Abstract
Ciliary neurotrophic factor (CNTF) is produced by astrocytes and promotes neurogenesis and neuroprotection. Little is known about the role of CNTF in affective behavior. We investigated whether CNTF affects depressive- and anxiety-like behavior in adult mice as tested in the forced swim, sucrose preference and elevated-T maze tests. Female wild type CNTF+/+ mice more readily developed behavioral despair with increased immobility time and decreased latency to immobility in the forced swim test than male CNTF+/+ littermates. The lack of CNTF in CNTF−/− mice had an opposite effect on depressive-like behavior in female mice (reduced immobility time and increased sucrose preference) vs. male mice (increased immobility time). Female wildtype mice expressed more CNTF in the amygdala than male mice. Ovariectomy increased CNTF expression, as well as immobility time, which was significantly reduced in CNTF−/− mice, suggesting that CNTF mediates overiectomy induced immobility time, possibly in the amygdala. Progesterone but not 17-β estradiol inhibited CNTF expression in cultured C6 astroglioma cells. Progesterone treatment also reduced CNTF expression in the amygdala and decreased immobility time in female CNTF+/+ but not in CNTF−/− mice. Castration did not alter CNTF expression in males nor their behavior. Lastly, there were no effects of CNTF on the elevated T-maze, a behavioral test of anxiety, suggesting that a different mechanism may underlie anxiety-like behavior. This study reveals a novel CNTF-mediated mechanism in stress-induced depressive-like behavior and points to opportunities for sex-specific treatments for depression, e.g. progesterone in females and CNTF-stimulating drugs in males.
Keywords: depression, anxiety, progesterone, amygdala, ovariectomy, castration
1. INTRODUCTION
Affective disorders, such as depression and anxiety, are highly prevalent in stress-related disorders and can be life-threatening (Ressler and Mayberg, 2007). Depression and anxiety are often linked together, with 85% of patients with depression have significant anxiety, and 90% of patients with anxiety disorder have depression (Tiller, 2013). Sex differences in the prevalence, incidence and treatment of affective disorders have been well established (Kessler et al., 2003). Women are approximately twice as likely as men to develop depression and anxiety (Essau et al., 2010) and have improved treatment responses than men to selective serotonin reuptake inhibitors (Berlanga and Flores-Ramos, 2006). Rodent models have been widely used to investigate the neurobiology of affective disorders and discover new treatments. However, male animals have mostly been used as subjects in these studies, and there has not been a focus on sex differences in these models (Beery and Zucker, 2011). A few studies have included both male and female rodents, and revealed sex differences in affective behavior (Donner and Lowry, 2013; Kokras et al., 2015). In the present study, the forced swim test and sucrose preference test were used to assess behavioral despair (Yin et al., 2016) and behavioral anhedonia (Cryan et al., 2002), and elevated T-maze was used to evaluate conflict anxiety (Donner and Lowry, 2013). With these tests, several studies found that females displayed higher levels of depressive-like behavior and lower levels of anxiety than male controls, whereas others reported opposite results or no sex differences (Donner and Lowry, 2013; Kokras et al., 2015).
Ciliary neurotrophic factor (CNTF) belongs to the interleukin-6 (IL-6) cytokine family and is a neural cytokine which is almost exclusively expressed in the nervous system (Stockli et al., 1989). In the brain, CNTF is produced by astrocytes and increased following brain injury (Kang et al., 2012). The CNTF-specific receptor, CNTFRα, is expressed by astrocytes and neurons (Lee et al., 1997). CNTF promotes neurite outgrowth in the hypothalamus and hippocampus that are involved in stress responses (Askvig et al., 2012; Guthrie et al., 1997). CNTF also promotes adult neurogenesis (Kang et al., 2013a), increases neuronal survival after injury (Hagg et al., 1992; Kang et al., 2012) and enhances cognitive and memory function in rodent models (Garcia et al., 2010). Knockout of CNTF reduces the number of hippocampal GABAergic interneurons, as well as 5-HT levels and 5-HT1A receptor expression in female mice (Peruga et al., 2012), suggesting that CNTF may influence affective behavior in females through regulation of neurotransmission.
Approximately 2–3% of human population is CNTF deficient due to a frameshift mutation in exon 2, and the correlation of CNTF deficiency and psychiatric disorders remains ambiguous. Initial studies found that the frequency of mutations in the CNTF gene was higher in patients with psychiatric diseases than in healthy controls (Thome et al., 1996). Later studies failed to show the association of psychiatric phenotypes, especially schizophrenia, with the CNTF mutation (Nishiyama et al., 2006; Nothen et al., 1996), however, the gender differences were not systematically investigated in such studies. There has only been one study analyzing CNTF-mediated affective behavior in rodent models and using only female mice revealed that CNTF−/− mice displayed increased anxiety levels and depressive-like behaviors (Peruga et al., 2012).
In the present study, we investigated the role of CNTF on depressive- and anxiety-like behavior using both male and female CNTF+/+ and CNTF−/− mice, and how ovarian hormones regulate these behaviors through CNTF in the brain. Identifying such mechanisms could lead to sex-specific prevention, diagnosis and treatment of depression.
2. METHODS AND MATERIALS
2.1. Animals
A total of 248 mice were used. Heterozygous CNTF knockout mice were bred to produce F1 sex-matched littermates for experiments. The CNTF mice (original breeders provided by Regeneron Pharmaceuticals) are on a C57BL/6 background and we have backcrossed them seven times into the JAX C57BL/6 line. Genotyping was performed according to a protocol provided by the Regeneron. Both male and female mice were 3–4 months old at the time of behavioral testing. All mice were housed with food and water available ad libitum, and maintained on a 12 h light:12 h dark cycle. All animal procedures were approved by our University Committee on Animal Care complied with the NIH Guide on Care and Use of Animals.
2.2. Ovariectomy (OVX), Castration and in vivo progesterone treatment
Both male and female mice were anesthetized with an intraperitoneal (ip) injection of Avertin (0.4 mg 2,2,2-tribronoethanol in 0.02 ml of 2% 2-methyl-2-butanolin saline per gram body weight). For OVX, two 0.5 cm incisions were made bilaterally on the back. A sterile silk ligature was placed around the oviduct, and the ovary and the oviduct were removed through the incisions. For castration, an approximately 0.5–1 cm midline incision was made in the scrotum. A ligation was performed around the vas deferens and spermatic blood vessels and testes were removed. The skin and muscle were sutured. All experiments started 14 days after surgery. The sham mice received the same operation without ligation and removal of ovary and testis. Daily weight measurement over the first week was performed to monitor the health condition. Two weeks after OVX, both CNTF+/+ and CNTF−/− mice were injected subcutaneously with progesterone (10 mg/kg, Cat# 57-83-0, Tocris) for 4 days and the forced swim tests or open field tests were performed 2 h following last progesterone injection.
