Abstract
Over the past two decades considerable advances in our understanding of inflammatory and immune pathways have allowed for the growing use of targeted biologic therapy. Most notably, the introduction of tumor necrosis factor (TNF) inhibitors has dramatically changed the management of autoimmune inflammatory disorders, including ankylosing spondylitis (AS). Despite the efficacy of TNF inhibitors documented in multiple clinical trials, anti-TNF therapy in AS is far from foolproof; it is associated with serious adverse effects and limited response to therapy in some patients. Moreover, specific questions regarding the role of TNF as a mediator of AS remain unanswered. Therefore, additional efforts are needed in order to better understand the role of TNF in the pathogenesis of AS and to develop safer and more effective treatment strategies. The purpose of this review is to better the understanding of the physiologic and pathogenic roles of TNF signaling in the course of AS. Relevant TNF biology and novel approaches to TNF blockade in AS are discussed.
Keywords: ankylosing spondylitis, TNFR1, TNFR2, TNF inhibitors, progranulin
Graphical abstract
Tumor necrosis factor (TNF) inhibitors have dramatically improved the management of autoimmune inflammatory disorders such as ankylosing spondylitis (AS). However, anti-TNF therapy in AS can be associated with serious adverse effects and limited response to therapy. This review seeks to improve the understanding of the physiologic and pathogenic roles of TNF signaling in the course of AS. Relevant TNF biology and novel approaches to TNF blockade in AS are discussed.
Introduction
Ankylosing spondylitis
Ankylosing spondylitis (AS) is a progressive rheumatic disease that primarily affects the axial skeleton. Advanced AS is characterized by osteoproliferation leading to irreversible bony fusion of vertebral and sacroiliac joints, with limited spinal mobility and associated pain and loss of function.1 AS belongs to a more encompassing group of chronic inflammatory diseases affecting the spine collectively referred to as spondyloarthritis. In addition to AS, the archetype of the group, spondyloarthropathy encompasses psoriatic arthritis, arthritis/spondylitis associated with inflammatory bowel disease (IBD), reactive arthritis, and undifferentiated spondyloarthritis.2 In recent years, the Assessment of Spondyloarthritis International Society (ASAS) has classified spondyloarthritis as either axial or peripheral depending on the predominant regions of involvement. In line with the ASAS classification, the diagnosis of axial spondyloarthritis encompasses non-radiographic axial spondyloarthrits and classic AS (i.e., radiographic axial spondyloarthrits), based on the absence or presence of radiographic sacroilitis, respectively3, 4
The estimated prevalence of axial spondyloarthritis in the United States is similar to that of rheumatoid arthritis (RA), affecting 0.9 to 1.4% of the adult population.5 Men are estimated to be twice as likely to be affected by AS relative to women, and AS characteristically affects young adults, with a peak age of onset between 20 and 30 years.6 In a subset of patients, extra-articular features of AS, including acute anterior uveitis, psoriasis, and inflammatory bowel disease, manifest following or contemporaneous with onset of AS symptomology.1, 7 Additionally, the existence of one concomitant inflammatory disorder enhances the severity of AS, as well as the probability of an AS patient presenting additional inflammatory co-morbidities.7 These observations suggest potential overlap and synergistic effects of susceptibility genes associated with commonly co-occurring inflammatory conditions.7
AS has a strong genetic component and is associated with human leukocyte antigen-B27 (HLA-B27), a major histocompatibility complex (MHC) class I molecule involved in presentation of antigenic peptides to CD8+ cytotoxic T cells. Upwards of 90% of patients with either nonradiographic axial spondyloarthritis or ankylosing spondylitis are HLA-B27-positive.1, 8 The age-adjusted prevalence of HLA-B27 in unaffected individuals has been reported at 6.1% in the United States; by ethnicity, prevalence reaches 7.5% among Caucasians and 3.5% among all other US ethnicities combined.9 A person who is HLA-B27-positive may have a risk as low as 1.3–5%+ of developing AS1, 8, 10; however, there is a 5–16 fold increase in AS incidence among individuals with an affected first-degree relative.10–12 Importantly, HLA-B27 accounts for approximately 1/3 of the genetic risk for AS.10 More recently, genome-wide association studies have identified a number of non-MHC genes, including those in the IL-23–IL-17 signaling axis, as contributory to AS heritability, further indicating the complex polygenic nature of the disease.13
The pathogenesis of AS is not fully understood, but several mechanisms involving abnormalities in HLA-B27 folding have been hypothesized. Research has demonstrated that IL-17-producing CD4+ T cells may play a pivotal role in initiating the abnormal immune response. CD4+ T cells are hypothesized to interact with HLA-B27 dimers on antigen presenting cells (APCs) and stimulate the production of chemokines and cytokines responsible for the inflammation and structural damage seen in AS. Misfolded HLA-B27 oligomers may also accumulate in the endoplasmic reticulum (ER) of APCs to trigger the ER stress response and the production of IL-23 to potentiate the inflammatory process. Although no arthrogenic peptide has been implicated and the role of CD8+ T cells has not been conclusively demonstrated, peptide presentation by HLA-B27 may also play a role in the pathogenesis of AS.1, 14–16
There is a general consensus that enthesitis, or inflammation at the tendon-cartilage/bone insertion site, is the earliest recognizable sign of AS. However, a number of reports suggest that the disease may actually originate in the gut. Studies have shown that mucosal breach and gut inflammation may serve as additional triggers in AS, highlighting the importance of gut microbiota in disease genesis.17–20 Interestingly, it is also possible that mechanical strain contributes to the pathogenesis of AS. Evidence for this hypothesis comes from a study using transgenic mice overexpressing tumor necrosis factor (TNF), which suggests that mechanical strain drives inflammation and bone formation at entheseal sites.21
TNF
TNF (also known as TNF-α) has long been recognized as a pro-inflammatory cytokine and a master orchestrator of systemic immune responses. TNF is primarily produced by activated macrophages and monocytes in response to injury, extracellular pathogens, and other inflammatory triggers. TNF is produced in excess in disease states and has been implicated as the major mediator of acute and chronic inflammation, tissue destruction, and cachexia.22 The development of anti-TNF agents in the early 2000s has dramatically changed the management of inflammatory diseases such as AS. Multiple clinical trials have demonstrated TNF inhibitors to be dramatically effective in reducing disease activity and debilitating symptoms in patients with AS. Despite the documented efficacy of these agents, the use of TNF inhibitors in AS is limited by several factors. Inhibition of TNF is associated with serious adverse effects, an inadequate response in about 40% of patients, and it is unclear whether anti-TNF therapy can affect radiographic progression of the disease.23
Herein, we review relevant TNF biology, including its distinct receptors, signaling outcomes, as well as pathogenic and physiologic roles. We also discuss the role of TNF throughout the course of AS progression, provide an update on the use of TNF inhibitors in AS, and explore a selective approach to TNF blockade in the treatment of AS.
