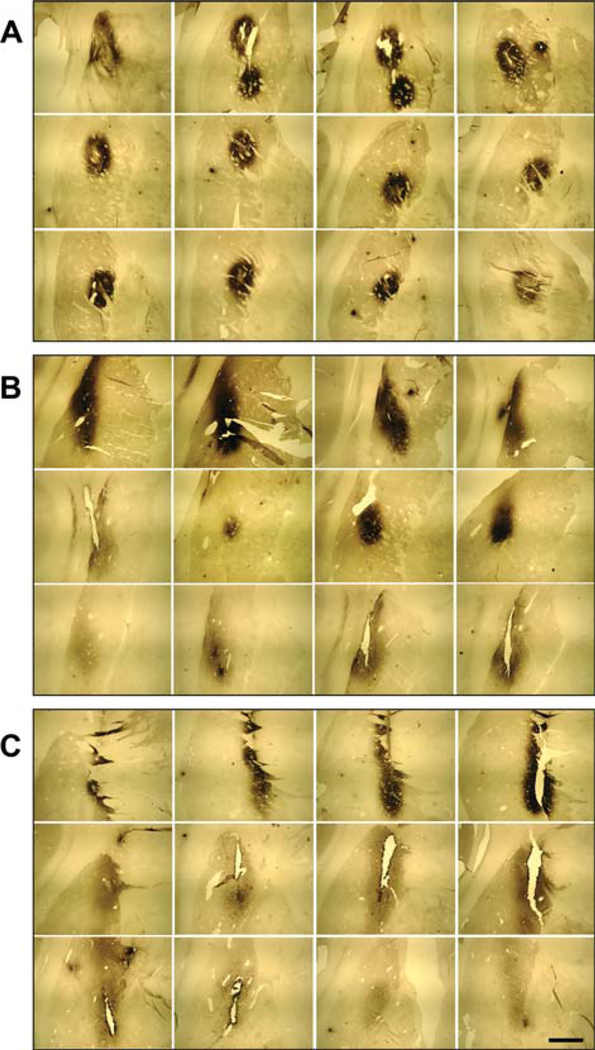
FIG. 1.
Photomicrographs, illustrating neurturin (NRTN) immune-histochemical staining throughout sections of the PD putamen. A: Case 1904-left hemisphere; B: Case 1802-right hemisphere; C: Case 1802-left hemisphere. Scale bar represents 5 mm.
In contrast to the strong NRTN expression in the putamen (i.e., terminal field of the dopamine nigral neurons), very little NRTN staining was seen in the SNc (i.e., neuronal cell bodies of these same neurons) despite appreciable, surviving dopaminergic, melanin-positive neurons (Fig. 2A–C).
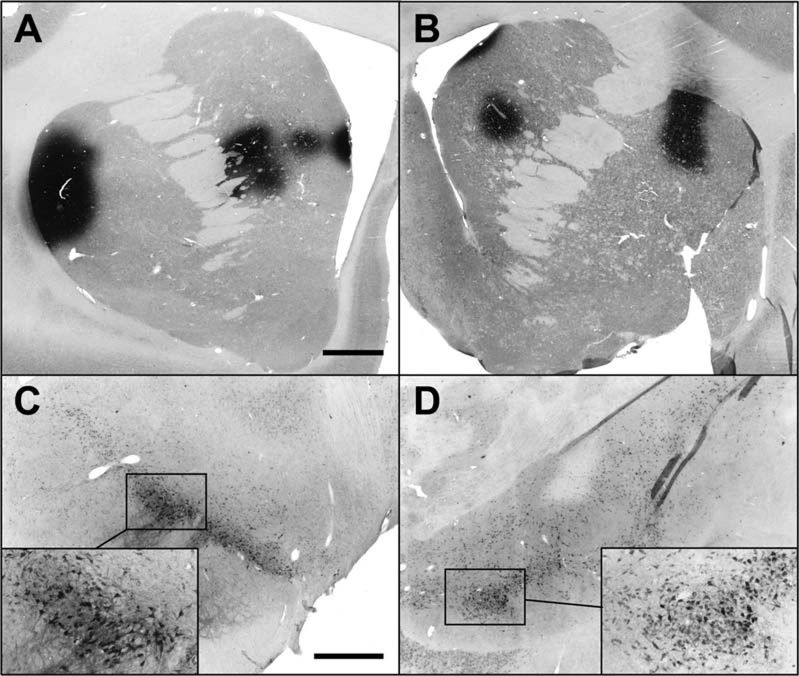
FIG. 2.