2.3. Behavioral tests
2.3.1. Forced swim test
The Forced swim test (i.e. the Porsolt swim test) was used to measure behavioral despair, the behavioral response to an acute stressor. The increased immobility time and lower immobility latency are considered as measures of behavioral despair. Mice were placed into a round black tub 55 cm in diameter and 42 cm deep with 25°C water. Each mouse was tested in one 6-min trial, similar to past work (Costa et al., 2013). All behavior was recorded by a digital camera mounted above the apparatus with only the last 4 min used for data analysis in which the number of bouts of immobility and the duration of immobility in seconds were scored live using AnyMaze behavioral scanning software (Stoelting Co., Wood Dale, IL). Immobility was defined as the cessation of all movements except those necessary to stay afloat.
2.3.2. Sucrose preference test
The Sucrose preference test was used to measure behavioral anhedonia. Mice were singly caged and first acclimated to two bottles of drinking water for 4 days. Then mice were presented with one bottle of drinking water and one bottle of 1% sucrose for 4 days. The water bottle was switched (left and right) twice (morning and afternoon) on each day. The water and sucrose bottles were weighed prior to the experiment and during each position switch and at the end of test. The differences during the 4 day trial with drinking water and sucrose were used to calculate the volume intake from each bottle. The liquid intake was expressed as the average amount of water and sucrose intake per day. The sucrose preference was expressed as a percentage of sucrose intake relative to the total liquid intake.
2.3.3. Elevated T-maze test
The Elevated T-maze test was used to evaluate both conflict anxiety and panic-like escape response (Donner and Lowry, 2013). The apparatus stands 50 cm above the floor with two open arms and one closed arm and two open arms of equal dimensions (50 × 12 cm). The closed arm was enclosed by Plexiglas lateral walls (40 cm high) perpendicular to the two open arms. A Plexiglas rim (1 cm high) was designed to reduce the possibility of falls in the open arms. Each mouse was given four trials. On the first three trials, the animal was placed into the closed arm and the latency to visit the open arm was recorded. The first trial was a habituation trial, and trials 2 and 3 were scored as acquisition trials. When the animal exited the closed arm into the open arm with all four paws, the latency was recorded and the trial ended. On the fourth trial, the animal was placed on either end of the open arm (the side chosen was balanced across subjects), and the latency to enter the closed arm was recorded. If the animal did not leave the closed arm or exit the open arm within 5 min, the trial was ended. The apparatus was cleaned with an 8% alcohol solution in between trials.
2.3.4. Open field test
The Open field test was used to measure locomotor activity, and serve as a control for possible motor deficits that may confound performance on the forced swim stress or elevated T-maze. All open field testing was performed in square white Plexiglas box (72 cm on all sides) in a 10 min session. The open field test was administered one day after forced swim or elevated T-maze and the distance traveled in meter was recorded.
2.4. RT-qPCR
Mice were euthanized by decapitation under Avertin anesthesia. The brain was dissected and flash frozen in wet dry ice with isopentane and stored at −80 °C. The frontal cortex was collected before sectioning the brain using a cryostat. The hypothalamus including paraventricular nucleus, hippocampus and amygdala were punched out from a 700 μm cryostat coronal brain section as shown in figure 3A. All samples were stored at −80 °C for mRNA and protein analysis. RT-qPCR was performed as described previously (Kang et al., 2013a). Briefly, total RNA from frontal cortex, hypothalamus, amygdala and hippocampus was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA). The 100 ng RNA/μl reaction was reverse transcribed using random primers and MMLV-reverse transcriptase (Promega). The qPCR was performed using Taqman Gene Expression Master Mix Kit (Applied Biosystems) and the following primers (Applied Biosystems), mouse GAPDH (4352932E), CNTF (Mm00446373_m1), IL-6 (Mm00446190-m1) and Leukemia inhibitory factor (LIF, Mm00434762-g1) with a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). Reactions were performed in triplicate. The number of cycles for each gene was subtracted from GAPDH within the same sample. We used the 2−ΔΔCt method to analyze the data were analyzed, expressing the values as fold change compared to male mice or sham control mice.
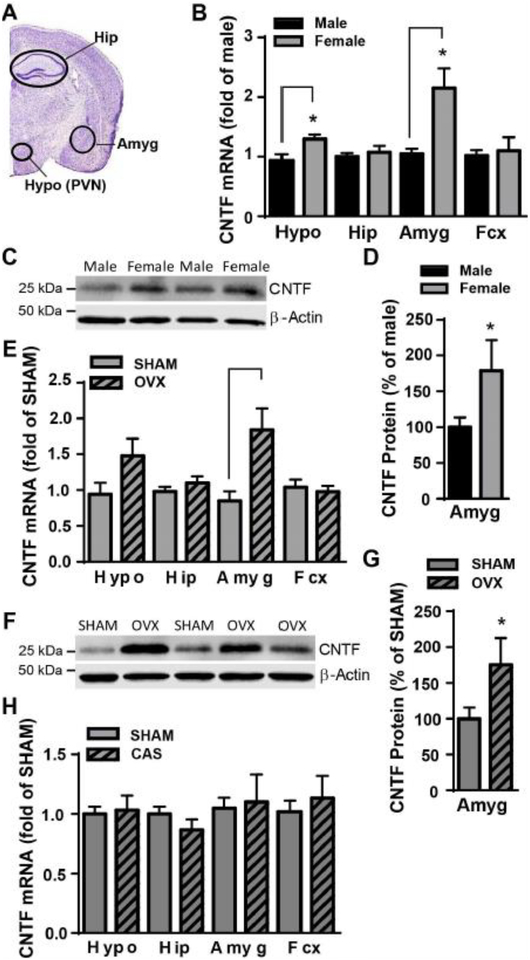
Figure 3. Female mice had high levels of CNTF in the hypothalamus and amygdala, and OVX increased CNTF in the amygdala.
A) A coronal section of the mouse brain stained with cresyl violet (Allen Brain Atlas) showing the dissected brain regions: hypothalamus (Hypo, including paraventricular nucleus, PVN), amygdala (Amyg), hippocampus (Hip). The frontal cortex (Fcx) is not shown. Levels of CNTF mRNA and protein were measured by RT-qPCR and western blot. Naïve female mice had more CNTF mRNA B) in the hypothalamus and amygdala than males. Female mice also had more CNTF protein in the amygdala C, D) than males. Two weeks after OVX females had more CNTF mRNA E) and protein F, G) in the amygdala. H) Castration (CAS) did not alter CNTF mRNA in the male brain. N=4–5 mice/group. * p<0.05 (Student t test).
