Abstract
Objectives:
The frontal lobe hypothesis of age-related cognitive decline suggests that the deterioration of the prefrontal cortical regions that occurs with aging leads to executive function deficits. Photobiomodulation (PBM) is a newly developed, non-invasive technique for enhancing brain function, which has shown promising effects on cognitive function in both animals and humans. This randomized, sham-controlled study sought to examine the effects of PBM on the frontal brain function of older adults.
Methods/Designs:
Thirty older adults without a neuropsychiatric history performed cognitive tests of frontal function (i.e., the Eriksen flanker and category fluency tests) before and after a single 7.5-min session of real or sham PBM. The PBM device consisted of three separate light-emitting diode cluster heads (633 nm and 870 nm), which were applied to both sides of the forehead and posterior midline, and delivered a total energy of 1349 J.
Results:
Significant group (experimental, control) × time (pre-PBM, post-PBM) interactions were found for the flanker and category fluency test scores. Specifically, only the older adults who received real PBM exhibited significant improvements in their action selection, inhibition ability, and mental flexibility after vs. before PBM.
Conclusions:
Our findings support that PBM may enhance the frontal brain functions of older adults in a safe and cost-effective manner.
Keywords: photobiomodulation, low level laser light therapy, randomized sham-controlled trial, executive function, Eriksen flanker test, category fluency test
Introduction
The frontal lobe hypothesis suggests that the cognitive deficits in older adults are mainly related to the anatomical and functional deterioration of the prefrontal cortical regions of the brain.1,2Studies on the neurobiological changes in older adults have shown that these individuals exhibit significantly greater reductions in cortical volume,3 greater neuronal atrophy4 and synapse loss,5 and more senile plaques6 in the prefrontal lobe than in other regions of the brain. Consistent with these structural changes in the prefrontal regions of the brain, most of the cognitive deficits that are associated with aging are in executive function (EF), which is mediated by the frontal lobe.7 Specifically, EF refers to a set of administrative and decision-making abilities that are important for behavior, namely the planning and initiation of actions, selection of relevant information, inhibition of irrelevant information, and flexible thinking. Empirical evidence has shown that compared to younger adults, older adults perform less well on some standardized neuropsychological tests of EF, such as the Wisconsin Card Sorting Test8 and the verbal fluency test.9–11 To identify the EF deficits in older adults, several experimental computerized paradigms have been employed, including the divided attention,12 n-back,13,14 and Eriksen flanker tests.15,16 Prior studies have shown that deficits in some aspects of EF, especially inhibition and mental flexibility, can predict subsequent global cognitive decline,17 future falls,18,19 and poor functional status20 in healthy older adults without dementia. Thus, training procedures or interventions that can effectively improve these frontal functions are clinically important.
Photobiomodulation (PBM) is a newly developed, non-invasive interventional technique that has been found to exert positive effects on cognitive function according to both animal21–23 and human24–26 studies. The mechanism of PBM is based on bioenergetics, photochemistry, and photobiology. Specific molecules inside the neurons absorb the photons and change the rate of metabolic reactions within the cells, ultimately activating signaling pathways and transcription factors.27 The primary molecular photoacceptor of red and near-infrared light is cytochrome c oxidase, which is a key enzyme in the mitochondrial respiratory chain.28–31 Photon absorption increases oxygen consumption,32 leading to increased oxidative phosphorylation and mitochondrial activity.33 In turn, more adenosine triphosphate is synthesized,32 thus providing extra metabolic energy for neural transduction.34 Cerebral blood flow is increased due to the vasodilation that occurs after the release of nitric oxide.35 The increased adenosine triphosphate and cerebral blood flow work together to enhance brain function.36–38 This mechanism is supported by previously reported animal experiments, which identified increases in cytochrome c oxidase and metabolic capacity following PBM.37,39,40 Other studies using various mouse models showed that PBM effectively provided neuroprotection,41,42 improved memory function,43 and reduced brain tissue loss.22
The abovementioned neuroenhancement effects of PBM have also been found in humans.25,26,44–46 Although PBM has been around since the 1960s47,48 (originally called “low level laser therapy”49), it has only recently been applied in different populations as a neuropsychological intervention for enhancing brain function. For instance, PBM improved the EF, verbal learning, and memory of patients with traumatic brain injuries,25 as well as the cognitive and functional abilities for daily living in patients with mild to moderately-severe dementia.26 In addition, the remission of symptoms of patients with major depression and anxiety was observed following PBM.45,46 Apart from clinical patient populations, PBM has been applied to healthy college students, whereby 8 min of PBM treatment to the right prefrontal pole (FP2) successfully improved their memory, sustained attention, and emotional state.44 Besides, Vargas et al. has conducted a PBM study to 21 elderly with subjective memory complaint and reported a significant effect on some frontal cognitive function, such as sustained attention, and short term memory.50
The major objective of the present study was to examine the effects of PBM on frontal executive function in older adults. We hypothesized that PBM would facilitate frontal executive function, which would be indicated by improved inhibition ability and greater mental flexibility in older adults. To assess the effects of PBM on these functions, we employed the Eriksen flanker and category fluency tests. The Eriksen flanker test51 is widely used to measure inhibition ability and selective attention. It requires individuals to judge the direction of a target stimulus, which is surrounded by flanker stimuli. Consistent with the notion that this test evaluates frontal lobe function, functional magnetic resonance imaging studies have shown that the Eriksen flanker test is primarily mediated by the right ventrolateral prefrontal cortex, supplementary motor area, and left parietal cortex.52 Moreover, many research studies have found that older adults respond slower than do younger adults to the congruent and incongruent conditions of this test.15,16,53 We also used the category fluency test, a common test for mental flexibility and lexical/semantic access, which requires individuals to generate as many examples of a category (e.g., animals) as they can in 1 min. Positron emission tomography and functional magnetic resonance imaging studies have shown that performing this test is primarily mediated by the left ventrolateral and dorsomedial prefrontal cortex and the temporal cortex.54 Similar to the findings for the Eriksen flanker test, research studies using the category fluency test revealed that older adults have poorer category fluency than do younger adults.9,11,55
Materials and methods
Participants
The study sample consisted of 30 older adults (≥60 years) without dementia, who were recruited through campus advertisements and the subject database of the neuropsychology laboratory at the Department of Psychology of The Chinese University of Hong Kong. The older adults were initially screened by a research assistant and excluded if they had a history of traumatic head injury or any neurological and/or psychiatric disorders. In addition, participants were excluded from the present study if they exhibited signs of dementia, as indicated by a total raw score <112 on the Chinese version of the Dementia Rating Scale (CDRS),56 or a high level of depression, as indicated by a total score >7 on the Chinese version of the Geriatric Depression Scale (CGDS).57 All participants had normal or corrected-to-normal vision during the experiment. Each older adult was paid 100 HKD for participating in this study. The study was conducted in accordance with the Helsinki Declaration of the World Medical Association Assembly and approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee. All participants provided written informed consent.