Example of melanin-positive neurons and NRTN-immunostaining in PD substantia nigra, pars compacta (SNc). While many clusters of melanin-positive, dopaminergic perikarya were observed in nigra (A: low magnification and B: high magnification), only rarely did any nigral neurons show evidence for NRTN staining (e.g., C: high magnification). Panels A and B illustrate the general failure of nigrostriatal neurons to retrogradely transport NRTN to nigral perikarya following intraputaminal delivery of AAV2-NRTN, whereas Panel C illustrates a rare example of NRTN in the nigra from successful retrograde transport. Scale bar represents 220 μm in panel A and 22 μm in panels B and C.
Two young monkeys were administered a particularly low dose of CERE-120 (less than 4% of the human PD dose, by relative volume of each targeted structure) and euthanized only 1 month later, to provide a conservative estimate of the early-onset bioactivity of CERE-120 with low NRTN expression levels. The volume of striatal NRTN expression in these two monkeys was estimated to be only 5.6% and 1.8%. Despite this low level of striatal NRTN expression, and in contrast to the PD cases, NRTN retrograde labeling was easily seen within SNc perikarya (see Fig. 3) and anterogradely transported NRTN+ fibers were seen coursing within the globus pallidus and SN pars reticulata.
FIG. 3.
NRTN staining in young monkeys administered low dose of AAV2-NRTN to striatum, intentionally covering only 5.6% and 1.8% of striatal volume (panels A and B, respectively). Figures represent the coronal section with greatest area of NRTN protein for each monkey. Despite modest NRTN coverage of striatum, NRTN was clearly seen in nigra only 1 month after AAV2-NRTN administration (panels C and D). Scale bar represents 2 mm in panels A and B. Scale bar in panel C represents 1 mm in lower magnification photos in panels C and D, and in higher magnification photos in C and D, represents 400 μm.
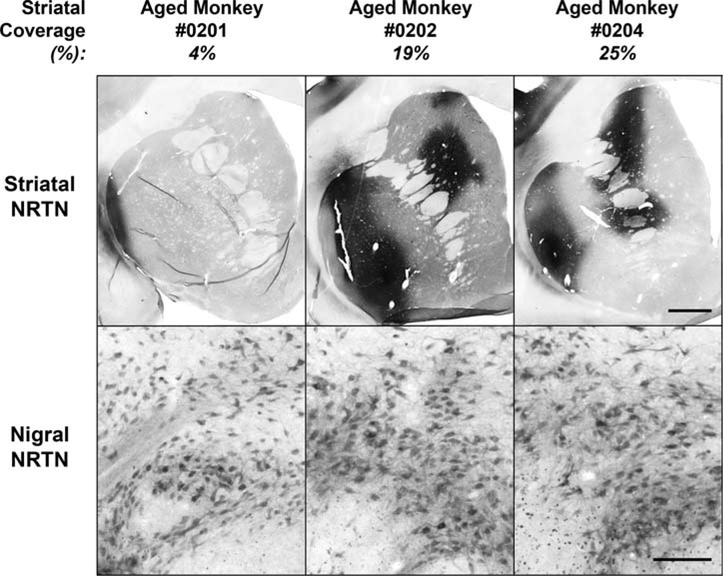
For the three aged monkeys administered AAV2-NRTN 8 months earlier, NRTN was estimated to cover 4, 19, and 25% (mean 16%) of the entire striatum, by volume. Despite the variation in striatal NRTN coverage, NRTN was consistently observed in the SNc (see Fig. 4), in contradistinction to the PD tissue.
FIG. 4.
NRTN in 3 aged monkeys treated with AAV2-NRTN administration to striatum. Note abundant NRTN-positive cells in substantia nigra in all 3 cases (bottom panels) following NRTN expression in striatum (top panels), even in case when volume of NRTN coverage was only 4%. Scale bar in top panels represents 2.0 mm, and 0.2 mm in lower panels.
For the five MPTP-treated monkeys (administered AAV2-NRTN 4 days following MPTP treatment euthanized 10 months later), a mean of ~13% of the entire putamen by volume stained for NRTN (8%, 8%, 13%, 15%, and 23%, respectively). Similar to other primate studies, extensive evidence for retrogradely and anterogradely transported NRTN within the substantia nigra pars compacta and reticulata, respectively, was F5 observed (see Fig. 5).
