Abstract
Background:
Evidence-based, patient-specific estimates of abusive head trauma probability can inform physicians’ decisions to evaluate, confirm, exclude, and/or report suspected child abuse.
Objective:
To derive a clinical prediction rule for pediatric abusive head trauma that incorporates the (positive or negative) predictive contributions of patients’ completed skeletal surveys and retinal exams.
Participants and Setting:
500 acutely head-injured children under three years of age hospitalized for intensive care at one of 18 sites between 2010 and 2013.
Methods:
Secondary analysis of an existing, cross-sectional, prospective dataset, including (1) multivariable logistic regression to impute the results of abuse evaluations never ordered or completed, (2) regularized logistic regression to derive a novel clinical prediction rule that incorporates the results of completed abuse evaluations, and (3) application of the new prediction rule to calculate patient-specific estimates of abusive head trauma probability for observed combinations of its predictor variables.
Results:
Applying a mean probability threshold of >0.5 to classify patients as abused, the 7-variable clinical prediction rule derived in this study demonstrated sensitivity 0.73 (95% CI: 0.66-0.79) and specificity 0.87 (95% CI: 0.82-0.90). The area under the receiver operating characteristics curve was 0.88 (95% CI: 0.85-0.92). Patient-specific estimates of abusive head trauma probability for 72 observed combinations of its seven predictor variables ranged from 0.04 (95% CI: 0.02-0.08) to 0.98 (95% CI: 0.96-0.99).
Conclusions:
Seven variables facilitate patient-specific estimation of abusive head trauma probability after abuse evaluation in intensive care settings.
Keywords: abusive head trauma, child abuse, clinical prediction rule, prediction tool, non-accidental trauma
INTRODUCTION
Abusive head trauma (AHT) is the leading cause of traumatic death and disability during early childhood, with an estimated incidence of 20 to 30 cases per 100,000 infants and young children per year (Duhaime, Christian, Rorke, & Zimmerman, 1998; Keenan, Runyan, Marshall, Nocera, Merten, & Sinai, 2003; Ellingson, Leventhal, & Weiss, 2008; Minns, Jones, & Mok, 2008; Barlow & Minns, 2000; Eisele, Keglar, Trent, & Coronado, 1999; Jayawant et al., 1998). Along the continuum of their acute clinical care, physicians face three vital decisions that can impact whether or not young victims of AHT are recognized, evaluated, reported, and protected. These decision points include (1) the early decision to obtain or forgo neuroimaging, (2) the subsequent decision to launch or forgo an abuse evaluation if/when neuroimaging confirms trauma, and (3) the decision to reasonably confirm or exclude abuse. Reporting of suspected abuse can occur anywhere along this continuum, and should be accomplished as soon as possible when suspected.
To inform the first of these decisions, Berger and colleagues (2016) derived and validated a clinical decision rule to identify infants at increased risk for brain injury or AHT who might benefit from neuroimaging in the emergency department; and Pierce et al. (2010) derived a clinical decision rule to identify young children with bruising who need further evaluation for abuse. To inform physicians’ second decision along the continuum of AHT acute clinical care, PediBIRN (Pediatric Brain Injury Research Network) investigators derived and validated a 4-variable clinical decision rule (the PediBIRN-4) for application in pediatric intensive care unit (PICU) settings (Hymel et al., 2013, 2014). To inform the third decision, Maguire and colleagues derived and validated their 6-variable PredAHT prediction tool (Maguire, Kemp, Lumb, & Farewell, 2011; Cowley, Morris, Maguire, Farewell, & Kemp, 2015).
This report describes the derivation of a novel clinical prediction rule for pediatric AHT. Like the PredAHT, our objective was to derive a prediction rule that incorporates the (positive or negative) predictive contributions of patients’ completed abuse evaluations, to inform providers’ pending decisions to confirm, exclude, and/or report suspected AHT. Unlike the PredAHT, the prediction rule described in this study was derived using data captured prospectively by investigators applying uniform methods and definitional criteria across 18 sites.
METHODS
Overview.
This was a retrospectively designed, secondary analysis of de-identified, cross-sectional data from the PediBIRN research network with detailed methods described previously (Hymel et al., 2013, 2014). All participating sites obtained approval for the parent studies with waiver of informed consent from their local institutional review board. This secondary analysis was determined to be exempt from review by the Institutional Review Board at Penn State Health Hershey Medical Center.
The PediBIRN dataset.