Procedure
Participants were assigned randomly to either the experimental group (n = 15) or the control group (n = 15). Participants in each group completed neuropsychological assessments and a PBM session. The whole experimentation was administered by trained research assistants and a graduate student, who were supervised by a clinical neuropsychologist. The neuropsychological assessments included the (1) CDRS,56 which estimates the level of global cognitive functioning; (2) CGDS,57 which measures the level of depressive symptoms; (3) Beck Anxiety Inventory (BAI),58 which measures the level of anxiety symptoms; and (4) the Hong Kong List Learning Test (HKLLT),59 which is a standardized verbal list learning test that can be used to measure verbal memory in local Chinese populations.
The PBM session consisted of the application of PBM using a painless, non-invasive light-emitting diode (LED) device (Model 1100; MedX Health, Toronto) and two computerized assessments evaluating the effects of PBM. The PBM device contained three LED cluster heads. Each LED cluster head had the following parameters: diameter, 5.35 cm (9 red diodes [633 nm] and 52 near infrared diodes [870 nm] were embedded into each LED cluster head); total area, 22.48 cm2; total power, 999 mW; power density, 44.4 mW/cm22; and continuous wave. The detailed specification and calculation of parameters is stated in Table 1. This device was approved by The United States Food and Drug Administration as imposing insignificant risk (Food and Drug Administration-cleared for home treatment, 2005). In the present study, the three LED cluster heads were placed on the participant’s head. According to the International 10–20 system,60 two cluster heads were fixed to the left (Fp1) and right (Fp2) frontopolar regions, while the remaining cluster head was fixed to Pz.
Table 1.
Parameters of each LED cluster of the PBM device used in the present study.
| Model | Model 1100; MedX Health, Toronto |
|---|---|
| Diameter | 5.35 cm |
| Area | 22.48 cm2 ((5.35cm ÷ 2)2 × π) |
| Energy Density | 20 J/cm2 |
| Duration | 7.5 mins (450 sec) |
| Power Density | 44.4 mW/cm2 (20 J/cm2 ÷ 450 sec) |
| Total Power | 999mW (44.4mW/cm2 × 22.48 cm2) |
| Total Energy | 449.6 J (999mW × 450 sec) |
During the PBM administration, participants were instructed to keep their eyes closed and sit still in an office chair. They were also asked to wear a pair of eye protection glasses. In the experimental group, PBM consisting of 20 J/cm2 and a total energy dose of 1349 J was applied simultaneously at each cluster LED head to the head of the participants by the three LED cluster heads. In the control group, the PBM device was turned off before the application of light. We arranged for the device to beep before it was turned off to give the control participants the impression that the device was operating. All participants were blinded to their group assignment. None of the participants reported any adverse side effects. The duration of the PBM administration was 7.5 min.
Before and after PBM, participants performed computerized assessments of frontal function. The experimental procedure and head setup are illustrated in Figure 1. The sections below describe the details of each test.
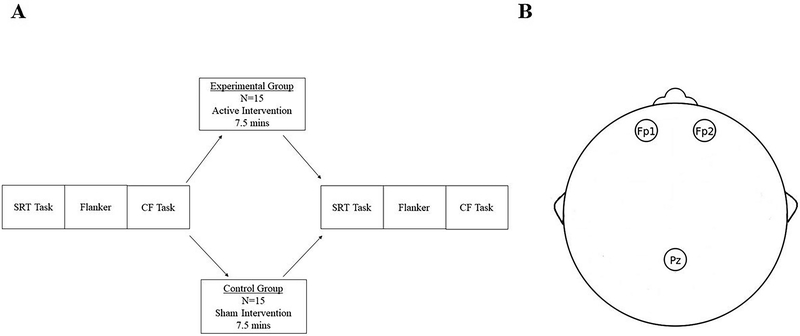
Figure 1.
Experimental setup of the photobiomodulation session. (A) Procedures for photobiomodulation. Note. SRT = simple reaction time task; CF Task = category fluency task. (B) Sites of photobiomodulation according to International 10–20 system.60
Tests
Modified Eriksen flanker test.
