Abstract
Aims.
A 5-point change in the Kansas City Cardiomyopathy Questionnaire (KCCQ) is commonly considered to be a clinically significant difference in health status in patients with HF. We evaluated how the magnitude of change relates to subsequent clinical outcomes.
Methods and Results.
Using data from the HF-ACTION trial of exercise training in chronic HF (N=2331), we used multivariable Cox regression with piecewise linear splines to examine the relationship between change in KCCQ overall summary score from baseline to 3 months (range 0–100; higher scores reflect better health status) and subsequent all-cause mortality/hospitalization. Among 2038 patients with KCCQ data at the 3 month visit, KCCQ scores increased from baseline by ≥5 points for 45%, scores decreased by ≥5 points for 23%, and scores for the remaining 32% of patients changed by <5 points. There was a nonlinear relationship between change in KCCQ and outcomes. Worsening health status was associated with increased all-cause mortality/hospitalization (adjusted HR 1.07 per 5-point KCCQ decline; 95% CI: 1.03–1.12; p <.001). In contrast, improving health status, up to an 8-point increase in KCCQ, was associated with decreased all-cause mortality/hospitalization (adjusted HR 0.93 per 5-point increase; 95% Confidence Interval [CI]: 0.90–0.97; p < .001). Additional improvements in health status beyond an 8-point increase in KCCQ was not associated with all-cause death or hospitalization (p=0.42).
Conclusion.
In patients with heart failure, small changes in KCCQ are associated with changing future risk, but more research will be necessary to understand how different magnitudes of improving health status affect outcomes.
Keywords: health status, quality of life, PRO
Over the last 2 decades, health-related quality of life—and the related concept of “health status”—has increasingly been used as an outcome measure in clinical trials and comparative effectiveness research.1–6 Indeed, in 2009 the United States Food and Drug Administration provided guidance on the usage of patient-reported outcomes such as health status to support labeling claims.7 In heart failure (HF), the Kansas City Cardiomyopathy Questionnaire (KCCQ) is one of the most commonly used and rigorously studied instruments for quantifying health status, having been validated in multiple HF-related disease states.8–11 In fact, baseline health status measured by the KCCQ predicts HF prognosis, and the U.S. Food and Drug Administration is considering the KCCQ for official recognition as a validated clinical outcome for HF.12–14
Interpreting the clinical meaning of statistically significant changes in health status measures, however, remains relatively unknown. A 5-point change in KCCQ overall summary score is widely considered to be the minimally noticeable clinical difference experienced by patients.4, 15–17 In an observational cohort of 476 outpatients with HF followed over a 6-week period, a mean of −5.4 ± 10.8 points and +5.7 ± 16.1 points corresponded to the treating cardiologist’s assessment of a small deterioration or improvement in heart failure, respectively.18 Change was determined by the physician’s assessment of whether a patient’s health status had changed based on a single 7-point scale question: large, moderate, or small deterioration; no change; or small, moderate, or large improvement. In the same analysis, patients deemed changes of as many as 1 to 5 KCCQ points to be clinically meaningful.19
In recent randomized trials, HF interventions from ivabradine to sacubitril/valsartan have been associated with small changes in health status from 1 to 3 KCCQ points.5, 14 To better characterize the potential clinical benefit associated with smaller changes in health status, we used data from Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) to describe patient groups who experienced a change in health status from baseline to 3 months and estimated the relationship between changing health status and subsequent clinical outcomes.
Methods
The study design and primary results of HF-ACTION have been previously published.4, 20 HF-ACTION remains the largest multicenter, randomized clinical trial to have investigated the safety and efficacy of aerobic exercise training compared to optimal medical therapy—in combination or alone—in ambulatory patients with reduced ejection fraction (EF ≤35%) and New York Heart Association (NYHA) class II-IV symptoms. The primary endpoint was a composite of all-cause death or all-cause hospitalization, and secondary endpoints included all-cause death and cardiovascular (CV) death or heart failure hospitalization. Exercise training consisted of supervised aerobic exercise (walking, treadmill, or stationary cycling) 3 times weekly for 36 sessions, followed by transition to a home-based exercise program. Follow-up occurred over a median of 2.6 years. The protocol was approved by the institutional review board or ethics committee at each of the 82 participating clinical study centers and the coordinating center. All patients provided written informed consent.
In HF-ACTION, the KCCQ questionnaire was used to measure HF-specific health status.8 The KCCQ transforms patient answers to 23 Likert-scale items into a 0 to 100-scaled overall summary score. Higher scores represent better health status. Several health domains specific to heart failure patients are tested including physical functioning, symptoms frequency and severity, social function, self-efficacy, and overall quality of life. Patients self-administered the KCCQ at the baseline visit, at 3-month intervals for the first 12 months, and annually thereafter for up to 4 years. However, despite the protocol, there was increasing missingness in KCCQ data capture after the 3-month visit. Additionally, patients separated by treatment group did not experience significant changes in KCCQ after 3 months.4 French-speaking participants in France and Quebec used a certified French translation of the KCCQ. Patients also completed the Beck Depression Inventory II (BDI) at same intervals as the KCCQ. Higher BDI-II scores indicate worse depressive symptoms; scores ≥ 10 are considered to represent clinically significant depressive symptoms.21
We categorized change in overall KCCQ summary score from baseline to 3 months as decrease (≥ 5 points decrease), no change (absolute change < 5 points), and increase (≥ 5 points increase). We describe baseline characteristics by the categorized change from baseline to 3 months. Categorical variables were presented as frequencies and percentages, and continuous variables were reported as medians with 25th and 75th percentiles. We compared baseline differences using the Kruskal-Wallis test for continuous variables and Pearson chi-square or Fisher’s exact tests for categorical variables.
