Abstract
Progress in prostate cancer research is presently limited by a shortage of reliable in vitro model systems. We describe a novel self-assembling peptide, bQ13, which forms nanofibers and gels useful for the 3D culture of prostate cancer spheroids, with improved cytocompatibility compared to related fibrillizing peptides. The mechanical properties of bQ13 gels could be controlled by adjusting peptide concentration, with storage moduli ranging between 1–10 kPa. bQ13’s ability to remain soluble at mildly basic pH considerably improved the viability of encapsulated cells compared to other self-assembling nanofiber-forming peptides. LNCaP cells formed spheroids in bQ13 gels with similar morphologies and sizes to those formed in Matrigel or RADA16-I. Moreover, prostate-specific antigen (PSA) was produced by LNCaP cells in all matrices, and PSA production was more responsive to enzalutamide treatment in bQ13 gels than in other fibrillized peptide gels. bQ13 represents an attractive platform for further tailoring within 3D cell culture systems.
Keywords: Supramolecular, hydrogel, fibrillizing, biomaterial
Graphical Abstract
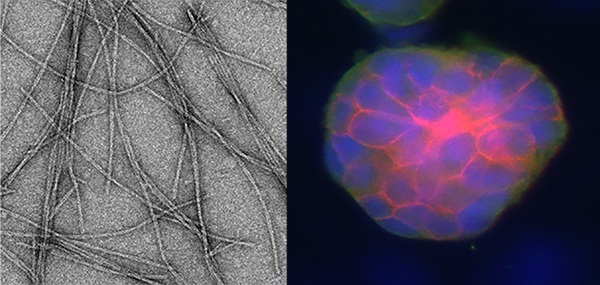
A fibrillizing peptide was designed that self-assembles into β-sheet nanofiber gels with improved cytocompatibility compared to related peptides (nanofibers shown, left). These gels supported the culture of prostate cancer cell spheroids (right).
Introduction
The development of effective treatments for prostate cancer has been slowed by a lack of reliable in vitro and in vivo models for studying the basic biological processes of the disease. Progress has been particularly hindered by the limited availability of human-derived and hormone-naïve model systems, particularly cell lines. Whereas early stages of prostate cancer are largely treatable presently, later stages of the disease tend to be metastatic, drug resistant, castration-unresponsive, and without effective therapies.1 The shortcomings of 2D cell culture in preclinical prostate cancer research are well acknowledged, as this method is limited in its capacity to model a vast range of centrally important processes such as migration, invasion, metastasis, microenvironmental aspects, and others.2,3 Additionally, 2D cultures are poorly predictive in drug screening efforts.4–6 However, current methods to culture prostate cancer cells in 3D are limited in their success to recapitulate the tumor microenvironment. Matrigel, a commonly used 3D matrix, is an extracellular matrix-like material secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma, composed of approximately 60% laminin, 30% collagen IV, 8% entactin, traces of perlecan, and growth factors such as transforming growth factor β (TGF-β), epidermal growth factor (EGF), insulin-like growth factor 1, and others.6 Despite its essential contribution to research progress over the past several decades, Matrigel has considerable limitations that are widely recognized. It suffers from batch-to-batch variability, incomplete definition, xenogeneic sourcing, and an inability to tailor its composition. Its batch-to-batch variability alone has contributed to the common practice of individual laboratories reserving specific lots of the material with the manufacturer, with obvious ramifications for experimental replication by independent researchers. In an effort to reduce variability, growth factor-reduced Matrigel has been developed, but its content is still not fully defined. Nevertheless, owing to its unparalleled ability to support a broad range of cellular processes in 3D, Matrigel has been favored as the standard for the 3D culture of most cancer cell types.7–10
In response to the shortcomings of Matrigel, multiple synthetic 3D culture systems have received considerable interest.11 Synthetic matrices may avoid the most limiting aspects of Matrigel but must be designed to recapitulate the extracellular matrix’s mechanical properties, transport properties, specific biological interactions, and degradation/remodeling processes that occur within the tumor microenvironment.12–19 A subset of these materials, self-assembled peptides, have shown utility in 3D culture and promise to provide a chemically defined matrix that can be tailored with co-assembled factors for different experimental purposes.20–22 The peptide RADA16-I, sold commercially as PuraMatrix23, can exist in liquid form at acidic pH and is induced to form gels of physically entangled peptide nanofibers upon mixing with neutrally buffered saline, thus encapsulating cells within the material. Derivatives of RADA16-I and peptides extended with various cell-adhesive ligands have additionally been investigated.24–27 However, owing to the acidic character of the pre-gelled precursor solutions of these materials, cell survival during the encapsulation process is negatively affected.28 Another fibrillizing peptide previously investigated as a cell culture substrate is Q11 (Ac-QQKFQFQFEQQ-Am).21,22,29 Like RADA16-I, the termini of this peptide are amenable to functionalization with a range of small chemical moieties or short peptides. Upon fibrillization of the Q11 domain, these functional components are displayed on the surface of the nanofibers. Modifications of the Q11 domain allow for various applications including mimicry of the ECM and delivery of immunogenic epitopes.30–33 In one example, peptide mixtures of RDGS- and IKVAV-Q11 have been shown to support the growth of human umbilical vein endothelial cells (HUVECs)22,34,35, and microgels of Q11 created via emulsion processing have been used for the encapsulation and culture of 3T3 fibroblasts and C3H10T-1/2 mouse pluripotent stem cells.36 Despite these successes, like RADA16-I, Q11 is unable to remain as a liquid above acidic pH, thus causing cytotoxicity when cells are suspended within the un-gelled precursor.36 This property favors applications where cells can be cultured on top of the gels rather than within them, or where droplets of the peptide solutions can be very rapidly neutralized, for example in emulsion-processed microgels.22,34 The requirement for immediate gelation of cell/peptide mixtures limits the utility of these peptides as 3D cell culture matrices, because significant cell death occurs during routine cell handling procedures, and serial processes such as the filling of multi-well plates cannot be accomplished without ongoing cell death.
We sought to improve upon these issues by designing a modified version of the Q11 peptide, bQ13 (Ac-QQKFQFQFEQEQQ-Am), so named because of its 13-amino acid length and because it can remain ungelled at basic pH. We hypothesized that this peptide would fibrillize to form self-supporting physical hydrogels like Q11, yet the addition of a Gln-Glu dipeptide would maintain the alternating sequence necessary for self-assembly but result in a negatively charged peptide at neutral pH, thus allowing it to remain in the liquid state at a larger pH range. We demonstrate that this peptide exhibits improved cytocompatibility for human prostate cancer cells compared to alternative 3D culture matrices and is amenable to drug sensitivity assays.
Methods
Peptides.