All of the data used in this analysis were captured prospectively in strictly observational studies conducted between 2010 and 2013 at 18 participating sites (Hymel et al., 2013, 2014). The dataset contains uniform, patient-specific, historical, clinical, and radiological data regarding 500 previously healthy children under three years of age hospitalized for intensive care of acute, cranial or intracranial injuries confirmed on CT or MRI. Victims of motor vehicle collisions were excluded. None were diagnosed with a medical mimic. Prospective study design facilitated complete capture of required data regarding every patient. Because the parent studies were strictly observational, physicians were free to launch or forgo abuse evaluations based on their patient-specific assessments of abuse probability and cost vs. benefit. Thus, some patients did not undergo skeletal survey and/or retinal examination.
Definitional criteria.
Gold standard definitional criteria for AHT and non-AHT do not exist. For this analysis, we applied a priori definitional criteria for AHT, non-AHT, an abnormal skeletal survey, and an abnormal retinal examination (Table 1) that were based on prior, peer-reviewed studies (Hymel et al., 2013, 2014; Duhaime et al, 1992; Kleinman, 1998).
Table 1.
A priori definitional criteria for AHT, non-AHT, an abnormal skeletal survey, and an abnormal retinal exam.
| A patient’s head trauma was classified as abusive IF… |
| • The primary caregivera admitted abusive acts, OR… |
| • Abusive acts by the primary caregivera were witnessed by an unbiased, independent observer, OR… |
| • The primary caregivera specifically denied that the pre-ambulatory infant or young child in his/her care had experienced any head trauma, OR… |
| • The primary caregivera provided an account of the child’s head injury event that was clearly historically inconsistent with repetition over time, OR… |
| • The primary caregivera provided an account of the child’s head injury event that was clearly developmentally inconsistent with the child’s known (or expected) gross motor skills, OR… |
| • Abuse evaluation revealed patterned bruising or dry contact burns, hot water immersion burns, or CT-confirmed intra-abdominal injury. |
| A patient’s head trauma was classified as non-abusive IF… |
| • The child’s head injury event was witnessed by an unbiased, independent observer who described the event as accidental (non-abusive), OR… |
| • The primary caregivera provided an account of the child’s head injury event that was both historically consistent with repetition over time and developmentally consistent with the child’s known (or expected) gross motor skills…AND…abuse evaluation failed to reveal patterned bruising or dry contact burns, hot water immersion burns, or CT-confirmed intra-abdominal injury |
| A patient’s skeletal survey was classified as abnormal IF… |
| • The survey revealed rib fracture(s), classic metaphyseal lesion fracture(s), epiphyseal separation(s), fracture(s) of the scapula or sternum, fracture(s) of digit(s), vertebral body fracture(s) or dislocation(s) OR fracture(s) of spinous process(es) |
| A patient’s retinal examination was classified as abnormal IF… |
| • The exam by an ophthalmologist revealed retinoschisis OR retinal hemorrhages described as dense, extensive, covering a large surface area, and/or extending to the ora serrata |
Abbreviations: AHT=abusive head trauma
The primary caregiver was defined a priori as the person responsible for the child when he/she was acutely head-injured or first became clearly and persistently ill with clinical signs linked to acute traumatic head injuries confirmed on neuroimaging.
Imputation.
Applied before abuse evaluation as an AHT screening tool, the PediBIRN-4’s patient-specific estimates of AHT probability correlate positively and strongly with the results of patients’ subsequent, completed, abuse evaluations (Hymel et al. 2015). Therefore, to impute the results of abuse evaluations never ordered or completed in some patients, we invoked a multiple imputation process via multivariable logistic regression, applying the PediBIRN-4’s four predictor variables to impute the dichotomous (positive vs. negative) results of skeletal surveys and/or retinal exams never ordered or completed across 1,000 imputed datasets (Groenwald, Donders, Roes, Harrell, & Moons, 2012).
Prediction rule derivation.
To achieve our overall objective, the actual/imputed results of skeletal survey and retinal exam were incorporated into the new AHT prediction rule by design. Other candidate predictor variables considered for inclusion included 20 highly discriminating, highly reliable clinical variables previously identified in the dataset (Hymel et al., 2013). To identify an expanded subset of these variables that performed with high predictive accuracy (i.e., area under the receiver operating characteristics curve [ROC-AUC], sensitivity, specificity), we fit a regularized logistic regression with a least absolute shrinkage and selection operator (lasso) penalty (Tibshirani, 1996) across the 1,000 imputed datasets. The frequencies of candidate predictor variables selected across the 1,000 imputed datasets were ranked. Ultimately, an optimal cluster of predictor variables was selected for inclusion in the new AHT prediction tool based on high rank, predictive accuracy, and perceived ease of clinical application.
Estimation of prediction rule performance.