Cox proportional hazards models were used to assess the association between change in overall KCCQ summary score (as a continuous independent variable) and rate of clinical outcomes. These analyses were landmarked at the 3-month visit. We tested the proportional hazards assumption and the linearity assumption for the change in overall KCCQ summary score. To assess the linearity assumption, we modeled the relationship between change in KCCQ summary score and outcomes using restricted cubic splines. The linearity assumption was not satisfied. To aid in interpretability, we modeled the nonlinear relationship between change in KCCQ summary score and outcomes using piecewise linear splines with a single knot at the value of 8 for the change in KCCQ summary score. This knot value was selected by fitting the model over a range of potential knots, and selecting the model that had the lowest Akaike Information Criteria. To examine the robustness of the association, we employed three nested adjustment models: Model 1 – unadjusted; Adjusted Model 2 – adjusted for baseline KCCQ overall score; and Adjusted Model 3 – Model 2 in addition to randomized treatment assignment and additional clinical covariates known to predict outcomes in HF-ACTION.22 Additional sensitivity analyses were performed with KCCQ change from 3- to 6-months to study these associations at a more stable time period after clinical trial enrollment.
Given the clinical interpretability of a 5-point change to represent minimal change, we also used multivariable Cox proportional hazards models to assess the association between change in KCCQ summary score as a categorical variable (≥ 5 point improvement, ≥ 5 point deterioration, and <5 point change) and risk of clinical outcomes. In addition to estimating hazard ratios and 95% confidence intervals, two-sided tests were also conducted at a significance level of 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
This analysis included 2038 patients with complete KCCQ data at baseline and 3 months. Patients who experienced an event in the first 3 months were excluded from the analysis (N=293). Online supplemental Table 1 compares the characteristics between patients excluded and included in the primary analysis. There were minor differences between the populations. Excluded patients were more likely to be African American (42.9% vs 31.1%), less likely to have an ICD (32.1% vs 41.4%), and reported lower KCCQ summary scores (62 vs 69).
Table 1.
Baseline characteristics by change in KCCQ overall summary score
| Characteristic | Overall n = 2038 |
Decrease (≥ 5 point) n = 462 |
No change (< 5 point) n = 658 |
Increase (≥ 5 point) n = 918 |
p-value |
|---|---|---|---|---|---|
| Age (years) | 60 (52, 68) | 60 (52, 70) | 61 (52, 70) | 59 (51, 67) | 0.04 |
| Female | 581/2038 (28.5%) | 123/462 (26.6%) | 205/658 (31.2%) | 253/918 (27.6%) | 0.18 |
| Race | 0.16 | ||||
| Black | 625/2007 (31.1%) | 155/455 (34.1%) | 180/649 (27.7%) | 290/903 (32.1%) | |
| White | 1277/2007 (63.6%) | 278/455 (61.1%) | 437/649 (67.3%) | 562/903 (62.2%) | |
| BMI, (kg/m2) | 30 (26, 35) | 30 (26, 35) | 29 (26, 34) | 30 (26, 36) | 0.04 |
| HF status | |||||
| Resting systolic blood pressure (mm Hg) | 111 (100, 126) | 110 (100 ,124) | 112 (100, 127) | 110 (100, 124) | 0.43 |
| Resting heart rate (beats per minute) | 70 (63, 77) | 70 (62, 78) | 68 (62, 76) | 70 (64, 78) | 0.02 |
| NYHA class III-IV | 741/2038 (36.4%) | 173/462 (37.4%) | 205/658 (31.2%) | 363/918 (39.5%) | 0.003 |
| Ischemic etiology of HF | 1061/2038 (52.1%) | 249/462 (53.9%) | 362/658 (55.0%) | 450/918 (49.0%) | 0.04 |