We employed a modified arrow version of the Eriksen flanker test50 to measure inhibition ability and selective attention. To increase the degree of conflict induced by flanker stimuli, the task was modified such that the flanker stimuli (i.e., “<< <<“ or “>> >>“) were always presented 200 ms before the central target was observed (i.e., “>“ or “<“).62–64 Specifically, each trial began with the presentation of horizontally oriented flanker stimuli. After 200 ms, the target stimulus was presented at the center of a computer screen for 800 ms, and the flankers remained on screen until the target disappeared. Participants were instructed to judge the direction of the central target and to respond as quickly as possible by pressing a button on a computer mouse, with the left direction being assigned to the left button and the right direction being assigned to the right button. The direction of the central target was equiprobable. The flanker and target stimuli were followed by an inter-stimulus interval (ISI) of 1000 ms, during which a blank screen was shown.
The flanker task consisted of two conditions, the congruent and incongruent conditions. The task consisted of 100 trials in total, and took 3 min 20 s for each individual to complete. Prior to the actual experimental session, participants performed a practice session to familiarize themselves with the task. The accuracy and mean RT on correct trials were recorded separately for the congruent and incongruent conditions.
Category fluency test.
The category fluency test is a widely used frontal lobe test that requires the controlled retrieval of lexical/semantic information. In the present study, participants were instructed to generate as many words belonging to a given category as possible within a time limit of 1 min. Participants were also asked to avoid repetitions. Two categories were employed for word selection; the first category was “animals” and the second category was “means of transportation”61. The total number of animal and transportation words produced was calculated for each individual.
Simple RT test.
A simple RT test, adapted from previous studies,44 was employed as a control task for response speed. It consisted of 40 trials in total. On each trial, a white plus sign (+) was presented for 1000 ms against a black background at the center of a computer screen. Participants were instructed to respond by clicking the left button of a computer mouse as fast as possible upon stimulus presentation. The valid response period was 1000 ms. The stimulus presentation was followed by an ISI of 2–10 s that increased in 2-s steps, during which a blank screen was presented. There were 8 trials for each ISI, and the ISI order was pseudo-randomized for each individual. The primary dependent variable was the mean RT.
Data Analysis
The demographic and clinical characteristics (except for gender) and neuropsychological test scores were compared between the experimental and control groups using independent sample t-tests (two-tailed). Gender was compared using a chi-squared test.
Next, participants’ scores on the frontal function assessments that were performed before and after PBM were analyzed. To calculate the mean RT on the flanker and simple RT tests, incorrect trials and correct trials with a RT <150 ms or three standard deviations above the respective group mean were excluded. To check whether the experimental and control groups differed before PBM, independent sample t-tests were first performed to compare the pre-PBM test scores between the groups. Then, to compare the changes in test scores from before to after PBM between the two groups, repeated measures analyses of variance (ANOVAs), with time (pre-PBM, post-PBM) as the within-subjects factor and group (experimental, control) as the between-subjects factor, were conducted on the dependent variables (e.g., mean RT, accuracy). Paired-samples t-tests (two-tailed) were then used to evaluate the changes in test scores from before to after PBM in the experimental and control groups separately.
All statistical analyses were performed using SPSS 24.0 software (IBM Corporation, Armonk, NY, USA). Because all statistical tests were planned, the significance level was set at p < .05 for all tests.
Results
Demographic characteristics, neuropsychological functioning, and mood state
The demographics, clinical characteristics, and neuropsychological assessment scores of the experimental and control groups, as well as the related statistics, are presented in Table 2. No significant differences in age, gender, handedness, or years of education were identified between the groups (ps > .092). Furthermore, no significant group differences in the neuropsychological measures, including the HKLLT trial 3 recall and 10-min delayed recall scores and the total raw score of the CDRS, were observed (ps > .49). No group differences in the levels of depressive and anxiety symptoms, as indicated by the CGDS and BAI scores, respectively, were identified (ps > .18).
Table 2.
Comparisons of the means and standard deviations of the demographic variables, neuropsychological functioning scores, and mood state scores between the groups, as conducted using independent-samples t-tests and chi-squared tests.
| Group |
||||||
|---|---|---|---|---|---|---|
| Control (N = 15) |
Experimental (N = 15) |
|||||
| M | SD | M | SD | t/χ2 | p | |
| Demographic variables | ||||||
| Age (years) | 68.77 | 4.66 | 66.29 | 2.93 | −1.74 | 0.09 |
| Gender (M/F) | 1/14 | 2/13 | 0.37 | 0.54 | ||
| Handedness (L/R) | 1/14 | 1/14 | 0.00 | 1.00 | ||
| Education (years) | 6.27 | 3.9 | 8.17 | 3 | 1.50 | 0.15 |
| Neuropsychological functioning | ||||||
| CDRS (raw score; out of 144) | 131.4 | 8.42 | 131.2 | 7.94 | −0.07 | 0.95 |
| HKLLT | ||||||
| Trial 3 recall (raw score; out of 16) | 10.47 | 2.70 | 11.13 | 2.53 | 0.70 | 0.49 |
| 10-min delayed recall (raw score; out of 16) | 9.13 | 3.87 | 9.73 | 3.35 | 0.45 | 0.65 |
| Mood state | ||||||
| BAI (total score; out of 63) | 7.73 | 10.12 | 3.4 | 6.91 | −1.37 | 0.18 |
| CGDS (total score; out of 15) | 2.2 | 2.54 | 2.4 | 2.06 | 0.24 | 0.82 |
Note. CDRS = Chinese version of the Dementia Rating Scale; HKLLT = Hong Kong List Learning Test; BAI = Beck Anxiety Inventory; CGDS = Chinese version of the Geriatric Depression Scale (short form); Trial 3 refers to the last learning trial of the HKLLT. No significant group differences were found for any of the variables.
