Abstract
Neuronal death and replacement, or neuronal turnover, in the adult brain is one of many fundamental processes of neural plasticity. The adult avian song control circuit provides an excellent model for exploring mature neuronal death and replacement by new neurons. In the song control nucleus HVC of adult male Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelli) nearly 68,000 neurons are added each breeding season and die during the subsequent nonbreeding season. To accommodate large seasonal differences in HVC neuron number, the balance between neuronal addition and death in HVC must differ between seasons. To determine whether maintenance of new HVC neurons changes within and between breeding and nonbreeding conditions, we pulse-labeled two different cohorts of new HVC neurons under both conditions and quantified their maintenance. We show that the maintenance of new HVC neurons, as well as new non-neuronal cells, was higher at the onset of breeding conditions than at the onset of nonbreeding conditions. Once a steady-state HVC volume and neuronal number was attained in either breeding or nonbreeding conditions, neuronal and non-neuronal maintenance were similarly low. We found that new neuronal number correlated with new non-neuronal number within each cohort of new neurons. Together, these data suggest that sex steroids promote the survival of an initial population of new neurons and non-neuronal cells entering HVC. However, once HVC is fully grown or regressed, neuronal and non-neuronal cell turnover is regulated by a common mechanism likely independent of direct sex steroid signaling.
Keywords: adult neurogenesis, degeneration, neuronal death, seasonal plasticity, songbird, steroid hormone, testosterone, anti-BrdU (RRID: AB_2536432), anti-NeuN (RRID: AB_2532109)
Graphic Abstract
Introduction
Neuronal death is a necessary concomitant of adult neurogenesis in the majority of vertebrate species. In mammals and birds, the total volume and maximum neuronal number in neurogenic brain regions do not increase throughout life, as opposed to the continuous growth of the CNS in indeterminate growing vertebrates like fish. Rather, even brain regions that routinely incorporate adult-born neurons reach a stable size in adulthood of vertebrates with determinate growth (Wang et al., 2002; Heine et al., 2004). The processes of neuronal birth and death are often tightly coordinated and may be causally linked (Scharff et al., 2000; Thompson and Brenowitz, 2009; Larson et al., 2014). For example, neurological conditions that result in neuronal death such as ischemia, injury, and depression, can induce a neurogenic response by themselves or in conjunction with therapeutic interventions (Fossati et al., 2004; Sairanen et al., 2005). Likewise, in the telencephalic sensorimotor nucleus HVC (proper name; (Reiner et al., 2004)) of songbirds, neuronal death in response to loss of trophic support has been proposed to create “vacancies” for subsequent neuronal recruitment (Nottebohm, 2004). Although critically important for proper development and homeostasis of the brain and for normal behavior (reviewed in (Dekkers et al., 2013)), non-pathogenic neuronal death in the context of adult neurogenesis remains under examined.
The seasonally-induced changes in neuronal death and addition of newborn neurons within HVC of adult songbirds provide a robust model for examining the processes and mechanisms of seasonally plastic neuronal turnover, defined as the process by which older neurons in the adult brain are replaced over time by younger neurons, consistent with prior use of this term (Wang et al., 1999; Spalding et al., 2013). The sensorimotor song nucleus HVC (Figure 1A) adds nearly 68,000 adult-born neurons, an increase of about 25%, within four days of exposure to breeding systemic testosterone levels and a long day photoperiod (Smith et al., 1995; Smith et al., 1997; Tramontin and Brenowitz, 1999). Most new neurons project to the target nucleus, the robust nucleus of the archopallium (RA; (Scott and Lois, 2007; Tokarev et al., 2016)). Conversely, within four days of a transition from breeding to nonbreeding conditions, HVC rapidly regresses in size (Thompson et al., 2007) with the same number of neurons undergoing apoptosis (Thompson and Brenowitz, 2008; Larson et al., 2014).
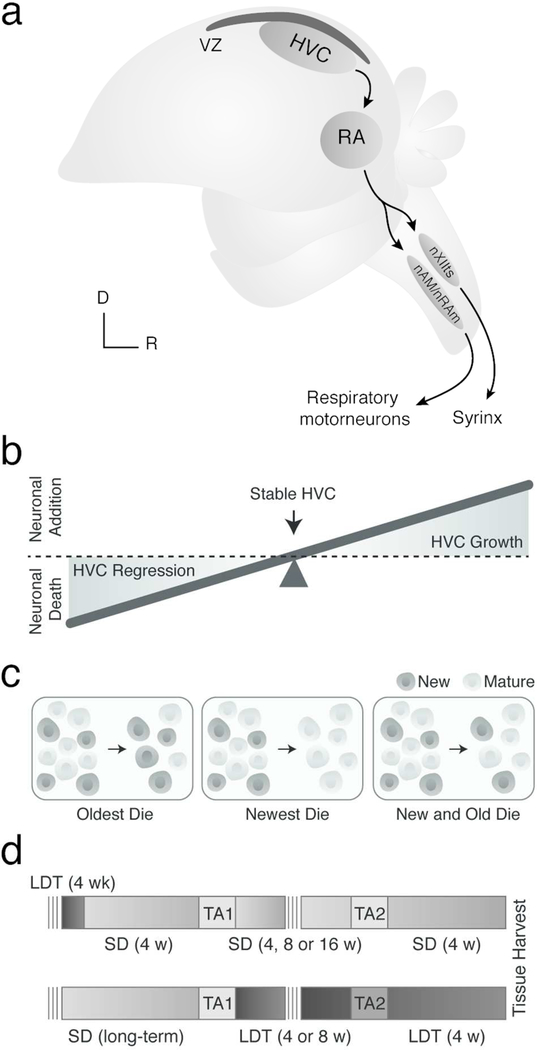
Figure 1.
(A) Schematic illustrating the major projections within the song production circuit. Note the ventricular zone (VZ) in black directly dorsal to HVC. RA = robust nucleus of the arcopallium, nXII = tracheosyringeal portion of hypoglossal nucleus, nAM/nRAm = nucleus ambiguus and nucleus retroambigualis, D = dorsal, and R = rostral. HVC grows and regresses each breeding and nonbreeding season by about 68,000 neurons as a result of increased new neuronal survival and neuronal apoptosis, respectively. (B) Schematic illustrating how the balance between neuronal birth and death hypothetically contributes to HVC growth and regression. (C) Predictive models for HVC neuronal turnover. New neurons that enter HVC might: entirely replace oder neurons (left), temporarily contribute to HVC growth and then die (middle), or serve to replace some older neurons, but also die (right). (D) Experimental design for testing the predictive models above in nonbreeding (i.e., top) and breeding (bottom) conditions. SD = short day photoperiods to promote nonbreeding physiological condition (light grey), LDT = long day photoperiods and exogenous testosterone pellet to stimulate breeding-like physiological condition (dark grey), TA1 and TA2 = thymidine analog 1 and thymidine analog 2. Note: darker grey TA2 background (bottom) indicates injections were administered in LDT conditions rather than SD conditions.
To accommodate this growth and regression of HVC, the balance between neuronal addition and death must also change seasonally. During the transition into breeding conditions, neuronal addition must exceed neuronal death, allowing HVC volume and total neuronal number to increase (Figure 1B). Upon the subsequent transition to nonbreeding condition, neuronal death must exceed neuronal addition for HVC volume and total neuronal number to decrease. During stable-state periods of both breeding and nonbreeding conditions, when HVC volume and total neuron number remain constant, neuronal addition and death must become balanced in order to maintain a constant HVC volume and neuronal number.