2.5. Western blotting
The protein portion of hypothalamus, hippocampus, amygdala and frontal cortex from RNA isolation was suspended by sonication in 50, 100, 100 and 400 μl RIPA buffer supplemented with protease and phosphatase inhibitors, respectively. Following centrifugation, the protein concentration in the supernatant was measured using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, #23228). A total of 15 μg protein were resolved by electrophoresis and transferred to a PVDF membrane, which was incubated with blocking buffer containing 0.1% Tween-20 and 5% non-fat milk. The membranes were then incubated overnight at 4 °C with anti-CNTF antibody (1:500, MAB338, RRID:AB_2083064) and anti-β actin (1:2000, #4970,RRID:AB_2223172). Following washing with buffer, the membranes were incubated with HRP-labeled secondary antibody (1:1000, Cell signaling) which was detected with an ECL through Odyssey® Fc Imaging System (LI-COR Biotechnology).
2.6. In vitro astroglioma C6 cells
C6 astroglioma cells were used due to their ability to produce CNTF and maintained as previously described (Keasey et al., 2013). C6 cells were plated at 320,000/ml and maintained for 24 h. Progesterone (Cat # 57-83-0, Tocris) and 17β estradiol (Cat #50-28-2, Tocris) were dissolved in 0.2–2% DMSO (Vehicle) and added directly to cell culture medium 24 h after plating. Cells were maintained for a further 4 h to measure CNTF, IL-6 and LIF mRNA.
2.7. Statistical analyses.
Statistical significance was determined by p < 0.05 using graphpad Prism (version 7). Student t test was performed when two groups were compared. A one-way ANOVA was used when there was three or more groups with testing for one factor. A two-way ANOVA was used when there were two factors to be tested, such as genotype and sex. The Newman-keuls tests were used for post hoc multiple comparisons.
3. RESULTS
3.1. CNTF had a sex-specific role in the behavioral despair in adult mouse
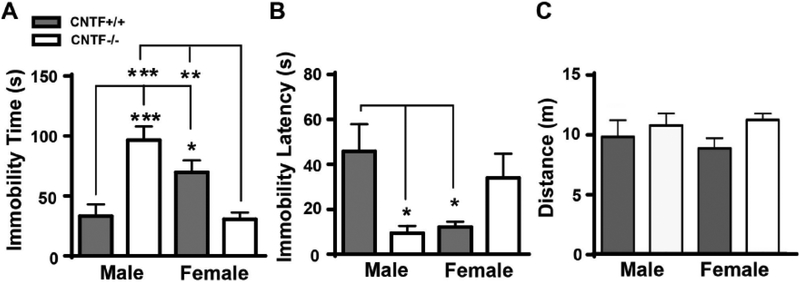
A two-way ANOVA revealed a significant interaction between genotype and sex on both latency to immobility (F1, 42 = 10.3, p = 0.003) and overall immobility time (F1, 42 = 26.54, p < 0.0001). Post hoc comparisons revealed that male CNTF+/+ mice displayed significantly less immobility time and higher latency to immobility compared to female CNTF+/+ mice (Fig. 1A–B, gray bars), suggesting that male mice of this strain are overall more resistant to behavioral despair than females. CNTF deficiency had an opposite effect in male as compared to female mice. Female CNTF−/− mice demonstrated significantly shorter immobility time compared to female CNTF+/+ littermates, whereas male CNTF−/− mice demonstrated an increased immobility time and a decreased latency to immobility compared to male CNTF+/+ littermates (Fig. 1A–B, compare gray to white bars), This suggests that a lack of CNTF yields a depressive-like phenotype in males, but produces an anti-depressive phenotype in female mice in a forced swim test. These data suggest that CNTF may have anti-depressive effects in males but pro-depressive effects in female mice in this behavioral test. These effects were not due to motor deficits since there were no group differences in the total locomotor activity calculated as distance in an open field test (Fig. 1C, all main effects and interactions, p > 0.10).
Figure 1. CNTF has a sex-specific role in learned helplessness behavior in mice.
Both male and female CNTF+/+ and CNTF−/− were subjected to a forced swim test. Male CNTF+/+ mice displayed a less immobility time A) and longer immobility latency B) than female CNTF+/+ littermates, indicating a sex difference and female mice have more prone to develop learned helplessness. CNTF deficiency led to an opposite effect on learned helplessness. Female CNTF−/− mice displayed a significantly reduced immobility time A) than female CNTF+/+ littermates, whereas male CNTF−/− had a longer immobility time A) and less immobility latency B) than male CNTF+/+ littermates. C) There was no motor deficit among the groups tested by locomotor activity in the open field test. N=10 mice/group. * p<0.05, ** p<0.01, *** p<0.001 (Two-way ANOVA followed by Newman-keuls multiple comparisons).
3.2. CNTF deficiency increased sucrose preference in female mice
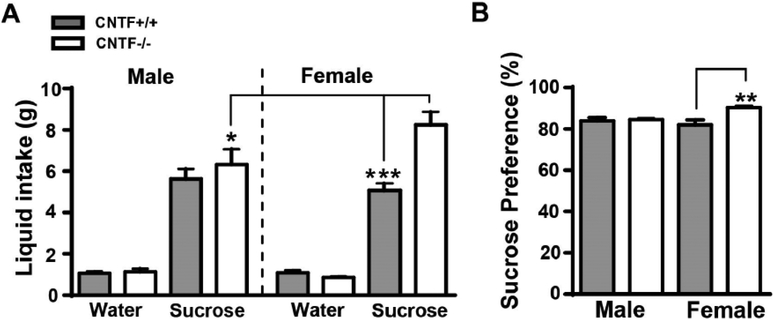
All mice showed a significantly higher consumption of sucrose (F1, 46 = 350.7, p < 0.0001) compared to water, and a significant main effect of sex and genotype (F3, 46 = 5.596, p = 0.002). Female CNTF−/− mice consumed substantially more sucrose (Fig. 2A) than female CNTF+/+ littermates or male CNTF−/− mice. We also calculated sucrose preference as percentage of sucrose intake to the total intake including water and sucrose. There was a significant main effect of genotype (F1, 23 = 7.041, p = 0.014) and the interaction of sex with genotype (F1, 23 = 5.233, p = 0.032) on sucrose preference. Female CNTF−/− mice had a significantly higher preference for sucrose (Fig. 2B) than female CNTF+/+ littermates, suggesting that female CNTF−/− mice have an increased affinity for a natural reward as tested by sucrose preference test.
Figure 2. Female CNTF−/− mice had reduced behavioral anhedonia.
Both male and female CNTF+/+ and CNTF−/− mice were tested for sucrose preference to evaluate behavioral anhedonia. Female CNTF−/− mice consumed a substantially more sucrose A), and had a significantly higher sucrose preference B) than female CNTF+/+ littermate. No differences were detected in water consumption. N= 8, 5, 7 and 7 mice in male and female CNTF+/+ vs. CNTF−/− mice. ** p<0.01, *** p<0.001 (Two-way ANOVA followed by Newman-keuls multiple comparisons).