Performance on the modified Eriksen flanker test
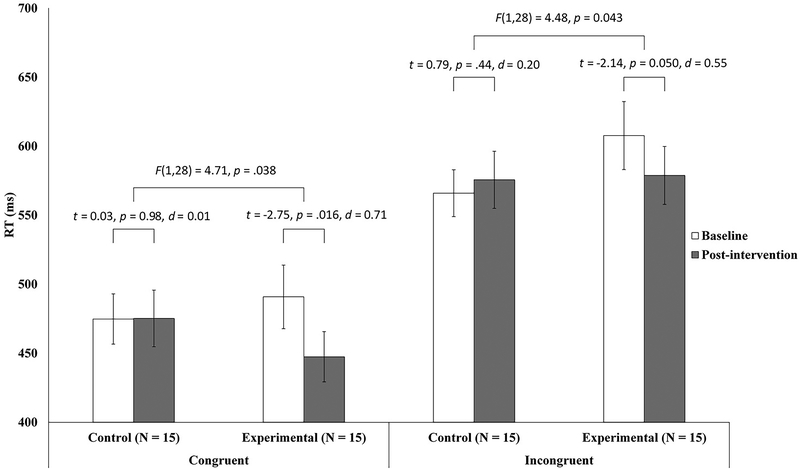
We first analyzed the congruent condition of the flanker test (Figure 2). No group differences in mean RT (p = .59) or accuracy (p = .72) were identified before PBM. Following this, a repeated measures ANOVA, with time (pre-PBM, post-PBM) as the within-subjects factor and group (experimental, control) as the between-subjects factor, was performed to compare the changes in mean RT between the experimental and control groups. Confirming our initial hypothesis, we found a significant group × time interaction (F[1,28] = 4.72, p = .038). Paired-samples t-tests showed that only the experimental group exhibited a significant decrease in the mean RT (t = −2.75, p = .016, d = 0.71). No significant change in the mean RT was identified in the control group (p = .98). Thus, only those participants who received real PBM responded faster on the congruent condition of the flanker test. In addition, probably due to a ceiling effect, an independent sample t-test showed no significant group difference in the pre- to post-PBM changes in accuracy (p > .50).
Figure 2.
Reaction times of the experimental and control groups for both the congruent and the incongruent conditions of the flanker test. Paired-samples t-tests were performed to detect any pre-to-post reaction time differences after real or sham photobiomodulation (PBM) in the experimental and control group, respectively. Repeated measures analyses of variance were performed to investigate the group (experimental, control) × time (pre-PBM, post-PBM) interaction. The error bars represent one standard error.
The incongruent condition yielded similar findings (Figure 2). No group differences in the mean RT (p = .18) or accuracy (p = .42) were identified before PBM. Compared with baseline, the mean RT after PBM was significantly shorter in the experimental group, but not in the control group. The repeated measures ANOVA showed a significant group × time interaction (F[1,28] = 4.48, p = .043). Paired-samples t-tests showed a marginally significant decrease in the RT after PBM in the experimental group (t = −2.1, p = .050, d = 0.55), but not in the control group (p = .44). Therefore, PBM led to a significant improvement in inhibitory control. In addition, no significant group differences were found for the pre- to post-PBM changes in task accuracy (p > .14).
Given that the experimental group showed faster RTs during both the congruent and incongruent conditions of the modified Eriksen flanker test, it was necessary to exclude the possibility that this effect was due to an improvement in response speed, rather than to an improvement in inhibitory ability. To do this, we analyzed the data from the simple RT task. The repeated measures ANOVA did not reveal a significant group × time interaction (p = .44). Additionally, paired-samples t-tests did not show significant pre- to post-PBM changes in the mean RT in the experimental (pre- vs. post-PBM: 378.39 ms vs. 399.74 ms, p = .059) or control groups (pre- vs. post-PBM: 374.89 ms vs. 385.27 ms, p = .29). Furthermore, independent-samples t-tests did not reveal significant differences in the number of hits before and after PBM in either group (ps > .16). Hence, the results of the flanker test were not due to a simple increase in response speed. In summary, older adults appeared have improved mental flexibility and inhibition ability after real, but not sham, PBM.
Performance on the category fluency test
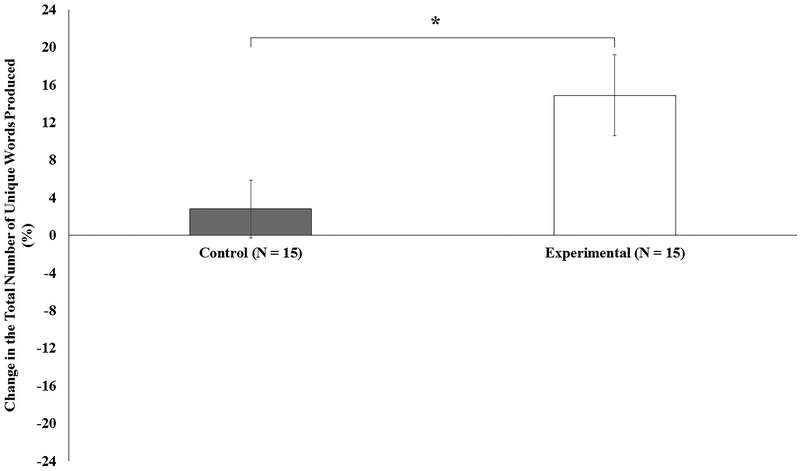
The category fluency test was employed to test mental flexibility. Before PBM, no significant group difference in the total number of unique words produced during the category fluency test was identified (p = .38). A repeated measures ANOVA, with time (pre-PBM, post-PBM) as the within-subjects factor and group (experimental, control) as the between-subjects factor, was performed. The results showed a significant group × time interaction (F[1,28] = 5.43, p = .027). Paired-samples t-tests revealed a significant increase in the total number of words produced after PBM in the experimental group (t = 3.25, p = .006, d = 0.84), but not in the control group (p = .89). The effect of PBM on the percentage change in the total number of unique words produced is shown in Figure 3. In summary, older adults generated significantly more examples within a category only after receiving PBM. These findings again suggest that PBM enhances flexible thinking and facilitates frontal function.