| Left ventricular ejection fraction (%) | 25 (20, 30) | 24 (19, 30) | 25 (20, 30) | 25 (20, 30) | 0.32 |
| Comorbidities | |||||
| Hypertension | 1201/2026 (59.3%) | 275/459 (59.9%) | 399/656 (60.8%) | 527/911 (57.8%) | 0.47 |
| Diabetes | 643/2038 (31.6%) | 158/462 (34.2%) | 192/658 (29.2%) | 293/918 (31.9%) | 0.20 |
| Previous MI | 867/2038 (42.5%) | 195/462 (42.2%) | 301/658 (45.7%) | 371/918 (40.4%) | 0.11 |
| Atrial Fibrillation/Flutter | 432/2038 (21.2%) | 120/462 (26.0%) | 127/658 (19.3%) | 185/918 (20.2%) | 0.02 |
| Labs | |||||
| ProBNP, pg/mL (n = 1225) | 829 (347, 1809) | 927 (361, 2301) | 849 (391, 1667) | 750 (318, 1748) | 0.08 |
| Blood urea nitrogen (mg/dL) | 21 (15, 28) | 22 (17, 30) | 21 (15, 27) | 20 (15, 27) | 0.002 |
| HF treatment | |||||
| Beta-blocker | 1926/2038 (94.5%) | 438/462 (94.8%) | 618/658 (93.9%) | 870/918 (94.8%) | 0.73 |
| Carvedilol dose equivalents (mg) | 38 (25–50) [1912] | 25 (25–50) [436] | 50 (25–50) [615] | 50 (25–50) [861] | 0.38 |
| ACE/ARB | 1933/2038 (94.8%) | 433/462 (93.7%) | 632/658 (96.0%) | 868/918 (94.6%) | 0.19 |
| Loop diuretic | 1592/2038 (78.1%) | 373/462 (80.7%) | 485/658 (73.7%) | 734/918 (80.0%) | 0.004 |
| Aldosterone antagonist | 920/2038 (45.1%) | 209/462 (45.2%) | 283/658 (43.0%) | 428/918 (46.6%) | 0.36 |
| Implantable Cardioverter Defibrillator | 844/2038 (41.4%) | 183/462 (39.6%) | 263/658 (40.0%) | 398/918 (43.4%) | 0.27 |
| HF function and quality of life | |||||
| Baseline exercise capacity | |||||
| Peak VO2 (mL/kg/min) | 14.5 (11.6, 17.7) | 14.0 (11.3, 17.3) | 15.0 (12.0, 17.9) | 14.6 (11.5, 17.8) | 0.08 |
| Peak heart rate with exercise (beats per minute) | 120 (104, 134) | 116 (101, 132) | 120 (106, 136) | 120 (105, 134) | 0.01 |
| CPX duration (min) | 10 (7, 12) | 9 (7, 12) | 10 (7, 13) | 10 (7, 12) | 0.002 |
| 6MWT distance (m) | 372 (300, 434) | 370 (304, 425) | 384 (314, 443) | 366 (290, 430) | 0.004 |
| KCCQ overall summary score | 69 (52, 83) | 74 (59, 86) | 80 (60, 92) | 60 (46, 74) | <.001 |
| Beck depression inventory II | 8 (4, 15) | 8 (5, 14) | 6 (3, 12) | 10 (5, 17) | <.001 |
| Social history | |||||
| Any college education | 1216/1995 (61.0%) | 266/451 (59.0%) | 394/641 (61.5%) | 556/903 (61.6%) | 0.62 |
| Married or living with partner | 1251/2024 (61.8%) | 289/459 (63.0%) | 408/650 (62.8%) | 554/915 (60.5%) | 0.57 |
| Income < $35,000* | 994/2031 (48.9%) | 217/460 (47.2%) | 318/658 (48.3%) | 459/913 (50.3%) | 0.49 |
| Unemployed | 104/1997 (5.2%) | 17/454 (3.7%) | 24/644 (3.7%) | 63/899 (7.0%) | 0.005 |
| Permanent employment disability | 622/1997 (31.1%) | 138/454 (30.4%) | 198/644 (30.7%) | 286/899 (31.8%) | 0.84 |
Values are median (25th, 75th) or number (%). CPX = cardiopulmonary exercise, HF = heart failure, KCCQ = Kansas city cardiomyopathy questionnaire, MI = myocardial infarction.
2003–2007 U.S. Dollars.
In the analysis population, the median baseline KCCQ overall summary score was 69 (Q1, Q3; 52, 83). From baseline to 3 months, 918 patients (45%) experienced a ≥5 point improvement in health status, while 23% (N=462) experienced a ≥5 point decline, and 32% (N=658) experienced a change of less than 5 points. The distribution of patients’ KCCQ overall summary scores at 3 months and as a change from baseline to 3 months is presented in Figure 1. Overall, 215 (11%) patients experienced a change between +5 to +8 KCCQ points, and 703 (35%) patients experienced an improvement in KCCQ overall score above 8 points.
Figure 1: Distributions of KCCQ in Analysis Cohort.