The ROC-AUC of the new AHT clinical prediction rule was calculated based on mean estimates of AHT probability from the 1,000 imputed datasets. Sensitivity, specificity, and predictive values were calculated by applying a probability threshold of >0.5 to classify patients as AHT. To ensure that AHT status was definitive, indeterminate cases that failed to meet definitional criteria for AHT or for non-AHT were excluded in this primary analysis of prediction rule performance. To obtain a best case and worst case performance scenario, we conducted sensitivity analyses reclassifying indeterminate cases as AHT and non-AHT, respectively. We also measured the new prediction rule’s performance against physicians’ final diagnoses of AHT and non-AHT, and completed equivalent sensitivity analyses.
Calculation of patient-specific estimates of AHT probability.
Mean estimates of AHT probability were calculated for every unique combination of the new rule’s predictor variables observed in the dataset, with 95% confidence intervals (CI) obtained applying multiple imputation variance estimators across the 1,000 imputed datasets. Additional information regarding statistical methods is available online (eMethods in the Supplement).
RESULTS
Patient sorting.
Applying a priori definitional criteria (Table 1), 187 (37%) of 500 patients in the dataset were classified as AHT and 269 (54%) as non-AHT. The remaining 44 “indeterminate” patients (9%) met neither criteria.
Imputation.
Three hundred twenty two (64%) of 500 patients underwent both skeletal survey and retinal examination, 69 patients (14%) underwent only one of these two abuse evaluations, and 109 patients (22%) underwent neither. Additional findings of abuse were revealed in 191 (49%) of the 391 patients who underwent one or both evaluations. Across 1,000 datasets, imputation predicted that 37 (mean) of the 178 patients lacking one or both abuse evaluations would have revealed additional findings of abuse, if both had been completed in every patient.
Prediction rule derivation.
Regularized logistic regression with lasso penalty applied to the 1,000 imputed datasets identified multiple clusters of candidate predictor variables that included the actual/imputed results of skeletal survey and retinal exam and performed with high predictive accuracy, as measured against AHT definitional criteria. Cross validation, ranking of selected variables, and serial measurements of predictive performance facilitated selection of an optimal 7-variable cluster that includes the PediBIRN-4’s four predictor variables, the results of skeletal survey and retinal exam, and “any brain hypoxia, ischemia or swelling” (Table 2).
Table 2.
The PediBIRN-7: Seven variables used to estimate AHT probability after abuse evaluation in acutely head-injured children <3 years hospitalized for intensive care.
| • Any clinically-significant respiratory compromise at the scene of injury, during transport, in the Emergency Department, or prior to admission |
| •Any bruising involving the child’s ear(s), neck OR torso |
| • Any subdural hemorrhage(s) or fluid collection(s) that are bilateral OR involve the interhemispheric space |
| • Any skull fracture(s) other than an isolated, unilateral, nondiastatic, linear, parietal skull fracture • Skeletal survey that revealed rib fracture(s), classic metaphyseal lesion fracture(s), epiphyseal separation(s), fracture(s) of the scapula or sternum, fracture(s) of digit(s), vertebral body fracture(s) or dislocation(s), OR fracture(s) of spinous process(es) • Retinal exam by an ophthalmologist that revealed retinoschisis OR retinal hemorrhages described as dense, extensive, covering a large surface area, and/or extending to the ora serrata • Any brain hypoxia, ischemia, OR swelling |
Abbreviations: AHT=abusive head trauma
Estimation of prediction rule performance.
Applying a mean probability threshold of >0.5 to classify patients as AHT, this new 7-variable clinical prediction rule (the PediBIRN-7) demonstrated overall sensitivity 0.73 (95% CI: 0.66-0.79), specificity 0.87 (95% CI: 0.82-0.90), positive predictive value (PPV) 0.79 (95% CI: 0.72-0.85), and negative predictive value (NPV) 0.82 (95% CI: 0.77-0.86). ROC-AUC was 0.88 (95% CI: 0.85-0.92) (Table 3).
Table 3.
Primary analysis of the PediBIRN-7’s predictive performance, applying definitional criteria to classify patients as AHT vs. non-AHT, and excluding all 44 indeterminate cases.
| Applying Definitional Criteria (excluding all 44 indeterminate cases) | |||
|---|---|---|---|
| PediBIRN-7 Estimate of AHT Probability | AHT | Non-AHT | |
| >0.50 | 136 | 36 | |
| ≤0.50 | 51 | 233 | |
| Value (95% CI) | |||
| Sensitivity | 0.73 (0.66-0.79) | ||
| Specificity | 0.87 (0.82-0.90) | ||
| Positive predictive value | 0.79 (0.72-0.85) | ||
| Negative predictive value | 0.82 (0.77-0.86) | ||
Abbreviations: AHT=abusive head trauma. ROC-AUC was 0.88 (95% CI: 0.85-0.92).