TNF Receptors and TNF Signaling Pathways
TNF is a homotrimeric cytokine that is initially expressed as a transmembrane protein. TNF may be released from its transmembrane conformation into a soluble structure via proteolytic cleavage by TNF-α-converting enzyme (TACE). Both soluble and transmembrane TNF are biologically active and exert their functions via two distinct receptors: TNF receptor 1 (TNFR1) and TNFR2. Notably, TNFR1 is activated by both soluble and transmembrane TNF, whereas TNFR2 is primarily activated by transmembrane TNF.24 TNFR1 is expressed constitutively on most cell types and functions primarily in inflammatory and innate immune responses, while TNFR2 is inducible, mainly expressed by immune, neuronal, and endothelial cells, and functions to mediate homeostatic and regulatory effects.25 A brief comparison between TNFR1 and TNFR2 is outlined in Table 1.
Table 1.
Comparison of TNFR1 and TNFR2
| TNFR1 | TNFR2 |
|---|---|
|
|
The binding of TNF to TNFR1 results in the recruitment of adaptor protein TNFR1-associated death domain (TRADD), receptor-interacting serine/threonine-protein kinase 1 (RIPK1), and TNRF-associated factor 2 (TRAF2), leading to the formation of complex I. This in turn triggers several phosphorylation and ubiquitination events leading to the activation of mitogen-activated kinase (MAPK) and nuclear factor κB (NFκB), the central mediator of the pro-inflammatory effects of TNF. Signaling via complex I causes upregulation of genes involved in inflammation, tissue degeneration, host defense against pathogens, and cell survival and proliferation.26 Notably, TRAF protein degradation regulates TNF-mediated NFκB activation.27 Recent studies have demonstrated that E3 ubiquitin ligase, carboxyl terminus of HSC70-interacting protein (CHIP)/STIP1 homology and U-Box containing protein 1 (STUB1) induces degradation of multiple TRAF proteins and regulates NFκB signaling.28, 29 CHIP knockout (KO) in vitro leads to increased osteoclast formation coincident with reduced osteoblast differentiation and function; CHIP KO mice exhibit an osteopenic phenotype resulting from increased TRAF activity and consequent osteoclastogenic activity.28, 29 Taken together, these studies suggest that CHIP may play an important role in TNF signaling.
An alternative signaling outcome following TNFR1 stimulation involves formation of cytoplasmic complex II, characterized by the binding of Fas-associated death domain (FADD) to TRADD and RIPK1, with downstream activation of caspase-8 and a signaling cascade culminating in programmed cell death. Under certain cellular conditions, the extent of which is largely unknown, complex II may also activate necroptosis via a mixed lineage kinase domain-like protein (MLKL)/RIPK3-dependent mechanism.26 TNF-induced necroptosis results in local inflammation that is central to the pathogenesis of a variety of disease states and is the focus of active research. Our knowledge concerning signaling through TNFR2 is a little more obscure, mostly due to its limited distribution compared to TNFR1. It has been shown that TNFR2 can recruit TRAF2 and activate NFκB, MAPKs, and AKT to promote transcription of genes involved in tissue regeneration, host defense against pathogens, and cell survival26. Unlike TNFR1, TNFR2 cannot recruit TRADD to induce apoptosis directly because it lacks a death domain. However, TNFR2 was shown to induce apoptosis indirectly in various cell types such as T cells and myeloid cells, demonstrating its ability as an immunoregulator.30, 31
Opposing roles of TNFRs
The intracellular domains of TNFR1 and TNFR2 are dissimilar and mediate distinct functional outcomes. TNF’s central role in host defense is largely mediated through TNFR1. TNFR1 was shown to be importantly involved in lymphatic organogenesis and immune responses to various microbial pathogens, including innate defenses against extracellular bacteria and the formation and maintenance of granulomas 25. TNFR1 has also been implicated in mediating TNF’s deleterious effects in autoimmunity and autoinflammation. For instance, research shows that TNFR1 knockout mice also exhibit significantly reduced susceptibility to CIA relative to wild type cohorts, indicating a critical role of TNFR1 signaling in initiation of the disease.32 Moreover, the absence of TNFR1 on hematopoetic cells markedly attenuated inflammation and bone destruction in a mouse model of erosive arthritis.33 TNFR1 has also been implicated in models of TNF-induced cardiomyopathy and IBD.34, 35 Taken together, the evidence reveals that TNFR1 is crucial to the innate and adaptive immune responses and that excessive TNFR1 signaling is a central culprit in inflammatory disease states.
In contrast to signaling through TNFR1, which when excessively stimulated is overwhelmingly deleterious, signaling through TNFR2 is mostly beneficial. TNFR2 has been shown to exert anti-inflammatory, anabolic, and protective effects in models of inflammatory arthritis, inflammatory bowel disease, central neurodegenerative disease, and inflammatory cardiomyopathy.34–37 The anti-inflammatory effects of TNFR2 partly stem from its ability to activate and expand T regulatory cells important in self-tolerance and suppression of inflammatory pathways. TNFR2-induced apoptosis of autoreactive T cells may also augment the anti-inflammatory role of this receptor, but the exact mechanism has not been fully elucidated and is likely complex. TNFR2 may also play a role in clearing of viral pathogens.38
TNF signaling in AS
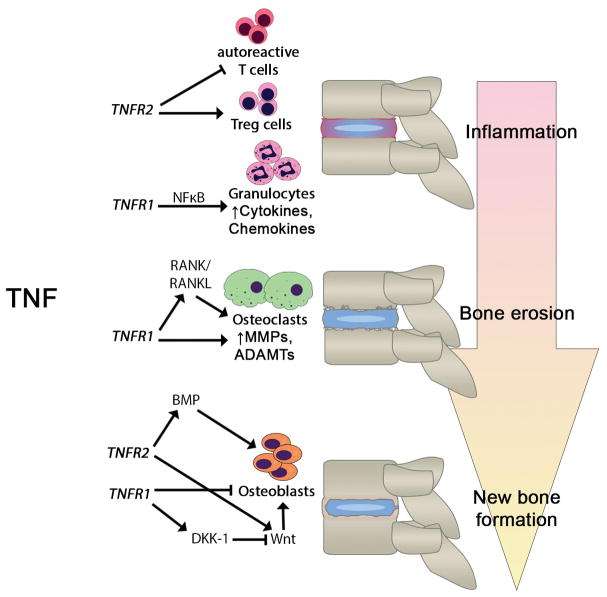
The pathological processes leading to structural joint changes seen in AS can be divided into three phases: (1) inflammation, (2) bone erosion, and (3) new bone formation.39 Effector pathways in each of the three phases may be linked to the pro-inflammatory cytokine TNF (Fig. 1).