Peptides Q11 (Ac-QQKFQFQFEQQ-Am), KKQ13 (Ac-QQKQKFQFQFEQQ-Am), KKKQ13 (Ac-QQKQKFKFQFEQQ-Am), and EEQ13 (Ac-QQKFQFQFEQEQQ-Am, subsequently named bQ13 for its ability to remain ungelled at basic pH) were synthesized on a 0.25 mmol scale using a CS Bio 136 automated peptide synthesizer on rink amide AM resin using standard Fmoc protocols and activation with HBTU/HOBt. All peptides were double-coupled and cleaved/deprotected with 95:2.5:2.5 TFA:triisopropylsilane (TIS):H2O. Peptides were precipitated and washed several times with cold diethyl ether, dissolved in water, lyophilized on a Labconco freeze-drying system, and stored as lyophilized powders below −20 °C. The peptide RADA16-I (Ac-RADARADARADARADA-Am) was purchased from Corning (PuraMatrix, Cat# 354250, Lot# 071224) as a 1% aqueous solution (5.8 mM). Peptide molecular weight was verified using MALDI-TOF-MS with an α-cyano-4-hydroxycinnamic acid matrix. Peptides were purified using reverse-phase HPLC, lyophilized, and stored at −20˚C until use.
Transmission Electron Microscopy.
Peptide nanofibers were stained with uranyl acetate and analyzed by TEM using previously reported methods.34 Briefly, peptides were dissolved in water at 2 mM and diluted with 1X PBS to 0.2 mM. Peptide solutions were applied to Formvar/carbon-coated 400 mesh copper grids, incubated for 1 minute, washed with water, and stained with 1% w/v uranyl acetate (UA). Imaging was performed on an FEI Tecnai G2 Twin transmission electron microscope. The edges of the nanofibers were stained darkly by UA, so nanofiber widths were measured between the centers of the dark UA layers on opposing sides of the nanofiber.
Rheometry.
Lyophilized bQ13 and Q11 peptides were initially dissolved in ultrapure water (resistivity >18.1 MΩ) at concentrations ranging between 5–15 mM. The pH of the solutions was adjusted using 1.0 M NaOH. bQ13 and Q11 peptide solutions were sonicated in bath sonicator for 15 minutes prior to use. RADA16-I, received from the manufacturer as a 5.8 mM aqueous solution, was diluted in ultrapure water at 1.45–5.8 mM and sonicated for 30 minutes prior to use. Matrigel (Corning, Cat# 354234, Lot# 5145009) was thawed at 4˚C overnight prior to use. The pH-adjusted aqueous peptide samples were basified and 100 μL was transferred immediately to the plate of the rheometer. All other rheometry samples were created by removing the bottom conical portion of 50 mL conical tubes, casting 100 μL of peptide precursor solutions into these molds, and overlaying the peptide with serum-free RPMI-1640 medium (Sigma-Aldrich) for 15 min at 37˚C. Gels were then transferred onto the lower plate of the rheometer. Viscoelastic properties were measured using an 8 mm parallel plate geometry (Malvern Instruments, Westborough MA, Kinexus pro+). After placing the sample on the platform, the stage was lowered onto the gel with a gap distance of 1 mm. Excess gel was removed from the edges of the samples. Measurements were performed at a frequency sweep from 0.01 to 5 Hz in a closed, humidified chamber at 0.1% strain. Three independent gels were measured for each sample type, and each experiment was repeated.
Cell Culture.
Mouse embryonic pluripotent C3H10T1/2 stem cells were cultured in basal medium Eagle (BME, Sigma B1522) containing 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, and 1X non-essential amino acids (Cellgro 25–025-cl). To initiate 3D culture of C3H10T1/2 cells, they were trypsinized and suspended in 10% sucrose containing 180 μg/mL penicillin, 300 μg/mL streptomycin, and 0.75 μg/mL amphotericin B, at a density of 1.2×107 cells/mL. Androgen-sensitive prostate cancer cells (LNCaP), a line of primary human prostate adenocarcinoma cells derived from a lymph node metastasis of a 50-year-old Caucasian male, were maintained in L-glutamine-enhanced RPMI-1640 containing 20 mM HEPES, 10% fetal bovine serum (Thermofisher Scientific), 100 U/mL Penicillin, and 100 μg/mL Streptomycin (Sigma-Aldrich). The cells were incubated at 37˚C and 5% CO2 and passaged after reaching 80% confluency. LNCaP cells were trypsinized and resuspended in 10% (w/vol) sucrose at 3.75 million cells/mL immediately prior to encapsulation in various gel materials. Cell line validation and mycoplasma testing was confirmed using DDC Medical services (Fairfield, OH).
Gel Encapsulation.
For initial encapsulation and cytotoxicity experiments using C3H10T1/2 cells, aqueous solutions of peptides were mixed 2:1 with 1.2×107 cells/mL C3H10T1/2 cells in 10% sucrose, achieving final peptide concentrations of 15 mM. 20 μL of cell/peptide suspensions were immediately pipetted into 200 μL culture media to neutralize the peptide and induce gelation. For LNCaP encapsulation experiments, peptides bQ13, Q11, and RADA16-I were prepared to achieve final concentrations of 15 mM, 5 mM, and 1.45 mM, respectively, as these concentrations produced gels of similar mechanical properties, and the RADA16-I concentration has been commonly employed in previous work37–39. Working solutions of bQ13 and Q11 peptides were dissolved in ultrapure water at 1.5 times the desired final concentrations: 22.5 mM for bQ13 and 7.5 mM for Q11. bQ13 peptide solutions were further adjusted to pH 9.8 with the addition of 1.0 M NaOH. Solutions were sonicated for at least 15 minutes prior to use. RADA16-I was diluted to 2.175 mM in ultrapure water and sonicated for at least 30 minutes prior to use. Peptide solutions were then mixed 2:1 with cells suspended in 10% sucrose (w/v) at a concentration of 3.75 million cells/mL to create a final concentration of 1.25 million cells/mL in the peptide precursor solutions. To form cell-encapsulating gels, 40 μL of the cell/peptide mixture was pipetted slowly into the well of a 96-well plate containing 200 μL of prewarmed RPMI-1640 medium. Matrigel was thawed at 4˚C overnight prior to use and kept cool until mixing with pelleted cells to a final concentration of 1.25 million cells/mL. 40 μL of the mixture were then pipetted into an empty well of a 96-well plate and allowed to gel in an incubator for 30 minutes before 200 μL of RPMI-1640 medium was added to each well. Four independent gels were used for each analysis.
Cell viability assessment.
Gels were stained with 4 μM calcein AM and 2 μM ethidium homodimer for 30 minutes at 37˚C covered from the light. After staining and immediately prior to counting, gels were then dissociated carefully with slow and repetitive pipetting, which rendered the cells in a more 2-dimensional arrangement that facilitated counting. Cells were counted on a fluorescent microscope. Three gels were made for each encapsulation time and two images were collected for each gel (a total of 6 images per encapsulation time). Live and dead cells were counted and the survival ratio was calculated.
MTS Cell Survival Assay.
Cell survival was quantified by measuring metabolic activity using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega). 20 μL of MTS solution was pipetted into each well containing the gels and 100 μL of medium. The samples were then incubated at 37˚C on a shaker set at 10 rpm for 4 hours. 75 μL each of the resulting solutions were then transferred to a duplicate plate, and their absorbance was read at 490 nm using a plate reader. Four independent gels were used for each analysis.