Sensitivity analysis revealed that the PediBIRN-7 performed with sensitivity 0.72 (95% CI: 0.66-0.78), specificity 0.87 (95% CI: 0.82-0.90), PPV 0.82 (95% CI: 0.76-0.87), NPV 0.78 (95% CI: 0.73-0.83), and ROC-AUC 0.87 (95% CI: 0.84-0.91) if/when we assumed that all 44 indeterminate cases were victims of AHT (eTable 1, online). If we assumed instead that they were not victims of AHT, the PediBIRN-7 performed with sensitivity 0.73 (95% CI: 0.66-0.79), specificity 0.79 (95% CI: 0.74-0.83), PPV 0.67 (95% CI: 0.60-0.74), NPV 0.83 (0.78-0.87), and ROC-AUC 0.84 (0.80-0.87) (eTable 2, online).
Physicians diagnosed 247 (49%) of 500 patients with AHT, 227 (45%) with non-AHT, and 26 (5%) with indeterminate head trauma (Table 4). Applying physicians’ final diagnoses to classify patients as AHT vs. non-AHT, the PediBIRN-7 performed with sensitivity 0.87 [95% CI: 0.83-0.91], specificity 0.90 [95% CI: 0.85-0.93]), PPV 0.90 [95% CI: 0.86-0.94), and NPV 0.87 (95% CI: 0.82-0.91). ROC-AUC was 0.94 (95% CI: 0.91-0.96) (Table 5). Sensitivity analyses reclassifying physicians’ 26 indeterminate cases as AHT or as non-AHT demonstrated minimal change in rule performance (eTables 3 and 4, online).
Table 4.
Patient classification based on definitional criteria vs. physicians’ final diagnoses.
| Applying Physicians’ Final Diagnoses | ||||
|---|---|---|---|---|
| Applying Definitional Criteria | Abusive Head Trauma | Nonabusive Head Trauma | Indeterminate Head Trauma | TOTALS |
| Abusive Head Trauma | 175 | 7 | 5 | 187 |
| Nonabusive Head Trauma | 35 | 216 | 18 | 269 |
| Indeterminate Head Trauma | 37 | 4 | 3 | 44 |
| TOTALS | 247 | 227 | 26 | 500 |
Table 5.
Primary analysis of the PediBIRN-7’s predictive performance, applying physicians’ final diagnoses to classify patients as AHT vs. non-AHT, and excluding all 26 indeterminate cases.
| Applying Physicians’ Final Diagnoses (excluding all 26 indeterminate cases) | |||
|---|---|---|---|
| PediBIRN-7 Estimate of AHT Probability | AHT | Non-AHT | |
| >0.50 | 216 | 23 | |
| ≤0.50 | 31 | 204 | |
| Value (95% CI) | |||
| Sensitivity | 0.87 (0.83-0.91) | ||
| Specificity | 0.90 (0.85-0.93) | ||
| Positive predictive value | 0.90 (0.86-0.94) | ||
| Negative predictive value | 0.87 (0.82-0.91) | ||
Abbreviations: AHT=abusive head trauma. ROC-AUC was 0.94 (95% CI: 0.91-0.96).
Patient-specific estimates of AHT probability.
Only 72 (56%) of 128 potential combinations of the new prediction rule’s seven predictor variables were observed in the study population of 500 acutely head injured infants and young children. Mean estimates of AHT probability (with 95% CIs) measured across the 1,000 imputed datasets for these 72 observed combinations are presented in Figure 1. These estimates: (1) spanned virtually the entire scale of estimated probability, from 0.04 (95% CI: 0.02-0.08) to 0.98 (95% CI: 0.96-0.99); (2) were ≥0.90 for 17 of 72 combinations; (3) were >0.90 for all patients who manifested bruising of the torso, ear(s) or neck; bilateral or interhemispheric subdural hemorrhages or fluid collections; and an abnormal skeletal survey; and (4) were <0.40 for all patients who presented with ≤1 of the 7 predictor variables. Patient-specific estimates of AHT probability—applying physicians’ final diagnoses rather than definitional criteria to classify patients as AHT vs. non-AHT—are presented in Figure 2. Numerical estimates of AHT probability (with 95% CIs and likelihood ratios) are available online (eTables 5 and 6, online).
Figure 1. Estimates of AHT probability (with 95% CIs) for 72 observed combinations of the PediBIRN-7’ seven predictor variables, applying definitional criteria to classify AHT vs. non-AHT.