Three alternative hypotheses can be proposed about which particular populations of neurons (i.e. newest versus mature) are maintained through breeding and nonbreeding conditions (Figure 1C). The first hypothesis posits that adult-born neurons entering HVC might serve entirely to replace older neurons (Kirn and Nottebohm, 1993), in which case the oldest neurons would die. Alternatively, new neurons that enter HVC could be a transient population that dies either shortly after birth in nonbreeding conditions or during active regression of HVC or in stable nonbreeding conditions. Previous work has shown, however, that some new neurons incorporated during breeding condition persist through HVC regression (Nottebohm et al., 1994; Larson et al., 2014), ruling out this model as the sole mechanism. The final and most likely model is that both new and older mature neurons die as new neurons enter HVC in both breeding and nonbreeding conditions, and that maintenance of a given neuron is due to factors such as blood plasma testosterone levels, synaptic strength, electrical activity, and neurotrophin signaling (for reviews see (Brenowitz, 2008; Brenowitz and Larson, 2015)). In this model HVC could grow upon transition into breeding condition even while losing mature neurons so long as new neuron maintenance was greater in breeding than nonbreeding conditions. Here, we exploit the natural seasonal growth and regression of HVC in adult male Gambel’s white-crowned sparrow (Zonotrichia leucophrys gambelii) to test these proposed models of neuronal turnover within and between breeding and nonbreeding conditions. Functionally, we measured neuronal turnover as a decrease in the number adult-born neurons added to HVC at the onset of breeding or non breeding conditions over time, and the increase in the number of a younger cohort of new neurons. As such, the number of new neurons from any given cohort of adult-born neurons present in HVC at the end of the experiment represent all of the processes of adult neurogenesis as broadly defined to include neural stem cell (NSC) proliferation, migration and differentiation of neuroblasts, incorporation of new neurons into existing circuits, and survival up to the time of observation.
Materials and Methods
Animals
Fifty male Gambel’s white-crowned sparrows were collected in eastern Washington during their pre- or post-breeding season migration. Prior to the initiation of experiments, all birds were housed in indoor group aviaries and exposed to short day photoperiods (SD; 8 hr light, 16 hr dark) for at least 10 weeks to ensure that the song and reproductive systems were fully regressed and sensitive to the stimulatory effects of long day photoperiods (LD; 20 hr light, 4 hr dark) and testosterone. All experiments followed NIH animal use guidelines and were approved by the University of Washington Institutional Animal Care and Use Committee.
Experimental procedures
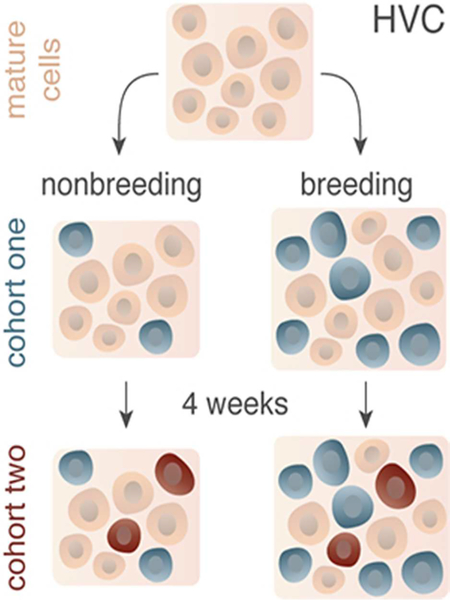
We synchronized the breeding condition of one group of birds by housing 30 birds in LD photoperiods and implanting a 12-mm Silastic capsule (1.47 mm inner diameter, 1.96 mm outer diameter) filled with crystalline testosterone (Sigma) above the scapula for four weeks. Four weeks of LD photoperiods and testosterone plasma level elevation (LDT) induces full breeding-like morphology and physiology of the song control circuit in the laboratory (Tramontin et al., 2000; Meitzen et al., 2009). To test neuronal turnover in nonbreeding conditions, we induced regression of the song control circuit by removing the subcutaneous testosterone pellet and transferring birds to a SD photoperiod for four weeks (Smith et al., 1997; Thompson et al., 2007; Larson et al., 2014). All SD birds received blank silastic pellets that were replaced every four weeks for the remainder of the experiment. To determine the effect of nonbreeding conditions on new neuronal turnover, we injected all birds once daily for five days with 3.33 μl/g of one of the two thymidine analogs (TA) randomly chosen and balanced across all groups at four weeks following the transition to nonbreeding conditions. The two thymidine analogs used at equimolar doses were bromodeoxyuridine (BrdU; 15 mg/ml in 0.09% NaCl and 0.012M NaOH; Sigma) and ethynyldeoxyuridine (EdU; 12 mg/ml in 0.09% NaCl and 0.012M NaOH; Invitrogen). At four (n=9), eight (n=12), or sixteen (n=9) weeks following the onset of the first series of randomly chosen TA injections (i.e., labeled cohort one), we injected 3.33 μl/g of the other thymidine analog daily for five days (i.e., cohort two). Four weeks after the start of the second thymidine analog pulses, all birds were killed.
To determine the effect of physiological condition on new neuronal turnover, we housed birds in LDT for either eight (n=11) or twelve (n=9) weeks, with testosterone pellet replacement every 4 weeks. We replaced implants to ensure that plasma testosterone levels remained in the breeding physiological range (Farner and Wingfield, 1978). Seven days prior to transitioning birds to LDT each bird received five daily intramuscular injections of 3.33 μl/g of one of thymidine analogs randomly chosen and balanced across all groups. Because neuroblasts take one to three weeks to migrate from their site of birth in the ventricular zone to HVC (Alvarez-Buylla et al., 1990), we chose to inject birds with the first thymidine analog prior to transfer to LDT so new neurons labeled at that time with thymidine analog would subsequently incorporate into HVC under breeding-like conditions. Four or eight weeks after onset of LDT birds were injected daily for five days with 3.33 μl/g of the other of the two thymidine analogs to label a second cohort of newborn neurons. Four weeks following the onset of the second thymidine analog injections birds were killed.
Importantly, all thymidine analog injections in both nonbreeding and breeding conditions were administered during periods that were previously described to have stable and similar amounts of neural stem cell proliferation (Larson et al., 2014). Moreover, all injections were made between five and seven hours after lights on. Thus, any differences in new cell maintenance would be due to differential incorporation, survival, or both, rather than differential neural stem cell proliferation rates.
Blood draw and hormone analysis
Blood samples were obtained from birds in SD and LDT every four weeks and upon termination of the experiment to measure circulating testosterone concentrations. Blood was also collected in birds of the SD group on the final day of LDT to confirm proper synchronization to breeding condition. We drew 250 μl of blood from the alar vein in the wing into heparinized collection tubes, immediately centrifuged the tubes, and stored the plasma at −80°C until assay. Using a Testosterone Enzyme Immunoassay kit (Enzo Life Sciences), we measured plasma testosterone concentration. Minimum and maximum detectable plasma testosterone concentrations were 0.03 ng/ml and 40.00 ng/ml, respectively. Samples with undetectable levels of testosterone were treated as having concentrations at the lower detection limit for statistical analyses. Intra-and inter-assay coefficients of variation were 0.59% - 41.25% and 10.00%, respectively.
Tissue collection and processing
Five to seven hours after lights-on, brains were removed quickly after birds were deeply anesthetized with isoflurane. Brains were sectioned in the coronal plane at 40 μm on a cryostat, and each section was thaw-mounted serially. Every third section was Nissl stained and the remaining slides were stored at −80°C for up to six months until immunolabeling.