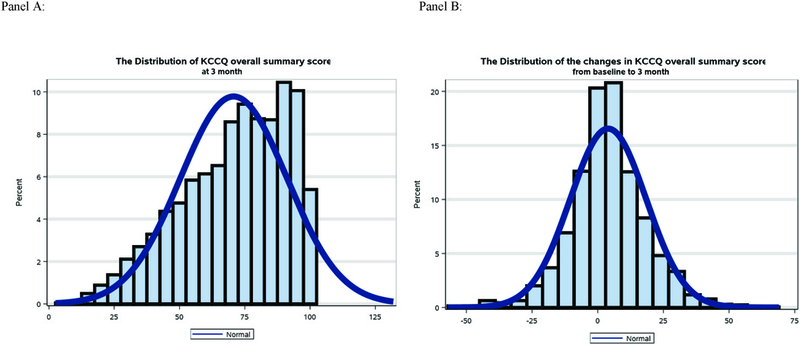
Panel A shows the distribution of patients’ Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score at 3 months in the analysis population. The median baseline KCCQ overall summary score was 69 (Q1, Q3; 52, 83). Panel B shows the distribution of patient’s change in KCCQ overall summary score from baseline to 3 months. As represented by the figure, 918 patients (45%) experienced a ≥5 point improvement in health status, while 23% (N=462) experienced a ≥5 point decline, and 32% (N=658) experienced a change of less than 5 points. Overall, 215 (11%) patients experienced a change between +5 to +8 KCCQ points and 703 (35%) patients experienced an improvement in KCCQ overall score above 8 points.
Table 1 lists baseline characteristics by change in KCCQ group. Groups were statistically different with respect to age, heart rate, BMI and history of atrial fibrillation and renal dysfunction, but most of these differences were not clinically meaningful. A higher proportion of patients who experienced improvement in KCCQ were assigned baseline NYHA class III-IV symptom limitations compared to those who reported minimal change (39.5% versus 31.2%). Baseline functional capacity as measured by peak VO2 was not significantly different among the 3 groups (median 14.5 mL/kg/min).
Baseline health status was significantly different among the 3 groups. Patients experiencing a ≥5 point improvement at 3 months had the lowest median baseline KCCQ score of 60 and a BDI-II score consistent with clinical depressive symptoms (Table 1). Patients experiencing minimal change had a 20-point higher median KCCQ of 80 and the lowest BDI-II of the three groups. Meanwhile, patients experiencing a decline in health status had intermediate KCCQ and BDI-II median scores of 74 and 6, respectively.
Figure 2 shows outcomes over a median follow-up of 31.2 months by change in KCCQ groups. Over follow-up, 68.9% of patients at risk had either died or been hospitalized. Those experiencing a decline in KCCQ had significantly greater rates of death or hospitalization than patients with minimal change (72.1% vs. 67.5%; p = 0.004). However, rates were similar between patients who reported a ≥ 5 point improvement and those with < 5 point change (68% vs. 67.5%). Similarly, rates of all-cause death and CV death or HF hospitalization were greatest among patients reporting a KCCQ decline compared to those with no change or a KCCQ increase.
Figure 2: Kaplan-Meier Curves of All-Cause Mortality or Hospitalization, All-Cause Mortality, and Cardiovascular Death or Heart Failure Hospitalization.
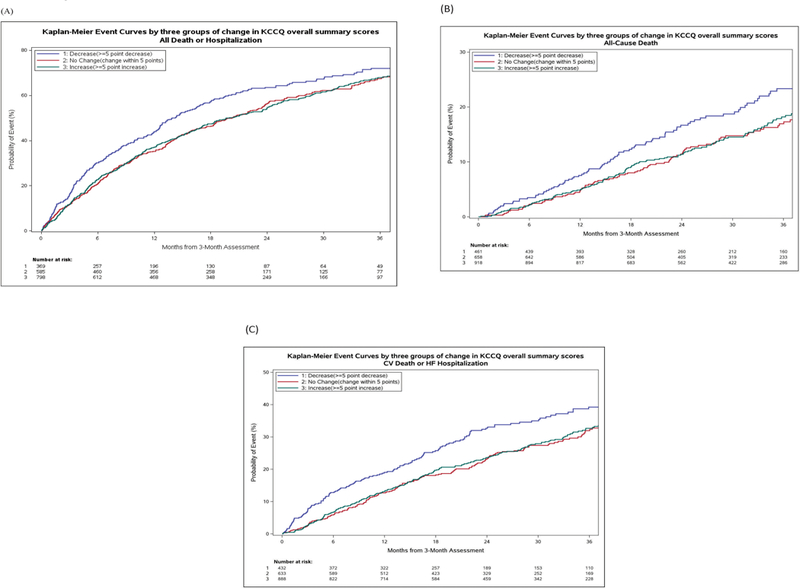
Unadjusted Kaplan-Meier curves for all-cause mortality or hospitalization (A), all-cause mortality (B), and cardiovascular mortality or heart failure hospitalization (C) in patients event-free for at least 3 months, stratified by changing Kansas City Cardiomyopathy Questionnaire overall summary score between baseline and 3 months. Blue line indicates ≥ 5 point decline; Red line indicates change within 5 points; Green line indicates ≥ 5 point increase.