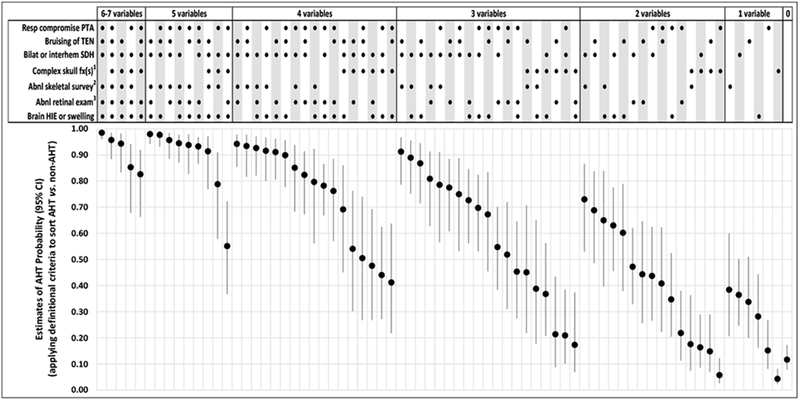
Abbreviations: AHT=abusive head trauma; CI-confidence interval; RESP=respiratory; PediBIRN=pediatric brain injury research network; PTA=prior to admission; TEN=torso, ear(s) or neck; SDH=subdural hemorrhage or fluid collection(s); fx(s)=fracture(s); HIE=hypoxic ischemic encephalopathy
1 Defined a priori as any skull fracture(s) other than an isolated, unilateral, nondiastatic, linear, parietal skull fracture
2 Defined a priori as skeletal survey that reveals rib fracture(s), classic metaphyseal lesion fracture(s), epiphyseal separation(s), fracture(s) of the scapula or sternum, fracture(s) of digit(s), vertebral body fracture(s) or dislocation(s), OR fracture(s) of spinous process(es)
3 Defined a priori as retinal exam by an ophthalmologist that reveals retinoschisis OR retinal hemorrhages described as dense, extensive, covering a large surface area, and/or extending to the ora serrate
[NOTE: Clinicians should not use these probability estimates when applying the PediBIRN-4, as doing so would be to assume that skeletal survey and retinal exam are normal.]
Figure 2. Estimates of AHT probability (with 95% CIs) for 72 observed combinations of the PediBIRN-7’s seven predictor variables, applying physicians’ final diagnoses to classify AHT vs. non-AHT.
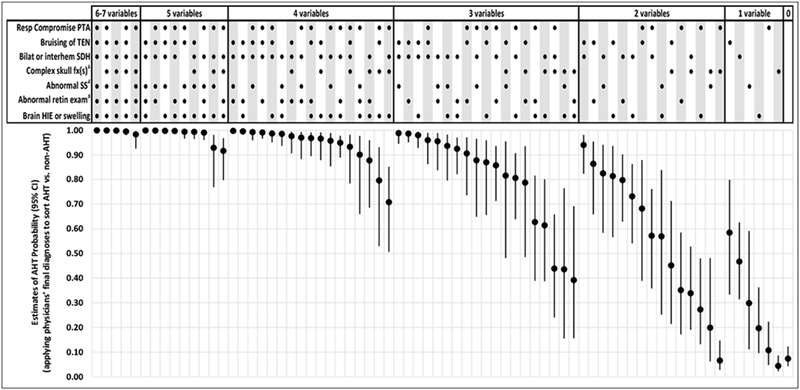
Abbreviations: AHT=abusive head trauma; CI-confidence interval; RESP=respiratory; PediBIRN=pediatric brain injury research network; PTA=priorto admission; TEN=torso, ear(s) or neck; SDH=subdural hemorrhage or fluid collection(s); fx(s)=fracture(s); HIE=hypoxic ischemic encephalopathy
1Defined a priori as any skull fracture(s) other than an isolated, unilateral, nondiastatic, linear, parietal skull fracture
2Defined a priori as skeletal survey that reveals rib fracture(s), classic metaphyseal lesion fracture(s), epiphyseal separation(s), fracture(s) of the scapula or sternum, fracture(s) of digit(s), vertebral body fracture(s) or dislocation(s), OR fracture(s) of spinous process(es)
3Defined a priori as retinal exam by an ophthalmologist that reveals retinoschisis OR retinal hemorrhages described as dense, extensive, covering a large surface area, and/or extending to the ora serrate
[NOTE: Clinicians should not use these probability estimates when applying the PediBIRN-4, as doing so would be to assume that skeletal survey and retinal exam are normal.]
DISCUSSION
As stated so clearly and succinctly by Guyatt and the other members of the original evidence-based medicine (EBM) working group: “In contrast to the traditional paradigm of medical practice, EBM acknowledges that intuition, unsystematic clinical experience, and pathophysiologic rationale are insufficient grounds for clinical decision making, and it stresses the examination of evidence from clinical research”(Guyatt et al., 2002).
Like the PredAHT (Maguire, Kemp, Lumb, & Farewell, 2011), the PediBIRN-7 presents evidence from clinical research that can inform physicians’ vital decisions to confirm, exclude, and/or report suspected AHT. Both clinical prediction rules facilitate patient-specific estimation of abuse probability that incorporates the predictive contributions of completed abuse evaluations, and both take us well beyond “the triad” (Narang, 2011) to reveal specific combinations of clinical findings associated with AHT.