Figure 3.
Percentage change in the total number of unique words generated during the category fluency test after photobiomodulation (PBM). An independent-samples t-test showed a significantly larger percentage change in the experimental group than in the control group (t = 2.28, p = .030, d = 0.83). The error bars represent one standard error. *p < .05.
Discussion
The present study examined the effectiveness of a 7.5-min session of PBM to improve frontal function in older adults without dementia. Our results showed improved action selection and inhibition ability, as indicated by significant RT decreases on the flanker test, in older adults who received real vs. sham PBM. In addition, the experimental group, but not the control group, produced significantly more words on the category fluency test, indicating an improvement in mental flexibility after PBM. Thus, the present findings suggest that PBM may enhance frontal function in older adults. The present results are in line with Vargas et al.’s findings, in which improvements in sustained attention and visual working memory of elderly has been reported.50
Our study preliminarily examined the effects of PBM on the cognitive function of healthy older adults. This is different to the conventional approach, whereby an intervention is employed to combat the cognitive decline that is associated with aging. Traditionally, this intervention involves some form of behavioral training. Previous research studies have reported positive results using behavioral interventions, with such interventions improving various cognitive functions in older adults including memory, problem solving, visual searching, task switching, inhibition, and reasoning.65–68 However, these training interventions often require multiple sessions to produce a significant effect and may be less applicable to older adults and those with limited educational levels. The present findings show that PBM may be considered as a supplementary or alternative intervention for improving or maintaining cognitive function in older adults. The relative ease of use and harmless nature of LED devices suggests they could function as home-use devices for this application. As studied by Disner et al., there is a significant interaction between attention bias modification, a cognitive intervention, and PBM.69 With the use of PBM, the beneficial effect of attention bias modification may be enhanced. Therefore, there is initial evidence that PBM may augment with other forms of behavioral intervention, in enhancing the treatment effects to the elderly.
Among the existing neuromodulation techniques, PBM is a relatively recent example that is capable of enhancing EF function. Other neuromodulation techniques, including transcranial direct current stimulation and transcranial magnetic stimulation, which utilize electrical currents and magnetic fields to stimulate the human brain, respectively,70,71 have a relatively longer history. Transcranial low intensity focused ultrasound is another modality that uses physical energy to stimulate the brain.72 Although previous studies using these transcranial brain stimulation approaches have also found beneficial effects on cognitive function in older adults,73–75 the mechanisms underlying these various manifestations of physical energy are quite different from those of PBM. While transcranial direct current stimulation and transcranial magnetic stimulation aim to induce very small electric currents in the cortex that facilitate the depolarization and hyperpolarization of neurons, PBM utilizes low-level light to generate cellular biochemistry and increase blood flow. Thus, further studies comparing the effects of various neuromodulation techniques to determine the optimal clinical interventions for improving cognitive function are required.
Several limitations of this study should be noted. Although the participants exhibited significant immediate improvements in inhibitory ability and mental flexibility after a single session of PBM, further studies are needed to investigate whether repeating PBM at yet-to-be-determined time intervals can produce long-lasting effects on EF. Vargas et al. compared the effects of repeating PBM at weekly intervals in the frontal cognitive function of older adults.50 By applying a single 8 min of PBM intervention, Vargas et al. reported improvement in the number of lapse in the psychomotor vigilance task. Furthermore, researchers have found greater effects after applying five 8 min of PBM intervention for 5 weeks (i.e. one for each week).50 Therefore, it is plausible that a longer PBM treatment may produce a long-lasting and greater treatment effects on EF. Moreover, the present study evaluated only two components of EF, namely inhibition ability and mental flexibility. Because different aspects of EF may be mediated by different parts of the frontal lobe (i.e., task setting based in the left hemisphere, and monitoring based in the right hemisphere),76 additional studies that conduct more-comprehensive assessments are needed to evaluate the specificity of the effects of PBM on frontal functions in older adults. Blanco et al. has found that rule-based learning, but not information-integration learning, is improved after PBM.77 This result provides some initial evidences in the specificity of the effects of PBM and highlights the clinical use of PBM, since rule-based category learning is impaired among elderly.78 Finally, our sample size was relatively small and most of the participants were females. Further investigations with larger sample sizes and more-balanced gender distributions are required.
Conclusion
In conclusion, the present study demonstrated that a single session of PBM specifically improved certain aspects of frontal function in older adults without dementia. Because a decline in frontal function may predict a subsequent decline in general cognitive function and future functional deterioration,17–20 PBM may serve as a potential neuroprotective agent for maintaining or improving cognitive function in older adults. Such neuroprotective approaches are becoming essential, given that the average age of the overall population continues to increase in recent decades.