Immunostaining.
After fixation with 4% paraformaldehyde (Electron Microscope Sciences, Hatfield, PA), cells were permeabilized using 0.25% Triton-X (Sigma-Aldrich) and blocked using 5% donkey serum (Sigma-Aldrich) in PBS overnight. Primary antibodies against E-cadherin (Abcam, Cambridge, MA, Cat# ab40772) and laminin-332 (Abcam, Cat# ab78286) were used at dilutions of 1:250 and incubated at 4˚C on an orbital shaker set at 10 rpm for 48 h. Secondary antibodies, Alexa Fluor 488 goat anti-mouse IgG1 (Invitrogen, Cat# A21121) or Alexa Fluor 546 goat anti-rabbit IgG (H+L) (Invitrogen, Cat# A11035), were used at 1:1000 dilution and incubated in the dark at 4˚C on an orbital shaker set at 10 rpm for 48 h. Cell nuclei were stained with DAPI during the secondary antibody incubation step. Three washes of one hour each at room temperature using PBS were performed between each step. To improve image quality by removing background-stained matrices, spheroids were dissociated from the matrices with slow repetitive pipetting before viewing with a fluorescent microscope. Morphological changes in the spheroids were not observed during this step. On a Life Technologies EVOS FL-Auto fluorescent microscope, z-stacks were collected through the middle of the spheroids (10 μm per stack, 12 slices collected). A slice corresponding to the center of the spheroid was selected, and the E-cadherin/laminin-332/DAPI channels were overlaid to generate merged images. Raw images are shown, without adjustment of brightness or contrast. Image analysis was performed using ImageJ software.
Drug Treatment.
Cells were cultured in peptide gels for 7 days prior to enzalutamide treatment (SelleckChem, Houston, TX) treatment. At day 7, the culture media was replaced with culture media containing 0, 10, 20, or 50 μM of enzalutamide. Owing to the poor solubility of enzalutamide, drug stocks were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich). Thus, all enzalutamide treatments and the negative control receiving no drug contained 0.5% DMSO for consistency. After 24 hours, the culture media was replaced with fresh drug solutions and the cells were treated for an additional 24 hours (48 hours total drug exposure). Culture supernatant samples were collected before and after the drug treatment and frozen at −20˚C. Secreted total Prostate-Specific Antigen (PSA) was measured using Roche Elecsys Total Prostate-Specific Antigen (PSA) Assay (Roche Molecular Biochemicals) at the University of Chicago Clinical Chemistry Core. Three independent gels were used for each sample type, and this experiment was repeated.
Statistical Analysis.
All statistical analyses were performed using GraphPad Prism 7 using one-way analysis of variance (ANOVA) with post-hoc Tukey’s multiple comparison test. Statistically significant differences are indicated as *p < 0.05, **p<0.01, ***p<0.001. All error bars represent standard errors of the mean.
Results
Nanofiber and Gel Characteristics
We performed an initial screen on 3 variants of Q11 (QQKFQFQFEQQ), each containing additional Lys or Glu residues within the alternating sequence of the peptide. We expected that peptides having a net positive or negative charge at neutral pH would have less tendency to gel, thus allowing cells to be mixed at pH values closer to neutral than Q11. We also maintained the pattern of alternating Gln residues in the new peptide designs (Table S1). To select the most promising of these for deeper investigation, we qualitatively studied their gelation behavior upon neutralization from acidic conditions, and we investigated the cytotoxicity of each. Each peptide was neutralized as much as possible without gelling, mixed with cells, and formed into hydrogels by mixing with culture media (Supplemental Information, Figure S1). Q11 formed gels above pH 3.7 and only supported the survival of 72% of encapsulated C3H10T1/2 cells. KKQ13 (QQKQKFQFQFEQQ) gelled at pH 4 and supported survival of 69.9% of cells. KKKQ13 (QQKQKFKFQFEQQ) gelled at pH 5.9, yet only supported the survival of 53% of encapsulated cells. However, EEQ13 (QQKFQFQFEQEQQ) became turbid upon neutralization but returned to a viscous, transparent solution when the pH was raised to 9.8. When mixed with cells at this pH value and gelled by adding to culture media, EEQ13 demonstrated excellent cytocompatibility, with 94.4% of C3H10T1/2 cells surviving the encapsulation process. We thus selected EEQ13 for deeper study and for comparison with other 3D matrices in the context of prostate cancer cell culture. We also renamed it bQ13 for its propensity to remain ungelled in basic conditions. Peptides KKQ13 and KKKQ13 were not studied further owing to their poor cytocompatibility.
The peptide bQ13 formed nanofibers in PBS, and TEM revealed morphologies similar to the nanofibers formed by Q11 and RADA16-I (Fig. 1). Each of the peptides produced well-formed nanofibers with comparable widths: 9.71 ± 0.101 nm for bQ13, 10.55 ± 0.103 nm for RADA16-I, and 9.21 ± 0.097 nm for Q11. RADA16-I did not stain as darkly with UA as bQ13 or Q11, potentially owing to its higher surface charge density. The lengths of the nanofibers were similarly polydisperse.
Figure 1.
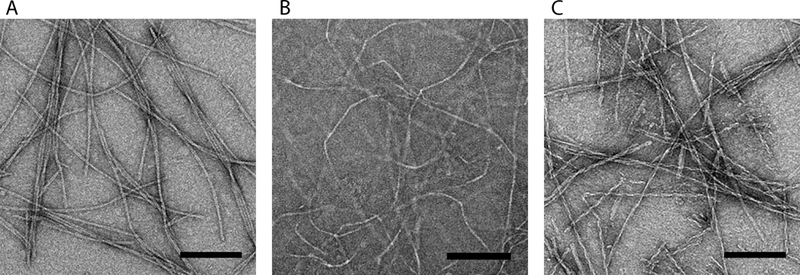
Negative stained TEM images of A) bQ13 B) RADA16-I and C) Q11 nanofibers. All scale bars = 100 nm.
Aqueous solutions of bQ13, RADA16-I, and Q11 were all soluble at pH 3.5, but each peptide behaved differently as the pH was raised via the addition of NaOH (Fig. 2A). As RADA16-I was neutralized, the storage modulus (G’) progressively increased until a maximum was reached at a pH of about 7, indicating gelation. Similarly, the storage modulus of Q11 also increased upon neutralization, although the maximum G’ was not reached until pH 9.5. This gelation behavior upon neutralization is a key confounding property of peptides such as Q11 and RADA16-I, as it hinders the production of ungelled cell/peptide stocks at neutral pH. In contrast, bQ13 remained ungelled throughout the pH range tested, up to pH 9.5 (Fig. 2A). As occurred during the initial cytotoxicity screening, bQ13 became turbid between pH 4–8, above which it returned to solution (Supplemental Information, Figure S2). Despite its useful non-gelling behavior in pure aqueous conditions, bQ13 formed hydrogels with concentration-dependent stiffness once the aqueous solutions were added to phosphate-buffered saline (Fig. 2C). Storage moduli between 1–10 kPa were achievable with bQ13 concentrations ranging between 5–15 mM. These stiffness values were similar to the range of stiffness exhibited by RADA16-I and Q11, whose gels in PBS also had storage moduli between 1–10 kPa (Fig. 2B). At equivalent concentrations, bQ13 gels were slightly less stiff than Q11, and all peptide gels were considerably stiffer than Matrigel.