Though similar in purpose, the PediBIRN-7 and PredAHT have numerous differences. They were derived in similar but unequal patient populations, using very different methodologies, applying very different AHT definitional criteria, with dissimilar requirements to impute data, and ultimately incorporating different predictor variables (Hymel et al., 2013; Maguire, Kemp, Lumb, & Farewell, 2011). Unlike the PredAHT, in deriving the PediBIRN-7, outcome variables were defined—and the reliability of candidate predictor variables was confirmed—a priori. In light of such extensive differences, we deemed derivation of the PediBIRN-7 to be both reasonable and necessary rather than duplicative, and predict that the PediBIRN-7 and PredAHT will play complementary roles in informing clinicians’ vital third decision along the continuum of AHT acute clinical care.
The evidence-based, patient-specific, post-evaluation estimates of AHT probability presented in this study (Figures 1 and 2; eTables 5 and 6, online) must be interpreted in the context of other relevant findings and data (e.g., the presenting history, past and family medical history, familial psychosocial risk factors, the results of tests that confirm or exclude medical mimics, and input from outside investigators). In isolation, they should not be considered a sufficient foundation upon which to base expert medical opinion or courtroom testimony.
Within this context, physicians who find reasonable concordance between their own diagnostic impressions and the patient-specific estimates of AHT probability facilitated by the PediBIRN-7 and/or the PredAHT (Maguire, Kemp, Lumb, & Farewell, 2011) will likely feel more confident that their impressions are valid. Physicians who instead find discordance may elect to press for further investigation, and to explore the possibilities of a false positive or negative result, an error in their clinical judgment, AHT masquerading as accidental trauma, and/or their own inexperience or implicit bias.
Only 72 of 128 possible combinations of the PediBIRN-7’s seven predictor variables were observed in the study population (Figures 1 and 2; eTables 5 and 6, online). Very likely, patho-physiological relationships predict that many of the remaining predictor combinations absent from the dataset will not occur clinically (e.g., “any brain hypoxia, ischemia or swelling” is unlikely to occur in the absence of “acute respiratory compromise”). Deeming it speculative, we elected to not report final estimates of AHT probability for specific combinations of the new prediction rule’s seven predictor variables that were not observed at any of our 18 participating sites.
Gold standard definitional criteria for AHT and non-AHT do not exist. In their absence, we elected to classify patients as AHT vs. non-AHT in two different ways: applying definitional criteria (Table 1), and applying physicians’ final diagnoses. Each approach has its advantages (e.g., physicians’ freedom to consider non-medical data, such as assessments of psychosocial risk, witness statements, and the results of scene investigation) and disadvantages (e.g., the potential introduction of circular reasoning and variability associated with differences in physician training, experience, and/or bias).
Across the 72 combinations of its predictor variables observed in the study population, the PediBIRN-7’s estimates of AHT probability based on physicians’ final diagnoses (Figure 2 and eTable 6, online) were generally higher than the equivalent, patient-specific estimates of AHT probability based on definitional criteria (Figure 1 and eTable 5, online). We speculate that the observed differences could represent the positive predictive contributions of non-medical and medical data not captured in our AHT definitional criteria (Table 1), but available to physicians (including the results of skeletal survey and retinal exam). The observed differences might also represent the impact of physicians’ bias and/or circular reasoning.
The strengths of this study and the new PediBIRN-7 prediction tool include the following: (1) the dataset used for analysis contains uniform data captured prospectively across 18 PICU sites; (2) the reliability of 20 candidate predictor variables was previously verified (Hymel et al., 2013); (3) definitional criteria for AHT, non-AHT, and for the results of skeletal survey and retinal exam that would be deemed abnormal, were each defined a priori (Table 1); and (4) the PediBIRN-7 performed well (Tables 3 and 5) applying definitional criteria—or physicians’ final diagnoses—to classify patients as AHT vs. non-AHT.
This secondary analysis has numerous limitations. The patient population was relatively small. Our definitional criteria for AHT, non-AHT, an abnormal skeletal survey, and an abnormal retinal exam are very likely imperfect. Variability in the timing, number, and modalities of neuroimaging may have decreased the accuracy of physicians’ assessments regarding “any brain hypoxia, ischemia or swelling” (Table 2). Because the PediBIRN-7’s (posttest) estimates of AHT probability (Figures 1 and 2, eTables 5 and 6) were based on a (pretest) measurement of AHT prevalence in the study population, they fail to capture or reflect patient-specific modifiers of pretest probability, and may not be generalizable to non-PICU settings or to PICU settings with significantly higher or lower AHT prevalence (pretest likelihood). Finally, the PediBIRN-7’s estimates of AHT probability were calculated through analysis of the same patient cohort used for rule derivation. Thus, until its predictive performance is validated in a novel, equivalent, PICU patient population, the PediBIRN-7 is not yet ready for widespread clinical application.