Antibody Characterization
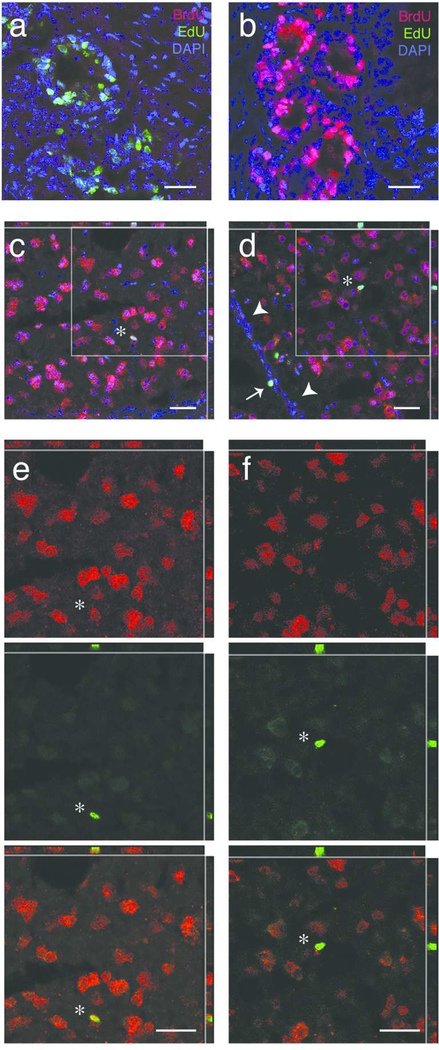
For all primary antibodies we observed temporal and spatial patterns of labeling consistent with previous publications, as listed in Table 1. With the MOBu-1 BrdU antibody we did not observe cross reaction with EdU labeled DNA ((Liboska et al., 2012); Figure 2A and 2B) or labeling in brain regions that lack high levels of adult cell division (e.g., RA, LMAN, optic tectum; (Alvarez-Buylla et al., 1994)). BrdU and EdU labeling was occasionally observed in brain regions known to have active mitosis (e.g., ventricular zone). As a positive control, all brain sections were run for immunohistochemistry (below) with intestinal tissue (sectioned and stored similar to brain tissue) obtained from birds injected with either BrdU or EdU two hours prior to tissue collection. Intestinal tissue was the control tissue of choice given constantly high numbers of mitotic cells present. With the NeuN antibody we observed strong labeling in nuclei with occasional weak labeling in the cytoplasm of mature neuronal cells (Mullen et al., 1992; Mao et al., 2016; Saito et al., 2018). The rabbit-anti-NeuN antibody we use (Table 1) was previously validated and shown to not cross react with A60 or Synapsin I (Mao et al., 2016). With all antibodies, we did not observe labeling when the primary antibody was omitted. Moreover, we did not observe BrdU or EdU labeling in tissue from animals that did not have thymidine analog injections prior to tissue harvesting.
Table 1.
Primary antibodies and labeling systems used for this study
| Antibody | Host/Isotope | Antigen (Immunogen) | Manufacturer and Cat. No. | RRID | Concentration; Dilution | Characterization |
|---|---|---|---|---|---|---|
| anti-BrdU | mouse/IgG | clone, MoBU-1 (BrdU) | Invitrogen B35128 | RRID: AB_2536432 | 0.1 mg/ml; 1 to 200 | Liboska et. al, 2012 |
| anti-NeuN | rabbit / IgG2b, kappa | synthetic peptide, unspecified immunogen within human NeuN aa 1–100 (neuronal marker) | Abcam ab177487 | RRID: AB_2532109 | 0.789 mg/ml; 1 to 500 | Mullen et. al., 1992; Mao et. al., 2016; Saito et. al., 2018 |
| AlexaFluor azide 488 | N/A | N/A | EdU Click-It Kit, Invitrogen C10337 | manufacturer’s resuspension; 1 to 2000 | Salic & Mitchinson, 2008 |
Figure 2.
Photomicrographs of thymidine labeled cells. (A) Image of intestinal tissue labeled for EdU (green), BrdU (red), and DAPI (blue) from bird injected with EdU two hours prior to tissue harvest. The BrdU antibody did not cross react with EdU positive cells, confirming that the MoBU BrdU antibody against BrdU does not react with EdU labeled DNA. (B) Image of intestine labeled for EdU (green), BrdU (red), and DAPI, a nuclear marker (blue) from bird injected with BrdU two hours prior to tissue harvest. The MoBU antibody labels BrdU positive cells, whereas the EdU click reaction does not. (C) Representative image of a thymidine analog positive neuron in HVC. Thymidine analog label, in this case, EdU, is shown in green, while label for NeuN, a neuronal marker, is shown in red, and DAPI, a nuclear marker, is shown in blue. The white star indicates a new neuron co-labeled with EdU, NeuN, and DAPI in the nucleus, as also observed in the cut outs of the X-Z and Y-Z planes. The grey box indicates the area of zoom in (E). (D) Representative image of thymidine analog positive non-neuronal cells in the proliferative ventricular zone (VZ) and HVC. Thymidine analog label, in this case, BrdU, is shown in green, NeuN in red, and DAPI in blue. The white arrows heads indicate the VZ, whereas the white arrow indicates a BrdU-positive cell that retained label (a very slowly dividing NSC or a postmitotic non-neuronal cell) within the VZ. The white star indicates a BrdU-positive non-neuronal cells within HVC. The box highlights the region of zoom in (F). (E) Magnified region from (B) showing an EdU-positive neuron in HVC. Single channel images for NeuN positive cells (top, red), the EdU positive cell (middle, green), and co-label (bottom). The presence of NeuN label both within and immediately outside of the area labeled with EdU (see X-Z and Y-Z cutouts) confirm neuronal identity of this new cell. (F) Magnified region from (C) showing an BrdU-positive non-neuronal cell in HVC. Single channel images for NeuN positive cells (top, red), the BrdU positive cell (middle, green), and co-label (bottom). The absence of NeuN label within the area labeled with BrdU (see X-Z and Y-Z cutouts) suggests that this BrdU labeled cell is nonneuronal. All scale bars (white) are 20 μm.
Immunohistochemistry and analysis
To visualize BrdU-labeled cells, we processed brain sections as follows. Briefly, sections were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS), rinsed in PBS with 0.5% DMSO and 0.5% Triton-X (PDTX; pH 7.4), dipped in distilled water, incubated with 2N HCl at 37°C for 30 minutes, and rinsed with PDTX. After blocking in 5% heat inactivated goat serum, slides were incubated with mouse-anti-BrdU (1:200; Invitrogen MOBu-1, B35128) and rabbitanti-neuN (1:500; Abcam, ab177487) antibodies. Importantly, the MOBu-1 BrdU antibody does not cross-react with EdU labeled DNA (Liboska et al., 2012). NeuN is a neuron-specific protein expressed in the soma of fully differentiated neurons of the central and peripheral nervous systems (Mullen et al., 1992; Mao et al., 2016). Labeling was visualized with the fluorescent secondary antibodies goat-anti-mouse Alexa Fluor 488 (Invitrogen) and goat-anti-rabbit Alexa Fluor 568 (Invitrogen). Slides were mounted with Fluoromount-G with DAPI. All BrdU-positive cells double labeled with DAPI and triple labeled with DAPI and NeuN were counted in each HVC section.
To visualize Edu-labeled cells, sections fixed in 4% paraformaldehyde were rinsed in PDTX, blocked in 3% heat inactivated bovine serum, and incubated with Alexa Fluor azide 488 (1:2000; EdU Click-It Kit, Invitrogen; (Salic and Mitchison, 2008)). Slides were rinsed in PDTX and blocked again in 5% heat inactivated goat serum. Sections were incubated with rabbit-anti-neuN (1:500; Abcam, ab177487), which was visualized with goat-anti-rabbit Alex Flour 568 (Invitrogen). Slides were mounted with Fluoromount-G with DAPI. All EdU-positive cells double labeled with DAPI and triple labeled with DAPI and NeuN in each HVC section were counted. Results are reported as unilateral data obtained from the averages of counts across both hemispheres.
Morphometric measurements in HVC and RA
The volume of HVC in the randomly chosen hemisphere was determined by tracing the borders of the nuclei identified in Nissl stained tissue onto paper, scanning the drawings, and using Image J Software (Version 1.46; NIH; http://rsb.info.nih.gov/ij/) to determine the area of each section of the nuclei. Total volume was calculated from these areas using the formula for a truncated cone (Tramontin et al., 1998). To estimate neuron density in HVC used a customized random systematic method as previously described and validated (Tramontin et al., 1998). Briefly, we counted all NeuN positive neurons that fell within an ocular grid (ca. 1.9 X 103 mm2 at 1000X) randomly positioned in each section of HVC. Samples were collected from each section through the full extent of HVC and counts were averaged across sections and divided by the volume under the ocular grid (grid area X 40 μm) to obtain neuronal density. We used Konigsmark’s formula number 4 to correct for oversampling due to cell splitting between sections (Konigsmark, 1970). Neuronal number was calculated by multiplying the neuronal density for each bird by that bird’s unilateral HVC volume. Histological measurements described below are unilateral data, reported as averaged volume across both hemispheres.