Key-points:
PBM may enhance frontal brain function in older adults after a single treatment.
Older adults who received sham PBM did not show executive function improvements.
Acknowledgements
The authors would like to thank Henry Lee and Rex Wang in the neuropsychology laboratory at the Department of Psychology of The Chinese University of Hong Kong for their efforts in data collection and management. Further appreciation is extended to all participants for their assistance in the recruitment process.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. MRH is supported by US NIH grants R01AI050875 and R21AI121700.
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Moscovitch M, Winocur G. The neuropsychology of memory and aging. The handbook of aging and cognition. 1992:315–372. [Google Scholar]
- 2.Raz N The aging brain in vivo: Differential changes and their modifiers In: Cabeza R, Nyberg L, Park D, ed. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. New York: Oxford University Press; 2004:19–57. [Google Scholar]
- 3.Haug H, Eggers R. Morphometry of the human cortex cerebri and corpus striatum during aging. Neurobiol Aging. 1991;12(4):336–8; discussion 352–5. doi: 10.1016/0197-4580(91)90013-A [DOI] [PubMed] [Google Scholar]
- 4.Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Exp Neurol 1975;47(3):392–403. doi: 10.1016/0014-4886(75)90072-2 [DOI] [PubMed] [Google Scholar]
- 5.Huttenlocher PR. Synaptic density in human frontal cortex-developmental changes and effects of aging. Brain Res 1979;163(2):195–205. [DOI] [PubMed] [Google Scholar]
- 6.Struble RG, Price DL Jr, Cork LC, Price DL. Senile plaques in cortex of aged normal monkeys. Brain Res 1985;361(1–2):267–275. doi: 10.1016/0006-8993(85)91298-3 [DOI] [PubMed] [Google Scholar]
- 7.Zelazo PD, Craik FI, Booth L. Executive function across the life span. Acta Psychol. 2004;115(2–3):167–183. doi: 10.1016/j.actpsy.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 8.Kramer AF, Humphrey DG, Larish JF, Logan GD. Aging and inhibition: Beyond a unitary view of inhibitory processing in attention. Psychol Aging. 1994;9(4):491–512. [PubMed] [Google Scholar]
- 9.Brickman AM, Paul RH, Cohen RA, et al. Category and letter verbal fluency across the adult lifespan: Relationship to EEG theta power. Archives of Clinical Neuropsychology. 2005;20(5):561–573. doi: 10.1016/j.acn.2004.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomer R, Levin BE. Differential effects of aging on two verbal fluency tasks. Percept Mot Skills. 1993;76(2):465–466. doi: 10.2466/pms.1993.76.2.465 [DOI] [PubMed] [Google Scholar]
- 11.Troyer AK. Normative data for clustering and switching on verbal fluency tasks. Journal of clinical and experimental neuropsychology. 2000;22(3):370–378. doi: 10.1076/1380–3395(200006)22:3;1-V;FT370 [DOI] [PubMed] [Google Scholar]
- 12.Naveh-Benjamin M, Craik FI, Guez J, Kreuger S. Divided attention in younger and older adults: Effects of strategy and relatedness on memory performance and secondary task costs. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31(3):520–537. doi: 10.1037/0278-7393.31.3.520 [DOI] [PubMed] [Google Scholar]
- 13.Dobbs AR, Rule BG. Adult age differences in working memory. Psychol Aging. 1989;4(4):500–503. [DOI] [PubMed] [Google Scholar]
- 14.Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55(4):352. doi: 10.1037/h0043688 [DOI] [PubMed] [Google Scholar]
- 15.Hillman CH, Motl RW, Pontifex MB, et al. Physical activity and cognitive function in a cross-section of younger and older community-dwelling individuals. Health psychology. 2006;25(6):678–687. doi: 10.1037/0278-6133.25.6.678 [DOI] [PubMed] [Google Scholar]
- 16.Zhu DC, Zacks RT, Slade JM. Brain activation during interference resolution in young and older adults: An fMRI study. Neuroimage. 2010;50(2):810–817. doi: 10.1016/j.neuroimage.2009.12.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark LR, Schiehser DM, Weissberger GH, Salmon DP, Delis DC, Bondi MW. Specific measures of executive function predict cognitive decline in older adults. Journal of the International Neuropsychological Society. 2012;18(1):118–127. doi: 10.1017/S1355617711001524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearney FC, Harwood RH, Gladman JR, Lincoln N, Masud T. The relationship between executive function and falls and gait abnormalities in older adults: A systematic review. Dement Geriatr Cogn Disord. 2013;36(1–2):20–35. doi: 10.1159/000350031 [DOI] [PubMed] [Google Scholar]
- 19.Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: New findings from a five-year prospective study link fall risk to cognition. PloS one. 2012;7(6):e40297. doi: 10.1371/journal.pone.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell‐McGinty S, Podell K, Franzen M, Baird AD, Williams MJ. Standard measures of executive function in predicting instrumental activities of daily living in older adults. Int J Geriatr Psychiatry. 2002;17(9):828–834. doi: 10.1002/gps.646 [DOI] [PubMed] [Google Scholar]
- 21.Farfara D, Tuby H, Trudler D, et al. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer’s disease. Journal of Molecular Neuroscience. 2015;55(2):430–436. doi: 10.1007/s12031-014-0354-z [DOI] [PubMed] [Google Scholar]
- 22.Oron A, Oron U, Streeter J, et al. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2007;24(4):651–656. doi: 10.1089/neu.2006.0198 [DOI] [PubMed] [Google Scholar]