Future studies are needed to validate the PediBIRN-7’s predictive performance in an independent cohort; to compare its estimates of AHT probability to those facilitated by the PredAHT; and to assess its acceptance, utilization, and impact on physicians’ decisions to confirm, exclude, and/or report suspected AHT.
CONCLUSION
Different combinations of seven clinical variables facilitate patient-specific estimation of AHT probability after abuse evaluation in PICU settings. If validated, application of the PediBIRN-7 as an AHT prediction tool could inform physicians’ pending decisions to confirm, exclude, and/or report suspected AHT.
Supplementary Material
What is known:
Multiple studies have demonstrated disparity and bias in physicians’ decisions to evaluate, diagnose, and/or report suspected child abuse.
What this study adds:
The 7-variable clinical prediction rule derived in this study incorporates the predictive contributions of completed abuse evaluations to facilitate patient-specific estimation of abusive head trauma probability that can inform pending decisions to confirm, exclude, and/or report suspected abuse.
ACKNOWLEDGMENTS
Dr. Hymel conceptualized and directed the overall analysis and interpretation of data, drafted portions of the initial manuscript, and authored revisions of the manuscript to address important intellectual content. Drs. Wang and Chinchilli designed and executed the statistical analyses, interpreted the results of those analyses, and drafted portions of the initial manuscript. Dr. Karst contributed substantially to the overall design of the study and the interpretation of results. Drs. Willson, Dias, Herman, Carroll, Haney and Isaac contributed substantially to data acquisition and to the interpretation of results. All authors critically reviewed the original and revised manuscripts for important intellectual content, approved this revision of the manuscript, and agree to be accountable for all aspects of the work.
The authors would like to acknowledge and thank the remaining PediBIRN investigators who helped to capture the data used in this secondary analysis: Antoinette Laskey, MD, MPH (Primary Children’s Medical Center, Salt Lake City, UT); Robin Foster, MD (The Children’s Hospital of Richmond, Richmond, VA); Veronica Armijo-Garcia, MD and Sandeep K. Narang, MD, JD (University of Texas Health Sciences Center at San Antonio, San Antonio, TX); Deborah A. Pullin, BSN, APRN (Dartmouth-Hitchcock Medical Center, Lebanon, NH); Jeanine M. Graf, MD (Texas Children’s Hospital, Houston, TX); Terra N. Frazier, DO and Kelly S. Tieves, DO, MS (Children’s Mercy Hospital, Kansas City, MO); Edward Truemper, MD (Children’s Hospital of Omaha, Omaha, NE); Kerri Meyer, MD and Lindall E. Smith, MD (Wesley Medical Center, Wichita, KS); Renee A. Higgerson, MD and George A. Edwards, MD (Dell Children’s Medical Center of Central Texas, Austin, TX); Nancy S. Harper, MD, FAAP and Karl L. Serrao, MD, FAAP, FCCM (Driscoll Children’s Hospital, Corpus Christi, TX); Andrew Sirotnak, MD, Joseph Albietz, MD, and Antonia Chiesa, MD (Children’s Hospital Colorado, Denver, CO); Stephen C. Boos, MD and Christine McKiernan, MD (Baystate Medical Center, Springfield, MA); Michael Stoiko, MD, Debra Simms, MD, FAAP, and Sarah J. Brown, DO, FACOP, FAAP (Helen DeVos Children’s Hospital, Grand Rapids, Ml); Amy Ornstein, MD, FRCPC (IWK Health Centre, Halifax, Nova Scotia) AND Phil Hyden, MD (Children’s Hospital of Central California, Madera, CA)
Funding: This work was supported by Dartmouth-Hitchcock Medical Center, a private family foundation, The Gerber Foundation, Penn State University, Penn State Health Milton S. Hershey Medical Center, and the National Institutes of Health [grant number P50HD089922]. These funding organizations had no role in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the manuscript for publication. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None
REFERENCES
- 1.Barlow KM, & Minns RA (2000). Annual incidence of shaken impact syndrome in young children. The Lancet, 356, 1571–1572. [DOI] [PubMed] [Google Scholar]