Figure 1.
The role of TNF in the progression of ankylosing spondylitis. This figure illustrates the opposing roles of two TNF receptors, TNFR1 and TNFR2, in inflammation, bone erosion, and new bone formation in ankylosing spondylitis. In general, TNFR1 is pro-inflammatory and mediates tissue catabolism, whereas TNFR2 is anti-inflammatory and mediates tissue anabolism. The pathways and molecular mediators involved are indicated. Abbreviations: ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; BMP, bone morphogenetic protein; DKK-1, dickkopf-related protein-1; MMP, matrix metalloproteinase; NFκB, nuclear factor kappa-B, RANK/RANKL, receptor activator of nuclear factor kappa-B/RANK ligand; TNFR, tumor necrosis factor receptor.
Inflammation
Early stages of AS are dominated by a strong inflammatory process characterized by back pain, joint swelling, morning stiffness, and fatigue. TNF has previously been reported to be abundant in the sacroiliac joints of patients with AS, and it is believed to be the main cytokine contributing to the early disease.40 The pathogenic role of TNF at this stage of disease is further supported by the dramatic reduction in disease and symptom severity in AS patients treated with TNF inhibitors. TNFR1 is known to be the primary receptor conveying the pro-inflammatory effects of TNF, but the stimulus for TNF induction and its target cells are largely unknown.15 Furthermore, a considerable number of patients do not enter remission when treated with TNF inhibitors, suggesting that additional cytokines may play a pathogenic role.41
Bone erosion
In contrast to RA, bone erosion in AS is relatively mild and is not a defining feature of the pathology. The catabolic process seen at this stage of the disease is a direct consequence of local and systemic inflammation and can be largely attributed to TNF’s well-documented ability in stimulating osteoclastogenesis. Early hematopoietic progenitors differentiate into mature bone-resorbing osteoclasts under the influence of macrophage-colony stimulating factor (M-CSF) and receptor-activator of nuclear factor kappa-B ligand (RANKL). In the presence of RANKL, TNF synergistically enhances osteoclastogenesis to promote the local bone erosion observed in AS. 42 In addition, TNF was shown to induce the expression of extracellular digestive enzymes such as matrix metalloproteinases (MMPs) and aggrecanases (ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs)).43 These enzymes can degrade collagen, aggrecan, and other cartilage and intervertebral disc components and contribute to the destructive process.
TNFR1 is the main receptor that mediates these catabolic effects. Activation of NFκB via complex I was shown to be critically important for osteoclastogenesis and regulation of the expression of MMP1 and MMP344. Additionally, TNFR1 further contributes to the structural damage seen at this stage of the disease via complex II. Complex II mediates tissue destruction by inducing apoptosis and necroptosis, the latter of which results in plasma membrane rupture and leakage of cellular contents into the extracellular space45. TNF-induced necroptosis propagates local inflammation that further contributes to the bone, intervertebral disc, and cartilage damage seen in AS.
Inflammatory bone loss may also be attributable to TNF-mediated destabilization of the master osteoblast transcription factor, runt-related gene 2 (RUNX2), and bone morphogenetic signaling proteins, including mothers against decapentaplegic homolog 1 (SMAD1), through upregulation of HECT-domain E3 ligases SMAD ubiquitin regulatory factor (SMURF)1 and SMURF2.46 TNF increases SMURF1 expression in osteoblast cell lines47 and SMURF1 expression is elevated in long bones from TNF transgenic mice (TNF-tg), concomitant with reductions in RUNX2 and SMAD1 at the protein level.48 Accordingly, TNF may limit bone formation in inflammatory pathologies through enhancing SMURF E3 ligase-mediated proteasomal degradation of key modulators of osteoblast differentiation and activity.
New bone formation
The hallmark feature of AS is the formation of new bone, which initially involves the entheses and later progresses to bridge whole joints, leading to ankylosis. The role of TNF in new bone formation is not entirely clear, although evidence indicates that TNF may have a mixed effect on osteoblastogenesis. In the classical sense, TNF is a known inhibitor of osteoblast differentiation. This effect has been attributed to TNF’s ability to suppress the expression of insulin-like growth factor-1 (IGF-1), osterix (also known as SP7), and RUNX2 and was shown to be mediated through TNFR1.46, 49–51 The resulting decrease in osteoblastogenesis, coupled with TNF’s ability to induce osteoclastogenesis, promotes a pattern of bone erosion and joint destruction that is characteristic of RA. This pattern of joint pathology is not seen in AS, where the initial inflammatory insult is followed by some degree of new bone formation.
Recent contradictory findings suggest that TNF may paradoxically stimulate osteoblastogenesis and thereby contribute to pathological bone formation seen in AS. Studies using human and rodent mesenchymal stem cells (MSCs) show that TNF contributes to osteogenic differentiation through the activation of NFκB, with downstream induction of bone morphogenic proteins (BMPs), osterix, RUNX2, osteocalcin (OCN), and alkaline phosphatase (AP).52–55 The opposing effects of TNF on osteoblastogenesis appear to depend on the concentration, exposure time, and the differentiation state of the responding cells.43 These finding are in sharp contrast to the classical interpretation of the effects of TNF on osteogenic differentiation and may contribute to anabolic joint changes in AS.