Statistical Analysis
Prior to presentation and statistical analyses of data, all cell counts were adjusted to prevent oversampling using Konigsmark formula (Konigsmark, 1970). Using analysis of covariance (ANCOVA), we tested the response variables condition, interval between pulses, and first thymidine analog injected for effects as well as interactions between these variables. We examined residuals for normality and homoscedasticity and transformed primary data to correct for variation in residuals among groups (Sokal and Rohlf, 2012). Transformed data included log transformation of HuC/D positive cell density and thymidine analog 2 positive neuron number; square root of HuC/D neuron number and the ratio of thymidine analog 1 to 2 positive neurons; and natural log of thymidine analog 1 positive neuron number, thymidine analog 1 positive non-neuron number, and the ratio of thymidine analog 1 to 2 positive nonneuronal cells. Neither effects of first thymidine analog or length of time between pulses nor interactions from the full factorial of interactions between these two variables and condition were significant (Table 2). Thus, first thymidine analog and length of time between pulses were not included in the final ANCOVA (Table 3). We also removed one variable at a time (i.e., either first analog or interval), performed an ANOVA with two response variables (i.e., first analog or interval within condition), and neither identified any signifciant effects of first thymidine analog injection or length between pulses or interactions (not presented).To test for correlations while also controlling for multiple comparisons, we performed a nonparametric Spearman’s rank correlation test with the following data sets: testosterone plasma levels, HVC volume, cohort one neuron number, cohort one non-neuronal cell number, cohort two neuron number, and cohort two non-neuronal cell number (Table 4). Because each of these factors differed between breeding and nonbreeding conditions, tests for correlation were only conducted within the same physiological condition, not across conditions. All statistical analyses were made using JMP Version 8.0.1 (SAS, Cary NC). All data are presented as mean ± SEM with an alpha level of 0.05.
Table 2.
Lack of effect of thymidine analog order and length between thymidine analog pulses
| F Ratio; p- value |
Condition (i.e., SD v. LDT) |
Length Between Thymidine Analogs (i.e., 4w, 8w, 16w) |
First Thymidine Analog Injection (i.e., BrdU v. EdU) |
Condition * Length |
Condition * First Thymidine Analog |
Length * First Thymidine Analog |
Condition * Length * First Thymidine Analog |
||
|---|---|---|---|---|---|---|---|---|---|
| Testosterone | |||||||||
| Final serum levels | F(7,27)=48.8197; p<0.0001 | F(1,27)=284.6605; p<0.0001 | F(1, 27)=0.1356; p=0.7156 | F(1, 27)=0.2588; p=0.6151 | F(1, 27)=0.0043; p=0.9485 | F(1, 27)=1.0260; p=0.3201 | F(1, 27)=3.2693; p=0.0817 | F(1,27)=3.5725; p=0.0695 | |
| HVC | |||||||||
| Unilateral Volume | F(7,55)=4.8715; p=0.0003 | F(1,55)=21.5070; p<0.0001 | F(1, 55)=0.8880; p=0.3501 | F(1, 55)=3.1390; p=0.0820 | F(1, 55)=0.4093; p=0.5250 | F(1,55)=1.6195; p=0.2085 | F(1, 55)=0.0423; p=0.8378 | F(1, 55)=0.3781; p=0.5412 | |
| Neuronal Density† | F (7,18)=1 −6326; p=0.1896 | F(1,18)=0.0211; p=0.8862 | F (1,18)=0.9922; p=0.3324 | F (1,18)=1.7569; p=0.2016 | F(1,18)=4.2054; p=0.0551 | F (1,18)=0.5571; p=0.4651 | F(1,18)=0.6636; p=0.4260 | F (1,18)=0.0331; p=0.8577 | |
| Unilateral Neuronal number† | F(7,16)=2.2963; | F(1,16)=8.2188; | F (1,16)=0.2319; | F (1,16)=0.0039; | F(1,16)=1.8628; | F (1,16)=0.1703; | F(1,16)=0.3218; | F(1,16)=2.0671; | |
| p=0.0799 | p=0.0112 | p=0.6367 | p=0.9511 | p=0.1912 | p=0.6853 | p=0.5784 | p=0.1698 | ||
| Cohort One | |||||||||
| Neuronal number† | F(7,34)=4.5634; p=0.0011 | F(1,34)=13.1990; p=0.0009 | F(1, 34)=1.1839; p=0.2842 | F(1, 34)=0.0104; p=0.9195 | F(1,34)=0.2747; p=0.6063 | F(1, 34)=0.1005; p=0.7532 | F(1,34)=0.4701; p=0.4976 | F(1,34)=0.0714; p=0.7909 | |
| Non-neuronal number | F(7, 32)=2.0059; p=0.0852 | F(1, 32)=0.4958; p=0.4864 | F(1,32)=2.1199; p=0.1551 | F(1, 32)=0.2355; p=0.6308 | F(1, 32)=0.1786; p=0.6754 | F(1, 32)=2.5835; p=0.1178 | F(1, 32)=0.1784; p=0.6756 | F(1, 32)=0.9164; p=0.3456 | |
| Cohort Two | |||||||||
| Neuronal number | F(7,39)=1.0227; p=0.4309 | F(1, 39)=0.0195; p=0.8897 | F(1, 39)=2.0180; p=0.1634 | F(1, 39)=0.0369; p=0.8487 | F(1, 39)=0.1710; p=0.6815 | F(1, 39)=0.3420; p=0.5621 | F(1, 39)=0.2718; p=0.8897 | F(1, 39)=0.0660; p=0.7987 | |
| Non-neuronal number | F(7,39)=0.2734; p=0.9606 | F(1, 39)=0.0981; p=0.7558 | F(1, 39)=0.6978; p=0.4086 | F(1, 39)=0.0183; p=0.8930 | F(1, 39)=1 4065; p=0.2428 | F(1, 39)=0.0119; p=0.9138 | F(1, 39)=0.0069; p=0.9344 | F(1, 39)=0.1013; p=0.7520 |
Data transformed to adjust for residuals
Table 3.
Effect of condition on physiology, morphology, and cell addition to HVC
| SD |
LDT |
One-way ANOVA: Condition |
|||||
|---|---|---|---|---|---|---|---|
| 4 | 8 | 16 | 4 | 8 | |||
| Testosterone | Final serum levels (ng/mL) | 0.38 ± 0.10 | 0.57 ± 0.22 | 1.18 ± 0.59 | 17.74 ± 2.25 | 18.59 ± 1.41 | F(1,33)=339.5762; p<0.0001 |
| HVC | Unilateral Volume (mm3) | 0.35 ± 0.03 | 0.48 ± 0.05 | 0.38 ± 0.04 | 0.53 ±0.03 | 0.57 ± 0.03 | F(1, 61)=25.4217; p<0.0001 |
| Neuronal Density (x103/mm3)† | 126 ± 12 | 96 ± 5 | 99 ± 2 | 97 ± 6 | 111 ± 3 | F(1, 24)=0.4314; p=0.5176 | |
| Unilateral Neuronal number (x103)† | 38 ± 7 | 42 ± 5 | 30 ± 6 | 51 ± 7 | 59 ± 6 | F(1, 22)=11.1158; p=0.0030 | |
| Cohort One | Neuronal number (x103)† | 87 ± 17 | 74 ± 11 | 44 ± 15 | 176 ± 34 | 144 ± 17 | F(1, 40)=23.7029; p<0.0001 |
| Non-neuronal number (x103)† | 84 ± 22 | 101 ± 12 | 52 ± 12 | 163 ± 33 | 98 ± 15 | F(1, 38)=4.4650; p=0.0412 | |
| Cohort Two | Neuronal number (x103)† | 127 ± 24 | 77 ± 13 | 64 ± 13 | 122 ± 26 | 89 ± 12 | F(1, 45)=1.0291; p=0.3158 |
| Non-neuronal number (x103) | 100 ± 33 | 99 ± 15 | 84 ± 12 | 81 ± 17 | 108 ± 16 | F (1, 45)=0.0000; p=0.9975 | |
| Cohort One : Cohort Two | Neuronal number† | 0.73 ± 0.19 | 0.98 ± 0.17 | 0.32 ± 1.06 | 1.63 ± 0.58 | 1.74 ± 0.30 | F(1, 26)=11.6996; p=0.0021 |
| Non-neuronal number† | 0.98 ± 0.15 | 1.23 ± 0.29 | 1.06 ± 0.55 | 2.28 ± 0.72 | 1.36 ± 0.62 | F(1, 24)=0.4395; p=0.5137 | |
| All values are mean ± S.E.M. | |||||||
Data transformed during statistical analyses to adjust for residuals
Table 4.