Figure 2.
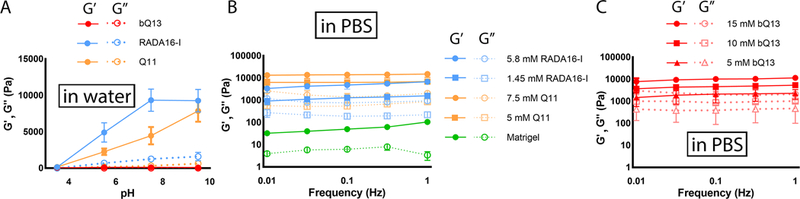
Rheological properties of peptide solutions and gels. Aqueous solutions of peptides were neutralized from pH 3.5, and storage moduli were measured using oscillating rheometry; whereas bQ13 remained ungelled, RADA16-I and Q11 formed hydrogels (A). Gels formed by adding aqueous peptide solutions to PBS had concentration-dependent storage moduli: RADA16-I, Q11, and Matrigel are shown in (B), bQ13 at various concentrations is shown in (C). Means +/− s.e.m. shown, n=3 experimental replicates per group.
Collectively, these studies indicated that aqueous solutions of bQ13 could be maintained in an ungelled state at mildly basic pH, yet hydrogels with tunable mechanics were formed upon mixing with phosphate buffered saline.
Cell Encapsulation and Survival
We next compared the survival of LNCaP human prostate cancer cells after the process of mixing them with ungelled aqueous solutions of peptides and transferring into culture media to generate cell-laden gels. At time points from 30 to 900 sec, live and dead cells were stained with calcein-AM and ethidium homodimer, respectively. To avoid premature gelation upon mixing with LNCaP cells, Q11 and Puramatrix required a pH no higher than 3.5 in the cell/precursor solution. bQ13, however, enabled the formation of cell/precursor mixtures at pH 9.8, at which the peptide/cell mixture was neither gelled nor turbid. For cells that were mixed with peptides and immediately pipetted into media, encapsulation in bQ13 showed considerably improved survival compared to RADA16-I or Q11 (Fig. 3A-D). The mean LNCaP survival rate was 96% for bQ13 encapsulation, whereas it was markedly lower for RADA16-I (50%) or for Q11 (66%). We next determined the extent to which LNCaP cells could survive in aqueous peptide solutions for extended periods of time, as would occur in situations where stock solutions of cells would be serially pipetted into multiwell plates of media, whether in automated liquid handling systems or by hand. In Q11 and RADA16-I, LNCaP viability continued to fall over time, with considerable cell death occurring between 30–300 seconds and comparatively less additional cell death occurring between 300–900 seconds. By 900 seconds, only 32% of LNCaP cells were still viable in RADA16-I, and only 49% were viable in Q11. In studies of LNCaP behavior, this rapid death of a large proportion of cells would select for the subpopulation of hardiest cells capable of withstanding the encapsulation procedure, thus biasing any subsequent in vitro investigation of cell behavior. Any serial pipetting of cells into culture media using these two peptides would produce heterogeneous experimental groups. In contrast, cells encapsulated in bQ13 maintained their viability to a much greater degree, even over 900 seconds. Viability at 60 seconds was 97%, and it was 77% after 900 seconds. LNCaP survival was also quantified at 24 h and 7 d using the MTS assay for metabolic activity (Fig. 3E, F). Using Matrigel as a standard, as it is commonly used for LNCaP culture6,9,40, it was found that bQ13 gels supported equivalent or better cell survival and growth. By 24 h, LNCaP cells cultured in RADA16-I or Q11 showed significantly reduced metabolic activity compared to bQ13 or Matrigel, presumably owing the poor survival during encapsulation (Fig. 3E). The cells in RADA16-I and Q11 did not recover, as this pattern of reduced viability persisted until at least 7 days (Fig. 3F). These experiments collectively established that LNCaP cells survived encapsulation within bQ13 gels to a much greater degree than Q11 or RADA16-I, enabling cell growth to a similar degree as Matrigel, the current matrix favored for the culture of this cell type.
Figure 3.
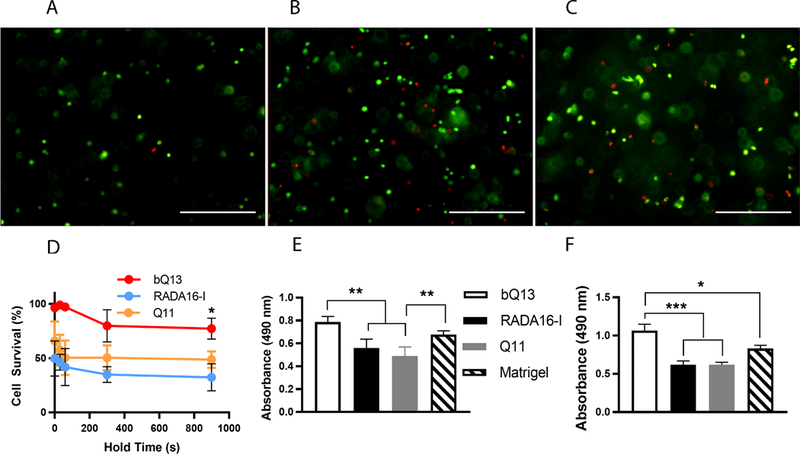
Survival of LNCaP cells after encapsulation in peptide gels. Representative live/dead staining (A-C) of LNCaP cells within bQ13 (A), RADA16-I (B), and Q11 (C) after incubating the cells in the peptide precursors for 300 sec before neutralization/gelation in media. Scale bars = 400 μm. D) Cell survival for cells suspended in ungelled precursors of bQ13, RADA16-I, and Q11 for variable amounts of time (hold time, indicated) and then subsequently gelled by pipetting into media. E) Metabolic activity (MTS assay) of LNCaP cells cultured for 24 hours in various matrices. F) Metabolic activity (MTS assay) of cells cultured for 7 days. *p<0.05, **p<0.01, ***p<0.001 by ANOVA with Tukey’s post-hoc test. Means +/− s.e.m. shown. n=4 experimental replicates per group.
Spheroid formation
When cultured for 7 days, LNCaP cells formed spheroids in bQ13, RADA16-I, Q11, and Matrigel (Fig. 4). These were spherical and had similar average diameters between culture materials (112.2 ± 5.0 μm in bQ13, 114.6 ± 5.5 μm in RADA16-I, 111.6 ± 4.7 μm in Q11 and 120.2 ± 3.8 μm in Matrigel). RADA16-I, in particular, exhibited significantly more cellular debris, presumed to be dead single cells. These diameters were similar to those reported previously for LNCaP spheroids cultures using liquid overlay on round-bottom wells41 or polyethylene glycol gels42, and slightly larger than spheroids cultured in hyaluronic acid/acrylate copolymers bearing RGD ligands18. Additionally, a one-way ANOVA analysis indicated no significant difference between the means of the groups and no significant variance between groups (F=0.523).