TNF can also affect bone formation through the wingless (WNT)/β-catenin pathway, a regulatory pathway of bone homeostasis that governs osteoblast differentiation. The interaction between TNF and the WNT/β-catenin signaling pathway is not well-established; however, emerging reports show that a complex interplay may exist. It has previously been shown that TNF upregulates dickkopf-1 (DKK-1), a natural inhibitor of WNT signaling, in patients with RA, effectively suppressing signaling via this pathway.56 In contrast to RA, WNT signaling may be abnormally enhanced in AS, particularly in its late stages. In line with this hypothesis, lower functional levels of DKK-1 were reported in sera of AS patients who developed syndesmophytes compared to those who did not demonstrate syndesmophyte development on follow up radiographs.57 Using human MSCs, TNF was shown to increase the levels of WNT5a and promote tissue non-specific AP-mediated mineralization in an autocrine manner.58 Furthermore, a recent study analyzing gene transcriptional profiles in patients with AS shows that genes in the WNT signaling pathway were differentially expressed following therapy with the TNF inhibitor adalimumab.59
Activation of WNT signaling facilitates nuclear translocation of β-catenin and activation of target genes driving osteoblast formation and bone remolding. Immunohistochemical analyses of disc tissue obtained from spondyloarthropathy patients reveal elevated nuclear β-catenin expression.60 Moreover, transgenic mice with conditional activation of β-catenin in cartilage display a progressive phenotype similar to that of AS, including extensive osteophyte formation, vertebral fusion, as well as defects in growth plate and disc structure.60 Several reports have demonstrated IL-1β- and TNF-mediated stimulation of β-catenin signaling in various cell types, including those of the intervertebral disc.61–63 In turn, TNF and WNT/β-catenin signaling have been demonstrated to form a positive-feedback loop in nucleus pulposus cells in vitro, as activation of β-catenin signaling leads to up-regulation of TNF expression in disc cells.62. Taken together, these reports suggest that that activation of TNF inflammatory cascade may directly or indirectly induce WNT/β-catenin signaling and contribute to pathogenic bone formation seen in phase three of AS.
TNF inhibitors in treatment of AS
The major goals of AS treatment strategies are to minimize inflammation, its associated symptoms, and to inhibit new bone formation (the pharmacological treatment options for AS are summarized in Table 2). Nonsteroidal anti-inflammatory drugs (NSAIDs), including selective inhibitors of cyclooxygenase 2, are the mainstay of treatment. For patients with symptoms insufficiently responsive to NSAID therapy or for whom NSAIDs cause intolerable side effects, the use of TNF inhibitors is indicated. The TNF inhibitors currently approved for use in AS include infliximab, entanercept, adalimumab, certolizumab, and golimumab. These agents were shown to be equally efficacious in AS; however, some are preferred when extra articular manifestations such as uveitis accompany axial disease.
Table 2.
Pharmacologic treatment options for ankylosing spondylitis
| NSAIDs (including selective COX-2 inhibitors) |
|
| Analgesics |
|
| Glucocorticoids |
|
| DMARDs |
|
| TNF inhibitors |
|
| Secukinumab |
|
NSAID, nonsteroidal anti-inflammatory drugs; COX-2, cyclooxygenase-2; DMARDs, disease modifying anti-rheumatic drugs
The use of TNF inhibitors in AS and other TNF-mediated diseases has several limitations, the most important being low rates of disease remission and the development of serious adverse effects necessitating the issue of a black box warning for these drugs. The harmful effects of TNF inhibitors include an increased risk of opportunistic infections such as disseminated fungal infections and reactivation of latent tuberculosis. The use of TNF inhibitors is also associated with an increased risk of malignancies, and paradoxically, the development of additional autoimmune disorders.25
The effect of TNF inhibitors on radiographic progression of AS remains a matter of controversy. Extensions of pivotal randomized clinical trials (RCT) failed to show an inhibitory effect of adalimumab, infliximab, and entanercept on spinal structural changes over a period of two years.64–66 However, accumulating evidence from retrospective analyses of well-characterized cohorts shows that TNF inhibitors are associated with a reduction in spinal radiographic progression, an effect attributed to their ability to suppress disease activity.67–69 The need for an extended prospective RCT against placebo that could prove causality cannot be overstated; however, such studies would be impossible to conduct due to ethical considerations. The working hypothesis is that current TNF inhibitors may be marginally effective in reducing radiographic progression in AS, especially when initiated early and administered over a long time period.
Selective TNF blockade
The lack of TNFR specificity of current TNF inhibitors may limit their effectiveness and potential as disease-modifying drugs. Nonspecific TNFR inhibition may also mechanistically contribute to some of the adverse effects seen with current TNF inhibitors, especially the development of autoimmune disorders, as TNFR2 has been shown to be involved in T cell function and survival. The next generation of TNF inhibitors should ideally inhibit the effects of TNFR1 while preserving those of TNFR2. This inhibition strategy is conceptually superior to global TNF inhibition because it selectively targets the pro-inflammatory pathogenic pathway and leaves essential signals for tissue homeostasis and immunocompetence intact. Indeed, a number of drugs have undertaken this approach and a number of selective TNFR1 antagonists37, 70–73, selective TNFR2 agonists74, and mixed TNFR1 antagonists and TNFR2 agonists are currently in development (Table 3).
Table 3.
Next generation of TNF drugs
| Class | Mechanism | Agent | Description | Reference |
|---|---|---|---|---|
| Selective TNFR1 antagonists | TNFR1-specific antibody | ATROSAB | IgG1 against specific TNFR1 epitope | 56 |
| TROS | Trivalent nanobody comprising two anti-human TNFR1 domains linked with an anti-albumin domain | 57 | ||
| MDS5541 | Fusion antibody composed of an anti-TNFR1 domain and an albumin-specific domain | 58 | ||
| TNF mutein | XENP345 and Pro1595 | Dominant-negative TNF muteins that bind soluble TNF to form inactive heteromers | 29 | |
| R1antTNF | TNFR1-antagonistic TNF mutein that also has dominant negative function | 59 | ||
| Selective TNFR2 agonists | TNF mutein | STAR2 | Three linker-connected mouse TNF (D221N-A223R) promoters fused to chicken TNC trimerization domain | 60 |
| Mixed TNFR1 antagonists & TNFR2 agonists | Endogenous protein | PGRN | A growth factor comprising of 7 ½ repeats of cysteine-rich motifs that binds to TNFR1 and TNFR2 to exert anti-inflammatory effects | 28 |
| Engineered peptide | Atsttrin | A molecule composed of three TNFR2-binding domains of PGRN; shows better anti-inflammatory activity than PGRN | 28 |
ATROSAB, antagonistic TNF receptor one-specific antibody; Atsttrin, antagonist of TNF/TNFR signaling via targeting to TNF receptors; PRGN; progranulin; TORS, TNF receptor-one silencer; R1antTNF, TNF receptor 1 antagonist; STAR2, selective mouse TNF-based agonist of TNF receptor 2; TNC, tenascin C
A novel treatment strategy that may prove effective in the treatment of autoimmune diseases may utilize the homeostatic effects mediated by TNFR2. Previous studies have shown TNFR2 to be importantly involved in immune modulation and defects that disturb TNF-TNFR2 signaling have been identified in autoreactive T-cells in models of autoimmune diseases, including AS.75, 76 Autoreactive T cells appear sensitive to the apoptotic effects of TNF and the administration of exogenous TNF may be effective in suppressing their pathogenic functions. Interestingly, exogenous TNF was shown to reverse autoimmune diabetes mellitus in mice by targeting and killing autoreactive T cells.77 This approach however is only experimental and is not intended for humans due to likely systemic toxicity resulting from widespread TNFR1 stimulation. Selective TNFR2 agonism has emerged as a more viable therapeutic strategy. Notably, several studies have now established the safety and efficacy of this approach, through promoting apoptosis of autoreactive T cells in autoimmune animal models.