Figure 3 shows the nonlinear relationship between change in KCCQ and all-cause death or hospitalization. In unadjusted analysis, improvement in KCCQ up to 8 points increase was associated with lower risk of clinical outcomes (Hazard ratio [HR]: 0.91 per 5 point increase in KCCQ up to 8; 95% CI: 0.88 to 0.95; p < 0.001); larger increases above 8 points were not associated with a change in risk of clinical outcomes (Table 2; Figure 2). Worsening KCCQ was associated with an increased risk of death or hospitalization (HR: 1.10 per 5 point decrease in KCCQ; 95% confidence ratio [CI]: 1.06 to 1.14; p < 0.001). After adjustment for randomized treatment assignment, baseline KCCQ score, and other clinical covariates found to predict outcomes in this trial population, overall results were similar.22 The modeling of the other CV endpoints demonstrated similar overall relationships (Table 2). Additional sensitivity analyses evaluating KCCQ change between the 3- and 6- month interval also demonstrated a non-linear and similar overall risk relationships (Online Supplemental Table 2).
Figure 3: Relationship between Change in KCCQ at 3 Months and All-cause Mortality or Hospitalization.
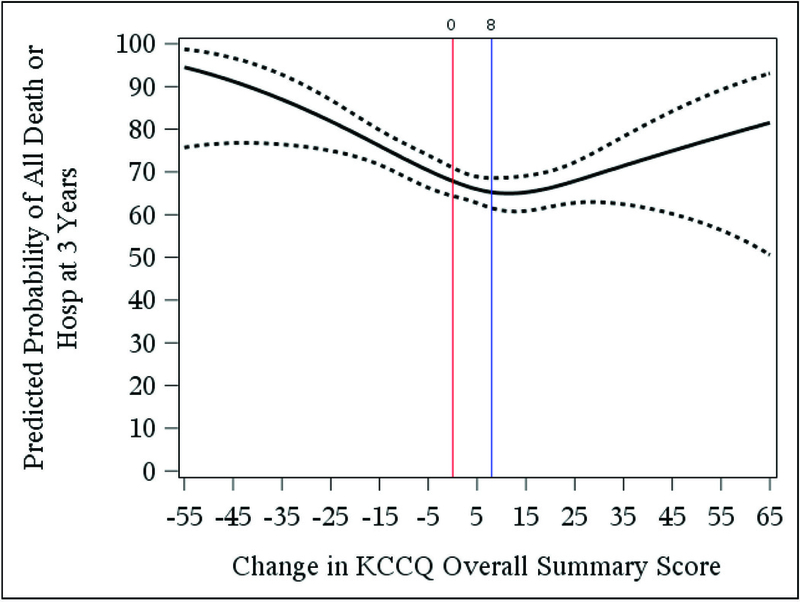
Among patients event-free at 3 months, this plot demonstrates the nonlinear relationship between 3-month change in Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score and predicted probability of all-cause death or hospitalization within 3 years. In our data, we observe a single inflection point where change in KCCQ is equal to 8. Increases in KCCQ from 0 to 8 was associated with a significant reduction in risk (p<0.001) but increases in KCCQ greater than 8 were not significantly associated with an increase in risk (p=0.183). Vertical reference bars identify these inflection points. Decreases in KCCQ between baseline and 3 months was associated with increased risk (p <0.001). Solid line indicates the predicted probability of death/hospitalization; dotted lines indicates point-wise 95% confidence intervals.
Table 2.
Association between Change in KCCQ at 3 months (Per 5 KCCQ Point Change from 0) and Study Outcomes
| Unadjusted | Adjusted for Baseline KCCQ | Adjusted for Clinical Variables | |||||
|---|---|---|---|---|---|---|---|
| Outcome | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| All-cause mortality or all-cause hospitalization |
|||||||
| KCCQ | Per 5 point decline from 0 | 1.10 (1.06, 1.14) | <.001 | 1.11 (1.06, 1.15) | <.001 | 1.07 (1.03, 1.12)* | <.001 |
| Per 5 point increase from 0 to 8 | 0.91 (0.88, 0.95) | <.001 | 0.91 (0.87, 0.94) | <.001 | 0.93 (0.90, 0.97)* | <.001 | |
| Per 5 point increase above 8 | 1.04 (0.98, 1.09) | 0.18 | 0.97 (.92, 1.03) | 0.28 | 0.98 (0.92, 1.03)* | 0.42 | |
| All-cause mortality | |||||||
| KCCQ | Per 5 point decline from 0 | 1.12 (1.06, 1.18) | <.001 | 1.13 (1.07, 1.19) | <.001 | 1.10 (1.04, 1.17)† | <.001 |
| Per 5 point increase from 0 to 8 | 0.90 (0.85, 0.94) | <.001 | 0.89 (0.84, 0.94) | <.001 | 0.91 (0.86, 0.96) † | <.001 | |
| Per 5 point increase above 8 | 1.07 (0.99, 1.16) | 0.08 | 1.00 (0.92, 1.09) | 0.96 | 1.02 (0.94, 1.10) † | 0.72 | |
|
Cardiovascular mortality or heart failure hospitalization |
|||||||
| KCCQ | Per 5 point decline from 0 | 1.11 (1.06, 1.16) | <.001 | 1.19 (1.07, 1.17) | <.001 | 1.09 (1.04, 1.14)‡ | <.001 |
| Per 5 point increase from 0 to 8 | 0.90 (0.86, 0.95) | <.001 | 0.89 (0.85, 0.94) | <.001 | 0.92 (0.87, 0.97)‡ | <.001 | |
| Per 5 point increase above 8 | 1.05 (0.98, 1.12) | 0.18 | 0.97 (0.90, 1.04) | 0.41 | 0.98 (0.91, 1.05)‡ | 0.49 | |
Model for all-cause mortality or all-cause hospitalization adjusted for baseline KCCQ score, randomized treatment assignment, dosage of beta-blocker, KCCQ symptom stability, ejection fraction (LVEF), country (US vs non-US), sex, ventricular conduction, Weber class, blood urea nitrogen (BUN), and mitral regurgitation.