Figure 4.
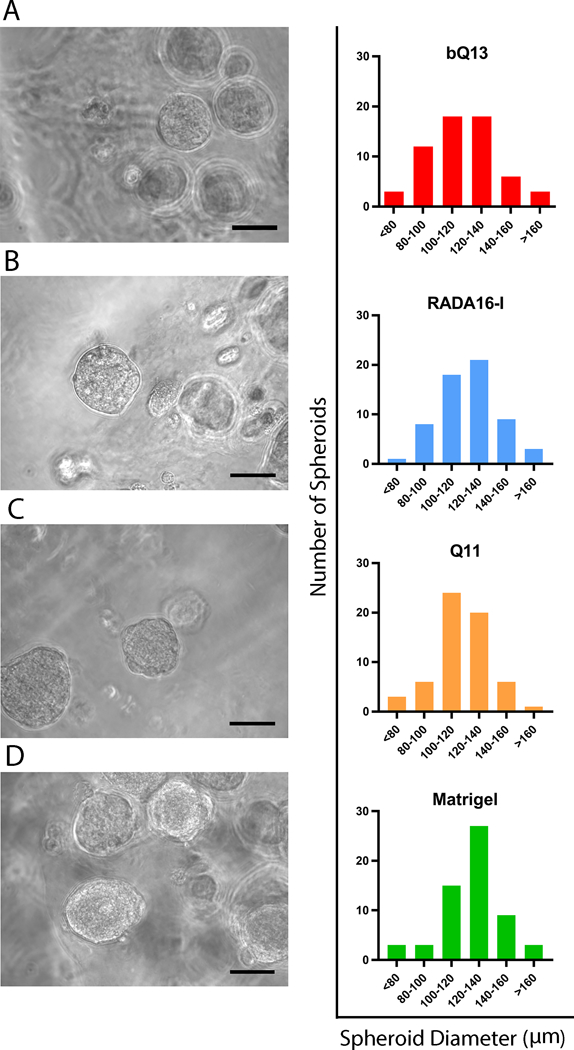
Representative spheroids formed in A) bQ13, B) RADA16-I, C) Q11, and D) Matrigel and the distribution of spheroid diameters. Images acquired after 7 days of culture. 60 spheroids were measured from 10 representative images for each material. Scale bars = 100 μm.
Immunofluorescence staining of E-cadherin and laminin-332 were used to investigate the multicellular organization of cultured LNCaP spheroids in various matrices after 7 d of culture. E-cadherin is a transmembrane protein that connects adjacent epithelial cells together at adherens junctions, and laminin-332 is a secreted extracellular matrix protein that localizes to the basolateral side of polarized epithelia. To diminish background staining from the matrices, spheroids were fixed, permeabilized, and recovered from the matrices by mechanical dissociation via pipetting. Shape and size was not noticeably affected by this process. Overall spheroid morphology was similar between matrices (Fig. 5). Neither E-cadherin nor laminin-332 showed clear evidence of apical-basolateral polarization in any of the matrices investigated, indicating disorganized tumor-like masses. In all matrices, E-cadherin was enriched at cell-cell boundaries, and laminin-332 appeared to be interspersed throughout the spheroid, with slight concentrations at cell-cell boundaries (Fig. 5). These results indicated that the LNCaP cells assume a disorganized, non-polarized morphology within the spheroids, consistent with the behavior of LNCaP cells in previously investigated synthetic matrices such as functionalized hyaluronic acid.18,43
Figure 5.
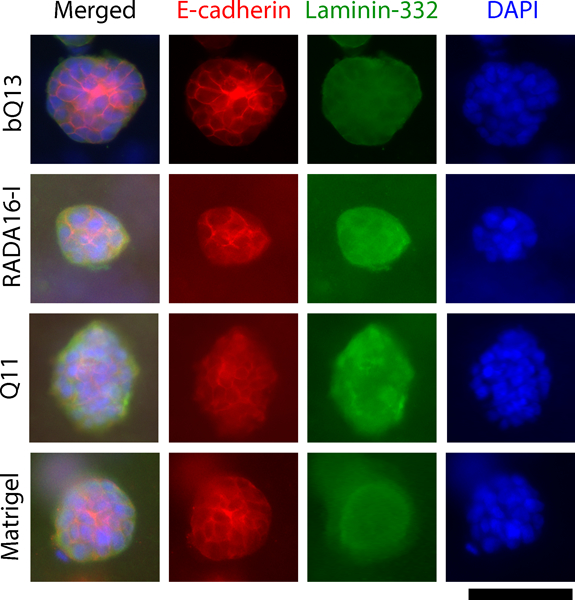
Immunofluorescence of spheroids collected from dissociated matrices. E-cadherin (located at adherens junctions) and laminin-332 (secreted extracellular matrix) indicated that LNCaP spheroids exhibited an unpolarized, disorganized structure in bQ13, Q11, Matrigel, and RADA16-I. Scale bar = 100 μm.
PSA production and drug sensitivity
In gels of bQ13, RADA16-I, and Matrigel, the ability of LNCaP cells to produce prostate-specific antigen (PSA) was investigated, and their susceptibility to the androgen receptor inhibitor enzalutamide was evaluated. PSA is an established AR target gene and thus secreted PSA is associated with AR pathway activity. LNCaP cells were cultured for 7 days and allowed to form into spheroids. After the cells had established spheroids, at day 7 PSA levels were measured in the media to establish a baseline, as the cells produced different amounts of PSA in different matrices. The influence of enzalutamide on spheroid growth and PSA production was then assessed by applying the drug in various concentrations and measuring PSA concentrations in the media two days later (9 days total from the initial seeding of cells into the matrices). The amount of PSA in the media on Day 9 was divided by the amount of PSA in the media immediately prior to drug treatment at Day 7. Thus, this normalization indicates deviation from the PSA production at Day 7. In the absence of drug treatment, PSA secretion increased nearly 300% between the 7th and 9th days in culture when they were grown in bQ13 gels (Fig. 6), which we interpreted as an indication that the spheroids continued to grow and develop in bQ13 over this time frame. In Matrigel, PSA secretion also increased over this time period, though to a lesser degree than in bQ13. Contrastingly, spheroids cultured in RADA16-I showed only slightly increased PSA production over the 48 h period. This result suggested that spheroids cultured in Matrigel and bQ13 proliferated, increased their capacity to produce PSA, or both, while those in RADA16-I remained comparatively unchanged over the window of time measured. This suggests that bQ13 is a more favorable medium for spheroid growth and development compared to RADA16-I, but without additional study it is not clear what properties of each peptide led to these differences. When treated with enzalutamide, LNCaP cells cultured in bQ13 gels exhibited considerable sensitivity to the drug (Fig. 6), as did those cultured in Matrigel. In contrast, LNCaP spheroids cultured in RADA16-I were comparatively less affected by enzalutamide. The DMSO carrier necessary to dissolve the drug (0 μM enzalutamide group) had a small influence on PSA production in bQ13 and RADA16-I. Overall, the dynamic range exhibited in enzalutamide sensitivity was greatest for LNCaP cells cultured in bQ13. Collectively these results indicated that LNCaP spheroids cultured in bQ13 were able to reveal distinctions in PSA production between drug-treated and untreated groups comparably with Matrigel and to a better degree that RADA16-I.