- 23.Xuan W, Vatansever F, Huang L, Hamblin MR. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J Biomed Opt. 2014;19(10):108003. doi: 10.1117/1.JBO.19.10.108003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berman MH, Halper JP, Nichols TW, Jarrett H, Lundy A, Huang JH. Photobiomodulation with near infrared light helmet in a pilot, placebo controlled clinical trial in dementia patients testing memory and cognition. Journal of neurology and neuroscience. 2017;8(1):176. doi: 10.21767/2171-6625.1000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naeser MA, Zafonte R, Krengel MH, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: Open-protocol study. J Neurotrauma. 2014;31(11):1008–1017. doi: 10.1089/neu.2013.3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saltmarche AE, Naeser MA, Ho KF, Hamblin MR, Lim L. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: Case series report. Photomedicine and laser surgery. 2017;35(8):432–441. doi: 10.1089/pho.2016.4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE Journal of selected topics in quantum electronics. 2016;22(3):348–364. doi: 10.1109/JSTQE.2016.2561201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. In Seminars in cutaneous medicine and surgery. 2013;32(1):41–52. Frontline Medical Communications. [PMC free article] [PubMed] [Google Scholar]
- 29.Hamblin MR. Shining light on the head: Photobiomodulation for brain disorders. BBA clinical. 2016;6:113–124. doi: 10.1016/j.bbacli.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karu TI. Mechanisms of low-power laser light action on cellular level. Paper presented at the Effects of Low-Power Light on Biological Systems V. 2000;4159:1–18. doi: 10.1117/12.405918 [DOI] [Google Scholar]
- 31.Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J Biol Chem 2005;280(6):4761–4771. doi: 10.1074/jbc.M409650200 [DOI] [PubMed] [Google Scholar]
- 32.Lane N Cell biology: power games. Nature. 2006;443:901–993. doi: 10.1038/443901a [DOI] [PubMed] [Google Scholar]
- 33.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: Phosphorylation and translocation during priming and activation. J Leukoc Biol 2005;78(5):1025–1042. doi: 10.1189/jlb.0804442 [DOI] [PubMed] [Google Scholar]
- 34.Tafur J, Mills PJ. Low-intensity light therapy: Exploring the role of redox mechanisms. Photomedicine and laser surgery. 2008;26(4):323–328. doi: 10.1089/pho.2007.2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salgado AS, Zângaro RA, Parreira RB, Kerppers II. The effects of transcranial LED therapy (TCLT) on cerebral blood flow in the elderly women. Lasers in medical science. 2015;30(1):339–346. doi: 10.1007/s10103-014-1669-2 [DOI] [PubMed] [Google Scholar]
- 36.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5′-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res 2010;1306:100–105. doi: 10.1016/j.brainres.2009.10.022 [DOI] [PubMed] [Google Scholar]
- 37.Rojas JC, Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol 2013;86(4):447–457. doi: 10.1016/j.bcp.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 38.Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near‐infrared laser irradiation. Lasers Surg Med 2010;42(6):566–576. doi: 10.1002/lsm.20938 [DOI] [PubMed] [Google Scholar]
- 39.Rojas JC, Lee J, John JM, Gonzalez-Lima F. Neuroprotective effects of near-infrared light in an in vivo model of mitochondrial optic neuropathy. J Neurosci 2008;28(50):13511–13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michalikova S, Ennaceur A, van Rensburg R, Chazot P. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: Effects of low infrared light. Neurobiol Learn Mem 2008;89(4):480–488. doi: 10.1016/j.nlm.2007.07.014 [DOI] [PubMed] [Google Scholar]
- 41.Rojas JC, Gonzalez-Lima F. Mitochondrial optic neuropathy: In vivo model of neurodegeneration and neuroprotective strategies. Eye and brain. 2010;2:21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojas JC, Gonzalez-Lima F. Low-level light therapy of the eye and brain. Eye and brain. 2011;3:49–67. doi: 10.2147/EB.S21391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojas JC, Bruchey AK, Gonzalez-Lima F. Low-level light therapy improves cortical metabolic capacity and memory retention. J Alzheimer’s Dis 2012;32(3):741–752. doi: 10.3233/JAD-2012-120817 [DOI] [PubMed] [Google Scholar]
- 44.Barrett D, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13–23. doi: 10.1016/j.neuroscience.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 45.Cassano P, Cusin C, Mischoulon D, et al. Near-infrared transcranial radiation for major depressive disorder: Proof of concept study. Psychiatry journal. 2015. doi: 10.1155/2015/352979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiffer F, Johnston AL, Ravichandran C, et al. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: A pilot study of 10 patients with major depression and anxiety. Behavioral and Brain Functions. 2009;5(1):46. doi: 10.1186/1744-9081-5-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg 1971;122(4):532–535. doi: 10.1016/0002-9610(71)90482-X [DOI] [PubMed] [Google Scholar]
- 48.Mester E, Szende B, Tota J. Effect of laser on hair growth of mice. Kiserl Orvostud 1967;19:628–631. [Google Scholar]
- 49.Anders JJ, Lanzafame RJ, Arany PR. Low-level light/laser therapy versus photobiomodulation therapy. Photomedicine and Laser Surgery. 2015;33(4):183–184. doi: 10.1089/pho.2015.9848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vargas E, Barrett DW, Saucedo CL, et al. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers in medical science. 2017;32(5):1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16(1):143–149. doi: 10.3758/BF03203267 [DOI] [Google Scholar]