Model of all-cause mortality adjusted for baseline KCCQ score, randomized treatment assignment, sex, body mass index, loop diuretic dose, creatinine, exercise duration, ventricular conduction, Canadian Cardiovascular Society angina classification, and LVEF.
Model for cardiovascular mortality or heart failure hospitalization adjusted for baseline KCCQ score, randomized treatment assignment, loop diuretic dose, LVEF, mitral regurgitation, ventricular conduction, KCCQ symptom stability, BUN, race, sex, age, Weber class, VE/VCO2.
In analyses evaluating the association between categorized change in KCCQ and risk of clinical outcomes, those experiencing a decline in health status had significantly higher risk of death or hospitalization, all-cause death, and CV mortality or HF hospitalization in unadjusted analyses (Table 3; Figure 2). However, after adjustment for clinical variables, while a consistent trend for increased risk persisted across all endpoints, the association was only statistically significant between health status decline and CV mortality or HF hospitalization (adjusted HR 1.36; 95% CI: 1.06–1.76; p = 0.02). In contrast, the group of patients reporting a ≥ 5 point improvement in KCCQ experienced similar rates of death or hospitalization as patients reporting minimal change (unadjusted HR: 1.0; 95% CI: 0.86–1.17; p = 0.99; adjusted HR: 0.88; 95% CI: 0.74–1.04; p = 0.12). There was no significant interaction between change in KCCQ and outcomes by randomized treatment assignment (p = 0.22 for all cause death/hospitalization).
Table 3.
Association between KCCQ Categorical Change Groups at 3 Months and Study Outcomes (Reference = No change [<5 point group])
| Unadjusted | Adjusted for Baseline KCCQ | Adjusted for Clinical Variables | |||||
|---|---|---|---|---|---|---|---|
| Outcome | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
|
All-cause mortality or all-cause hospitalization |
|||||||
| KCCQ | No change (<5 point) | Reference | Reference | Reference | |||
| Decrease (≥ 5 point) | 1.33 (1.11, 1.60) | 0.002 | 1.28 (1.07, 1.54) | 0.008 | 1.19 (0.99, 1.43)* | 0.07 | |
| Increase (≥5 point) | 1.0 (0.86, 1.17) | 0.99 | 0.84 (0.71, 0.99) | 0.04 | 0.88 (0.74, 1.04)* | 0.12 | |
| All-cause mortality | |||||||
| KCCQ | No change (<5 point) | Reference | Reference | Reference | |||
| Decrease (≥ 5 point) | 1.39 (1.04, 1.86) | 0.03 | 1.37 (1.03, 1.84) | 0.03 | 1.26 (0.94, 1.69) † | 0.12 | |
| Increase (≥5 point) | 0.97 (0.74, 1.26) | 0.80 | 0.81 (0.61, 1.06) | 0.12 | 0.87 (0.66, 1.15) † | 0.33 | |
|
Cardiovascular mortality or heart failure hospitalization |
|||||||
| KCCQ | No change (<5 point) | Reference | Reference | Reference | |||
| Decrease (≥ 5 point) | 1.42 (1.11, 1.82) | 0.006 | 1.39 (1.09, 1.79) | 0.009 | 1.36 (1.06, 1.76)‡ | 0.02 | |
| Increase (≥5 point) | 1.01 (0.81, 1.26) | 0.95 | 0.83 (0.66, 1.05) | 0.12 | 0.88 (0.70, 1.11)‡ | 0.29 | |
Model for all-cause mortality or all-cause hospitalization adjusted for baseline KCCQ score, randomized treatment assignment, dosage of beta-blocker, KCCQ symptom stability, ejection fraction (LVEF), country (US vs non-US), sex, ventricular conduction, Weber class, blood urea nitrogen (BUN), and mitral regurgitation.
Model of all-cause mortality adjusted for baseline KCCQ score, randomized treatment assignment, sex, body mass index, loop diuretic dose, creatinine, exercise duration, ventricular conduction, Canadian Cardiovascular Society angina classification, and LVEF.
Model for cardiovascular mortality or heart failure hospitalization adjusted for baseline KCCQ score, randomized treatment assignment, loop diuretic dose, LVEF, mitral regurgitation, ventricular conduction, KCCQ symptom stability, BUN, race, sex, age, Weber class, VE/VCO2.