Nonparmetric correlations between testosterone levels, HVC morphology, and cell numbers
| SD Groups |
||||
|---|---|---|---|---|
| Testosterone | HVC Volume | Cohort One Neurons | Cohort Two Neurons | |
| HVC Volume | −0.1916; | |||
| 0.5116 | - | - | - | |
| Cohort One Neurons | −0.2394; | 0.1168; | ||
| 0.4535 | 0.6240 | - | - | |
| - | ||||
| Cohort Two Neurons | 0.1156; | 0.1533; | 0.2328; | |
| 0.6940 | 0.5188 | 0.3685 | - | |
| - | ||||
| Cohort One Non-neurons | −0.1296; | 0.0835; | 0.5971; | 0.0535; |
| 0.6881 | 0.7191 | 0.0026 | 0.8383 | |
| - | ||||
| Cohort Two Non-neurons | 0.3991; | 0.0617; | 0.3575; | 0.5124; |
| 0.1574 | 0.7961 | 0.1589 | 0.0088 | |
|
LDT Groups |
||||
| HVC Volume | 0.4018; | |||
| 0.1735 | - | - | - | |
| Cohort One Neurons | −0.1138; | 0.0354; | ||
| 0.07542 | 0.8964 | - | - | |
| Cohort Two Neurons | 0.2906; | 0.0070; | −0.0721; | |
| 0.3860 | 0.9772 | 0.8149 | - | |
| - | ||||
| Cohort One Non-neurons | −0.2504; | 0.3494; | 0.6571; | −0.0424; |
| 0.5497 | 0.2420 | 0.0057 | 0.9074 | |
| Cohort Two Non-neurons | 0.1761; | 0.1761; | −0.4672; | 0.7046; |
| 0.6045 | 0.4707 | 0.1074 | 0.0003 | |
All results from Spearman’s rank correlation tests presented as Spearman Rho (ρ)and the associated p-value (i.e., ρ; p).
Results
Validation of breeding and nonbreeding condition
We confirmed that our experimental manipulations induced breeding or nonbreeding-like physiological condition by comparing blood plasma testosterone levels and morphological changes within HVC with those previously reported (Smith et al., 1995; Smith et al., 1997; Tramontin et al., 2000). As expected, LDT birds had significantly elevated plasma testosterone levels when compared to testosterone levels of SD birds (Table 3). Birds in the SD groups had elevated testosterone levels of 16.42 ± 2.17 ng/mL while exposed to LDT, confirming breeding-like condition synchronization (Smith et al., 1997). Moreover, HVC morphology was consistent with previous reports: unilateral HVC volume and HVC neuronal number were greater in LDT than SD birds (Table 3).
Neuronal turnover during breeding and nonbreeding conditions
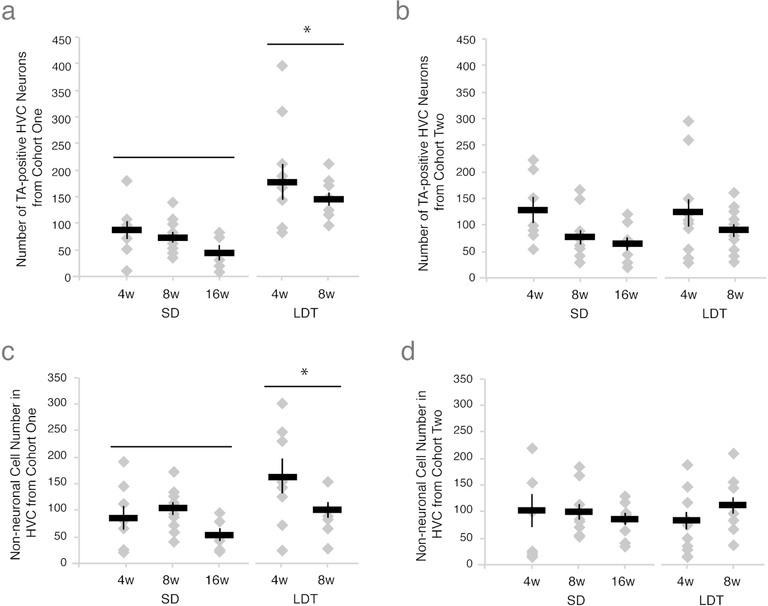
To test whether breeding condition promoted the maintenance of new HVC neurons added during HVC growth, we labeled an initial cohort of new HVC neurons with either BrdU or EdU (i.e., cohort one) in birds transitioned into SD or LDT conditions. Thymidine analog-labeled neuron number varied with condition (i.e. breeding and nonbreeding), but not the number of weeks birds were housed in a given condition (Tables 2 and 3 and Figures 2 and 3A). Birds in LDT maintained more new neurons in HVC than birds in SD (Table 3). This result suggests that breeding condition promotes the maintenance of new neurons incorporated into HVC during initial HVC growth. The number of new neurons did not differ with length of time between pulses within or between breeding and non-breeding groups (Table 2).
Figure 3.
Breeding conditions promote maintenance of an initial cohort of new neurons and non-neuronal cells, but do not alter subsequent neuronal turnover. (A) Number of thymidine analog positive neurons in one hemisphere of HVC from cohort one across all survival times in nonbreeding and breeding condition. Depicted results include both BrdU and EdU positive cell data pooled within experimental group (see Table 2 for lack of thymidine analog order effect), with data from individual birds represented as grey diamonds and the group mean as a black bar with S.E.M.. The number of new HVC neurons maintained from the initial cohort of thymidine analog label are significantly higher under breeding conditions, suggesting that LDT conditions promote greater maintenance of an initial cohort of new neurons than in SD. Because in both nonbreeding and breeding conditions neurons from the first cohort are maintained, these data further indicate that some new neurons replace older neurons, while other new neurons die (see Figure 1C, right panel). (B) Number of new neurons maintained in HVC on one side of the brain from the second thymidine analog label in all experimental groups. Physiological condition and interval between injection of the two thymidine analogs did not significantly affect the number of new neurons from the second cohort maintained in HVC. These data suggest that during stable conditions, new neurons enter HVC to replace older neurons in equal amounts regardless of condition (Tables 2 and 3). (C-D) The number of new non-neuronal HVC cells maintained from the cohort labeled with the first thymidine analog (C) and the second thymidine analog (D) across all survival times in nonbreeding and breeding condition. LDT conditions lead to greater maintenance of new non-neuronal cells from the first thymidine analog-labeled cohort but not the second when compared to SD conditions (Table 3). These data suggest that breeding conditions not only promote the increased maintenance of an initial population of new neurons but also new non-neuronal cells, which together contribute to the growth of HVC.
To test the role of breeding condition on the maintenance of new HVC neurons incorporated during stable breeding or nonbreeding condition, we labeled a second cohort of new HVC neurons with other of the two thymidine analogs (i.e., cohort two) in birds maintained in SD or LDT conditions for 4 or more weeks. We found that the number of new neurons labeled from the second cohort did not differ between conditions, suggesting that neuronal turnover of cohort two neurons returned to baseline levels once HVC attained steady breeding or nonbreeding state. The condition and length of time birds spent in either condition did not affect the number of new HVC neurons belonging to the second cohort (Tables 2 and 3 and Figure 3B).
To asses the relative turnover rates of neurons from cohort one and cohort two we calculated the ratio between the two neuronal populations. In all nonbreeding condition groups the number of neurons from cohort one was less than cohort two (i.e., a ratio less than one). Given that neuronal number remains constant in nonbreeding conditions (Table 3), this finding suggests that the second cohort replaced some of the first cohort neurons. By contrast, in breeding condition birds the ratio of cohort one number of neurons from cohort one was higher than cohort two (i.e, a ratio greater than one). Together these data suggest that turnover of cohort one labeled neurons is greater in nonbreeding birds.