Notably, the endogenous growth factor progranulin (PGRN) was identified as a natural ligand of TNFRs in 2011.36, 78, 79 This ubiquitous molecule has multiple functions, including significant roles in cell proliferation, wound healing, and regulation of immune responses.80–90 Interestingly, PGRN functions as a physiological antagonist of TNFR1 and a potent agonist of TNFR2 (with approximately 600-fold higher binding affinity to TNFR2 than TNF). PGRN and Atsttrin, an engineered peptide consisting of the domains and adjacent linker regions of PGRN responsible for interacting with TNFRs, appear to have strong anti-inflammatory properties and were shown to mediate protective and anabolic effects in mouse models of osteoarthritis and inflammatory arthritis.36, 91 Moreover, PGRN was shown to have protective and therapeutic effects in additional inflammatory disease models, including IBD, psoriasis, diabetes mellitus, SLE, and others.92 A very recent publication has also demonstrated the ability of Atsttrin to inhibit TNF-mediated catabolism in a murine ex vivo model of intervertebral disc degeneration, supporting the protective effect of Atsttrin in TNF-driven inflammatory conditions impacting the axial skeleton.93 These observations suggest that PGRN might ameliorate inflammatory disease states by simultaneously blocking the pro-inflammatory effects of TNFR1 and activating protective signaling mediated by TNFR2. This unique pharmacodynamic property has made PGRN a promising drug for TNF-mediated diseases. More excitingly, the development of Atsttrin, as a non-oncogenic PGRN-derived small engineered molecule, offers an exciting early candidate for evaluation of the therapeutic potential of selective TNFR modulation in treatment of inflammatory pathologies, including AS.
Selective targeting of TNFRs may prove to be the optimal therapeutic strategy for AS. Early inflammatory and catabolic stages of AS have clearly benefited from inhibition of the pro-inflammatory effects mediated by TNFR1. It is unclear, however, whether boosting TNFR2 signal would confer a benefit to patients with AS. On one hand, TNFR2-mediated suppression of autoreactive T cells and expansion of Treg cells may prove effective in controlling autoimmunity and the inflammatory process in AS. On the other hand, downstream effects of TNFR2 may worsen the late anabolic process of the disease. This potentially harmful effect of TNFR2 was indicated in transgenic mice overexpressing transmembrane TNF, a primary ligand of TNFR2, which developed axial and peripheral joint inflammation and new bone formation typical of spondyloarthritis.94, 95 Interestingly, these mice did not develop systemic inflammation, additional evidence that TNFR1, and not TNFR2, is the principal disease mediator of AS. This model strongly argues for a deleterious role of TNFR2 in the late stages of AS; however, human data describing this phenomenon are lacking. Additional efforts are necessary in order to elucidate the molecular mechanism of transmembrane TNF and TNFR2 in the pathogenesis of AS.
Conclusion
Therapies targeting TNF have proven to be a huge success and a scientific breakthrough in the treatment of TNF-mediated diseases such as AS. However, our knowledge regarding the role of TNF throughout the course of AS is incomplete. Evidence strongly implicates TNF and its dominant pro-inflammatory receptor TNFR1 to be the central mediators of early inflammation and catabolic joint changes. Notably, however, the specific role of TNF in new bone formation is not clear. Future studies are needed in order to elucidate the molecular pathways leading to the structural joint changes in AS. Additionally, our efforts should focus on the specific roles of TNFR2 in inflammatory disease states and new treatment strategies targeting TNF should facilitate the beneficial signaling mediated by this receptor.