Discussion
Prior studies have shown the sensitivity of KCCQ to reflect clinical change and risk for clinical outcomes in individual patients with heart failure.12–14, 18 In a large trial population of stable outpatients with heart failure with reduced ejection fraction, we show a non-linear relationship between changing KCCQ health status scores and clinical outcomes. Worsening patient-reported health status was associated with significant increases in all-cause death/hospitalization per point change of KCCQ, and small improvements in KCCQ between 0 and 8 points was associated with a similar reduction in risk of future outcomes. However, larger short-term improvements in KCCQ above 8 points were no longer associated with any further risk reduction. These results extend our understanding of KCCQ as a tool to prognosticate disease in HF, especially for patients at risk for decompensation.
Patient-reported quality of life as it relates to health—or health status—encompasses broad concepts of physical and social functioning, mental and general health, as well as overall perceptions of energy or vitality, pain, and cognitive function. Adding to the complexity, these factors can be influenced by varying personal values, preferences, and motivation, as well as available psychological and social support.23 Our results support prior findings that the KCCQ measures patient features not captured by traditional clinical variables.13, 17
Prior authors have evaluated serial KCCQ measurement changes over 2 months and shown its association with mortality in the EPHESUS clinical trial population of patients with HF after an MI.24 In that population, each 5-point decrease in KCCQ was associated with an 11% increased risk of 1-year all-cause mortality. Our analysis corroborates and expands these results to a wider population of stable outpatients with HF and with longer follow-up. In contrast, though, our analysis suggests a non-linear relationship in how changing KCCQ predicts future adverse events.
In our analysis, declines in KCCQ were consistently associated with increased risk. Patients experiencing a decline in health status showed some trends for having greater disease severity (higher median NT-proBNP markers, higher likelihood of having atrial fibrillation, and worse renal function) but differences were modest. They also reported fair baseline health status by KCCQ, where a median score of 74 has been shown to be associated with NYHA class II symptoms in prior reports.13 In these patients, a decline in KCCQ may serve as a more reliable prognosticator of clinical worsening and need for heightened monitoring, as previously prescribed by other authors.13, 24 In clinical settings where the signal to noise ratio of patient data is declining, the consistency of this relationship suggests that “declining KCCQ score” has value as an actionable risk assessment.
In our analysis, experiencing an improvement in KCCQ score from 0 to 8 was associated with a significant reduction in risk of future adverse outcomes. However, with larger degrees of positive change (8 KCCQ points in our sample), we no longer show a prognostic relationship. Given the subjective and qualitative nature of improving health status, large improvements may have incrementally smaller effects on future outcomes, suggesting a theoretical “ceiling effect.” This nonlinear relationship has not been seen in other analyses and requires better characterization in other populations. In our study population, important differences differentiated patients who experienced ≥ 5 point improvement from other groups. Patients who experienced ≥ 5 point improvement had the lowest median baseline KCCQ scores by 20 points compared to the minimal change group; prior research estimated a discrepancy of around 30 points to be associated with the difference between NYHA class I and class III symptoms.13 For these patients, their low baseline KCCQ may have as much or more prognostic importance as their health status improvement. Indeed, recent authors have suggested that current KCCQ score is more strongly associated with future outcomes than change in KCCQ.25
While quality of life improvements have inherent value to patients, our analysis suggests we do not yet know what range of effect sizes are enough to meaningfully impact their welfare and treatment decisions. Based on the study by Spertus et al, a 5-point change in the KCCQ overall summary score is widely recognized to be the minimally noticeable clinical difference and prognostic of future events.18, 24 Recent randomized trials studying HF interventions have reported KCCQ changes in the range of 1–5 points.4, 5, 14 More analysis is necessary to better discriminate how patients interpret these small KCCQ changes. In the context of our current analysis, this study suggests changes smaller than a 5-point improvement may be prognostic of clinical outcomes. For clinical trials that use HF health status as primary or secondary outcomes, these observations have important implications.
We make note of potential limitations in this analysis. First, HF-ACTION represents a clinical trial population of disproportionately younger and more male patients with HF; some selection bias is inherent. Second, patients responded to serial health status surveys in this analysis in the setting of a clinical trial. While it is uncertain if our findings will generalize to a different clinical setting, recent systematic reviews have not supported the idea that patients enrolled in clinical trials receive inherently better care.26 Third, a smaller proportion of patients experienced large magnitudes of change, which may have limited our power to define the association of improvements in KCCQ with outcomes. Fourth, while we adjusted for multiple clinical factors known to predict outcomes in HF-ACTION, the complex nature of quantifying patient health status certainly allows for the possibility of residual confounding. These clinical variables are based on prior analyses,22 and some variables may be collinear with KCCQ. Lastly, our findings are exploratory in nature, and we did not adjust for multiplicity of statistical testing.
After adjustment for clinical variables, changing health status did not have a linear relationship with clinical outcomes. As reported by the KCCQ scale, worsening patient-reported health status and improvements in KCCQ up to 8 points was associated with risk of future clinical events in patients with HF. These results affirm the use of KCCQ as a clinical tool to monitor changes in health status in HF patients. Further investigation is still needed to understand the best clinical and research utility for these complex measures.