Figure 6.
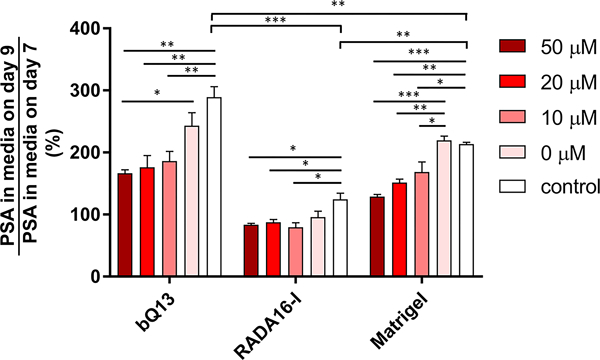
LNCaP cells cultured within various 3D matrix materials exhibited differential sensitivity to Enzalutamide treatment. Secreted PSA levels after exposure to the drug were normalized to levels of PSA in the same gels prior to drug exposure. *p<0.05, **p<0.01, ***p<0.001 by ANOVA with Tukey post-hoc test. n = 3 replicates per treatment group.
Discussion
Peptides that form hydrogels via β-sheet fibrillization have been studied extensively as matrices for cell culture over the past 2 decades.44 The design rules originally developed by Zhang and co-workers based on charge complementarity and patterns of alternating hydrophilic and hydrophobic residues23 spurred interest in a variety of different peptides with varying length, charge distribution, and sequences that produce gels with varied mechanical and biological properties. These include the peptides RADA1623,45,46, EAK1647, KFE848, and their variants35,49–51. Other well-studied peptides that form supramolecular nanofiber gels include the multidomain fibrillizing peptides developed by Hartgerink and coworkers52,53, glutamine-rich self-assembling peptides originally reported by Aggeli and coworkers54, and the subsequently studied related peptides such as Q1122,34,55,56 and bQ13, the focus of the present work. Several β-sheet fibrillizing peptides have been available commercially, notably Puramatrix from Corning, HydroMatrix from Sigma-Aldrich, and PGD-HydroGels from PeptiGelDesign. Considering additional designs beyond linear fibrillizing peptides such as β-hairpins57,58; aromatic peptide amphiphiles59–63; peptide amphiphiles64–66, and others67,68, supramolecular peptide matrices for 3D cell culture represent a diverse family.
In many fibrillar peptide culture systems that are commercially available, the issue of compromised cell viability driven by the low pH of un-gelled precursor solutions remains an issue. Our own exploration of the peptide Q11 has similarly been hindered. Despite Q11’s reliable assembly into hydrogels with acceptable mechanical properties for cell culture34 and its ability to be functionalized with a variety of different cell binding ligands34, it must be maintained near pH 3 to remain un-gelled, and this complicates the formation of 3D cell cultures with embedded cells. This issue drove us previously to focus on applications where the matrices could be used as planar substrates, for example as synthetic basement membranes for endothelial cells.21–22 In this way, the materials could be gelled and neutralized prior to placing them in contact with cells. Another strategy for rapidly neutralizing Q11 gels has been the formation of microgels in emulsion processes, where short diffusion distances allow for rapid neutralization.36
To render the Q11 system more amenable to 3D cell encapsulation, we developed bQ13, and we investigated bQ13 gels in the context of human prostate cancer cell culture. Ex vivo cultured prostate cancer organoids from both primary tumor cells and cell lines are receiving considerable interest, both for modeling the phenotypes of various forms of the disease and evaluating therapeutics.69 The vast majority of these studies have employed Matrigel, despite speculations that minor components may variably influence phenotype or drug responses.70 bQ13 compared favorably with Matrigel, possessing good cytocompatibility, tunable mechanics in an appropriate stiffness regime for synthetic ECMs, considerably reduced cell death compared to other self-assembling peptides, and the ability to support the formation of spheroids. Human LNCaP cells cultured in bQ13 gels formed spheroids that were sensitive to the nonsteroidal antiandrogen drug enzalutamide to a similar degree as LNCaP cells cultured in Matrigel. LNCaP cells were less sensitive to enzalutamide in gels of RADA16-I, potentially owing to reduced initial cell viability.
Previous fibrillar peptides such as Q11 and RADA16-I have been amenable to further modification with various biofunctional ligands including cell-binding peptides,22,34,71 and folded proteins can be installed within Q11 fibers using previously reported strategies for tagging expressed proteins with co-assembling polypeptide tails.31 The availability of such tools suggests potentially fruitful future work with prostate cancer organoids in bQ13 gels. It may be interesting to investigate functionalized bQ13 peptides with factors expected to modulate the growth or phenotype of prostate cancer cells such as cell-binding ligands, growth factors, or proteolytic cleavage sites expected to be operative in the tumor microenvironment. Such studies would be facilitated by the modularity of self-assembled peptide systems, which could be easily optimized by studying the factors in combination22 and would be considerably more defined than existing biologically sourced 3D culture matrices such as Matrigel.
Conclusion
Here we introduced the peptide bQ13, which forms nanofibers and gels useful for the 3D culture of human prostate cancer spheroids. Owing to its ability to remain soluble at a wide range of pH, the viability of encapsulated cells could be improved in comparison with other self-assembling nanofiber-forming peptides. The mechanical properties of bQ13 gels could be controlled by adjusting the peptide’s concentration, with storage moduli ranging between 1–10 kPa. LNCaP human prostate cancer cells formed spheroids in bQ13 gels with similar morphologies and sizes to those formed in Matrigel or RADA16-I. The cells remained disorganized in each material, not exhibiting any signs of apical/basolateral polarization. LNCaP cells produced PSA in all matrices, and PSA production was more sensitive to enzalutamide treatment in bQ13 than in other fibrillized peptide gels. Owing to the chemical definition and modifiability of peptide culture materials, properties that biologically derived matrices such as Matrigel lack, bQ13 represents an attractive platform for further modification and tailoring as a defined 3D matrix for prostate cancer cell culture.
Supplementary Material
Acknowledgements
This research was supported by the National Institutes of Health (NCI R21 CA196434, NIBIB R01 EB009701). KMH was supported by NIH T32 GM008555. MALDI was performed with support from the North Carolina Biotechnology Consortium, Grant #2017-IDG-1018. The contents are solely the responsibility of the authors and do not necessarily represent the official views of these agencies.