3.3. Female mice had higher levels of CNTF in the amygdala than male mice and OVX increased CNTF expression
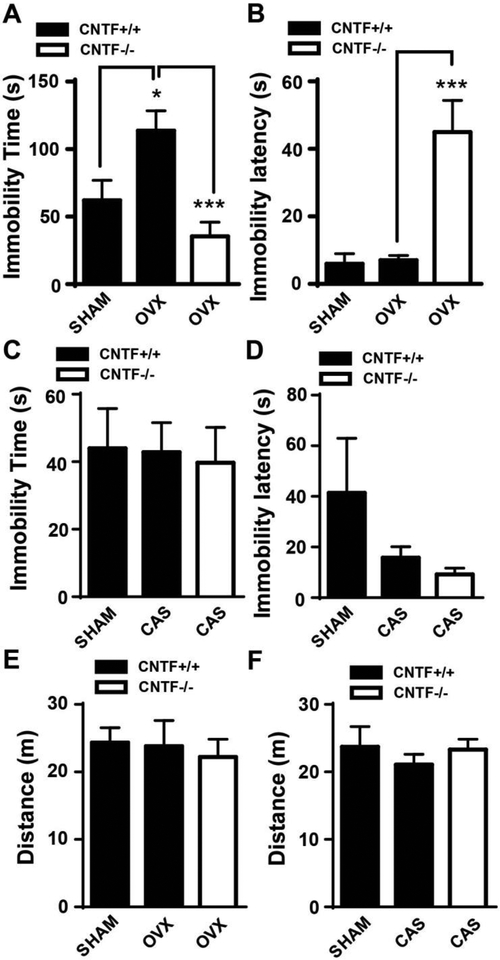
CNTF mRNA levels in different brain areas involved in mood regulation were measured using RT-qPCR in both male and female CNTF+/+ mice. These brain areas included the hypothalamus including paraventricular nucleus, a key element of the HPA axis, hippocampus, amygdala (Fig. 3A) and frontal cortex. Female mice had significantly higher levels of CNTF mRNA than males in the hypothalamus (t(7) = 2.722, p =0.030, Fig. 3B) and amygdala (t(7) = 3.220, p =0.018) but not in the hippocampus (t(7) = 0.620, p =0.555) or frontal cortex (t(7) = 0.355, p =0.733). The increase was more prominent in amygdala with an over 2-fold increase as compared to male controls. CNTF protein levels in the amygdala was significantly greater in female than male mice (t(7) = 1.931, p =0.047, Fig.3C, D). The mRNA levels of IL-6 and leukemia inhibitory factor that belong to the same cytokine family with CNT were also measured in these samples and no difference was found between male and female (data not shown). We further investigated whether ovarian hormones contributed to high levels of CNTF in female brain by measuring CNTF mRNA and protein in different brain areas 2 weeks after OVX. Surprisingly, OVX substantially increased CNTF mRNA (t(7) = 2.746, p =0.029, Fig. 3E) and protein (t(11) = 1.966, p =0.038, Fig. 3F,G) expression in the amygdala but not in the hypothalamus, hippocampus and frontal cortex (t(7) = 1.895 (Hyp), 1.029 (Hip) and 0.454 (Fcx), p > 0.1), suggesting ovarian hormones inhibit CNTF expression in the amygdala of female mice. Castration in male CNTF+/+ mice did not alter brain CNTF in male mice (Fig. 3H, t(6) = 0.274 (Hyp), 1.257 (Hip), 0.229 (Amyg) and 0.621 (Fcx), p > 0.10), suggesting a non-hormonal mechanism for its regulation.
3.4. Progesterone selectively reduced CNTF expression in cultured C6 astroglioma cells
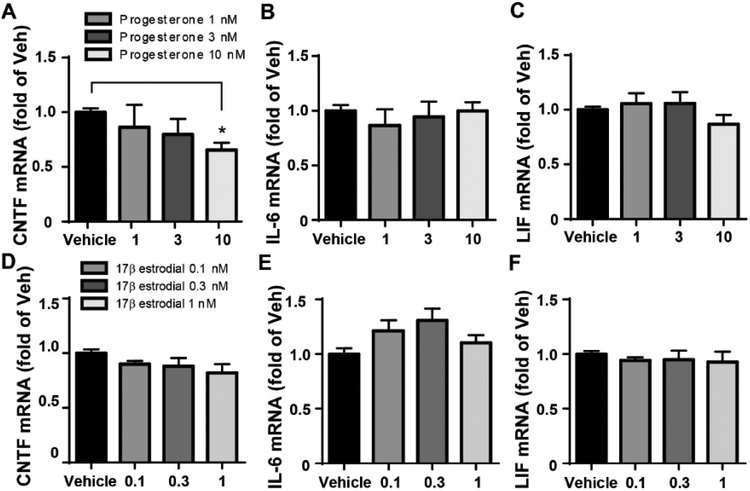
In order to test ovarian hormone-mediated inhibition of CNTF we incubated C6 astroglioma cell, a cell model of astrocytes which produce CNTF, with 17-β estradiol or progesterone at different concentrations for 4 h and then measured CNTF mRNA levels. These concentrations of 17-β estradiol or progesterone have been shown to successfully affect astrocyte functions (De Marinis et al., 2013; Weber et al., 2016). Progesterone produced a dose-dependent reduction of CNTF (Fig. 4A, F3, 12 = 4.253, p = 0.029). Compared to vehicle, progesterone at 10 nM significantly reduced CNTF mRNA. Progesterone did not affect the mRNA levels of IL-6 and LIF (Fig. 4B–C, F3, 12 = 0.3798 (IL-6) and 1.111 (LIF), p >0.10). Further, 17-β estradiol at 0.1, 0.3 or 1 nM did not change the mRNA levels of CNTF, IL-6 and LIF (Fig. 4D–F, F3, 20 = 1.801 (CNTF), 0.055 (IL-6) and 0.413 (LIF), p > 0.10). Together, these data suggest progesterone but not estrogen specifically inhibits CNTF, but not other highly related cytokine, expression in the astrocytes.
Figure 4. Progesterone inhibited CNTF mRNA expression in C6 cells.
The C6 astroglioma cells, a cell model of astrocyte, were incubated with progesterone or 17β estradiol at different concentrations for 4 h and then the levels of CNTF mRNA were measured RT-qPCR. A) Progesterone at 10 nM significantly reduced CNTF (A) without affecting IL-6 (B) and LIF (C) mRNA. N=5, 3, 3 and 5 experiments. * p<0.05 (One-way ANOVA followed by Newman-keuls multiple comparisons). 17β estradiol did not alter these cytokines (D, E, F). N= 6, 3, 3 and 6 experiments.