Acknowledgments
We apologize to the colleagues whose publications are not included due to the space limitation. This work was supported by NIH research grants R01AR062207, R01AR061484, 1R01NS103931, and a DOD research grant W81XWH-16-1-0482.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Taurog JD, Chhabra A, Colbert RA. Ankylosing Spondylitis and Axial Spondyloarthritis. The New England journal of medicine. 2016;374:2563–2574. doi: 10.1056/NEJMra1406182. [DOI] [PubMed] [Google Scholar]
- 2.Baeten D, Breban M, Lories R, et al. Are spondylarthritides related but distinct conditions or a single disease with a heterogeneous phenotype? Arthritis and rheumatism. 2013;65:12–20. doi: 10.1002/art.37829. [DOI] [PubMed] [Google Scholar]
- 3.Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Annals of the rheumatic diseases. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 4.Rudwaleit M, van der Heijde D, Landewe R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Annals of the rheumatic diseases. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 5.Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis care & research. 2012;64:905–910. doi: 10.1002/acr.21621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet (London, England) 2017;390:73–84. doi: 10.1016/S0140-6736(16)31591-4. [DOI] [PubMed] [Google Scholar]
- 7.Brophy S, Pavy S, Lewis P, et al. Inflammatory eye, skin, and bowel disease in spondyloarthritis: genetic, phenotypic, and environmental factors. The Journal of Rheumatology. 2001;28:2667–2673. [PubMed] [Google Scholar]
- 8.Reveille JD. The genetic basis of spondyloarthritis. Current rheumatology reports. 2004;6:117–125. doi: 10.1007/s11926-004-0056-6. [DOI] [PubMed] [Google Scholar]
- 9.Reveille JD, Hirsch R, Dillon CF, et al. The Prevalence of HLA–B27 in the US: Data From the US National Health and Nutrition Examination Survey, 2009. Arthritis and rheumatism. 2012;64:1407–1411. doi: 10.1002/art.33503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ABM, Gail KL, MAJ, et al. Susceptibility to ankylosing spondylitis in twins the role of genes, HLA, and the environment. Arthritis & Rheumatism. 1997;40:1823–1828. doi: 10.1002/art.1780401015. [DOI] [PubMed] [Google Scholar]
- 11.van der Linden SM, Valkenburg HA, de Jongh BM, et al. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. A comparison of relatives of spondylitis patients with the general population. Arthritis and rheumatism. 1984;27:241–249. doi: 10.1002/art.1780270301. [DOI] [PubMed] [Google Scholar]
- 12.Baron M, Zendel I. HLA-B27 testing in ankylosing spondylitis: An analysis of the pretesting assumptions. 1989 [PubMed] [Google Scholar]
- 13.Cortes A, Hadler J, Pointon JP, et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nature genetics. 2013;45:730–738. doi: 10.1038/ng.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranganathan V, Gracey E, Brown MA, et al. Pathogenesis of ankylosing spondylitis - recent advances and future directions. Nature reviews Rheumatology. 2017;13:359–367. doi: 10.1038/nrrheum.2017.56. [DOI] [PubMed] [Google Scholar]
- 15.Tam LS, Gu J, Yu D. Pathogenesis of ankylosing spondylitis. Nature Reviews Rheumatology. 2010;6:399. doi: 10.1038/nrrheum.2010.79. [DOI] [PubMed] [Google Scholar]
- 16.Sieper J, Braun J, Dougados M, et al. Axial spondyloarthritis. Nature reviews Disease primers. 2015;1:15013. doi: 10.1038/nrdp.2015.13. [DOI] [PubMed] [Google Scholar]
- 17.Breban M, Tap J, Leboime A, et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Annals of the rheumatic diseases. 2017;76:1614–1622. doi: 10.1136/annrheumdis-2016-211064. [DOI] [PubMed] [Google Scholar]
- 18.Rehakova Z, Capkova J, Stepankova R, et al. Germ-free mice do not develop ankylosing enthesopathy, a spontaneous joint disease. Human immunology. 2000;61:555–558. doi: 10.1016/s0198-8859(00)00122-1. [DOI] [PubMed] [Google Scholar]
- 19.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. The Journal of experimental medicine. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Praet L, Van den Bosch F, Mielants H, et al. Mucosal inflammation in spondylarthritides: past, present, and future. Current rheumatology reports. 2011;13:409–415. doi: 10.1007/s11926-011-0198-2. [DOI] [PubMed] [Google Scholar]
- 21.Jacques P, Lambrecht S, Verheugen E, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Annals of the rheumatic diseases. 2014;73:437–445. doi: 10.1136/annrheumdis-2013-203643. [DOI] [PubMed] [Google Scholar]
- 22.Chu WM. Tumor necrosis factor. Cancer letters. 2013;328:222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callhoff J, Sieper J, Weiss A, et al. Efficacy of TNFalpha blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a meta-analysis. Annals of the rheumatic diseases. 2015;74:1241–1248. doi: 10.1136/annrheumdis-2014-205322. [DOI] [PubMed] [Google Scholar]
- 24.MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cellular signalling. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- 25.Van Hauwermeiren F, Vandenbroucke RE, Libert C. Treatment of TNF mediated diseases by selective inhibition of soluble TNF or TNFR1. Cytokine & growth factor reviews. 2011;22:311–319. doi: 10.1016/j.cytogfr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nature reviews Rheumatology. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funakoshi-Tago M, Kamada N, Shimizu T, et al. TRAF6 negatively regulates TNFα-induced NF-κB activation. Cytokine. 2009;45:72–79. doi: 10.1016/j.cyto.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Li S, Shu B, Zhang Y, et al. CHIP Regulates Osteoclast Formation through Promoting TRAF6 Protein Degradation. Arthritis & rheumatology (Hoboken, NJ) 2014;66:1854–1863. doi: 10.1002/art.38521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T, Li S, Yi D, et al. CHIP regulates bone mass by targeting multiple TRAF family members in bone marrow stromal cells. Bone Research. 2018;6:10. doi: 10.1038/s41413-018-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin RH, Hwang YW, Yang BC, et al. TNF receptor-2-triggered apoptosis is associated with the down-regulation of Bcl-xL on activated T cells and can be prevented by CD28 costimulation. Journal of immunology (Baltimore, Md : 1950) 1997;158:598–603. [PubMed] [Google Scholar]
- 31.Chan FK, Lenardo MJ. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. European journal of immunology. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 32.Mori L, Iselin S, de Libero G, Lesslauer W. Attenuation of collagen-induced arthritis in 55-kDa TNF receptor type 1 (TNFR1)-IgG1-treated and TNFR1-deficient mice. Journal of immunology. 1996;157:3178–3182. [PubMed] [Google Scholar]
- 33.Bluml S, Binder NB, Niederreiter B, et al. Antiinflammatory effects of tumor necrosis factor on hematopoietic cells in a murine model of erosive arthritis. Arthritis and rheumatism. 2010;62:1608–1619. doi: 10.1002/art.27399. [DOI] [PubMed] [Google Scholar]
- 34.Higuchi Y, McTiernan CF, Frye CB, et al. Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation. 2004;109:1892–1897. doi: 10.1161/01.CIR.0000124227.00670.AB. [DOI] [PubMed] [Google Scholar]