References
- 1.Shen MM & Abate-Shen C Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 24, 1967–2000 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, Farach-Carson MC & Jia X Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol. Adv. 32, 1256–1268 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurioka D, Takagi A, Yoneda M, Hirokawa Y, Shiraishi T & Watanabe M Multicellular Spheroid Culture Models: Applications in prostate Cancer Research and Therapeutics. J. Cancer Sci. Ther. 03, 60–65 (2011). [Google Scholar]
- 4.Edmondson R, Broglie JJ, Adcock AF & Yang L Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 12, 207–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmondson R, Adcock AF, Yang L, Liu G, Diot A & Xirodimas D Influence of Matrices on 3D-Cultured Prostate Cancer Cells’ Drug Response and Expression of Drug-Action Associated Proteins. PLoS One 11, e0158116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Härmä V, Virtanen J, Mäkelä R, Happonen A, Mpindi J-P, Knuuttila M, Kohonen P, Lötjönen J, Kallioniemi O & Nees M A Comprehensive Panel of Three-Dimensional Models for Studies of Prostate Cancer Growth, Invasion and Drug Responses. PLoS One 5, e10431 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee GY, Kenny PA, Lee EH & Bissell MJ Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 4, 359–65 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinman HK & Martin GR Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 15, 378–386 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ & Mooney DJ Engineering tumors with 3D scaffolds. Nat. Methods 4, 855–60 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Yamada KM & Cukierman E Modeling Tissue Morphogenesis and Cancer in 3D. Cell 130, 601–610 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Thakuri PS, Liu C, Luker GD & Tavana H Biomaterials-Based Approaches to Tumor Spheroid and Organoid Modeling. Adv. Healthc. Mater. 7, 1700980 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tibbitt MW & Anseth KS Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 103, 655–663 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worthington P, Pochan DJ & Langhans SA Peptide Hydrogels - Versatile Matrices for 3D Cell Culture in Cancer Medicine. Front. Oncol. 5, 92 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du X, Zhou J, Shi J & Xu B Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 115, 13165–13307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frantz C, Stewart KM & Weaver VM The extracellular matrix at a glance. J. Cell Sci. 123, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietras K & Östman A Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 316, 1324–1331 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD & Rowley DR Reactive Stroma in Human Prostate Cancer. Clin. Cancer Res. 8, (2002). [PubMed] [Google Scholar]
- 18.Hao Y, Zerdoum AB, Stuffer AJ, Rajasekaran AK & Jia X Biomimetic Hydrogels Incorporating Polymeric Cell-Adhesive Peptide To Promote the 3D Assembly of Tumoroids. Biomacromolecules 17, 3750–3760 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieh S, Taubenberger AV, Lehman ML, Clements JA, Nelson CC & Hutmacher DW Paracrine interactions between LNCaP prostate cancer cells and bioengineered bone in 3D in vitro culture reflect molecular changes during bone metastasis. Bone 63, 121–131 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Gelain F, Bottai D, Vescovi A & Zhang S Designer Self-Assembling Peptide Nanofiber Scaffolds for Adult Mouse Neural Stem Cell 3-Dimensional Cultures. PLoS One 1, e119 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung JP, Nagaraj AK, Fox EK, Rudra JS, Devgun JM & Collier JH Co-assembling peptides as defined matrices for endothelial cells. Biomaterials 30, 2400–2410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung JP, Moyano JV & Collier JH Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices. Integr. Biol. (Camb). 3, 185–96 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Holmes T, Lockshin C & Rich A Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc. Natl. Acad. Sci. U. S. A. 90, 3334–8 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horii A, Wang X, Gelain F & Zhang S Biological Designer Self-Assembling Peptide Nanofiber Scaffolds Significantly Enhance Osteoblast Proliferation, Differentiation and 3-D Migration. PLoS One 2, e190 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradshaw M, Ho D, Fear MW, Gelain F, Wood FM & Iyer KS Designer self-assembling hydrogel scaffolds can impact skin cell proliferation and migration. Sci. Rep. 4, 6903 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunha C, Panseri S, Villa O, Silva D & Gelain F 3D culture of adult mouse neural stem cells within functionalized self-assembling peptide scaffolds. Int. J. Nanomedicine 6, 943–55 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes TC, de Lacalle S, Su X, Liu G, Rich A & Zhang S Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc. Natl. Acad. Sci. U. S. A. 97, 6728–33 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eagle H The effect of environmental pH on the growth of normal and malignant cells. J. Cell. Physiol. 82, 1–8 (1973). [DOI] [PubMed] [Google Scholar]
- 29.Jung JP, Jones JL, Cronier SA & Collier JH Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials 29, 2143–2151 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collier JH & Messersmith PB Self-Assembling Polymer–Peptide Conjugates: Nanostructural Tailoring. Adv. Mater. 16, 907–910 (2004). [Google Scholar]
- 31.Hudalla GA, Sun T, Gasiorowski JZ, Han H, Tian YF, Chong AS & Collier JH Gradated assembly of multiple proteins into supramolecular nanomaterials. Nat. Mater. 13, 829–36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pompano RR, Chen J, Verbus EA, Han H, Fridman A, McNeely T, Collier JH & Chong AS Titrating T-Cell Epitopes within Self-Assembled Vaccines Optimizes CD4+ Helper T Cell and Antibody Outputs. Adv. Healthc. Mater. 3, 1898–1908 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collier JH & Messersmith PB Self-Assembling Polymer–Peptide Conjugates: Nanostructural Tailoring. Adv. Mater. 16, 907–910 (2004). [Google Scholar]
- 34.Jung JP, Nagaraj AK, Fox EK, Rudra JS, Devgun JM & Collier JH Co-assembling peptides as defined matrices for endothelial cells. Biomaterials 30, 2400–2410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho H, Balaji S, Sheikh AQ, Hurley JR, Tian YF, Collier JH, Crombleholme TM & Narmoneva DA Regulation of endothelial cell activation and angiogenesis by injectable peptide nanofibers. Acta Biomater. 8, 154–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian YF, Devgun JM & Collier JH Fibrillized peptide microgels for cell encapsulation and 3D cell culture. Soft Matter 7, 6005–6011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharya M, Malinen MM, Lauren P, Lou Y-R, Kuisma SW, Kanninen L, Lille M, Corlu A, GuGuen-Guillouzo C, Ikkala O, Laukkanen A, Urtti A & Yliperttula M Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 164, 291–298 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Nagrath D, Chen PC, Berthiaume F & Yarmush ML Three-Dimensional Primary Hepatocyte Culture in Synthetic Self-Assembling Peptide Hydrogel. Tissue Eng. Part A 14, 227–236 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Liedmann A, Rolfs A & Frech MJ Cultivation of human neural progenitor cells in a 3-dimensional self-assembling peptide hydrogel. J. Vis. Exp. 59, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Fournier MV, Ware JL, Bissell MJ, Yacoub A & Zehner ZE Inhibition of vimentin or beta1 integrin reverts morphology of prostate tumor cells grown in laminin-rich extracellular matrix gels and reduces tumor growth in vivo. Mol. Cancer Ther. 8, 499–508 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballangrud AM, Yang WH, Dnistrian A, Lampen NM & Sgouros G Growth and characterization of LNCaP prostate cancer cell spheroids. Clin. Cancer Res. 5, 3171s–3176s (1999). [PubMed] [Google Scholar]