FIG. 5.
NRTN in striatum and nigra of MPTP monkeys treated with AAV2-NRTN. Panels A and C: sections from a monkey with 8% NRTN coverage of striatum (lowest coverage in group). Panels B and D: monkey with ~23% coverage of putamen (highest coverage in group). Open arrow denotes edge of relatively small AAV2-NRTN nigra injection (note that NRTN-stained nigral neurons are seen several mm away, reflecting retrograde transport of NRTN from striatum). Scale bar (in B) represents 2 mm in panels A and B. Scale bar (in D) represents 1 mm in C and D lower magnification panels and 208 μm in C and D higher magnification insets.
Tyrosine Hydroxylase Immunohistochemistry
Examination of the human PD autopsy tissue found only scant evidence for TH induction following CERE-120. The clearest evidence for TH induction was observed in the targeted striatum, well within the boundaries of some of the most intense sites of NRTN staining (see Fig. 6A–C). This signal, reflecting only sparse TH-positive fibers, was observed on average in 50% of the NRTN-positive putaminal sites shown in Figure 1 (i.e., 0%, 62%, and 80%, respectively). No evidence for TH induction was found in the SNc of the PD brains, despite the presence of numerous melanin-containing, TH+ dopaminergic neurons (see Figs. 2A,B and 6D,E).
FIG. 6.
Representative photomicrographs from PD putamen (top panels A–C) and substantia nigra (bottom panels D–F). Panels B and C provide example of TH induction in putamen, relative to NRTN expression in adjacent section (A), following AAV2-NRTN administration. Note TH signal: (i) was observed only well within the area of NRTN staining, (ii) was sparse, and (iii) occurred only ~50% of the time NRTN was seen. Panels D–F depict representative example of TH+ neurons and fibers in PD substantia nigra (D and E) and nigrostriatal pathway of the same hemisphere (F). Scale bar (in A) represents 1332 μm in A and B, 100 μm in C, E, and F and 833 μm in D.
In contrast to the sparse TH-induction signal in the PD cases, TH-induction following AAV2-NRTN treatment in monkeys was consistently observed in nigrostriatal neurons, was generally robust and typically mirrored the extent and intensity of NRTN expression in the striatum and the nigra. This was evident even at 28 days postdosing, with substantially lower doses, and less NRTN expression, than in the PD cases. Finally, in further contrast to the TH response in PD brain, the area of TH induction in monkey striatum consistently exceeded that of NRTN staining in adjacent sections (Fig. 7).
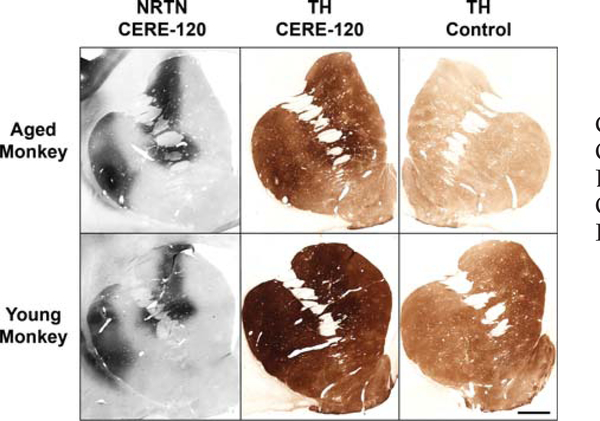
FIG. 7.
Typical examples of TH induction in aged (top) and young (bottom) monkeys, showing relationship between NRTN expression in striatum (left panels) and corresponding TH induction (middle panels). For the aged monkey, the right-hand panel depicts TH-ir on his contralateral (untreated) side. For young monkey, a representative section from a control monkey injected with formulation-buffer is depicted. Note that in these (and typically all) monkey studies, the area of TH induction extends well beyond boundaries of NRTN staining. Scale bar represents 2 mm.
DISCUSSION
Attempts to develop neurotrophic factors as therapeutic agents for neurodegenerative diseases have persisted for decades. While their potential for substantially advancing treatment is still generally acknowledged, significant obstacles with delivery continue to hamper their translation to clinical practice. The present novel data warn that the long-standing delivery issues are likely even more complex for human CNS diseases than previously appreciated, but also demonstrate that gene therapy may offer the means to overcome these issues, while providing valuable insight for a path forward.