Turnover of new non-neuronal cells during breeding and nonbreeding conditions
We examined maintenance of new non-neuronal cells in HVC, and found that, as with new neurons, non-neuronal cell maintenance was initially dependent on physiological condition but returned to homeostasis (i.e., nonbreeding levels of turnover) once stable HVC size was attained. The first cohort of thymidine analog-labeled non-neuronal cells was higher in birds in breeding condition than those in nonbreeding condition (Table 3 and Figure 3C). Non-neuronal cell number from the second cohort, however, did not differ between conditions (Table 3 and Figure 3D). These results suggest that, similar to new neurons, non-neuronal cell maintenance depends on breeding condition, but only during periods of active HVC growth, not when HVC size is stable. Unlike new neuronal turnover, the proportion of new non-neuronal cells from cohort one versus cohort two did not differ between breeding and nonbreeding physiological conditions (Table 3).
Factors mediating maintenance of new neuronal and non-neuronal cells in HVC
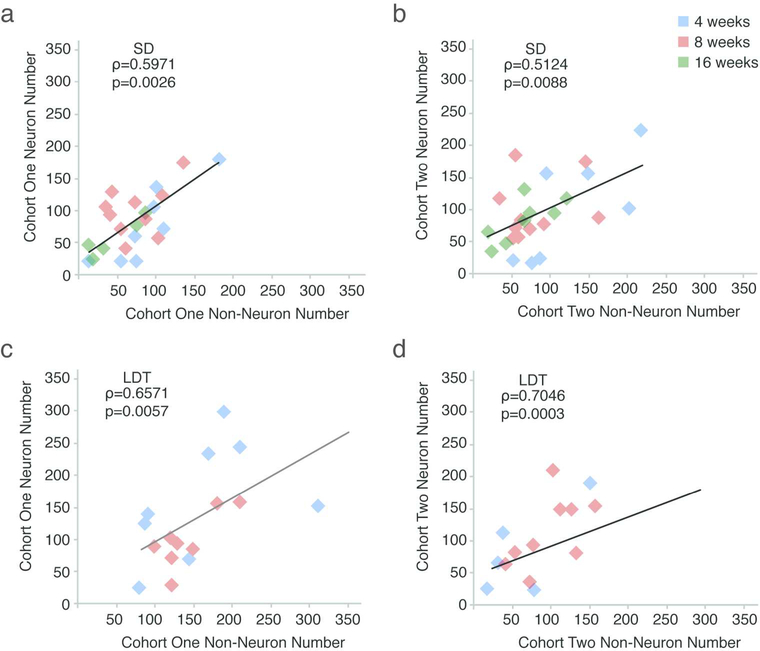
We observed variability in numbers of new neurons from both cohorts within all experimental groups, and so we asked which of the factors measured might have contributed to this variability using nonparametric Spearman’s rank correlation tests. Because condition (breeding vs. nonbreeding) significantly affected the experimental results, we performed correlation tests with all measured variables for the SD and LDT data sets independently (Table 4). Testosterone plasma levels did not significantly correlate with HVC volume or the number of new neuronal and non-neuronal cells from either cohorts in SD or LDT. This lack of correlation suggests that once testosterone levels exceed a threshold that stimulates seasonal growth of the song system (Smith et al., 1995; Smith et al., 1997; Brenowitz, 2008), individual differences in testosterone levels do not contribute to individual variation in HVC volume or new cell turnover. HVC volume also did not co-vary with new cell numbers from either cohort, suggesting variability in the maintenance of new cells within a given condition (i.e., breeding or nonbreeding) is not a major contributor to variability in HVC volume. The number of new neurons was significantly correlated with the number of new non-neuronal cells consistently within each of the two cohorts and conditions (i.e., SD and LDT; Table 4 and Figure 4). Neither the number of new neurons, nor the number of new non-neuronal cells, co-varied significantly across the two cohorts, suggesting that the number of new cells added and maintained from the first cohort did not significantly affect the number of new cells maintained from the second cohort. Taken together, these data suggest that although LDT conditions promote increased neuronal and non-neuronal cell maintenance of the first cohort of new cells in HVC, some additional factor not measured in the current study influenced addition and maintenance of all new cells in HVC, and did so independent of condition.
Figure 4.
The number of new neuronal and non-neuronal cells co-vary significantly within, but not across, cohorts (also see Table 4). The results of Spearman’s rank correlation from comparisons of: (A) neuronal and non-neuronal cells labeled with the first thymidine analog in SD, (B) neuronal and non-neuronal cells labeled with the second TA in SD, (C) neuronal and non-neuronal cells labeled with the first thymidine analog in LDT, (D) neuronal and non-neuronal cells labeled with the second thymidine analog in LDT. The correlation between new neuronal and non-neuronal cells within, but not across, thymidine analog-labeled cohorts suggests that a temporally controlled common factor present during both breeding and nonbreeding conditions mediates addition, survival, or both of new cell populations.
Discussion
Although neuronal birth and death are necessarily coordinated and are often causally linked in some systems, their relationship to each other is not well understood, especially in the context of the healthy adult brain. We described natural neuronal turnover in the intact song production nucleus HVC, and tested the role of breeding condition physiology, in modulating new cell maintenance. We showed that breeding conditions increased the maintenance of new neurons added to HVC at the onset of breeding conditions relative to new neurons added in nonbreeding conditions. Once HVC attained a steady breeding-like or nonbreeding-like state, new neuronal turnover returned to baseline levels. We observed a similar pattern of maintenance for non-neuronal cells, with greater turnover during periods of active breeding-like growth than periods when HVC volume was stable in either nonbreeding or breeding states. Finally, we found a positive correlation between the numbers of new neurons and new non-neuronal cells within, but not across, cohorts in both breeding and non-breeding conditions. However, neither the plasma testosterone level, nor HVC volume of individual birds accounted for the variability of new cell numbers between birds. Together, these data suggest that gross changes toward breeding physiological condition promote the survival of an initial population of new neuronal and non-neuronal cells added to HVC, but that once HVC is fully grown maintenance of later cohorts of new cells returns to the homeostatic levels of stable nonbreeding conditions.
Both new and older neural cells are replaced in actively growing and stable HVC
We presented three hypotheses regarding the specific populations of neuronal and non-neuronal cells that are lost and replaced to accommodate the growth, stabilization, and regression in size of HVC (Figure 1C). The first model was that only older cells in HVC die and are replaced by new neurons and non-neuronal cells. However, previous studies reported that some new neurons incorporated in breeding conditions die through regression (Walton et al., 2012; Larson et al., 2014). In context of the current study, this model might predict that the number of new cells maintained in HVC would not vary between breeding and nonbreeding conditions, as all new cells would be maintained, and that the growth of HVC during the transition into breeding condition would result from increased survival of older neurons. We found, however, that the maintenance of the new cell populations, especially neurons from the first thymidine labeled cohort, was higher in breeding conditions than in nonbreeding conditions. Given a constant rate of NSC proliferation during stable breeding and nonbreeding condition (Larson et al., 2014), this observation suggests that a number of older neurons are maintained at the expense of newer neurons in nonbreeding conditions, further refuting this first model of turnover.
The second hypothesis posited that new cells that enter HVC are a transient population that either die soon after birth in nonbreeding conditions, or later during active HVC regression for cells added to HVC under breeding conditions. Previous work showed that new neurons incorporated during growth of HVC in breeding birds can persist through its subsequent regression (Nottebohm et al., 1994; Larson et al., 2014). Here, we show that even when HVC volume is stable, new cells can persist as long as 4 weeks in stable breeding and 20 weeks in nonbreeding birds. In order for new cells to be maintained in HVC during these periods of stable total neuron number, older neurons must die and be replaced by younger cells. This observation is inconsistent with the second model of HVC neuronal turnover.
Our data support the third hypothesis for neuronal turnover – that both new and older neuronal and non-neuronal cells die as new cells enter HVC regardless of physiological condition. In this scenario, the growth of HVC in breeding birds results from a change in the balance between cell survival and death towards greater maintenance of both new and older cells. Indeed, we found greater maintenance of the first cohort of incoming new cells during the transition into breeding conditions than during stable nonbreeding conditions (e.g., cohort one of LDT 4w v. SD 4w). Moreover, once HVC growth stabilized in breeding conditions, turnover of new cells returned to an equilbirium similar to that observed in stable nonbreeding conditions. We also found that although a second cohort of new neurons is added to HVC in both SD and LDT, total HVC neuron number remains unchanged, further suggesting that both new and older neurons die in nonbreeding and breeding conditions.