3.5. OVX increased behavioral despair through upregulation of CNTF
OVX-induced increase of behavior despair, i.e. increasing immobility time in the forced swim test, has been well established in female rodents (Bekku et al., 2006). Here, we tested whether CNTF was involved in OVX-induced behavioral despair. Female CNTF+/+ and CNTF−/− mice were ovariectomized and two weeks later tested in the forced swim test. In CNTF+/+ mice, OVX significantly increased immobility time compared to sham littermates (Fig. 5A, F1, 24 = 3.548, p = 0.048), confirming OVX induces behavioral despair in CNTF wildtype mice. However, knockout of CNTF abolished OVX-induced immobility time (Fig. 5A, F1, 24 = 6.349, p = 0.0004) and increased latency to immobility (Fig. 5B, F1, 24 = 5.979, p = 0.0008) compared to CNTF+/+ mice, suggesting OVX increases behavior despair through upregulation of CNTF expression, possibly in the amygdala. Further studies will need to confirm the role of CNTF in the amygdala on behavioral despair in this task using conditional knockout of CNTF or selective blockade of CNTF in the amygdala. In male mice, castration did not alter immobility time (Fig. 5C, F3, 33 = 2.862, p = 0.071) and immobility latency (Fig. 5D, F3, 33 = 0.044, p = 0.957) in both CNTF+/+ and CNTF−/− mice, which may be due to a lack of change of CNTF by castration. Notably, OVX and castration did not affect locomotor activity tested by open field test (Fig. 5D and E, p > 0.10).
Figure 5. OVX increased depressive-like behavior through upregulation of CNTF.
Both female sham and OVX mice were subjected to a forced swim test. A) OVX increased immobility time in CNTF+/+ mice and OVX-induced immobility time in CNTF−/− mice was significantly reduced compared to that in CNTF+/+ mice, suggesting OVX-induced depressive-like behavior though CNTF. B) OVX significantly increased immobility latency in CNTF−/− mice. Castration did not alter immobility time C) and latency D) in male mice. D-E) OVX and castration did not alter locomotor activity tested by open field test. N= 6, 10 and 11 in A, B and E. N=8, 15 and 13 mice in C, D and F. * p<0.05, *** p<0.001 (One-way ANOVA followed by Newman-keuls multiple comparisons).
3.6. Progesterone rescued OVX-induced behavioral despair through inhibition of CNTF
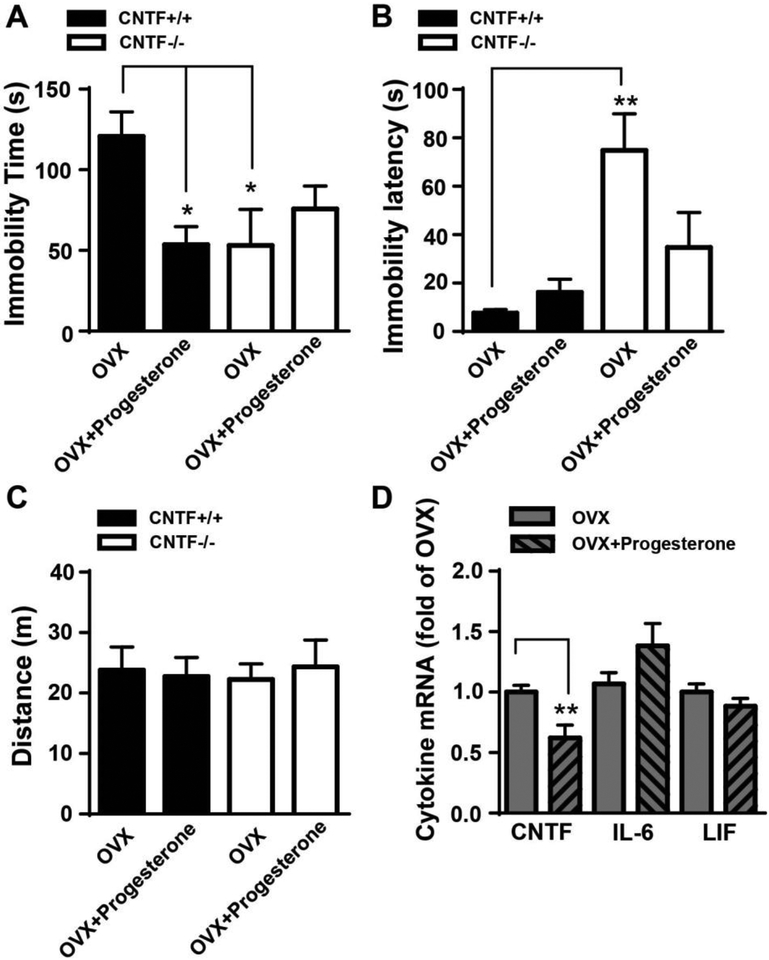
Progesterone inhibits OVX-induced increase of immobility time in the forced swim test as reported in rodents (Frye, 2011). Here we tested whether CNTF was involved in progesterone-mediated reduction of behavior despair. Two weeks after OVX, mice were treated with progesterone for 4 days and the forced swim test was performed 2 h after last progesterone injection. A two-way ANOVA revealed a significant interaction between genotype and progesterone treatment on immobility time (F1, 27 = 7.505, p = 0.011, Fig. 6A) as well as a significant main effect of genotype (F1, 27 = 13.32, p = 0.001, Fig. 6B) and a significant interaction of genotype and progesterone (F1, 27 = 4.293, p = 0.048, Fig. 6B) in latency to immobility. Progesterone reduced immobility time by more than 50% in OVX CNTF+/+ mice (Fig. 6A). In OVX CNTF−/− mice, both immobility time and latency were comparable with or without progesterone treatment (Fig. 6A, B), suggesting that CNTF may be involved in resistance to behavioral despair produced by progesterone. Importantly, progesterone did not affect locomotor activity in open field test (Fig. 6C, all main effects and interactions, p > 0.10) and these effects do not appear to be due to a motor effect of progesterone. Further, progesterone treatment significantly reduced CNTF mRNA (Fig. 6D, t(9) = 3.287, p =0.009) but not IL-6 and LIF (t(10) = 1.520 (IL-6) and 1.230 (LIF), p > 0.10) in the amygdala of CNTF+/+ mice. These data suggest progesterone decreases OVX-induced behavioral despair through inhibition of CNTF, possibly in the amygdala.
Figure 6. Progesterone rescued OVX-induced depressive-like behavior through inhibition of CNTF.
A) Progesterone significantly reduced immobility time in OVX CNTF+/+ mice, indicating an anti-depressant effect. Progesterone did not alter immobility time in OVX CNTF−/− mice, suggesting antidepressant effect of progesterone is through CNTF. B) Progesterone had no effect on immobility latency in both OVX CNTF+/+ and CNTF−/− mice. N= 9, 6, 6 and 10 mice. * p<0.05, ** p<0.01 (Two-way ANOVA followed by Newman-keuls multiple comparisons). C) Progesterone administration did not change locomotor activity tested by open field test. N= 9, 8, 6 and 5 mice. D) Progesterone reduced CNTF mRNA expression in the amygdala of OVX CNTF+/+ mice. N= 6 mice/group, * p<0.05 (Student t test).