We show that gene therapy can produce targeted expression of a potentially potent therapeutic protein deep within the brains of neurodegenerative patients. Administration of AAV2-NRTN (CERE-120) to the putamen in the PD brain resulted in clear expression of NRTN in the targeted putamen, conservatively estimated to cover ~15% of the structure by volume. While it is not known how much coverage is required for clinical benefit in PD patients, this amount is clearly within the range that provided biological benefit in several nonhuman primate models,4,12–14 and the accumulated animal and human safety data now provide the justification for responsibly expanding that coverage further. However, an equally important observation is that, in contrast to all prior animal studies, NRTN expression in the PD putamen did not result in labeling of the neuronal cell bodies in the SNc, despite putaminal coverage more than sufficient to produce this response in young, aged, and MPTP-parkinsonian monkeys. This distinction suggests a profound difference in the status and function of nigrostriatal neurons in advanced PD versus typical animal models used for PD translational research. Moreover, if future attempts to develop neurotrophic factors for diseases like PD are to be successful, they likely will have to take this difference into account.
Of equal interest is the modest AAV2-NRTN-mediated TH-induction seen in the putamen following AAV2-NRTN administration and subsequent NRTN expression. Since TH is a major enzyme for dopamine synthesis and a surrogate for functional enhancement of degenerating dopamine neurons, our data offer the first evidence that gene therapy delivering a neurotrophic factor can enhance impaired markers of dopamine function in the degenerating neurons of the PD brain. However, the lack of a robust and consistent TH signal in the putamen (in conjunction with the no detectable TH-induction in the SNc) suggests the biological response achieved was suboptimal and very likely insufficient to produce satisfactory clinical improvement. These findings therefore provide a plausible explanation for the modest and inconsistent clinical effects reported for patients enrolled in the controlled AAV2-NRTN study (Marks et al., in press).
The robust TH signal in nonhuman primates in response to AAV-NRTN is in marked contrast to the limited signal in PD in a number of important ways, including: (1) the intensity of TH signal was far less in PD, (2) it occurred with less frequency and reliability, and (3) it occurred within a much smaller portion of the putamen, well within the region of NRTN expression. This lack of robust TH-induction in PD is consistent with the lack of NRTN-labeled SNc neurons, for it is commonly recognized that to optimally activate cellular repair genes in degenerating neurons, neurotrophic proteins must be elevated in the cell bodies of those neurons. Yet, the observation that even a weak TH response was induced in the degenerating PD nigrostriatal neurons may be very important. Together with the evidence for a similarly weak NRTN signal in the SNc, it suggests that strategies assuring greater neurotrophic exposure to degenerating perikarya are likely required to gain a more rapid and robust neurotrophic response and thus more meaningful clinical benefit.
Conventional wisdom, based on considerable animal research with neurotrophic factors (primarily GDNF) in degenerating nigrostriatal neurons, argued that targeting the terminal fields of these neurons (i.e., the striatum) is both necessary and sufficient to gain optimal neurotrophic benefit (and that targeting the SNc is unnecessary or even counter-productive).18–20 This perspective was further supported by seminal research with neurotrophic factors demonstrating that they most often function by being taken-up by the neuron’s terminals and retrogradely transported to their cell bodies to induce trophic effects.21,22 Indeed, delivering GDNF or NRTN to the terminal field in the striatum has consistently been shown to be sufficient to elevate GDNF and NRTN levels in both the axon terminals as well as the cell bodies in the nigra via retrograde transport.8,23–25 We show here for the first time that this does not occur in a similar fashion in advanced PD, revealed by the paucity of NRTN-positive perikarya in the nigra, despite clear NRTN in the putamen and sufficient dopamine neurons in the SNc.
It is noteworthy that many of the studies demonstrating the protective effects of intrastriatal GDNF and NRTN against 6-OHDA neurotoxicity preadministered the neurotrophic factor before toxin exposure, and in so doing, assured that adequate protein had been transported to the cell bodies well before the neurons began to degenerate and axonal transport mechanisms became impaired (e.g., as shown in Refs. 20, 26, and 27). The data convincingly demonstrate that it is not necessary to directly administer the protein to the nigra when using an experimental paradigm that results in nigral exposure of the trophic factor prior to significant degeneration, but do not demonstrate that nigral exposure of the trophic factor is not essential. Similarly, data showing injections of GDNF into the nigra, in addition to the striatum, are without benefit and may be harmful20 also should be interpreted with care. These data merely show that when sufficient nigral GDNF exists from retrograde transport of striatal GDNF, the additional targeting of the nigra is unnecessary and may even produce dose-related side effects.