- 52.Hazeltine E, Poldrack R, Gabrieli JD. Neural activation during response competition. J Cogn Neurosci 2000;12(Supplement 2):118–129. doi: 10.1162/089892900563984 [DOI] [PubMed] [Google Scholar]
- 53.Salthouse TA. Selective review of cognitive aging. Journal of the International neuropsychological Society. 2010;16(5):754–760. doi: 10.1017/S1355617710000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner S, Sebastian A, Lieb K, Tüscher O, Tadić A. A coordinate-based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC neuroscience. 2014;15(1):19. doi: 10.1186/1471-2202-15-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of clinical neuropsychology. 1999;14(2):167–177. doi: 10.1016/S0887-6177(97)00095-4 [DOI] [PubMed] [Google Scholar]
- 56.Chan AS, Choi M, Salmon DP. The effects of age, education, and gender on the mattis dementia rating scale performance of elderly chinese and american individuals. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2001;56(6):P356–P363. doi: 10.1093/geronb/56.6.P356 [DOI] [PubMed] [Google Scholar]
- 57.Lee HB, Chiu HF, Kowk WY, Leung CM. Chinese elderly and the GDS short form: A preliminary study. Clinical Gerontologist: The Journal of Aging and Mental Health. 1993;14(2):37–42. [Google Scholar]
- 58.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 1988;56(6):893–897. doi: 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- 59.Chan A, Kwok I. Hong kong list learning test. Hong Kong: Chinese University of Hong Kong. 2006. [Google Scholar]
- 60.Jasper H The ten-twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology. 1958;10:367–380. [PubMed] [Google Scholar]
- 61.Chan AS, Poon MW. Performance of 7-to 95-year-old individuals in a chinese version of the category fluency test. Journal of the International Neuropsychological Society. 1999;5(6):525–533. [DOI] [PubMed] [Google Scholar]
- 62.Beste C, Konrad C, Uhlmann C, Arolt V, Zwanzger P, Domschke K. Neuropeptide S receptor (NPSR1) gene variation modulates response inhibition and error monitoring. Neuroimage. 2013;71:1–9. doi: 10.1016/j.neuroimage.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 63.Bombeke K, Langford ZD, Notebaert W, Boehler CN. The role of temporal predictability for early attentional adjustments after conflict. PloS one. 2017;12(4):e0175694. doi: 10.1371/journal.pone.0175694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bluschke A, Chmielewski WX, Roessner V, Beste C. Intact context-dependent modulation of conflict monitoring in childhood ADHD. Journal of attention disorders. 2016:1087054716643388. doi: 10.1177/1087054716643388 [DOI] [PubMed] [Google Scholar]
- 65.Anguera JA, Boccanfuso J, Rintoul JL, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Basak C, Boot WR, Voss MW, Kramer AF. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychol Aging. 2008;23(4):765–777. doi: 10.1037/a0013494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rapp S, Brenes G, Marsh A. Memory enhancement training for older adults with mild cognitive impairment: A preliminary study. Aging and Mental Health. 2002;6(1):5–11. doi: 10.1080/13607860120101077 [DOI] [PubMed] [Google Scholar]
- 69.Disner SG, Beevers CG, Gonzalez-Lima F. Transcranial laser stimulation as neuroenhancement for attention bias modification in adults with elevated depression symptoms. Brain stimulation. 2016;9(5):780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 2006;117(4):845–850. doi: 10.1016/j.clinph.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 71.Hallett M Transcranial magnetic stimulation and the human brain. Nature. 2000;406(6792):147–150. doi: 10.1038/35018000 [DOI] [PubMed] [Google Scholar]
- 72.Chen S, Su W, Wu C, Lan T, Yang F. Transcranial ultrasound stimulation improves long-term functional outcomes and protects against brain damage in traumatic brain injury. Mol Neurobiol 2018:1–11. doi: 10.1007/s12035-018-0897-z [DOI] [PubMed] [Google Scholar]
- 73.Cotelli M, Calabria M, Manenti R, et al. Improved language performance in alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry. 2011;82(7):794–797. doi: 10.1136/jnnp.2009.197848 [DOI] [PubMed] [Google Scholar]
- 74.Manenti R, Cotelli M, Miniussi C. Successful physiological aging and episodic memory: A brain stimulation study. Behav Brain Res 2011;216(1):153–158. doi: 10.1016/j.bbr.2010.07.027 [DOI] [PubMed] [Google Scholar]
- 75.Solé-Padullés C, Bartrés-Faz D, Junqué C, et al. Repetitive transcranial magnetic stimulation effects on brain function and cognition among elders with memory dysfunction. A randomized sham-controlled study. Cerebral cortex. 2005;16(10):1487–1493. doi: 10.1093/cercor/bhj083 [DOI] [PubMed] [Google Scholar]
- 76.Stuss DT. Functions of the frontal lobes: Relation to executive functions. Journal of the international neuropsychological Society. 2011;17(5):759–765. doi: 10.1017/S1355617711000695 [DOI] [PubMed] [Google Scholar]
- 77.Blanco NJ, Saucedo CL, Gonzalez-Lima F. Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol Learn Mem 2017;139:69–75. [DOI] [PubMed] [Google Scholar]
- 78.Maddox WT, Pacheco J, Reeves M, Zhu B, Schnyer DM. Rule-based and information-integration category learning in normal aging. Neuropsychologia. 2010;48(10):2998–3008. doi: 10.1016/j.neuropsychologia.2010.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]