The maintenance of new versus older cells in HVC during active regression could not be tested directly in this study because the neural stem cell proliferation rate increases transiently when neurons die during HVC regression (Larson et al., 2014). This temporary increase in proliferation complicates efforts to directly compare the maintenance of new cells when HVC is actively regressing with that when HVC reaches stable nonbreeding size.
Breeding physiological condition promotes the maintenance of new neurons during active HVC growth
Breeding conditions supported a higher number of the first cohort of new neurons than nonbreeding conditions or the second cohort of neurons in either condition. This increase in maintenance of the first cohort of new neurons contributes to the overall growth of HVC to its full breeding volume and total neuronal number. Our results are consistent with a previous model, which posits that HVC growth occurs once plasmic testosterone levels reach a threshold (Smith et al., 1995; Smith et al., 1997; Brenowitz, 2008). Once this threshold level is exceeded, no further increases in HVC volume or neuron number occur (Smith et al., 1995; Smith et al., 1997), suggesting a ceiling effect of testosterone induced growth of HVC. The combination of threshold and ceiling effects of testosterone can explain the lack of correlation between HVC volume and HVC total neuron number with individual testosterone levels. Breeding conditions, specifically high systemic T levels and downstream trophic effects including increased BDNF expression and activation of the PI3K-Akt-mTOR signaling cascade ((Brenowitz, 2013; Brenowitz and Larson, 2015) and Kranz, Lent, Miller, Chao, and Brenowitz unpublished observation), may “lock in” the first cohort of new neurons, and thus facilitate HVC growth.
The correlation between the numbers of new neuronal and non-neuronal cells within a given cohort suggests that the addition and maintenance of these different populations are functionally linked. Individual differences in the degree to which factors like neurotrophin expression, canonical signaling cascades, and others that are stimulated by testosterone may also contribute to individual differences in the correlated increase in new neurons and new non-neuronal cells. Such other factors may include arborization (Devoogd and Nottebohm, 1981; Devoogd et al., 1985), angiogenesis (Goldman and Nottebohm, 1983; Louissaint et al., 2002), intrinsic and synaptic neuronal activity (Piatti et al., 2011; Larson et al., 2013) and local inflammation from dying cells (Larson, 2018). Although no studies to date have directly examined the role of inflammation in HVC neuronal addition and death, studies in white-crowned sparrows have shown that acute induction of systemic inflammation suppressed hormone release and decreased singing behavior (Owen-Ashley et al., 2006). Previous research has suggested that singing activity can modulate the number of new neurons (Li et al., 2000; Pytte et al., 2011). Thus, the relationship between HVC neuronal cell number and singing behavior in sparrows suggests that inflammation might exert its influence on singing behavior through modulation of neurogenesis, neural cell death, and neuronal turnover.
Breeding physiological condition does not directly regulate the turnover of new or older neural cells during the period of stable HVC total neuron number
The null hypothesis regarding which HVC cells are maintained longer, new versus older cells, is that maintenance is stochastic and not dependent on any intrinsic or extrinsic properties of HVC or seasonal physiology in general. Under stable breeding and stable non breeding conditions, the turnover of both new and older neuronal and non-neuronal cells appears consistent with a stochastic model. During the period of active growth of HVC at the onset of breeding conditions, however, the addition and maintenance of the first cohort of new neurons is regulated by breeding physiological condition, specifically elevated plasma testosterone and its downstream effectors, in a non-stochastic manner (discussed above). We found that physiological conditions did not affect the maintenance of the second cohort of new neuronal or non-neuronal cells once HVC volume has stabilized in either the fully grown or regressed state. Moreover, neither individual variability in testosterone plasma levels nor HVC volume accounted for variability of new cell number within the second cohort. Although the turnover of neuronal and non-neuronal cells appears stochastic, the fate of a given cell is likely not random; factors such as neurotrophin and canonical signaling, intrinsic neuronal activity, and singing behavior (all discussed above) among others potentially determine the turnover, or lack thereof, of individual neural cells.
Precise timing of TA pulses controls for temporal changes in NSC proliferation rate
Increased survival of new neurons added to HVC during the early stages of breeding condition growth seems to contradict a previous report in male canaries that new neurons persisted longer in nonbreeding conditions than breeding conditions (Nottebohm et al., 1994). Nottebohm et. al. reported that canaries pulsed with 3H-thymidine in fall (nonbreeding season) had a significantly higher number of labeled cells 40 to 120 days later than canaries pulsed in spring (breeding season). However, a direct comparison of our results to the canary work can not be made because: 1) canaries exhibit reduced seasonality compared to white-crowned sparrows (Bentley et al., 2003), 2) the breeding season group of canaries was allowed to transition into nonbreeding conditions as shown by a reduction in HVC volume in their spring 140 day experimental group (Nottebohm et al., 1994), and most importantly, 3) the timing of 3H-thymidine pulses was not precisely controlled to ensure that neural stem cell proliferation rates were similar across canary experimental groups. As white-crowned sparrows transition from breeding to nonbreeding conditions, neural stem cell proliferation increases as a direct consequence of HVC neuronal death (Larson et al., 2014). The reported increase in survival of 3H-thymidine positive cells in nonbreeding canaries therefore might be due to a similar temporary increase in neural stem cell proliferation during HVC regression. For our study we purposely timed all BrdU and EdU injections to occur during periods known to have stable and similar amounts of neural stem cell proliferation (Larson et al., 2014). Thus, our observation of breeding condition modulating new cell number in sparrows is not complicated by differential neural stem cell proliferation rates, but rather is due to differential maintenance of new cells.
Conclusion
We show that a telencephalic nucleus in adult songbirds undergoes neuronal turnover such that during breeding conditions new neurons and non-neuronal cells enter HVC and become “locked in” to support the seasonal growth of HVC. Once HVC total neuron number stabilizes, subsequent cohorts of new cells enter HVC to replace older neurons at a rate similar to that of nonbreeding conditions. Individual differences in the rate at which new cells are maintained under stable breeding and nonbreeding states do not appear dependent on variation in breeding condition physiology including testosterone levels or HVC morphology. The pronounced and dynamic nature of neuronal turnover in HVC provides an excellent system for future studies examining mechanisms regulating adult neurogenesis, neural cell survival, natural neural cell loss and replacement.
Sex steroids promote the maintenance of an early cohort of new neurons within the avian sensorimotor nucleus HVC. This addition of new HVC neurons at the onset of breeding season contributes to growth of HVC and an increase in singing behavior. Once HVC volume and neuron number is stable, neuronal turnover, or the balance between neuronal birth and death, returns to homeostasis. These results confirm that both new and older neurons die as HVC grows and regresses across seasons.