A major observation of this article is the failure to achieve evidence of retrograde transport of NRTN from the striatum to the SNc in humans with advanced PD, in contradistinction to several nonhuman primate models, involving a range of dosing parameters. One possible explanation may relate to differences in tissue fixation, since the monkey tissue was fixed by intracardiac perfusion, while both PD brains were immersion-fixed following a postmortem interval of 6 or 13 hours. However, multiple converging lines of evidence support a conclusion that the different results are indeed due to a transport deficiency in advanced PD and not due to difficulties with staining the human tissue. First, despite the huge difference in NRTN staining pattern in the nigra between PD patients and nonhuman primates, the NRTN staining pattern seen in striatum is indistinguishable. Second, NRTN-expressing neurons in the PD striatum were detectable well outside the major sphere of intense NRTN staining, suggesting that even lower levels of NRTN can be detected with immunostaining. Third, monkey SNc neurons consistently displayed TH-upregulation secondary to retrograde NRTN transport, but no such upregulation was seen in the PD cases. Since TH-staining was seen in many unaffected brain regions in the PD cases (e.g., hypothalamus), the lack of evidence for TH-induction in SNc neurons argues for a lack of NRTN protein and against problems with detection. Finally, a weak TH induction pattern was observed in the putamen of the PD cases, also arguing against a detection issue. All these pieces of evidence support the conclusion that reduced NRTN protein in the PD nigra is most likely due to impaired transport of protein from the striatum to the SNc. Another difference between the monkey tissue and the human PD tissue was that the monkeys were injected with CERE-120 into both structures of the striatum (i.e., caudate and putamen), whereas the human PD patients were given CERE-120 into the putamen, only. However, this difference also cannot easily explain the profound differences in NRTN observed in the nigra between the two. In nonhuman primates, NRTN was easily detected in all regions of the substantia nigra including the ventrolateral portion, which preferentially innervates the postcommissural putamen (and not the caudate). Thus, this protein could only exist in the nigra via retrograde transport from the putamen. The absence of similar NRTN in the human PD cases following CERE-120 administration to the putamen argues for a clear difference in the bioactivity of CERE-120 in the nigrostriatal system of nonhuman primates versus moderately advanced PD patients.
While nonhuman primate models have proven to be invaluable in identifying and elucidating novel treatment approaches for neurodegenerative diseases like PD3,4,28–31 the novel data presented in this article, coupled with an emerging thesis in the literature regarding axonal transport deficiencies (see below) warn that it may likely be important to further refine models of neurodegeneration intended to support translational R&D efforts. The search for new means of inducing neuronal death far more slowly than does MPTP and 6-OHDA will likely be required, thus providing a longer “window of opportunity” to study and/ or exploit functional deficits that likely accompany the pathogenesis that eventually kills the neurons. Recent efforts by Chung et al.32 inducing axonal transport deficiencies by over-expressing alpha-synuclein raise intriguing possibilities into novel approaches for possibly developing such models.
Though we did not perform any measurements of axonal transport in our study, a loss of retrograde transport in nigrostriatal neurons undergoing severe degeneration in Parkinson’s disease represents the most parsimonious explanation for the lack of NRTN protein in the SNc. Others have previously noted that deficient axonal transport frequently precedes dying back of axon terminals in animal neurodegeneration models, while similar evidence is accumulating that disruptions in axonal transport occur well in advance of cell death and frank axonal degeneration in many neurodegenerative diseases (e.g., ALS, AD, and HD).33–36 Early-stage deficiencies in axonal transport in these other diseases have been linked to many of the same axonal pathogenic events observed in PD,33,35,36 suggesting the plausibility for a similar loss of axonal transport in PD. Moreover, several gene mutations causally linked to PD (e.g., parkin, PINK1, DJ-1, and LRRK) are known to impair mitochondrial-energy function, and axonal transport is highly energy-dependent, further supporting the plausibility of an axonal transport deficiency in PD. Additionally, at least 2 familial α-synuclein mutations implicated as causal in PD have been directly associated with impaired axonal transport in cultured neurons, while rats over-expressing α-synuclein (via administration of AAV-α-synuclein to the SN) display frank axonal transport loss prior to dying back of terminals and SNc cell death.32 Finally, α-synuclein deposits in PD axons have been coupled to transport impairments in PD brains,35 with the authors stating that “such deposits almost certainly will interfere with axon function and severely or completely suppress transport of substances through the axon”. Thus, the lack of NRTN protein in the SNc cell bodies, despite sufficient expression of NRTN within the terminal fields of those neurons, offers additional empirical support for the emerging concept that impairments in axonal transport may be an important, (possibly early) pathogenic event in many human neurodegenerative diseases, including PD.