3.7. CNTF was not involved in anxiety-like behavior in adult mice
Anxiety behavior was measured by latency to enter an open arm or to escape from an open arm in the elevated T-maze (Donner and Lowry, 2013). There was no difference in latency to enter the open arm on trial 2 and 3 (Supplemental Fig. 1A, both main effects and interaction, p > 0.10) or leave the open arm on the escape trial (Supplementary Fig. 1B, both main effects and interaction, p > 0.10) in both male and female CNTF+/+ and CNTF−/− mice, suggesting CNTF was not involved in anxiety-like behavior tested by elevated-T maze.
4. DISCUSSION
We identified a striking sex-specific opposite role of CNTF in depressive-like behavior in adult mice using the forced swim stress test and the sucrose preference test. Collectively, our data suggest that CNTF enhances depressive-like behaviors in female mice but inhibits depressive-like behaviors in male mice. They point to new opportunities for sex-specific strategies for treatment and possibly identify a novel pharmaceutical target for depression. Further, progesterone treatment prevented OVX-induced behavioral despair through CNTF, with implications for post-menopausal depression.
Little is known about CNTF-mediated affective behavior in rodent models. In apparent contrast to our findings, a single study, using only females, showed that CNTF−/− mice displayed increased depressive-like behaviors, and that they also had motor deficits at 8–15 weeks of age (Peruga et al., 2012). We did not find any group differences in two locomotor tests, consistent with the findings that CNTF−/− mice exhibit minor muscle weakness only in later adulthood at 28 weeks (Masu et al., 1993). It is difficult to explain how differences in locomotor function might explain the opposite effects of the previous study. The previous study used a shuttle box test, in which footshock was the stressful stimulus, to induce learned helplessness. In a clinical trial, CNTF caused hyperalgesia, cramps and muscle pain (Thoenen and Sendtner, 2002), suggesting that the lack of CNTF may reduce pain sensitivity, possibly explaining why the foot-shock test revealed a reduced response. Further, OVX induces thermal hyperalgesia in mice (Li et al., 2014), which may be due to upregulation of CNTF. One other study indirectly linked increased hippocampal CNTF caused by miRNA-155 knockout to reduced depressive behavior (Fonken et al., 2016), but we did not see changes in hippocampal CNTF after stress or OVX, both causing depressive behaviors.
4.1. CNTF contributes to high vulnerability of females to depression.
Women have a higher prevalence of depression than men (Aragam et al., 2011; Essau et al., 2010). However, the neurobiological mechanism(s) underlying sex differences of this disease is not fully understood. Most attention has been given to the functional roles of ovarian hormones, indicating that women are vulnerable to affective disorders when ovarian hormone fluctuation occurs. Estrogen-mediated modulation of neurotransmission is one of the underlying mechanisms of sex differences in depression (Douma et al., 2005). Estrogen-induced increase in hippocampal BDNF has been proposed as one of the mechanisms of beneficial estrogen therapy for post-menopausal depression (Numakawa et al., 2014). Our current study suggests that CNTF promotes depression in females and that this occurs in the amygdala and hypothalamus, the two brain areas mediating emotion and stress response (Bocchio et al., 2016; Mello et al., 2003). It is somewhat surprising to find a detrimental role of an otherwise beneficial neural cytokine, but this could explain why women are more prone to depressive-like behavior than men, whereas CNTF may play a protective effect against depression. In the brain, CNTF is produced by astrocytes (Stockli et al., 1989) and a sex dimorphism regarding astrocyte morphology and function in the hypothalamus and amygdala has been reported. For example, in the amygdala, male rats have more astrocytes and greater astrocyte process complexity than females (Johnson et al., 2008). Females have a greater intensity of GFAP staining in the amygdala (Acaz-Fonseca et al., 2016), perhaps indicating that these cells are more activated, which under injury conditions is associated with increased CNTF expression (Kang et al., 2012). However, the much higher levels of CNTF in the female amygdala may not be due to functional sex hormones since our data showed that castration did not alter CNTF expression in males and OVX increased CNTF while progesterone reduced CNTF expression in the female amygdala. In addition, our data were obtained from adult mice. Thus, it is possible that in adult mice, sex hormones may not directly contribute to the sex-dependent differential expression of CNTF in the amygdala. How and when the differential expression of CNTF in the amygdala is established is unknown. In puberty, androgen receptors mediate the sex dimorphism of astrocyte in rat amygdala (Johnson et al., 2013), suggesting that sex hormones in the development stage may contribute to the establishment of sex dimorphism in astrocytes in the amygdala.
If validated in humans, CNTF would be a good target for pharmacological treatments because CNTF expression can be regulated within hours to days in mice (Kang et al., 2013b; Keasey et al., 2013; Yang et al., 2008). Therefore, such treatments are expected to be more rapid than current FDA approved treatments for depression, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs), which take 2–3 weeks to have an effect. The influence of CNTF deficiency in 2–3% of humans on psychiatric disorders is not straightforward with some reports revealing it as a risk factor for psychiatric disorders (Thome et al., 1996) and others failing to show this (Nishiyama et al., 2006; Nothen et al., 1996). It will be important to determine whether these apparent discrepancies were due to differences in the numbers of women and men studied. Depression and anxiety represent two major aspects of major depression disorder and are often linked together (Tiller, 2013). It is, therefore, surprising that we find that CNTF does not seem to play a role in anxiety-like behavior in the elevated T-maze in both male and male mice. It remains to be determined whether this is also true in other behavioral tests and forms of anxiety.
4.2. Progesterone reduces behavioral despair through inhibition of CNTF possibly in the amygdala
Women experience dramatic fluctuation, decline and deficits in the levels of ovarian hormones across their lifetime, which are correlated to negative mood and depression (Douma et al., 2005). Ovarian hormones are highly lipophilic and pass readily through the blood brain barrier and affect brain functions (Hampl et al., 2015). Reductions of ovarian hormones are thought to contribute to the greater incidence of affective disorders in women. For example, the incidence of depressive-like symptoms is higher when ovarian hormone levels are low, including during premenstrual, postpartum and peri-menopausal periods (Hiroi and Neumaier, 2011). It would suggest that high female hormone levels are protective and both estrogen and progesterone mitigate depressive-like behavior in rodent models by modulating neurotransmission and increasing hippocampal BDNF (Douma et al., 2005; Frye, 2011). Our data suggest that high progesterone, but not estrogen, levels are protective against depression due to its inhibition of CNTF as also shown in the cultured C6 cell in vitro astrocyte cell model. The CNTF mechanism appears to be mostly in the amygdala, which plays a key role in emotional processing (Bocchio et al., 2016). Dysregulation of amygdala function contributes to the etiology of many psychiatric disorders, including depression (Victor et al., 2010).