- 35.Dube PE, Punit S, Polk DB. Redeeming an old foe: protective as well as pathophysiological roles for tumor necrosis factor in inflammatory bowel disease. American journal of physiology Gastrointestinal and liver physiology. 2015;308:G161–170. doi: 10.1152/ajpgi.00142.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science (New York, NY) 2011;332:478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. Journal of neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen DX, Ehrenstein MR. Anti-TNF drives regulatory T cell expansion by paradoxically promoting membrane TNF-TNF-RII binding in rheumatoid arthritis. The Journal of experimental medicine. 2016;213:1241–1253. doi: 10.1084/jem.20151255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magrey MN, Khan MA. The Paradox of Bone Formation and Bone Loss in Ankylosing Spondylitis: Evolving New Concepts of Bone Formation and Future Trends in Management. Current rheumatology reports. 2017;19:17. doi: 10.1007/s11926-017-0644-x. [DOI] [PubMed] [Google Scholar]
- 40.Francois RJ, Neure L, Sieper J, et al. Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor alpha in two patients with early disease and transforming growth factor beta in three more advanced cases. Annals of the rheumatic diseases. 2006;65:713–720. doi: 10.1136/ard.2005.037465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLeod C, Bagust A, Boland A, et al. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health technology assessment (Winchester, England) 2007;11:1–158. iii–iv. doi: 10.3310/hta11280. [DOI] [PubMed] [Google Scholar]
- 42.Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. doi: 10.1016/s0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- 43.Osta B, Benedetti G, Miossec P. Classical and Paradoxical Effects of TNF-alpha on Bone Homeostasis. Frontiers in immunology. 2014;5:48. doi: 10.3389/fimmu.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn SJ, Rhim EM, Kim JY, et al. Tumor necrosis factor-alpha induces matrix metalloproteinases-3, -10, and -13 in human periodontal ligament cells. Journal of periodontology. 2014;85:490–497. doi: 10.1902/jop.2013.130063. [DOI] [PubMed] [Google Scholar]
- 45.Linkermann A, Green DR. Necroptosis. The New England journal of medicine. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert L, He X, Farmer P, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. The Journal of biological chemistry. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 47.Kaneki H, Guo R, Chen D, et al. Tumor Necrosis Factor Promotes Runx2 Degradation through Up-regulation of Smurf1 and Smurf2 in Osteoblasts. Journal of Biological Chemistry. 2006;281:4326–4333. doi: 10.1074/jbc.M509430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo R, Yamashita M, Zhang Q, et al. Ubiquitin Ligase Smurf1 Mediates Tumor Necrosis Factor-induced Systemic Bone Loss by Promoting Proteasomal Degradation of Bone Morphogenetic Signaling Proteins. Journal of Biological Chemistry. 2008;283:23084–23092. doi: 10.1074/jbc.M709848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilbert L, He X, Farmer P, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert LC, Rubin J, Nanes MS. The p55 TNF receptor mediates TNF inhibition of osteoblast differentiation independently of apoptosis. American journal of physiology Endocrinology and metabolism. 2005;288:E1011–1018. doi: 10.1152/ajpendo.00534.2004. [DOI] [PubMed] [Google Scholar]
- 51.Abbas S, Zhang YH, Clohisy JC, et al. Tumor necrosis factor-alpha inhibits pre-osteoblast differentiation through its type-1 receptor. Cytokine. 2003;22:33–41. doi: 10.1016/s1043-4666(03)00106-6. [DOI] [PubMed] [Google Scholar]
- 52.Hess K, Ushmorov A, Fiedler J, et al. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45:367–376. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- 53.Yu RY, Zeng BJ, Liu YS, et al. Recombinant human tumor necrosis factor-alpha promotes human adipose-derived stromal cells transforming into osteoblast in vitro. Beijing da xue xue bao Yi xue ban = Journal of Peking University Health sciences. 2012;44:475–480. [PubMed] [Google Scholar]
- 54.Lu Z, Wang G, Dunstan CR, et al. Short-term exposure to tumor necrosis factor-alpha enables human osteoblasts to direct adipose tissue-derived mesenchymal stem cells into osteogenic differentiation. Stem cells and development. 2012;21:2420–2429. doi: 10.1089/scd.2011.0589. [DOI] [PubMed] [Google Scholar]
- 55.Cho HH, Shin KK, Kim YJ, et al. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. Journal of cellular physiology. 2010;223:168–177. doi: 10.1002/jcp.22024. [DOI] [PubMed] [Google Scholar]
- 56.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nature medicine. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 57.Heiland GR, Appel H, Poddubnyy D, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Annals of the rheumatic diseases. 2012;71:572–574. doi: 10.1136/annrheumdis-2011-200216. [DOI] [PubMed] [Google Scholar]
- 58.Briolay A, Lencel P, Bessueille L, et al. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-alpha in human mesenchymal stem cells. Biochemical and biophysical research communications. 2013;430:1072–1077. doi: 10.1016/j.bbrc.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 59.Dolcino M, Tinazzi E, Pelosi A, et al. Gene Expression Analysis before and after Treatment with Adalimumab in Patients with Ankylosing Spondylitis Identifies Molecular Pathways Associated with Response to Therapy. Genes. 2017:8. doi: 10.3390/genes8040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meina W, Dezhi T, Bing S, et al. Conditional activation of β-catenin signaling in mice leads to severe defects in intervertebral disc tissue. Arthritis & Rheumatism. 2012;64:2611–2623. doi: 10.1002/art.34469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jianxi W, Huajiang C, Peng C, et al. Inflammatory cytokines induce caveolin-1/β-catenin signalling in rat nucleus pulposus cell apoptosis through the p38 MAPK pathway. Cell Proliferation. 2016;49:362–372. doi: 10.1111/cpr.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiyama A, Yokoyama K, Nukaga T, et al. A complex interaction between Wnt signaling and TNF-alpha in nucleus pulposus cells. Arthritis research & therapy. 2013;15:R189. doi: 10.1186/ar4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wanqing X, Lijiang Z, Shan L, et al. Wnt/β-catenin signaling plays a key role in the development of spondyloarthritis. Annals of the New York Academy of Sciences. 2016;1364:25–31. doi: 10.1111/nyas.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Heijde D, Landewe R, Baraliakos X, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis and rheumatism. 2008;58:3063–3070. doi: 10.1002/art.23901. [DOI] [PubMed] [Google Scholar]
- 65.van der Heijde D, Landewe R, Einstein S, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis and rheumatism. 2008;58:1324–1331. doi: 10.1002/art.23471. [DOI] [PubMed] [Google Scholar]