Collectively, these data provide a plausible hypothesis for why the advanced PD patients treated with AAV2-NRTN in the double-blinded trial (Marks et al., in press) did not demonstrate a significant benefit in the primary endpoint at 12 months, but did show some benefit after a longer latency. We postulate that deficiencies in axonal transport resulted in far less NRTN than expected reaching the nigral cell bodies following CERE-120 administration to the putamen, thus blunting the neurotrophic response, especially within 12 months. At the same time, the limited amount of NRTN that did reach the nigral cell bodies likely induced a very mild trophic response (as evidenced by occasional NRTN-positive nigral neurons and the sparse TH induction in these PD cases). Over time, one might expect this initial NRTN signal and trophic response to become amplified, leading to a gradual induction of repair genes, improvement in cell function and the enhanced clinical response observed at the later time points.
In addition to offering a plausible explanation for the past clinical results observed with AAV2-NRTN, the data we report here also argue that targeting the striatal terminal fields only, is likely insufficient for achieving clinically meaningful effects in moderately advanced PD patients. Rather, it is likely necessary to also target nigral cell bodies to ensure that adequate NRTN is expressed there, so that genes to repair these degenerating cells can be induced more effectively, thus improving their function, perhaps including more efficient transport (i.e., via a positive, feed-forward process). These functional changes, in turn, might lead to more robust and rapid clinical improvement.
In conclusion, the data presented in this manuscript provide the first evidence that gene therapy can be used to target expression of a neurotrophic protein (NRTN), selectively to the brain region of major degeneration in advanced Parkinson’s patients. Further, we demonstrate that these degenerating neurons can respond to the neurotrophic-factor, for expression of NRTN is associated with a clear, albeit modest, trophic-response typically associated with improved dopaminergic function (i.e., TH-induction). Finally, our studies demonstrate that targeting the nigrostriatal system in PD must take into account deficiencies in the integrity of the nigrostriatal tract, which apparently limit axonal transport from the terminal fields of the degenerating neurons to their cell bodies. Thus, conventional, putaminal-only dosing (employed in several inconclusive trials in the past) likely provides suboptimal levels of neurotrophic factor in the cell body, significantly limiting the biological effects, and thus the potential clinical benefit of this therapeutic approach. In sum, our data collectively argue that gene therapy may provide the means to overcome the long-standing delivery issues associated with using neurotrophic factors for treating neurodegenerative diseases. However, to achieve optimal biological effects and therapeutic benefit, they also argue it is likely necessary to directly target the degenerating cell bodies (in the case of PD, the cells in the SNc), along with traditional targeting of their terminal field (in PD, the striatum). A Phase 1/ 2 clinical trial is underway to test the safety and efficacy of nigral and putaminal administration of CERE-120 in PD patients who experience motor complications despite adequate antiparkinsonian therapy (ClinicalTrials.gov identifier: NCT00985517).
Acknowledgments:
We appreciate a grant from the Michael J. Fox Foundation for Parkinson’s Research (to RTB) to help defray some of the costs of the clinical trial. We also thank the assistance of Amanda Sánchez Losada in preparing this manuscript, the technical assistance of Kathie Bishop, Gina Folino and Katrin Hofer for histological assistance, and the general support of Jeffrey Ostrove. Finally, the authors gratefully acknowledge the unselfish courage of patients and the immediate family of patients who volunteer to participate in experimental clinical trials and agree to donate their organs for scientific study after their death. In this regard, we are particularly indebted to DAB and JLC and their families, who donated the brains that provided the unique and crucial information comprising this paper, without which the CERE-120 (AAV2-NRTN) program would not likely have moved forward.