- 2.Berger RP, Fromkin J, Herman B, Pierce MC, Saladino RA, Flom L, Tyler-Kabara EC, McGinn T, Richichi R, & Kochanek PM (2016). Validation of the Pittsburgh infant brain injury score for abusive head trauma. Pediatrics, 138, e20153756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowley LE, Morris CB, Maguire SA, Farewell DM, & Kemp AM (2015). Validation of a prediction tool for abusive head trauma. Pediatrics, 136, 290–298. [DOI] [PubMed] [Google Scholar]
- 4.Duhaime AC, Alario AJ, Lewander WJ, Schut L, Sutton LN, Seidl TS, Nudelman S, Budenz D, Hertle R, Tsiaras W, & Loporchio S (1992). Head injury in very young children: mechanisms, injury types, and ophthalmologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics, 90, 179–185. [PubMed] [Google Scholar]
- 5.Duhaime AC, Christian CW, Rorke LB, & Zimmerman RA (1998). Nonaccidental head injury in infants--the “shaken-baby syndrome”. New England Journal of Medicine, 338, 1822–1829. [DOI] [PubMed] [Google Scholar]
- 6.Eisele JA, Keglar SR, Trent RB, & Coronado VG (1999). Nonfatal traumatic brain injury-related hospitalization in very young children--15 states. Journal of Head Trauma Rehabilitation, 21, 537–543. [DOI] [PubMed] [Google Scholar]
- 7.Ellingson KD, Leventhal JM, & Weiss HB (2008). Using hospital data to track inflicted traumatic brain injury. American Journal of Preventive Medicine, 34, S157–162. [DOI] [PubMed] [Google Scholar]
- 8.Groenwald RH, Donders AR, Roes KC, Harrell FE Jr., & Moons KG (2012). Dealing with missing outcome data in randomized trials and observational studies. American Journal of Epidemiology, 175, 210–217. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt G, Haynes B, Jaeschke R, Cook D, Greenhalgh T, Meade M, Green L, Naylor CD, Wilson M, McAlister F, & Richardson WS (2002). Introduction: The philosophy of evidence-based medicine In Guyatt G, & Drummond R (Eds.), Users’ guide to the medical literature. A manual for evidence-based practice (p. 4). Chicago, IL: AMA Press. [Google Scholar]
- 10.Hymel KP, Armijo-Garcia V, Foster R, Frazier TN, Stoiko M, Christie LM, Harper NS, Weeks K, Carroll CL, Hyden P, Sirotnak A, Truemper E, Ornstein AE, & Wang M (2014). Validation of a clinical prediction rule for pediatric abusive head trauma. Pediatrics, 134, el537–1544. [DOI] [PubMed] [Google Scholar]
- 11.Hymel KP, Herman BE, Narang SK, Graf JM, Frazier TN, Stoiko M, Christie LM, Harper NS, Carroll CI, Boos SC, Dias M, Pullin DA, & Wang M (2015). Potential impact of a validated screening tool for pediatric abusive head trauma. The Journal of Pediatrics, 167:1375–81. [DOI] [PubMed] [Google Scholar]
- 12.Hymel KP, Willson DF, Boos SC, Pullin DA, Homa K, Lorenz DJ, Herman BE, Graf JM, Isaac R, Armijo-Garcia V, & Narang SK (2013). Derivation of a clinical prediction rule for pediatric abusive head trauma. Pediatric Critical Care Medicine : A Journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies, 14, 210–220. [DOI] [PubMed] [Google Scholar]
- 13.Jayawant S, Rawlinson A, Gibbon F, Price J, Schulte J, Sharpies P, Sibert JR, & Kemp AM (1998). Subdural haemorrhages in infants: population based study. British Medical Journal, 317, 1558–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, & Sinai SH (2003). A population-based study of inflicted traumatic brain injury in young children. Journal of the American Medical Association, 290, 2542–2543. [DOI] [PubMed] [Google Scholar]
- 15.Kleinman PK (1998). Skeletal trauma: general considerations In: Kleinman PK (Ed), Diagnostic imaging of child abuse, 2nd edition (p. 9). St. Louis, MO: Mosby. [Google Scholar]
- 16.Maguire SA, Kemp AM, Lumb RC, & Farewell DM (2011). Estimating the probability of abusive head trauma: a pooled analysis. Pediatrics, 128, e550–e564. [DOI] [PubMed] [Google Scholar]
- 17.Minns RA, Jones PA, & Mok JY (2008). Annual incidence of shaken impact syndrome in young children. American Journal of Preventive Medicine, 34, S126–133. [DOI] [PubMed] [Google Scholar]
- 18.Narang SK (2011). A Daubert analysis of abusive head trauma/shaken baby syndrome. Houston Journal of Health Law and Policy, 11, 505–633. [Google Scholar]
- 19.Pierce MC, Kaczor K, Aldridge S, O’Flynn J, & Lorenz DJ (2010). Bruising characteristics discriminating physical child abuse from accidental trauma. Pediatrics, 125, 67–74. [DOI] [PubMed] [Google Scholar]
- 20.Tibshirani R (1996). Regression shrinkage and selection via the Lasso. Journal of the Royal Statistical Society, Series B (Methodological), 58, 267–288. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