- 66.van der Heijde D, Salonen D, Weissman BN, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis research & therapy. 2009;11:R127. doi: 10.1186/ar2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molnar C, Scherer A, Baraliakos X, et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Annals of the rheumatic diseases. 2018;77:63–69. doi: 10.1136/annrheumdis-2017-211544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maas F, Arends S, Brouwer E, et al. Reduction in Spinal Radiographic Progression in Ankylosing Spondylitis Patients Receiving Prolonged Treatment With Tumor Necrosis Factor Inhibitors. Arthritis care & research. 2017;69:1011–1019. doi: 10.1002/acr.23097. [DOI] [PubMed] [Google Scholar]
- 69.Baraliakos X, Haibel H, Listing J, et al. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Annals of the rheumatic diseases. 2014;73:710–715. doi: 10.1136/annrheumdis-2012-202698. [DOI] [PubMed] [Google Scholar]
- 70.Kontermann RE, Munkel S, Neumeyer J, et al. A humanized tumor necrosis factor receptor 1 (TNFR1)-specific antagonistic antibody for selective inhibition of tumor necrosis factor (TNF) action. Journal of immunotherapy (Hagerstown, Md : 1997) 2008;31:225–234. doi: 10.1097/CJI.0b013e31816a88f9. [DOI] [PubMed] [Google Scholar]
- 71.Steeland S, Puimege L, Vandenbroucke RE, et al. Generation and characterization of small single domain antibodies inhibiting human tumor necrosis factor receptor 1. The Journal of biological chemistry. 2015;290:4022–4037. doi: 10.1074/jbc.M114.617787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt EM, Davies M, Mistry P, et al. Selective Blockade of Tumor Necrosis Factor Receptor I Inhibits Proinflammatory Cytokine and Chemokine Production in Human Rheumatoid Arthritis Synovial Membrane Cell Cultures. Arthritis & Rheumatism. 2013;65:2262–2273. doi: 10.1002/art.38055. [DOI] [PubMed] [Google Scholar]
- 73.Shibata H, Yoshioka Y, Abe Y, et al. The treatment of established murine collagen-induced arthritis with a TNFR1-selective antagonistic mutant TNF. Biomaterials. 2009;30:6638–6647. doi: 10.1016/j.biomaterials.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 74.Chopra M, Biehl M, Steinfatt T, et al. Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion. The Journal of experimental medicine. 2016 doi: 10.1084/jem.20151563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nature reviews Drug discovery. 2010;9:482–493. doi: 10.1038/nrd3030. [DOI] [PubMed] [Google Scholar]
- 76.Faustman DL, Davis M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Frontiers in immunology. 2013;4:478. doi: 10.3389/fimmu.2013.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryu S, Kodama S, Ryu K, et al. Reversal of established autoimmune diabetes by restoration of endogenous beta cell function. The Journal of clinical investigation. 2001;108:63–72. doi: 10.1172/JCI12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu CJ. Progranulin: a promising therapeutic target for rheumatoid arthritis. FEBS Lett. 2011;585:3675–3680. doi: 10.1016/j.febslet.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu CJ, Bosch X. Progranulin: A growth factor, a novel TNFR ligand and a drug target. Pharmacology & therapeutics. 2012;133:124–132. doi: 10.1016/j.pharmthera.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jian J, Konopka J, Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. Journal of leukocyte biology. 2013;93:199–208. doi: 10.1189/jlb.0812429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu W, Hu W, Shi L, et al. Foxo4- and Stat3-dependent IL-10 production by progranulin in regulatory T cells restrains inflammatory arthritis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2017;31:1354–1367. doi: 10.1096/fj.201601134R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jian J, Li G, Hettinghouse A, et al. Progranulin: A key player in autoimmune diseases. Cytokine. 2016 doi: 10.1016/j.cyto.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jian J, Tian QY, Hettinghouse A, et al. Progranulin Recruits HSP70 to beta-Glucocerebrosidase and Is Therapeutic Against Gaucher Disease. EBioMedicine. 2016;13:212–224. doi: 10.1016/j.ebiom.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jian J, Zhao S, Tian Q, et al. Progranulin directly binds to the CRD2 and CRD3 of TNFR extracellular domains. FEBS Lett. 2013;587:3428–3436. doi: 10.1016/j.febslet.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jian J, Zhao S, Tian QY, et al. Association Between Progranulin and Gaucher Disease. EBioMedicine. 2016;11:127–137. doi: 10.1016/j.ebiom.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams A, Wang EC, Thurner L, et al. Novel insights into TNF receptor, DR3 and progranulin pathways in arthritis and bone remodeling. Arthritis & rheumatology (Hoboken, NJ) 2016 doi: 10.1002/art.39816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao YP, Liu B, Tian QY, et al. Progranulin protects against osteoarthritis through interacting with TNF-alpha and beta-Catenin signalling. Annals of the rheumatic diseases. 2015;74:2244–2253. doi: 10.1136/annrheumdis-2014-205779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao YP, Tian QY, Frenkel S, et al. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials. 2013;34:6412–6421. doi: 10.1016/j.biomaterials.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao YP, Tian QY, Liu B, et al. Progranulin knockout accelerates intervertebral disc degeneration in aging mice. Scientific reports. 2015;5:9102. doi: 10.1038/srep09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao YP, Tian QY, Liu CJ. Progranulin deficiency exaggerates, whereas progranulin-derived Atsttrin attenuates, severity of dermatitis in mice. FEBS Lett. 2013;587:1805–1810. doi: 10.1016/j.febslet.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei J-l, Fu W, Ding Y-j, et al. Progranulin derivative Atsttrin protects against early osteoarthritis in mouse and rat models. Arthritis research & therapy. 2017;19:280. doi: 10.1186/s13075-017-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jian J, Li G, Hettinghouse A, et al. Progranulin: A key player in autoimmune diseases. Cytokine. 2018;101:48–55. doi: 10.1016/j.cyto.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ding H, Wei J, Zhao Y, et al. Progranulin derived engineered protein Atsttrin suppresses TNF-alpha-mediated inflammation in intervertebral disc degenerative disease. Oncotarget. 2017;8:109692–109702. doi: 10.18632/oncotarget.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duivenvoorde LMv, Ambarus CA, Masdar H, et al. A2.15 Relative Overexpression of Transmembrane Versus Soluble TNF in Human and Experimental Spondyloarthritis. Annals of the rheumatic diseases. 2013;72:A9–A10. [Google Scholar]
- 95.Edwards CK, 3rd, Bendele AM, Reznikov LI, et al. Soluble human p55 and p75 tumor necrosis factor receptors reverse spontaneous arthritis in transgenic mice expressing transmembrane tumor necrosis factor alpha. Arthritis and rheumatism. 2006;54:2872–2885. doi: 10.1002/art.22077. [DOI] [PubMed] [Google Scholar]