The OVX-induced increase in behavioral despair did not occur in female CNTF−/− mice, suggesting OVX-induced behavioral despair is through upregulation of CNTF, possibly in the amygdala. Our sex-specific and gonadectomy data suggest that the hippocampus and frontal cortex do not play a role in CNTF-mediated affective behaviors. Thus, we proposed that inhibition of CNTF expression in the amygdala is a novel target for the known anti-depressant effects of progesterone. CNTF appears to be specific because progesterone did not affect IL-6 or LIF in the mice or C6 cells. The lack of anti-depressant effects of progesterone in OVX CNTF−/− mice suggests that progesterone acts through CNTF, again suggesting that it is an excellent molecular target. However, it is recognized there could be other mediators in this effect, such as GABA-gated chloride channels (Majewska et al., 1986). The lack of antidepressant effects of progesterone in OVX CNTF−/− mice could also possibly due to a floor effect since the levels of immobility time in OVX CNTF−/− mice were already low. We measured immobility using two dependent measurements, immobility time and latency to immobility. Thus even if there might have been a floor effect on immobility time there isn’t one on latency. Regardless, this is consistent with the findings that progesterone reduces injury-induced astrocytes activation (Djebaili et al., 2005). The two classic nuclear receptors that regulate gene expression through interaction of progesterone response elements in the promoters of target genes are broadly expressed in the brain (Brinton et al., 2008). The expression level of these receptors is prominent in the amygdala, especially in the central amygdala, compared to surrounding brain areas (Auger and De Vries, 2002).
4.3. CNTF appears to have anti-depressant effects in males
In remarkable contrast to the pro-depressive effect of CNTF in female mice, we found CNTF had an anti-depressive effect in male mice in a forced swim test. Male CNTF−/− mice had longer immobility time and less immobility latency than male CNTF+/+ littermate, suggesting CNTF deficiency increases behavioral despair, or sensitizes male mice to an acute stressor. This is consistent with the finding that male IL-6 knockout mice exhibit higher resistance to stress-induced behavior (Chourbaji et al., 2006) and generally, IL-6 counteracts the effects of CNTF, such as adult brain neurogenesis that is involved in depression (Bowen et al., 2011). The mechanism underlying the novel anti-depressant role of CNTF in males remains to be delineated. It is most likely not related to testosterone levels since castration did not affect depressive-like behavior in both CNTF+/+ and CNTF−/− mice. It is possible that the relatively low levels of progesterone in males compared to females may result in their higher CNTF levels. It also remains to be determined which cells mediate the effects of CNTF, but most likely include neurons, astrocytes and oligodendrocyte which express the CNTF-specific receptor. CNTF can affect neuropeptide expression (Rao et al., 1992), synaptic neurotransmission (Nai et al., 2010) and neuronal sprouting (Simon et al., 2010).
4.4. Conclusion
Taken together, our data indicate that CNTF plays an important sex-specific role in mouse depression-like behavior. This finding may lead to a new understanding of the biological substrate of affective disorders including depression, and to new ways of preventing, diagnosing and treating depression.
Supplementary Material
Highlights.
CNTF plays a sex-specific role in depressive-like behavior.
CNTF is pro-depressive in female mice but anti-depressive in male mice.
The anti-depressant effect of progesterone is through inhibition of CNTF in the amygdala.
ACKNOWLEDGEMENT:
We express our gratitude to Chiharu Lovins, Donald Lovins and Heath W. Shelton for technical support.
FUNDINGS: This work was supported by a grant from East Tennessee State University Research Development Committee-Major Grants Program (CJ), NIH grant AG029493 (TH) and in part by NIH grant C06RR0306551, and funds from Quillen College of Medicine (RWB, TH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interests or potential conflicts of interest.
REFERENCES
- Acaz-Fonseca E, Avila-Rodriguez M, Garcia-Segura LM, Barreto GE, 2016. Regulation of astroglia by gonadal steroid hormones under physiological and pathological conditions. Prog Neurobiol 144, 5–26. [DOI] [PubMed] [Google Scholar]
- Aragam N, Wang KS, Pan Y, 2011. Genome-wide association analysis of gender differences in major depressive disorder in the Netherlands NESDA and NTR population-based samples. J Affect Disord 133, 516–521. [DOI] [PubMed] [Google Scholar]
- Askvig JM, Leiphon LJ, Watt JA, 2012. Neuronal activity and axonal sprouting differentially regulate CNTF and CNTF receptor complex in the rat supraoptic nucleus. Exp Neurol 233, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger CJ, De Vries GJ, 2002. Progestin receptor immunoreactivity within steroid-responsive vasopressin-immunoreactive cells in the male and female rat brain. J Neuroendocrinol 14, 561–567. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I, 2011. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H, Araki H, 2006. Factors producing a menopausal depressive-like state in mice following ovariectomy. Psychopharmacology (Berl) 187, 170–180. [DOI] [PubMed] [Google Scholar]
- Berlanga C, Flores-Ramos M, 2006. Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J Affect Disord 95, 119–123. [DOI] [PubMed] [Google Scholar]
- Bocchio M, McHugh SB, Bannerman DM, Sharp T, Capogna M, 2016. Serotonin, Amygdala and Fear: Assembling the Puzzle. Front Neural Circuits 10, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen KK, Dempsey RJ, Vemuganti R, 2011. Adult interleukin-6 knockout mice show compromised neurogenesis. Neuroreport 22, 126–130. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J, 2008. Progesterone receptors: form and function in brain. Front Neuroendocrinol 29, 313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, Schwaninger M, Gass P, 2006. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis 23, 587–594. [DOI] [PubMed] [Google Scholar]
- Costa AP, Vieira C, Bohner LO, Silva CF, Santos EC, De Lima TC, Lino-de-Oliveira C, 2013. A proposal for refining the forced swim test in Swiss mice. Prog Neuropsychopharmacol Biol Psychiatry 45, 150–155. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I, 2002. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23, 238–245. [DOI] [PubMed] [Google Scholar]
- De Marinis E, Acaz-Fonseca E, Arevalo MA, Ascenzi P, Fiocchetti M, Marino M, Garcia-Segura LM, 2013. 17beta-Oestradiol anti-inflammatory effects in primary astrocytes require oestrogen receptor beta-mediated neuroglobin up-regulation. J Neuroendocrinol 25, 260–270. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG, 2005. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma 22, 106–118. [DOI] [PubMed] [Google Scholar]
- Donner NC, Lowry CA, 2013. Sex differences in anxiety and emotional behavior. Pflugers Arch 465, 601–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma SL, Husband C, O’Donnell ME, Barwin BN, Woodend AK, 2005. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv Nurs Sci 28, 364–375. [DOI] [PubMed] [Google Scholar]