Financial Disclosures: Raymond T. Bartus: Stock Ownership in medically-related fields: Ceregene, Inc; Advisory Boards: NIDA, BSC; Employment: Ceregene, Inc.; Honoraria: University of Kentucky; Grants: MJFF. Christopher D. Herzog: Stock Ownership in medically-related fields: Ceregene, Inc.; Employment: Ceregene, Inc. Alistair Wilson: Stock Ownership in medically-related fields: Ceregene, Inc.; Employment: Ceregene, Inc. Lamar Brown: Stock Ownership in medically-related fields: Ceregene, Inc.; Employment: Ceregene, Inc. Joao Siffert: Stock Ownership in medicallyrelated fields: Ceregene, Inc.; Advisory Boards: Orasi Medical; Employment: Ceregene, Inc.; Grants: MJFF. Eugene M. Johnson: Stock Ownership in medically-related fields: Ceregene, Inc. and Amarantus; Intellectual Property Rights: Inventor on patents licensed by Washington University to Ceregene for neurturin; Consultancies: Ceregene, Elan, Acorda, Amarantus; Advisory Boards: Ceregene SAB, MJFF, AT Children’s Project, Glaucoma Research Foundation; Employment: Washington University Medical School; Honoraria: MJFF; Royalties: R&D Research reagents (neuturin, persephin), Upstate Biological for antibody research reagents-these go through Washington University. C. Warren Olanow: Stock Ownership in medically-related fields: Ceregene, Inc., Clintrex; Consultancies: Ceregene, Novartis/Orion, Teva/Lundbeck, Solvay/Abbot; Expert Testimony: Welding litigation; Advisory Boards: Ceregene SAB; Employment: Mt. Sinai School of Medicine. Elliott J. Mufson: Stock Ownership in medically-related fields: Ceregene, Inc.; Consultancies: Ceregene, Inc.; Advisory Boards: Alz Forum; Employment: Rush; Contracts: Medtronics; Honoraria: NIH, Agence nationale de la Recherche, Paris, Springfield Geneva AD Conference; Grants: NIH PO1AG14449, RO1AG10688, RO1AG09466, RO1HD057564. Jeffrey H. Kordower: Stock Ownership in medically-related fields: Ceregene, Inc., Brainstorm; Consultancies: Ceregene, Inc.; Advisory Boards: Ceregene SAB; Grants: NIH, MJFF, Metronics.
Footnotes
Potential conflict of interest: Several of the authors are employees of Ceregene Inc., (see affiliations), the company developing AAV2-NRTN (CERE-120) for PD. The remaining authors (except for Y.C.) are consultants to the company. Employees and consultants receive financial remuneration and have been awarded stock options from Ceregene.
REFERENCES
- 1.Akerud P, Alberch J, Eketjall S, Wagner J, Arenas E. Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons. J Neurochem 1999;73:70–78. [DOI] [PubMed] [Google Scholar]
- 2.Horger BA, Nishimura MC, Armanini MP, et al. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci 1998;18:4929–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kordower JH, Emborg ME, Bloch J, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 2000;290:767–773. [DOI] [PubMed] [Google Scholar]
- 4.Kordower JH, Herzog CD, Dass B, et al. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol 2006;60:706–715. [DOI] [PubMed] [Google Scholar]
- 5.Kordower JH, Palfi S, Chen EY, et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann Neurol 1999;46:419–424. [DOI] [PubMed] [Google Scholar]
- 6.Nutt JG, Burchiel KJ, Comella CL, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 2003;60:69–73. [DOI] [PubMed] [Google Scholar]
- 7.Lang AE, Gill S, Patel NK, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 2006;59:459–466. [DOI] [PubMed] [Google Scholar]
- 8.Salvatore MF, Ai Y, Fischer B, et al. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol 2006;202:495–505. [DOI] [PubMed] [Google Scholar]
- 9.Sherer TB, Fiske BK, Svendsen CN, Lang AE, Langston JW. Crossroads in GDNF therapy for Parkinson’s disease. Mov Disord 2006;21:136–141. [DOI] [PubMed] [Google Scholar]
- 10.Gasmi M, Brandon EP, Herzog CD, et al. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol Dis 2007;27:67–76. [DOI] [PubMed] [Google Scholar]
- 11.Gasmi M, Herzog CD, Brandon EP, et al. Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson’s disease. Mol Ther 2007;15:62–68. [DOI] [PubMed] [Google Scholar]
- 12.Herzog CD, Dass B, Holden JE, et al. Striatal delivery of CERE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys. Mov Disord 2007;22:1124–1132. [DOI] [PubMed] [Google Scholar]