Acknowledgements:
Supported by NIH R01 MH53032 and R01 NS075331 to EAB; and National Research Service Award T32 GM007270, Washington Research Foundation - Hall Fellowship, and University of Virginia start-up funds to TAL. We thank David M Parichy and Brian P Flaherty for guidance with statistical analyses.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Alvarez-Buylla A, Ling CY, Yu WS. 1994. Contribution of neurons born during embryonic, juvenile, and adult life to the brain of adult canaries: regional specificity and delayed birth of neurons in the song-control nuclei. J Comp Neurol 347(2):233–248. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. 1990. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron 5(1):101–109. [DOI] [PubMed] [Google Scholar]
- Barnea A, Pravosudov V. 2011. Birds as a model to study adult neurogenesis: bridging evolutionary, comparative and neuroethological approaches. Eur J Neurosci 34(6):884–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley GE, Audage NC, Hanspal EK, Ball GF, Hahn TP. 2003. Photoperiodic response of the hypothalamo-pituitary-gonad axis in male and female canaries, Serinus canaria. J Exp Zool A Comp Exp Biol 296(2):143–151. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. 2008. Plasticity of the song control system in adult birds In: Zeigler HP, Marler P, eds. Neuroscience of birdsong. Cambridge: Cambridge University Press; p 332–349. [Google Scholar]
- Brenowitz EA. 2013. Testosterone and brain-derived neurotrophic factor interactions in the avian song control system. Neuroscience 239:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Larson TA. 2015. Neurogenesis in the Adult Avian Song-Control System. Cold Spring Harbor perspectives in biology 7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers MP, Nikoletopoulou V, Barde YA. 2013. Cell biology in neuroscience: Death of developing neurons: new insights and implications for connectivity. J Cell Biol 203(3):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoogd TJ, Nixdorf B, Nottebohm F. 1985. Synaptogenesis and changes in synaptic morphology related to acquisition of a new behavior. Brain Res 329(1–2):304–308. [DOI] [PubMed] [Google Scholar]
- Devoogd TJ, Nottebohm F. 1981. Sex-Differences in Dendritic Morphology of a Song Control Nucleus in the Canary - a Quantitative Golgi-Study. J Comp Neurol 196(2):309–316. [DOI] [PubMed] [Google Scholar]
- Farner DS, Wingfield JC. 1978. Environmental endocrinology and the control of annual reproductive cycles in passerine birds pp. 44–51. In: Assenmacher I, Farner DS ed Environmental endocrinology Berlin, Springer,. [Google Scholar]
- Fossati P, Radtchenko A, Boyer P. 2004. Neuroplasticity: from MRI to depressive symptoms. Eur Neuropsychopharmacol 14 Suppl 5:S503–510. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. 1983. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A 80(8):2390–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ. 2004. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging 25(3):361–375. [DOI] [PubMed] [Google Scholar]
- Kempermann G 2002. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci 22(3):635–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Nottebohm F. 1993. Direct evidence for loss and replacement of projection neurons in adult canary brain. J Neurosci 13(4):1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsmark B 1970. The counting of neurons In: Nauta W, Ebbeson S, eds. Contemporary research methods in neuroanatomy. New Yor: Springer-Verlag; p 315–340. [Google Scholar]
- Larson TA. 2018. Sex Steroids, Adult Neurogenesis, and Inflammation in CNS Homeostasis, Degeneration, and Repair. Front Endocrinol (Lausanne) 9:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson TA, Thatra NM, Lee BH, Brenowitz EA. 2014. Reactive neurogenesis in response to naturally occurring apoptosis in an adult brain. J Neurosci 34(39):13066–13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson TA, Wang TW, Gale SD, Miller KE, Thatra NM, Caras ML, Perkel DJ, Brenowitz EA. 2013. Postsynaptic neural activity regulates neuronal addition in the adult avian song control system. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XC, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. 2000. A relationship between behavior, neurotrophin expression, and new neuron survival. Proc Natl Acad Sci U S A 97(15):8584–8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liboska R, Ligasova A, Strunin D, Rosenberg I, Koberna K. 2012. Most anti-BrdU antibodies react with 2’-deoxy-5-ethynyluridine -- the method for the effective suppression of this cross-reactivity. PLoS One 7(12):e51679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Rao S Jr, Leventhal C, Goldman SA. 2002. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34(6):945–960. [DOI] [PubMed] [Google Scholar]
- Mao S, Xiong G, Zhang L, Dong H, Liu B, Cohen NA, Cohen AS. 2016. Verification of the Cross Immunoreactivity of A60, a Mouse Monoclonal Antibody against Neuronal Nuclear Protein. Front Neuroanat 10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Thompson CK, Choi H, Perkel DJ, Brenowitz EA. 2009. Time course of changes in Gambel’s white-crowned sparrow song behavior following transitions in breeding condition. Horm Behav 55(1):217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. 1992. NeuN, a neuronal specific nuclear protein in vertebrates. Development 116(1):201–211. [DOI] [PubMed] [Google Scholar]
- Nottebohm F 1989. From bird song to neurogenesis. Sci Am 260(2):74–79. [DOI] [PubMed] [Google Scholar]
- Nottebohm F 2004. The Road We Travelled: Discovery, Choreography, and Significance of Brain Replaceable Neurons. Ann N Y Acad Sci 1016:628–658. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, O’Loughlin B, Gould K, Yohay K, Alvarez-Buylla A. 1994. The life span of new neurons in a song control nucleus of the adult canary brain depends on time of year when these cells are born. Proc Natl Acad Sci U S A 91(17):7849–7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Ashley NT, Turner M, Hahn TP, Wingfield JC. 2006. Hormonal, behavioral, and thermoregulatory responses to bacterial lipopolysaccharide in captive and free-living white-crowned sparrows (Zonotrichia leucophrys gambelii). Horm Behav 49(1):15–29. [DOI] [PubMed] [Google Scholar]
- Piatti VC, Davies-Sala MG, Esposito MS, Mongiat LA, Trinchero MF, Schinder AF. 2011. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci 31(21):7715–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytte C, Yu YL, Wildstein S, George S, Kirn JR. 2011. Adult Neuron Addition to the Zebra Finch Song Motor Pathway Correlates with the Rate and Extent of Recovery from Botox-Induced Paralysis of the Vocal Muscles. J Neurosci 31(47):16958–16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED, Guturkun O. 2004a. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473(3):377–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Guturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. 2004b. The Avian Brain Nomenclature Forum: Terminology for a New Century in Comparative Neuroanatomy. J Comp Neurol 473:E1–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. 2005. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25(5):1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Koike T, Kawashima F, Kurata H, Shibuya T, Satoh T, Hata Y, Yamada H, Mori T. 2018. Identification of NeuN immunopositive cells in the adult mouse subventricular zone. J Comp Neurol. [DOI] [PubMed] [Google Scholar]
- Salic A, Mitchison TJ. 2008. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A 105(7):2415–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. 2000. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron 25(2):481–492. [DOI] [PubMed] [Google Scholar]
- Scott BB, Lois C. 2007. Developmental origin and identity of song system neurons born during vocal learning in songbirds. J Comp Neurol 502(2):202–214. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Wingfield JC. 1997. Roles of photoperiod and testosterone in seasonal plasticity of the avian song control system. J Neurobiol 32(4):426–442. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Wingfield JC, Baptista LF. 1995. Seasonal changes in song nuclei and song behavior in Gambel’s white-crowned sparrows. J Neurobiol 28(1):114–125. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. 2012. Biometry : the principles and practice of statistics in biological research. New York: W.H. Freeman. [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. 2013. Dynamics of hippocampal neurogenesis in adult humans. Cell 153(6):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Bentley GE, Brenowitz EA. 2007. Rapid seasonal-like regression of the adult avian song control system. Proc Natl Acad Sci U S A 104(39):15520–15525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Brenowitz EA. 2008. Caspase inhibitor infusion protects an avian song control circuit from seasonallike neurodegeneration. J Neurosci 28(28):7130–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Brenowitz EA. 2009. Neurogenesis in an adult avian song nucleus is reduced by decreasing caspasemediated apoptosis. J Neurosci 29(14):4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarev K, Boender AJ, Classen GA, Scharff C. 2016. Young, active and well-connected: adult-born neurons in the zebra finch are activated during singing. Brain Struct Funct 221(4):1833–1843. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. 1999. A field study of seasonal neuronal incorporation into the song control system of a songbird that lacks adult song learning. J Neurobiol 40(3):316–326. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Hartman VN, Brenowitz EA. 2000. Breeding conditions induce rapid and sequential growth in adult avian song control circuits: a model of seasonal plasticity in the brain. J Neurosci 20(2):854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Smith GT, Breuner CW, Brenowitz EA. 1998. Seasonal plasticity and sexual dimorphism in the avian song control system: stereological measurement of neuron density and number. J Comp Neurol 396(2):186–192. [DOI] [PubMed] [Google Scholar]
- Walton C, Pariser E, Nottebohm F. 2012. The zebra finch paradox: song is little changed, but number of neurons doubles. J Neurosci 32(3):761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Aviram R, Kirn JR. 1999. Deafening alters neuron turnover within the telencephalic motor pathway for song control in adult zebra finches. J Neurosci 19(23):10554–10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Hurley P, Pytte C, Kirn JR. 2002. Vocal control neuron incorporation decreases with age in the adult zebra finch. J Neurosci 22(24):10864–10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbrecht L, Kirn JR. 2004. Neuron Addition and Loss in the Song System: Regulation and Function. Ann N Y Acad Sci 1016:659–683. [DOI] [PubMed] [Google Scholar]