- Essau CA, Lewinsohn PM, Seeley JR, Sasagawa S, 2010. Gender differences in the developmental course of depression. J Affect Disord 127, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Gaudet AD, Gaier KR, Nelson RJ, Popovich PG, 2016. MicroRNA-155 deletion reduces anxiety- and depressive-like behaviors in mice. Psychoneuroendocrinology 63, 362–369. [DOI] [PubMed] [Google Scholar]
- Frye CA, 2011. Progesterone attenuates depressive behavior of younger and older adult C57/BL6, wildtype, and progesterone receptor knockout mice. Pharmacol Biochem Behav 99, 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia P, Youssef I, Utvik JK, Florent-Bechard S, Barthelemy V, Malaplate-Armand C, Kriem B, Stenger C, Koziel V, Olivier JL, Escanye MC, Hanse M, Allouche A, Desbene C, Yen FT, Bjerkvig R, Oster T, Niclou SP, Pillot T, 2010. Ciliary neurotrophic factor cell-based delivery prevents synaptic impairment and improves memory in mouse models of Alzheimer’s disease. J Neurosci 30, 7516–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie KM, Woods AG, Nguyen T, Gall CM, 1997. Astroglial ciliary neurotrophic factor mRNA expression is increased in fields of axonal sprouting in deafferented hippocampus. J Comp Neurol 386, 137–148. [PubMed] [Google Scholar]
- Hagg T, Quon D, Higaki J, Varon S, 1992. Ciliary neurotrophic factor prevents neuronal degeneration and promotes low affinity NGF receptor expression in the adult rat CNS. Neuron 8, 145–158. [DOI] [PubMed] [Google Scholar]
- Hampl R, Bicikova M, Sosvorova L, 2015. Hormones and the blood-brain barrier. Horm Mol Biol Clin Investig 21, 159–164. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF, 2011. Complex roles of estrogen in emotion: sex matters. Biol Psychiatry 70, 908–909. [DOI] [PubMed] [Google Scholar]
- Johnson RT, Breedlove SM, Jordan CL, 2008. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Comp Neurol 511, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT, Breedlove SM, Jordan CL, 2013. Androgen receptors mediate masculinization of astrocytes in the rat posterodorsal medial amygdala during puberty. J Comp Neurol 521, 2298–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Keasey MP, Arnold SA, Reid R, Geralds J, Hagg T, 2013a. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol Dis 49, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Keasey MP, Cai J, Hagg T, 2012. Loss of neuron-astroglial interaction rapidly induces protective CNTF expression after stroke in mice. J Neurosci 32, 9277–9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Keasey MP, Hagg T, 2013b. P2X7 receptor inhibition increases CNTF in the subventricular zone, but not neurogenesis or neuroprotection after stroke in adult mice. Transl Stroke Res 4, 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keasey MP, Kang SS, Lovins C, Hagg T, 2013. Inhibition of a novel specific neuroglial integrin signaling pathway increases STAT3-mediated CNTF expression. Cell Commun Signal 11, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, Howes MJ, Normand SL, Manderscheid RW, Walters EE, Zaslavsky AM, 2003Screening for serious mental illness in the general population. Arch Gen Psychiatry 60, 184–189. [DOI] [PubMed] [Google Scholar]
- Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C, 2015. Forced swim test: What about females? Neuropharmacology 99, 408–421. [DOI] [PubMed] [Google Scholar]
- Lee MY, Deller T, Kirsch M, Frotscher M, Hofmann HD, 1997. Differential regulation of ciliary neurotrophic factor (CNTF) and CNTF receptor alpha expression in astrocytes and neurons of the fascia dentata after entorhinal cortex lesion. J Neurosci 17, 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LH, Wang ZC, Yu J, Zhang YQ, 2014. Ovariectomy results in variable changes in nociception, mood and depression in adult female rats. PLoS One 9, e94312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM, 1986. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232, 1004–1007. [DOI] [PubMed] [Google Scholar]
- Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H, 1993. Disruption of the CNTF gene results in motor neuron degeneration. Nature 365, 27–32. [DOI] [PubMed] [Google Scholar]
- Mello AF, Mello MF, Carpenter LL, Price LH, 2003. Update on stress and depression: the role of the hypothalamic-pituitary-adrenal (HPA) axis. Rev Bras Psiquiatr 25, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Wang X, Jin Y, Sun D, Li M, Hu B, Zhang X, 2010. Ciliary neurotrophic factor enhances nicotinic synaptic transmission in sympathetic neurons. J Neurosci Res 88, 887–895. [DOI] [PubMed] [Google Scholar]
- Nishiyama J, Tochigi M, Itoh S, Otowa T, Kato C, Umekage T, Kohda K, Ebisawa T, Kato N, Sasaki T, 2006. No association between the CNTF null mutation and schizophrenia or personality. Psychiatr Genet 16, 217–219. [DOI] [PubMed] [Google Scholar]
- Nothen MM, Cichon S, Eggermann K, Propping P, Knapp M, Maier W, Rietschel M, 1996. CNTF and psychiatric disorders. Nat Genet 13, 142–143; author reply 144. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Richards M, Nakajima S, Adachi N, Furuta M, Odaka H, Kunugi H, 2014. The role of brain-derived neurotrophic factor in comorbid depression: possible linkage with steroid hormones, cytokines, and nutrition. Front Psychiatry 5, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruga I, Hartwig S, Merkler D, Thone J, Hovemann B, Juckel G, Gold R, Linker RA, 2012. Endogenous ciliary neurotrophic factor modulates anxiety and depressive-like behavior. Behav Brain Res 229, 325–332. [DOI] [PubMed] [Google Scholar]
- Rao MS, Tyrrell S, Landis SC, Patterson PH, 1992. Effects of ciliary neurotrophic factor (CNTF) and depolarization on neur opeptide expression in cultured sympathetic neurons. Dev Biol 150, 281–293. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS, 2007. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10, 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon CM, Jablonka S, Ruiz R, Tabares L, Sendtner M, 2010. Ciliary neurotrophic factor-induced sprouting preserves motor function in a mouse model of mild spinal muscular atrophy. Hum Mol Genet 19, 973–986. [DOI] [PubMed] [Google Scholar]
- Stockli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Gotz R, Lindholm D, Thoenen H, 1989. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature 342, 920–923. [DOI] [PubMed] [Google Scholar]
- Thoenen H, Sendtner M, 2002. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci 5 Suppl, 1046–1050. [DOI] [PubMed] [Google Scholar]
- Thome J, Kornhuber J, Baumer A, Rosler M, Beckmann H, Riederer P, 1996. CNTF and endogenous psychoses? Nat Genet 12, 123. [DOI] [PubMed] [Google Scholar]
- Tiller JW, 2013. Depression and anxiety. Med J Aust 199, S28–31. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC, 2010. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry 67, 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F, Endesfelder S, Buhrer C, Berns M, 2016. Effects of progesterone on hyperoxia-induced damage in mouse C8-D1A astrocytes. Brain Behav 6, e00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Arnold SA, Habas A, Hetman M, Hagg T, 2008. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J Neurosci 28, 2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Guven N, Dietis N, 2016. Stress-based animal models of depression: Do we actually know what we are doing? Brain Res 1652, 30–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.