Supplementary Material
Acknowledgments
Sources of Funding: RJM receives research support from the National Institutes of Health (U10HL110312 and R01AG045551–01A1). The HF-ACTION (NCT00047437) trial was funded by the National Heart, Lung and Blood Institute.
ABBREVIATIONS
- BMI
Body Mass Index
- EF
Ejection Fraction
- HF
Heart Failure
- HF-ACTION
Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- NYHA
New York Heart Association
Footnotes
Disclosures:
The authors report no relevant conflicts of interest or relationships with industry.
References
- 1.Croog SH, Levine S, Testa MA, Brown B, Bulpitt CJ, Jenkins CD, Klerman GL, Williams GH. The effects of antihypertensive therapy on the quality of life. N Engl J Med 1986;314(26):1657–64. [DOI] [PubMed] [Google Scholar]
- 2.Cleary PD, Epstein AM, Oster G, Morrissey GS, Stason WB, Debussey S, Plachetka J, Zimmerman M. Health-related quality of life among patients undergoing percutaneous transluminal coronary angioplasty. Med Care 1991;29(10):939–50. [DOI] [PubMed] [Google Scholar]
- 3.Lesperance F, Frasure-Smith N, Koszycki D, Laliberte MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P, Guertin MC, Investigators C. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007;297(4):367–79. [DOI] [PubMed] [Google Scholar]
- 4.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP, Investigators H-A. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301(14):1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P-H, Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371(11):993–1004. [DOI] [PubMed] [Google Scholar]
- 6.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, Group CTS. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377(9766):658–66. [DOI] [PubMed] [Google Scholar]
- 7.Administration USFaD. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35(5):1245–55. [DOI] [PubMed] [Google Scholar]
- 9.Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail 2005;7(2):235–42. [DOI] [PubMed] [Google Scholar]
- 10.Ortega T, Diaz-Molina B, Montoliu MA, Ortega F, Valdes C, Rebollo P, Almenar M, Iscar M, Research Network on T. The utility of a specific measure for heart transplant patients: reliability and validity of the Kansas City Cardiomyopathy Questionnaire. Transplantation 2008;86(6):804–10. [DOI] [PubMed] [Google Scholar]
- 11.Joseph SM, Novak E, Arnold SV, Jones PG, Khattak H, Platts AE, Davila-Roman VG, Mann DL, Spertus JA. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail 2013;6(6):1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation 2004;110(5):546–51. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, Conard MW, Williams RE, Cardiovascular Outcomes Research C. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol 2006;47(4):752–6. [DOI] [PubMed] [Google Scholar]
- 14.Ekman I, Chassany O, Komajda M, Bohm M, Borer JS, Ford I, Tavazzi L, Swedberg K. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J 2011;32(19):2395–404. [DOI] [PubMed] [Google Scholar]
- 15.Rumsfeld JS, Havranek E, Masoudi FA, Peterson ED, Jones P, Tooley JF, Krumholz HM, Spertus JA, Cardiovascular Outcomes Research C. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J Am Coll Cardiol 2003;42(10):1811–7. [DOI] [PubMed] [Google Scholar]
- 16.Veazie PJ, Noyes K, Li Q, Hall WJ, Buttaccio A, Thevenet-Morrison K, Moss AJ. Cardiac resynchronization and quality of life in patients with minimally symptomatic heart failure. J Am Coll Cardiol 2012;60(19):1940–4. [DOI] [PubMed] [Google Scholar]
- 17.Flynn KE, Lin L, Moe GW, Howlett JG, Fine LJ, Spertus JA, McConnell TR, Pina IL, Weinfurt KP. Relationships between changes in patient-reported health status and functional capacity in outpatients with heart failure. Am Heart J 2012;163(1):88–94 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS, Cardiovascular Outcomes Research C. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150(4):707–15. [DOI] [PubMed] [Google Scholar]
- 19.Dreyer RP, Jones PG, Kutty S, Spertus JA. Quantifying clinical change: discrepancies between patients’ and providers’ perspectives. Qual Life Res 2016;25(9):2213–20. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL, Investigators H-A. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301(14):1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ, Kitzman DW, Kraus WE, Rendall D, Pina IL, Cooper LS, Fiuzat M, Lee KL. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail 2012;5(1):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995;273(1):59–65. [PubMed] [Google Scholar]
- 24.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, Spertus JA. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation 2007;115(15):1975–81. [DOI] [PubMed] [Google Scholar]
- 25.Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG, Spertus JA. Association of Serial Kansas City Cardiomyopathy Questionnaire Assessments With Death and Hospitalization in Patients With Heart Failure With Preserved and Reduced Ejection Fraction: A Secondary Analysis of 2 Randomized Clinical Trials. JAMA Cardiol 2017. [DOI] [PMC free article] [PubMed]
- 26.Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet 2004;363(9405):263–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