- 13.Herzog CD, Dass B, Gasmi M, et al. Transgene expression, bioactivity, and safety of CERE-120 (AAV2-neurturin) following delivery to the monkey striatum. Mol Ther 2008;16:1737–1744. [DOI] [PubMed] [Google Scholar]
- 14.Herzog CD, Brown L, Gammon D, et al. Expression, bioactivity, and safety 1 year after adeno-associated viral vector type 2-mediated delivery of neurturin to the monkey nigrostriatal system support cere-120 for Parkinson’s disease. Neurosurgery 2009;64:602–612. [DOI] [PubMed] [Google Scholar]
- 15.Bartus RT, Herzog CD, Bishop K, et al. Issues regarding gene therapy products for Parkinson’s disease: the development of CERE-120 (AAV-NTN) as one reference point. Parkinsonism Relat Disord 2007;13 (Suppl 3):S469–S477. [DOI] [PubMed] [Google Scholar]
- 16.Marks WJ Jr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol 2008;7:400–408. [DOI] [PubMed] [Google Scholar]
- 17.Eriksdotter JM, Nordberg A, Amberla K, et al. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 1998;9:246–257. [DOI] [PubMed] [Google Scholar]
- 18.Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci 2004;7:105–110. [DOI] [PubMed] [Google Scholar]
- 19.Bjorklund A, Rosenblad C, Winkler C, Kirik D. Studies on neuroprotective and regenerative effects of GDNF in a partial lesion model of Parkinson’s disease. Neurobiol Dis 1997;4:186–200. [DOI] [PubMed] [Google Scholar]
- 20.Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci 2000;20:4686–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mufson EJ, Kroin JS, Sendera TJ, Sobreviela T. Distribution and retrograde transport of trophic factors in the central nervous system: functional implications for the treatment of neurodegenerative diseases. Prog Neurobiol 1999;57:451–484. [DOI] [PubMed] [Google Scholar]
- 22.Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS. Neurotrophic factors: from molecule to man. Trends Neurosci 1994;17:182–190. [DOI] [PubMed] [Google Scholar]
- 23.Ai Y, Markesbery W, Zhang Z, et al. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J Comp Neurol 2003;461:250–261. [DOI] [PubMed] [Google Scholar]
- 24.Su X, Kells AP, Huang EJ, et al. Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Human Gene Ther 2009;20:1627–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomac A, Lindqvist E, Lin LF, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 1995;373:335–339. [DOI] [PubMed] [Google Scholar]
- 26.Kirik D, Rosenblad C, Bjorklund A. Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur J Neurosci 2000;12:3871–3882. [DOI] [PubMed] [Google Scholar]
- 27.Choi-Lundberg DL, Lin Q, Schallert T, et al. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp Neurol 1998;154:261–275. [DOI] [PubMed] [Google Scholar]
- 28.Gash DM, Zhang Z, Ovadia A, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 1996;380:252–255. [DOI] [PubMed] [Google Scholar]
- 29.Jenner P From the MPTP-treated primate to the treatment of motor complications in Parkinson’s disease. Parkinsonism Relat Disord 2009;15 (Suppl 4):S18–S23. [DOI] [PubMed] [Google Scholar]
- 30.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol 2000;163:495–529. [DOI] [PubMed] [Google Scholar]
- 31.Bartus RT. Physostigmine and recent memory: effects in young and aged nonhuman primates. Science 1979;206:1087–1089. [DOI] [PubMed] [Google Scholar]
- 32.Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci 2009;29: 3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy S, Zhang B, Lee VM, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropath 2005;109:5–13. [DOI] [PubMed] [Google Scholar]
- 34.Morfini GA, Burns M, Binder LI, et al. Axonal transport defects in neurodegenerative diseases. J Neurosci 2009;29:12776–12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braak H, Sandmann-Keil D, Gai W, Braak E. Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by alpha-synuclein immunocytochemistry. Neurosci Lett 1999;265:67–69. [DOI] [PubMed] [Google Scholar]
- 36.De Vos KJ, Grierson AJ, Ackerley S, Miller CC. Role of axonal transport in neurodegenerative diseases. Ann Rev Neurosci 2008; 31:151–173. [DOI] [PubMed] [Google Scholar]