- 42.Sieh S, Taubenberger AV, Lehman ML, Clements JA, Nelson CC & Hutmacher DW Paracrine interactions between LNCaP prostate cancer cells and bioengineered bone in 3D in vitro culture reflect molecular changes during bone metastasis. Bone 63, 121–131 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Thakuri PS, Liu C, Luker GD & Tavana H Biomaterials-Based Approaches to Tumor Spheroid and Organoid Modeling. Adv. Healthc. Mater. 7, e1700980 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koutsopoulos S Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: Progress, design guidelines, and applications. J. Biomed. Mater. Res. A 104, 1002–16 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Zhang S, Lockshin C, Cook R & Rich A Unusually stable ?-sheet formation in an ionic self-complementary oligopeptide. Biopolymers 34, 663–672 (1994). [DOI] [PubMed] [Google Scholar]
- 46.Zhang S, Holmes TC, DiPersio CM, Hynes RO, Su X & Rich A Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials 16, 1385–93 (1995). [DOI] [PubMed] [Google Scholar]
- 47.Jun S, Hong Y, Imamura H, Ha B-Y, Bechhoefer J & Chen P Self-assembly of the ionic peptide EAK16: the effect of charge distributions on self-assembly. Biophys. J. 87, 1249–59 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marini DM, Hwang W, Lauffenburger DA, Zhang S & Kamm RD Left-Handed Helical Ribbon Intermediates in the Self-Assembly of a β-Sheet Peptide. Nano Lett. 2, 295–299 (2002). [Google Scholar]
- 49.Hong Y, Legge RL, Zhang S & Chen P Effect of Amino Acid Sequence and pH on Nanofiber Formation of Self-Assembling Peptides EAK16-II and EAK16-IV. Biomacromolecules 4, 1433–1442 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Lee NR, Bowerman CJ & Nilsson BL Effects of varied sequence pattern on the self-assembly of amphipathic peptides. Biomacromolecules 14, 3267–77 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Lee NR, Bowerman CJ & Nilsson BL Sequence length determinants for self-assembly of amphipathic β-sheet peptides. Biopolymers 100, 738–50 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Kang MK, Colombo JS, D’Souza RN & Hartgerink JD Sequence Effects of Self-Assembling MultiDomain Peptide Hydrogels on Encapsulated SHED Cells. Biomacromolecules 15, 2004–2011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galler KM, Aulisa L, Regan KR, D’Souza RN & Hartgerink JD Self-Assembling Multidomain Peptide Hydrogels: Designed Susceptibility to Enzymatic Cleavage Allows Enhanced Cell Migration and Spreading. J. Am. Chem. Soc. 132, 3217–3223 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aggeli A, Bell M, Boden N, Keen JN, Knowles PF, McLeish TC, Pitkeathly M & Radford SE Responsive gels formed by the spontaneous self-assembly of peptides into polymeric beta-sheet tapes. Nature 386, 259–62 (1997). [DOI] [PubMed] [Google Scholar]
- 55.Tian YF, Hudalla GA, Han H & Collier JH Controllably degradable β-sheet nanofibers and gels from self-assembling depsipeptides. Biomater. Sci. 1, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Restuccia A, Tian YF, Collier JH & Hudalla GA Self-Assembled Glycopeptide Nanofibers as Modulators of Galectin-1 Bioactivity. Cell. Mol. Bioeng. 8, 471–487 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L & Kretsinger J Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J. Am. Chem. Soc. 124, 15030–7 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Rajagopal K, Lamm MS, Haines-Butterick LA, Pochan DJ & Schneider JP Tuning the pH Responsiveness of β-Hairpin Peptide Folding, Self-Assembly, and Hydrogel Material Formation. Biomacromolecules 10, 2619–2625 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Jayawarna V, Ali M, Jowitt TA, Miller AF, Saiani A, Gough JE & Ulijn RV Nanostructured Hydrogels for Three-Dimensional Cell Culture Through Self-Assembly of Fluorenylmethoxycarbonyl–Dipeptides. Adv. Mater. 18, 611–614 (2006). [Google Scholar]
- 60.Harper MM, Connolly ML, Goldie L, Irvine EJ, Shaw JE, Jayawarna V, Richardson SM, Dalby MJ, Lightbody D & Ulijn RV in Methods Mol. Biol. 1777, 283–303 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Zhou M, Smith AM, Das AK, Hodson NW, Collins RF, Ulijn RV & Gough JE Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 30, 2523–2530 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Orbach R, Adler-Abramovich L, Zigerson S, Mironi-Harpaz I, Seliktar D & Gazit E Self-assembled Fmoc-peptides as a platform for the formation of nanostructures and hydrogels. Biomacromolecules 10, 2646–51 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Cheng G, Castelletto V, Jones RR, Connon CJ & Hamley IW Hydrogelation of self-assembling RGD-based peptides. Soft Matter 7, 1326–1333 (2011). [Google Scholar]
- 64.Matsuoka AJ, Sayed ZA, Stephanopoulos N, Berns EJ, Wadhwani AR, Morrissey ZD, Chadly DM, Kobayashi S, Edelbrock AN, Mashimo T, Miller CA, McGuire TL, Stupp SI & Kessler JA Creating a stem cell niche in the inner ear using self-assembling peptide amphiphiles. PLoS One 12, e0190150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Z, Sargeant TD, Hulvat JF, Mata A, Bringas P, Koh C-Y, Stupp SI & Snead ML Bioactive Nanofibers Instruct Cells to Proliferate and Differentiate During Enamel Regeneration. J. Bone Miner. Res. 23, 1995–2006 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui H, Webber MJ & Stupp SI Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers 94, 1–18 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang H, Ding Y, Sun XS & Nguyen TA Peptide hydrogelation and cell encapsulation for 3D culture of MCF-7 breast cancer cells. PLoS One 8, e59482 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu Z, Cai Z, Chen Q, Liu M, Ye L, Ren J, Liao W & Liu S Engineering β-sheet peptide assemblies for biomedical applications. Biomater. Sci. 4, 365–74 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Puca L, Bareja R, Prandi D, Shaw R, Benelli M, Karthaus WR, Hess J, Sigouros M, Donoghue A, Kossai M, Gao D, Cyrta J, Sailer V, Vosoughi A, Pauli C, Churakova Y, Cheung C, Deonarine LD, McNary TJ, Rosati R, Tagawa ST, Nanus DM, Mosquera JM, Sawyers CL, Chen Y, Inghirami G, Rao RA, Grandori C, Elemento O, Sboner A, Demichelis F, Rubin MA & Beltran H Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun. 9, 2404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weeber F, Ooft SN, Dijkstra KK & Voest EE Tumor Organoids as a Pre-clinical Cancer Model for Drug Discovery. Cell Chem. Biol. 24, 1092–1100 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Genové E, Shen C, Zhang S & Semino CE The effect of functionalized self-assembling peptide scaffolds on human aortic endothelial cell function. Biomaterials 26, 3341–3351 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


